1. Introduction
Understanding the wetting behavior of liquefied metal on solid surfaces of other metallic substrates is essential; for example, for laser brazing processes as well as for the thermal joining of dissimilar materials like aluminum and galvanized steel. Especially in case of a novel approach called laser keyhole brazing, overheated braze material must wet substrate surfaces which are not heated directly by the laser irradiation; see [
1].
The process efficiency of conventional laser beam brazing is limited due to the absorption efficiency. The absorption of laser energy in case of solid state laser sources like rod, fiber, or disk lasers by solid aluminum material is—depending on the surface topography and temperature—in the range of 4–8% (e.g., [
2]). In laser welding, the amount of absorbed energy can be increased up to 93% by changing the welding mode from heat conduction welding to deep penetration welding with keyhole (vapor capillary) formation, as shown for example in [
3]. The absorption of laser energy in keyhole welding depends, among other parameters, significantly on the keyhole shape (depth, diameter, and curvature). In turn, the keyhole formation and its shape depend on laser process parameters like focal diameter, focal position, power density distribution, and process velocity. The approach of keyhole formation known from laser deep penetration welding has been transferred to laser brazing by Radel et al. to increase the amount of absorbed laser energy [
1]. In doing so, full penetration of the wire (and thus melting of the base material) had to be prevented by limiting the penetration depth into the filler wire. The keyhole depth was affected by increasing the actual keyhole velocity by beam oscillation. In contrast to conventional laser brazing, the base material must not be irradiated directly by the laser beam to prevent melting due to the high laser power density in case of the keyhole utilization. Thus, the heating of the substrate to the working temperature—needed for suitable wetting and spreading—had to be realized by heat conduction from the overheated filler material in keyhole brazing. Hence, the wetting behavior of overheated filler material on non-heated substrates is one of the main processes for this novel approach of highly efficient laser keyhole brazing. Radel et al. showed for bead-on-plate brazing experiments with aluminum filler wire and galvanized (zinc-coated) steel sheets that laser keyhole brazing is principally possible and that the approach offers high potential for increasing the process efficiency in laser brazing [
1].
The joining of dissimilar materials like aluminum and steel by laser beam processes provides a huge potential to realize hybrid lightweight constructions in automotive and naval industries and in the case of aluminum-titanium joints also in the aerospace industry, as shown by Walther et al. [
4] and Kocik et al. [
5], respectively. Contrary to mechanical techniques such as riveting or clinching, thermal joining processes guarantee a direct material connection; see the overview by Martinsen et al. [
6]. The challenge in the thermal joining of aluminum and steel (for example, with brazing or welding) is the poor solubility of Al and Fe in solid state, which results in the growth of intermetallic compounds (see e.g., [
7]) during the joining processes. Intermetallic compounds are known to be brittle. Hence, excessive melting and mixing of both metals with a conventional welding process was found to be infeasible to produce joints with acceptable mechanical properties. However, the concept of melting only the joining partner with the lower melting temperature (here aluminum) and allowing it to wet the solid surface of the joining partner with the higher melting temperature (here steel) has become a promising approach. In this case, the joint is achieved by a short-time wetting process which is comparable to a continuous brazing process. The mechanical bonding is primarily generated by thermal-induced diffusion. The advantage of this approach is a comparatively low thermal impact on the fusion zone. Thus, the growth of intermetallic compounds can be reduced to a comparatively thin interfacial layer. For example, Achar et al. demonstrated that an intermetallic compound layer thickness of less than 10 µm is suitable to guarantee good joint strength for the industrial manufacturing of aluminum-steel joints [
8].
The precondition to realize keyhole brazing or such dissimilar joints is the ability of the liquefied metal to spread on the solid surface of the joining partner, meaning that the relation of surface tensions according to Young’s formula must be fulfilled. In the case of aluminum and steel, this condition is generally fulfilled, but the natural oxide layer—especially on the aluminum surface (even in liquid state)—inhibits a proper spreading in industrial environments [
9]. A possible way to overcome this issue is to apply flux to the fusion zone in order to dissociate oxides, modify the surface tension, and protect the fusion zone from re-oxidation. Most of these fluxes are toxic and corrosive, and must be applied to the fusion zone prior to joining and removed afterwards. These drawbacks limit the acceptance of the described joining technique for industrial applications. Moeller et al. reported an alternative method by using a laser-plasma-hybrid process that allows the removal of the oxide layer by cathodic cleaning and the wetting without any additional flux [
10].
It is also well known that zinc coatings on steel surfaces can improve the wetting of aluminum melts, even without flux, as Peyre et al. showed in [
11]. In fact, the beneficial effect of zinc coatings on steel surfaces has been observed for several different liquid metals, including copper [
12], magnesium [
13], or nickel [
14]. A mutual observation in each of these cases is an accumulation of zinc that appears near the outer boundary of the weld after solidification. According to Agudo et al. [
7], this phenomenon is caused by the fact that the zinc is liquefied and pushed to the side. However, little is known about the fluid dynamics taking place inside the melt once it hits the galvanized surface and starts to spread and mix up with the liquefied zinc. Thomy and Vollertsen demonstrated the beneficial effect of zinc coatings on a continuous laser-MIG (metal inert gas) joining process in terms of process stability and resulting seam properties [
15]. In [
16], the results of a series of single-droplet experiments are reported by Gatzen et al., showing the effect of short-time spreading and solidification of significantly over-heated AlSi12 droplets on different zinc-coated steel substrates. Gatzen et al. [
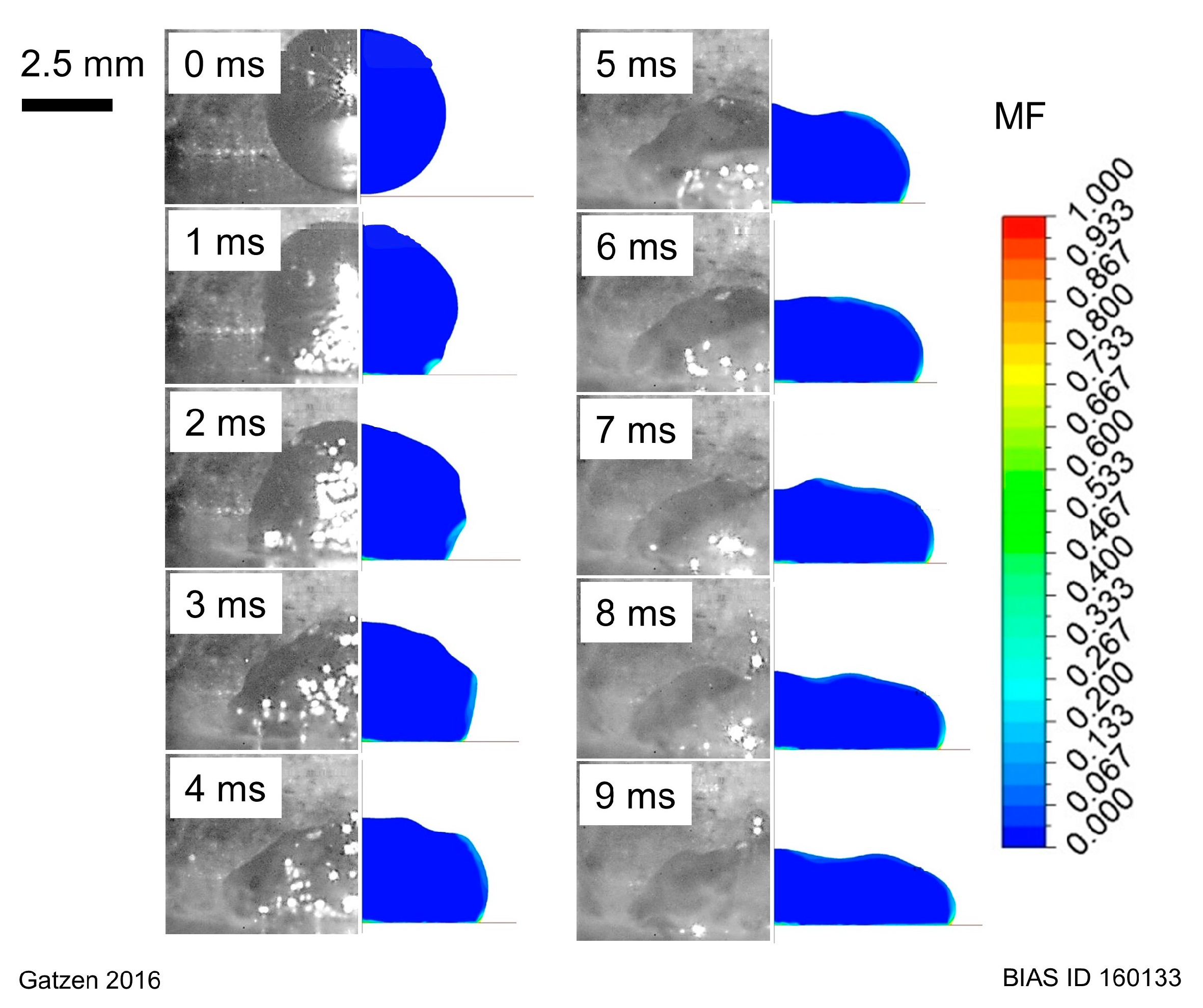
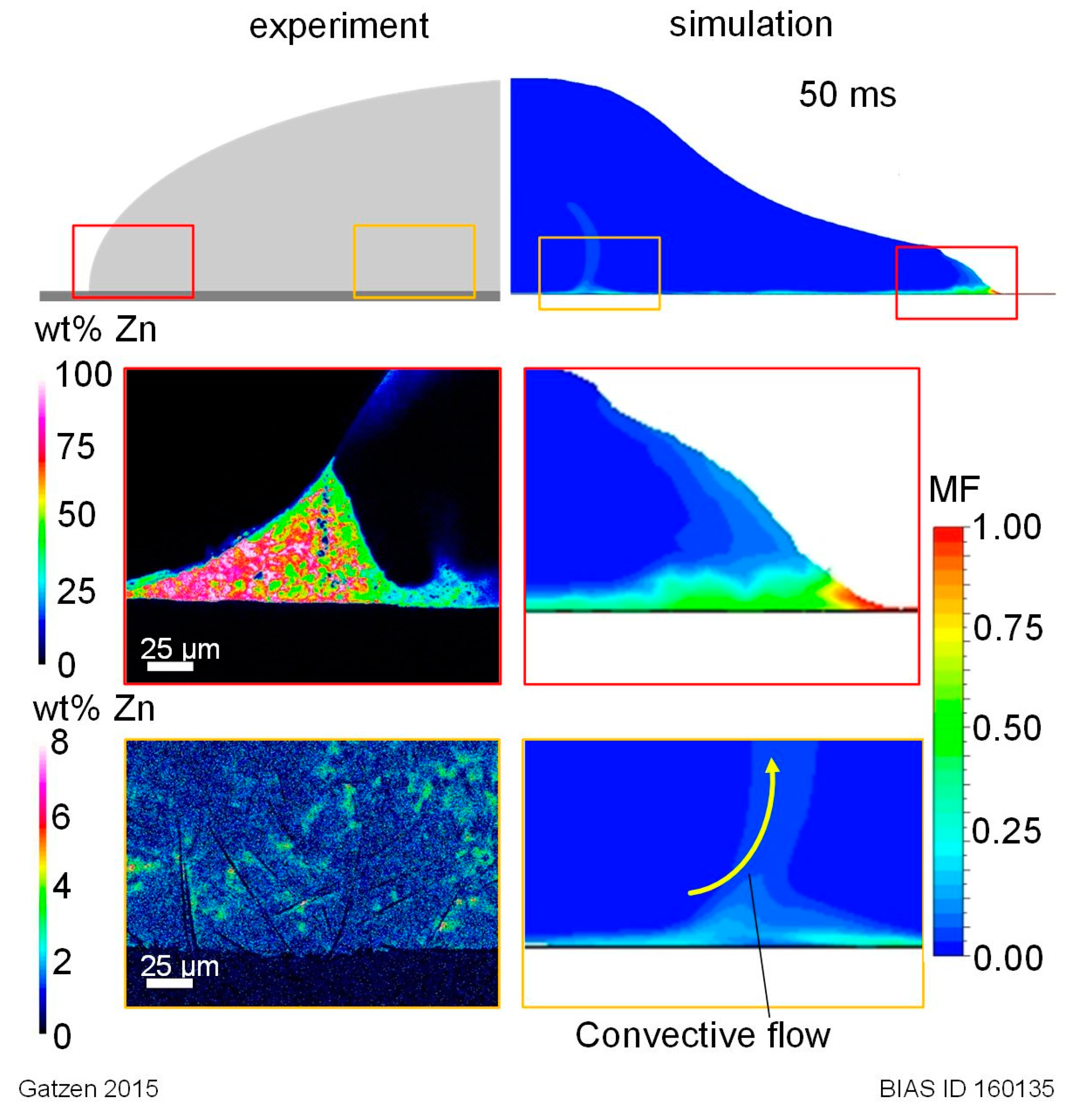
16] demonstrated that the coating type (electro- or hot-dip galvanized) does not affect the general wettability of zinc coatings, and suggested that the interaction between zinc and aluminum is originated by a fluid dynamic rather than by the classical surface tension relation described by Young’s formula. An accumulation of zinc along the toe of the solidified droplet was explained with fluid dynamic effect suggested by external observations of the droplets behavior only, and was substantiated by the findings of other authors.
In order to investigate the internal fluid dynamic behavior inside an individual aluminum droplet impinging and spreading on a zinc-coated steel surface and to further substantiate the experimental conclusions of [
16], a two-dimensional fluid-dynamic numerical model is applied in this study. The case of an overheated aluminum droplet spreading on a DX56 + Z140 galvanized steel substrate at room temperature (
TS = 23 °C) is observed experimentally and compared to the corresponding simulation results.
2. Experimental Method and Simulation Model
To investigate the spreading process of individual aluminum droplets on commercial galvanized steel substrates, a model experiment has been conducted that provides nearly isolated thermal and dynamic boundary conditions for the generation of liquid droplets. The results of a complete experimental series of single-droplet experiments were reported in [
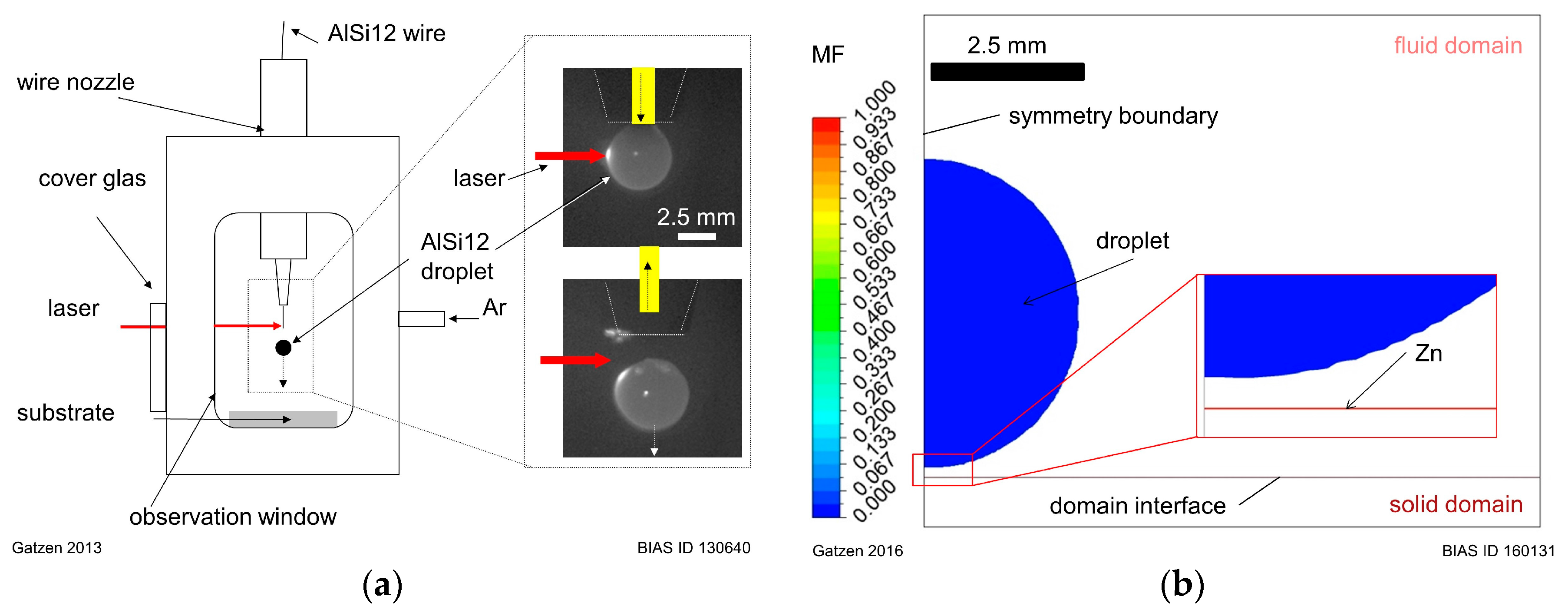
16]. The experimental model system (BIAS GmbH, Bremen, Germany) was designed to investigate short period spreading and solidification characteristics that are typical for laser brazing and dissimilar material joining processes. It consists of a process chamber that is filled with argon (see
Figure 1a). To produce liquid droplets, an AlSi12 aluminum wire was constantly fed vertically from the top of the chamber through a wire nozzle, and was horizontally irradiated through a cover glass by a laser beam (TruDisk8002, Trumpf GmbH + Co. KG, Ditzingen, Germany). The defocused laser beam melted the tip of the extending wire, forming a liquid droplet of aluminum. Once the droplet was formed and significantly overheated, it was detached by an abrupt pull-back of the remaining solid wire. Subsequently, the liquid droplet fell from a height of 22 mm down onto a zinc-coated substrate. The initial substrate temperature was kept at room temperature (
TS = 23 °C). The spreading process was observed by a high-speed camera (Phantom V5.1, Vision Research Inc., Wayne, NJ, USA) through the observation window in order to track the transient droplet deformation and melt propagation until complete solidification. A defocused two-color pyrometer-spot (IGAR-12 LO, IMPAC Infrared GmbH, Frankfurt, Germany) was also aligned on the substrate surface onto the region of impingement to measure the droplet temperature before its contact with the zinc-coated surface and until solidification. The maximum measured value was defined as the impingement temperature
Ts,im of the droplet.
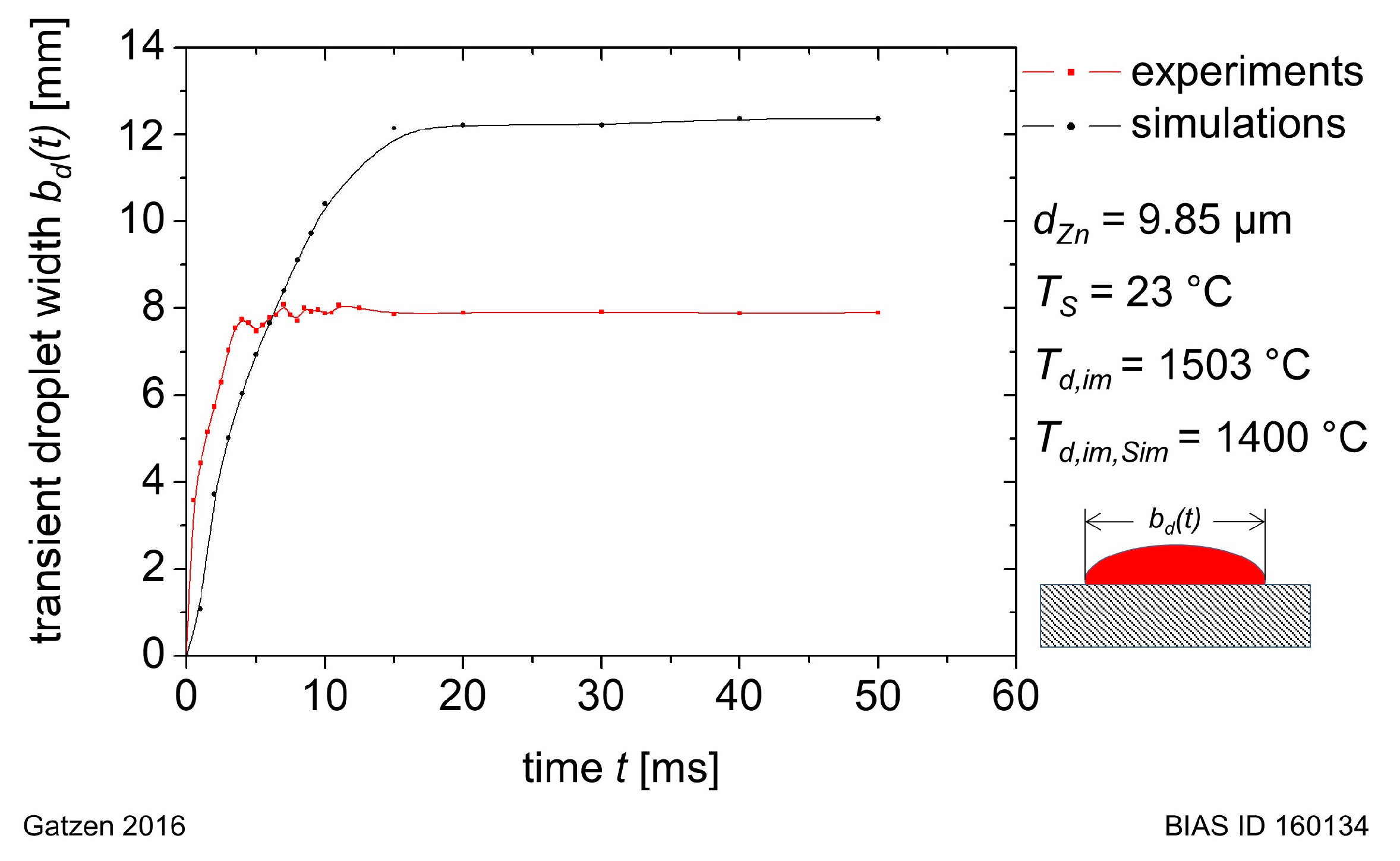
In this study, an aluminum droplet of 110 mg weight was generated and observed while spreading on a hot-dip galvanized steel sheet (DX56 + Z140) that was initially at room temperature.
In addition to the experimental observation, a numerical CFD-model (computational fluid dynamics) was designed to calculate the time-dependent fluid-dynamics of the liquid droplet on the zinc-coated substrate. To set up the model and simulate the spreading and solidification process including several different liquid and solid materials, the commercial CFD Toolbox ANSYS CFX
® (Ansys Inc., Canonsburg, PA, USA) was used. The software uses the finite-volume method to solve the conservation equations for momentum:
energy
and mass
The values are, respectively: the velocity field, the static enthalpy, the temperature field, the pressure, the density, the dynamic viscosity, and the thermal conductivity. describes an additional volume force to model solidification.
Using the symmetries of the problem, a 2D geometry was designed as shown in
Figure 1b. The model geometry represents a fluid and a solid domain, in which the fluid domain contains the liquid aluminum droplet, ambient gas, and the zinc coating which is initially in solid state but is molten during contact with the aluminum droplet. The solid domain contains the steel substrate that remains solid during the whole process, contributing only heat conduction in the energy equation. It is located under the liquid domain and is considered to be in full thermal contact.
In order to reduce the number of phases in the fluid domain to one gaseous and one liquid phase, aluminum and zinc are considered as a single fluid mixture that consists of two components. The composition of the mixture is calculated by an additional conservation equation by defining a mass fraction (MF) as scalar field with values between 0 and 1. A value of MF = 0 determines the liquid phase to be aluminum, while a value of MF = 1 is used to set the fluid properties to those of zinc. Since aluminum and zinc are fully mixable in liquid and solid state, the liquid phase was defined as a binary mixture. Hence, a linear dependency for the intermediate values of dynamic viscosity, density, and thermal conductivity on the MF is considered.
To simulate the free surface between liquid and gaseous phase, the so-called volume fraction field (VF) was calculated by an additional conservation equation. The VF is used to track the free interface and to apply surface tension forces to it.
The volume force
was added to the momentum equation to simulate solidification of the liquid phase by following the relation:
where
is the so-called liquid fraction that depends on the liquidus temperature
and the solidus temperature
of the mixture:
is a negative constant of high value that allows the volume force to dominate the momentum equation, reducing the velocity to zero once the maximum force is reached. A modified heat capacity was used to consider latent heat during solidification and melting:
is the original unmodified heat capacity and is the latent heat of melting.
Figure 1b also shows the initial conditions for the
VF and the
MF. The liquid phase with a
VF = 1 is represented by the colored areas in the domain. The liquid phase is divided in a droplet geometry placed about 0.2 mm above the surface and a small 9.85 µm-thick layer directly on the bottom surface of the fluid domain. The color of the areas represents the local
MF. For the droplet geometry, the
MF was set to zero, representing the liquid aluminum, and to 1 for the zinc. The layer thickness is comparable to the thickness of a Z140 zinc coating. The initial velocity of the droplet phase was set to a value of about 0.57 m/s to consider the gravitational acceleration of the droplet during its fall from of a height of 22 mm. The initial temperature of the droplet was set to 1400 °C, while the temperature of the zinc layer, gas and the solid domain was set to a value of 23 °C.
Instead of adhesive forces [
17], free slip boundary conditions were used at the interface between liquid and solid domains.
Table 1 gives the material and model parameters for the calculation.
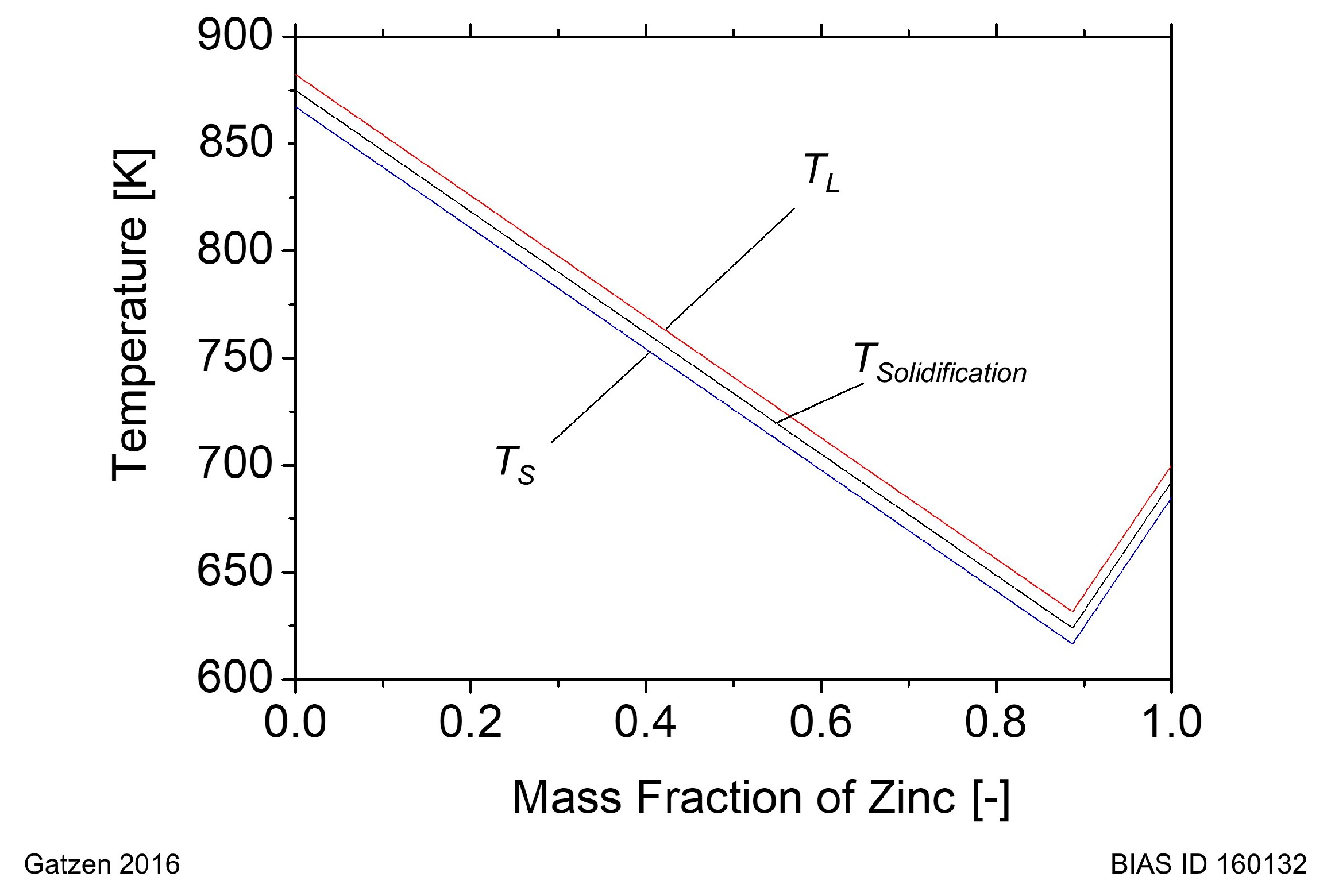
The liquidus temperature
and the solidus temperature
are determined according to the binary system Al-Zn. For simplification, both temperatures are considered as an offset value of the temperature
. They are shown in
Figure 2 in dependence of the
MF of zinc.
For this study, the model was used to simulate a liquid aluminum droplet spreading on a zinc layer which is initially at room temperature but molten during contact with the overheated melt. From the results of the transient simulation, the time-dependent droplet evolution (especially the wetting length) and the zinc distribution inside the melt until its solidification were analyzed.