Extraction of Palladium(II) from Hydrochloric Acid Solutions by Solvent Extraction with Mixtures Containing Either Cyanex 301 or LIX 63
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Chemicals
2.2. Solvent Extraction Procedure
3. Results and Discussion
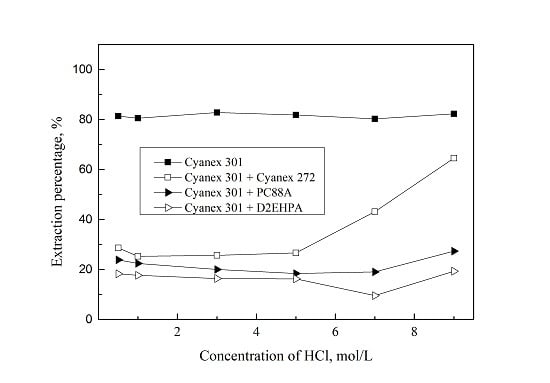
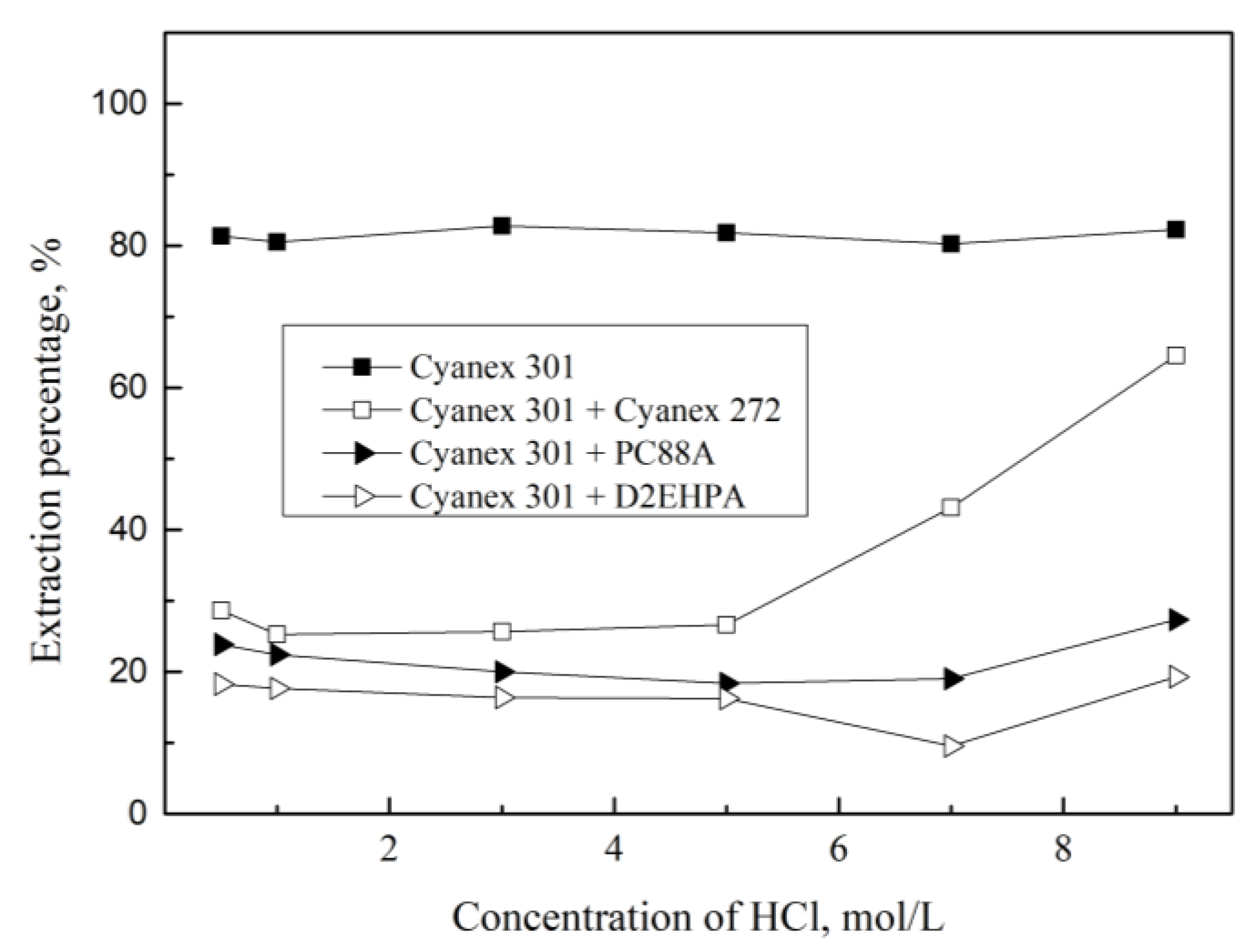
3.1. Extraction of Pd(II) with Mixture of Cyanex 301 and Various Extractants
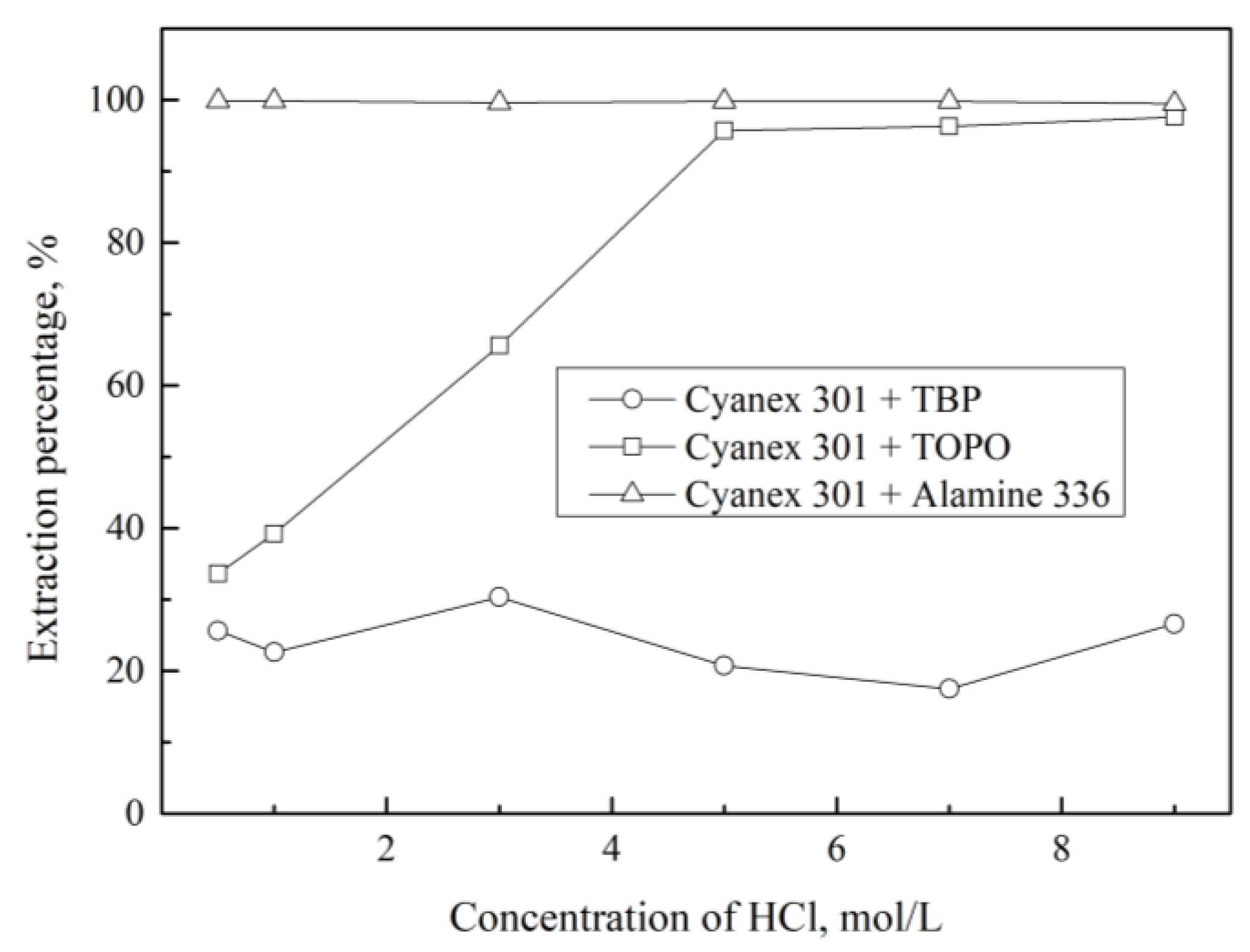
3.2. Extraction of Pd(II) with Mixture of LIX 63 and Various Extractants
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jimenez de Aberasturi, D.; Pinedo, R.; Ruiz de Larramendi, I.; Ruize de larramendi, J.I.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Ang, K.L. Review of the application of ion exchange resins for the recovery of platinum group metals from hydrochloric acid solutions. Miner. Process. Extr. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Rovira, M.; Cortina, J.L.; Amaldos, J.; Sastre, A.M. Recovery and separation of platinum group metals using impregnated resins containing Alamine 336. Solvent Extr. Ion Exch. 1998, 16, 1279–1302. [Google Scholar] [CrossRef]
- Anticó, E.; Hidalgo, M.; Masana, A.; Salvadó, V.; Muñoz, M.; Valiente, M. Study of a palladium mass accelerate transfer through a solid supported liquid membrane containing Kelex 100. Process. Metall. 1992, 7, 1505–1510. [Google Scholar]
- Barakat, M.A.; Mahomoud, M.H.H.; Mahrous, Y.S. Recovery and separation of palladium from spent catalyst. Appl. Catal. A 2006, 301, 182–186. [Google Scholar] [CrossRef]
- Kakoi, T.; Goto, M.; Nakashio, F. Solvent extraction of palladium with bis(2,4,4,-trimethylpentyl)dithiophosphinic acid and bis(2,4,4,-trimetylpentyl)monothiophosphinic acid. Solvent Extr. Ion Exch. 1994, 12, 541–555. [Google Scholar] [CrossRef]
- Chen, M.; Wu, S.; Huang, Z.; Chen, J.; Chen, M.J. Separation and recovery of Pd(II) and Pt(II) from cyanide liquors of Pd-Pt flotation concentrate via solvent extraction. J. Chem. Technol. Biotechnol. 2017, 92, 1699–1709. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, K.S. Synergistic extraction of palladium(II) with thenoyltrifluoroacetone and tri-n-octylphosphine oxide. Bull. Korean Chem. Soc. 1995, 16, 479–483. [Google Scholar]
- Cieszynska, A.; Wisniewski, M. Selective extraction of palladium(II) from hydrochloric acid solutions with phosphonium extractants. Sep. Purif. Technol. 2011, 80, 385–389. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.K.; Kim, S.K.; Lee, J.C. Separation of platinum and palladium from chloride solution by solvent extraction using Alamine 300. Hydrometallurgy 2010, 104, 1–7. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S. Separation of Pd(II) and Pt(IV) from hydrochloric acid solutions by solvent extraction with Cyanex 301 and LIX 63. Miner. Eng. 2018, 115, 13–20. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Lee, J.C.; Kim, E.Y.; Kim, M.S.; Kim, B.S.; Kumar, V. Leaching of platinum group metals (PGMs) from spent automotive catalyst using electro-generated chlorine in HCl solution. J. Chem. Technol. Biotechnol. 2013, 88, 1991–1999. [Google Scholar] [CrossRef]
- Rane, M.V.; Venugopal, V. Study on the extraction of palladium(II) and platinum(IV) using LIX 84I. Hydrometallurgy 2006, 84, 54–59. [Google Scholar] [CrossRef]
- Bandekar, S.V.; Dhadke, P.M. Solvent extraction separation of platinum(IV) and palladium(II) by 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC-88A). Sep. Purif. Technol. 1998, 13, 129–135. [Google Scholar] [CrossRef]
- Sun, P.P.; Lee, M.S. Separation of Pt(IV) and Pd(II) from the loaded Alamine 336 by stripping. Hydrometallurgy 2011, 109, 181–184. [Google Scholar] [CrossRef]
- Foulon, C.; Pareau, D.; Durand, G. Thermodynamic and kinetic studies of palladium(II) extraction by extractant mixtures containing LIX 63. Part I. Thermodynamic study. Hydrometallurgy 1999, 51, 139–153. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of Pt(IV), Pd(II), Rh(III), and Ir(IV) from concentrated hydrochloric acid solutions by solvent extraction. Hydrometallurgy 2016, 164, 71–77. [Google Scholar] [CrossRef]
- Marczenko, Z. Separation and Spectrophotometric Determination of Elements; John Wiley & Sons: Milton, Australia, 1986. [Google Scholar]
- Grigorieva, N.A.; Pavlenko, N.I.; Pashkov, G.L.; Flritlikh, I.Y.; Nikiforova, L.K. Investigation of the state of bis(2,4,4-trimethylpentyl)dithiophosphinic acid in nonane in the presence of electron-donar additives. Solvent Extr. Ion Exch. 2010, 28, 510–525. [Google Scholar] [CrossRef]
- Batchu, N.K.; Sonu, C.H.; Lee, M.S. Solvent extraction equilibrium and modeling studies of manganese from sulfate solutions by mixture of Cyanex 301 and TBP. Hydrometallurgy 2014, 144–145, 1–6. [Google Scholar] [CrossRef]
- Banda, R.; Min, S.H.; Lee, M.S. Selective extraction of Hf(IV) over Zr(IV) from aqueous H2SO4 solutions by solvent extraction with acidic organophosphorus based extractants. J. Chem. Technol. Biotechnol. 2014, 89, 1712–1719. [Google Scholar] [CrossRef]
- Whewell, R.J.; Foakes, H.J.; Hughes, M.A. Degradation in hydroxyoxime solvent extraction systems. Hydrometallurgy 1981, 7, 7–26. [Google Scholar] [CrossRef]
- Watson, E.K.; Rickelton, W.A. A review of the industrial and recent potential applications of trioctylphosphine oxide. Solvent Extr. Ion Exch. 1992, 10, 879–889. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Solvent extraction of Pr and Nd from chloride solution by mixtures of acidic extractants and LIX 63. Korean J. Met. Mater. 2016, 54, 592–597. [Google Scholar]
- Nguyen, T.H.; Lee, M.S. Effect of HCl concentration on the oxidation of LIX 63 and subsequent separation of Pd(II), Pt(IV), Ir(IV) and Rh(III) by solvent extraction. Korean J. Met. Mater. 2016, 54, 768–774. [Google Scholar]

| Extractant | Structure | R or R’ Group |
|---|---|---|
| D2EHPA | 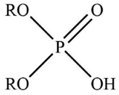 | R = C8H17– |
| PC 88A | 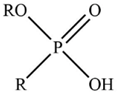 | R = C8H17– |
| Cyanex 272 | 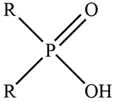 | R = C7H15– |
| Cyanex 301 | 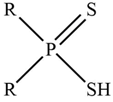 | R = C7H15– |
| LIX 63 | 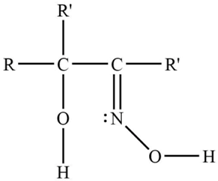 | R = H R’ = C7H15– |
| TBP |  | R = C4H9– |
| TOPO | 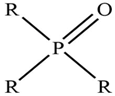 | R = C8H17– |
| Alamine 336 | 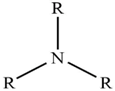 | R = C8H17– or C10H21– |
| [HCl], M | DCyanex 301 | DCyanex 301+Cyanex 272 | DCyanex 301+PC 88A | DCyanex 301+D2EHPA |
|---|---|---|---|---|
| 0.5 | 4.6 | 0.4 | 0.3 | 0.2 |
| 1.0 | 4.1 | 0.3 | 0.3 | 0.2 |
| 3.0 | 4.8 | 0.3 | 0.3 | 0.2 |
| 5.0 | 4.4 | 0.4 | 0.2 | 0.2 |
| 7.0 | 4.2 | 0.8 | 0.2 | 0.1 |
| 9.0 | 4.6 | 1.8 | 0.1 | 0.2 |
| [HCl], M | DCyanex 301+TBP | DCyanex 301+TOPO | DCyanex 301+Alamine 336 |
|---|---|---|---|
| 0.5 | 0.3 | 0.5 | 833.2 |
| 1.0 | 0.3 | 0.6 | 1000.0 |
| 3.0 | 0.4 | 1.9 | 262.4 |
| 5.0 | 0.3 | 19.3 | 555.1 |
| 7.0 | 0.2 | 25.9 | 499.5 |
| 9.0 | 0.4 | 29.9 | 191.5 |
| [HCl], M | RCyanex 272 | RPC 88A | RD2EHPA | RTBP | RTOPO | RAlamine 336 |
|---|---|---|---|---|---|---|
| 0.5 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 1.0 |
| 1.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 1.2 |
| 3.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.4 | 0.3 |
| 5.0 | 0.1 | 0.1 | 0.0 | 0.1 | 4.4 | 0.7 |
| 7.0 | 0.2 | 0.1 | 0.0 | 0.1 | 6.0 | 0.6 |
| 9.0 | 0.4 | 0.1 | 0.1 | 0.1 | 6.4 | 0.2 |
| Stripping Reagent | Pd(II) Stripping (%) |
|---|---|
| 0.5 mol/L HCl | 0.5 |
| 5.0 mol/L HCl | 1.0 |
| 0.5 mol/L NaSCN | 0.5 |
| 0.1 mol/L Na2S2O3 | 0.5 |
| 0.5 mol/L (NH2)2CS | 2.0 |
| 0.5 mol/L Na2CO3 | 0.5 |
| 0.5 mol/L Oxalic acid | 0.5 |
| 0.5 mol/L (NH2)2CS + 5.0 mol/L HCl | 1.0 |
| [HCl], M | DLIX 63 | DLIX 63+Cyanex 272 | DLIX 63+PC 88A | DLIX 63+D2EHPA |
|---|---|---|---|---|
| 0.5 | 5.2 | 2.2 | 1.0 | 0.7 |
| 1.0 | 4.3 | 1.1 | 0.7 | 0.4 |
| 3.0 | 3.8 | 1.2 | 0.8 | 0.5 |
| 5.0 | 3.1 | 0.8 | 0.5 | 0.3 |
| 7.0 | 0.1 | 0.2 | 0.1 | 0.1 |
| 9.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| [HCl], M | DLIX 63+TBP | DLIX 63+TOPO | DLIX 63+Alamine 336 |
|---|---|---|---|
| 0.5 | 0.4 | 1.3 | 140.4 |
| 1.0 | 0.3 | 1.5 | 66.6 |
| 3.0 | 0.3 | 9.5 | 57.9 |
| 5.0 | 0.4 | 16.9 | 57.1 |
| 7.0 | 0.4 | 1.1 | 3.3 |
| 9.0 | 0.0 | 0.5 | 0.5 |
| [HCl], M | RCyanex 272 | RPC 88A | RD2EHPA | RTBP | RTOPO | RAlamine 336 |
|---|---|---|---|---|---|---|
| 0.5 | 0.4 | 0.2 | 0.1 | 0.1 | 0.3 | 2.3 |
| 1.0 | 0.3 | 0.2 | 0.1 | 0.1 | 0.4 | 1.2 |
| 3.0 | 0.2 | 0.2 | 0.1 | 0.1 | 2.2 | 3.9 |
| 5.0 | 0.4 | 0.2 | 0.1 | 0.1 | 4.4 | 8.5 |
| 7.0 | 1.4 | 0.7 | 0.5 | 3.0 | 5.7 | 2.6 |
| 9.0 | 5.3 | 3.0 | 1.1 | 3.2 | 9.6 | 2.0 |
| Extractant | D |
|---|---|
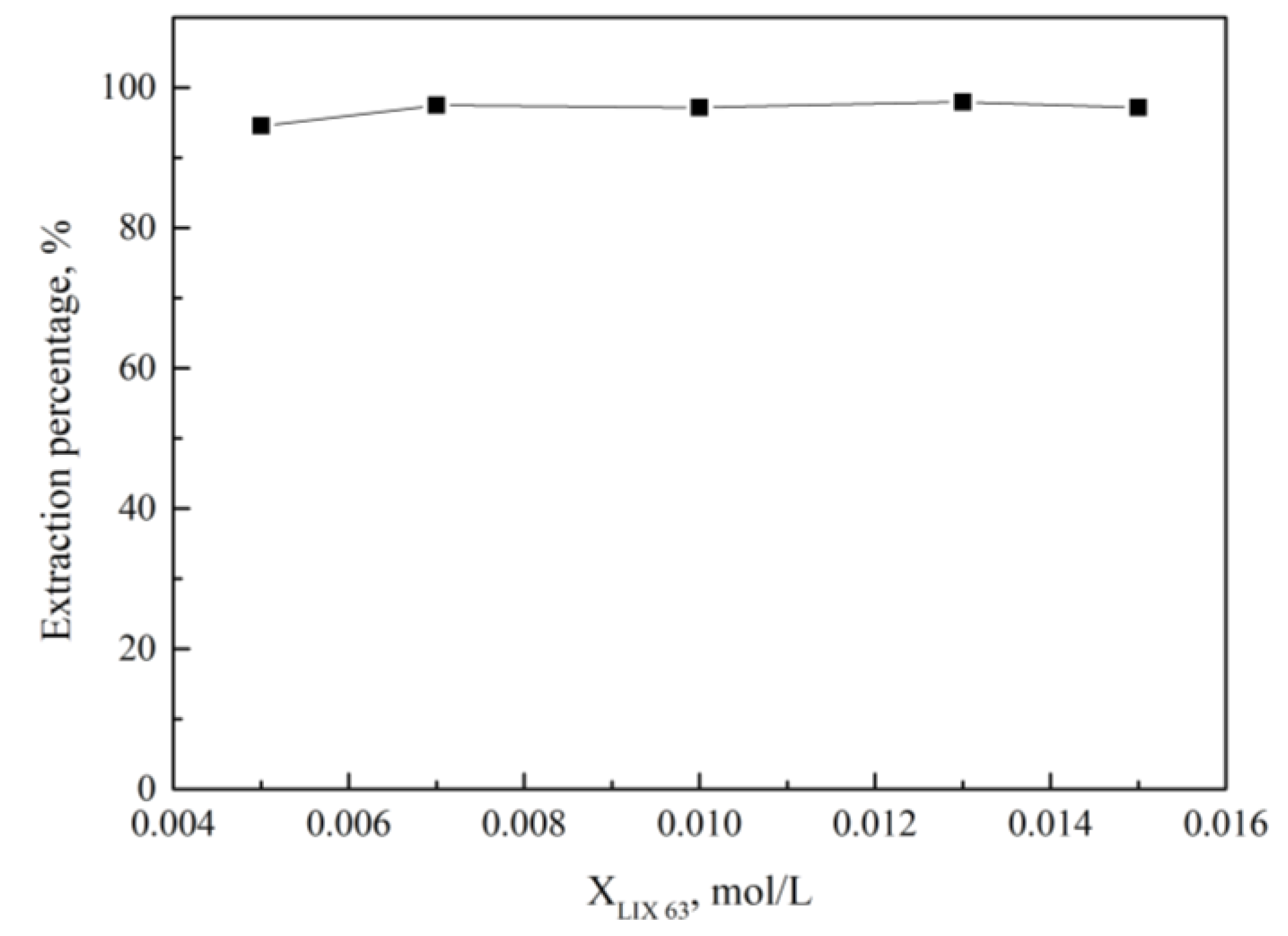
| 0.005 mol/L Alamine 336 + 0.015 mol/L LIX 63 | 34.6 |
| 0.007 mol/L Alamine 336 + 0.013 mol/L LIX 63 | 35.6 |
| 0.01 mol/L Alamine 336 + 0.01 mol/L LIX 63 | 34.6 |
| 0.013 mol/L Alamine 336 + 0.007 mol/L LIX 63 | 34.7 |
| 0.015 mol/L Alamine 336 + 0.005 mol/L LIX 63 | 17.4 |
| Stripping Reagent | Pd(II) Stripping (%) |
|---|---|
| 0.5 mol/L HCl | 0.0 |
| 5.0 mol/L HCl | 2.0 |
| 0.5 mol/L NaSCN | 3.0 |
| 0.1 mol/L Na2S2O3 | 0.9 |
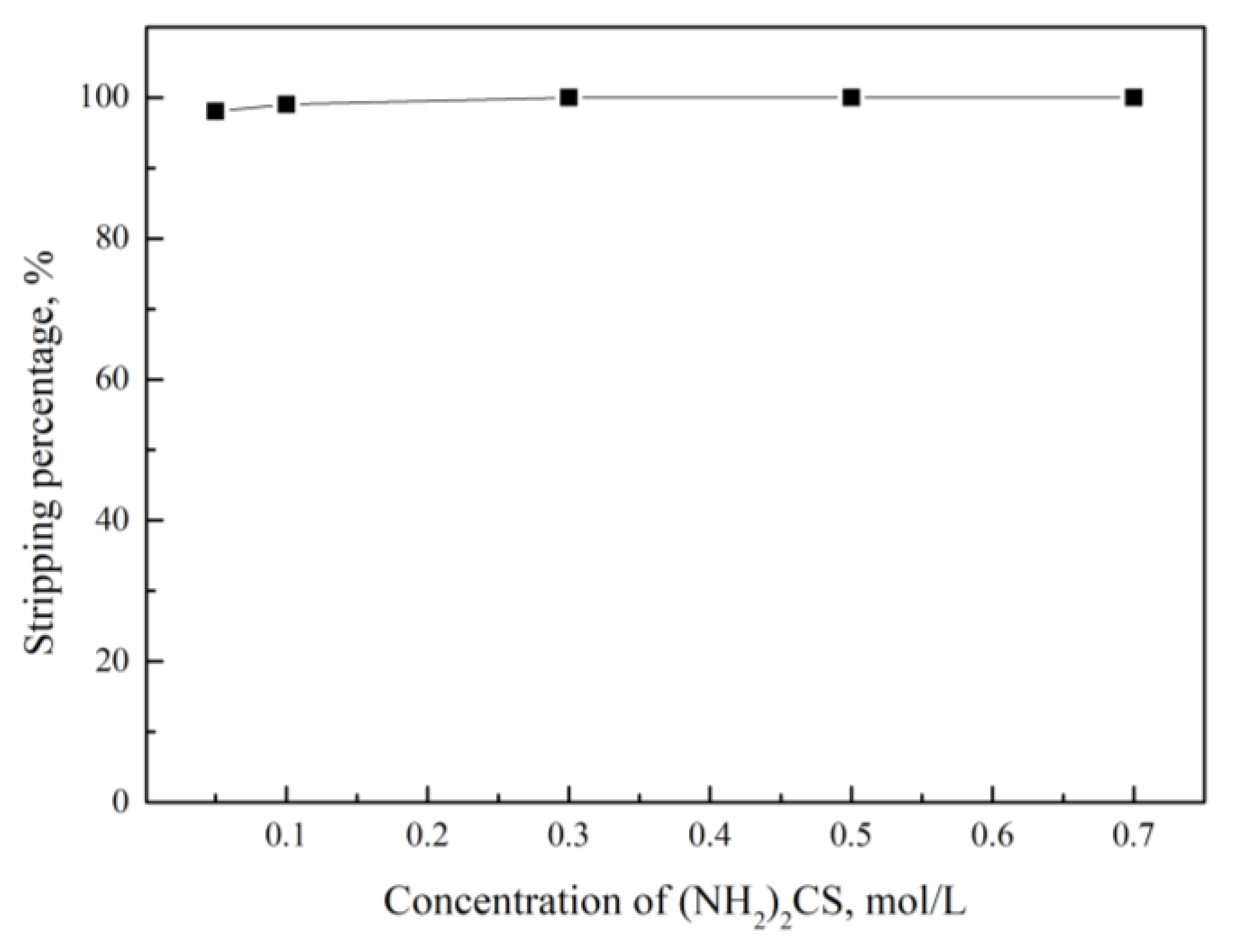
| 0.5 mol/L (NH2)2CS | 100.0 |
| 0.5 mol/L (NH2)2CS + 5.0 mol/L HCl | 27.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, H.T.; Lee, M.S.; Son, S.H. Extraction of Palladium(II) from Hydrochloric Acid Solutions by Solvent Extraction with Mixtures Containing Either Cyanex 301 or LIX 63. Metals 2017, 7, 541. https://doi.org/10.3390/met7120541
Truong HT, Lee MS, Son SH. Extraction of Palladium(II) from Hydrochloric Acid Solutions by Solvent Extraction with Mixtures Containing Either Cyanex 301 or LIX 63. Metals. 2017; 7(12):541. https://doi.org/10.3390/met7120541
Chicago/Turabian StyleTruong, Hoai Thanh, Man Seung Lee, and Seong Ho Son. 2017. "Extraction of Palladium(II) from Hydrochloric Acid Solutions by Solvent Extraction with Mixtures Containing Either Cyanex 301 or LIX 63" Metals 7, no. 12: 541. https://doi.org/10.3390/met7120541