Rhodium and Hafnium Influence on the Microstructure, Phase Composition, and Oxidation Resistance of Aluminide Coatings
Abstract
:1. Introduction
2. Experimental Procedure
- heating from room temperature up to 1040 °C;
- hafnizing with aluminizing at 1040 °C for 360 min;
- aluminizing at 1040 °C for 360 min;
- cooling samples with the furnace.
3. Results
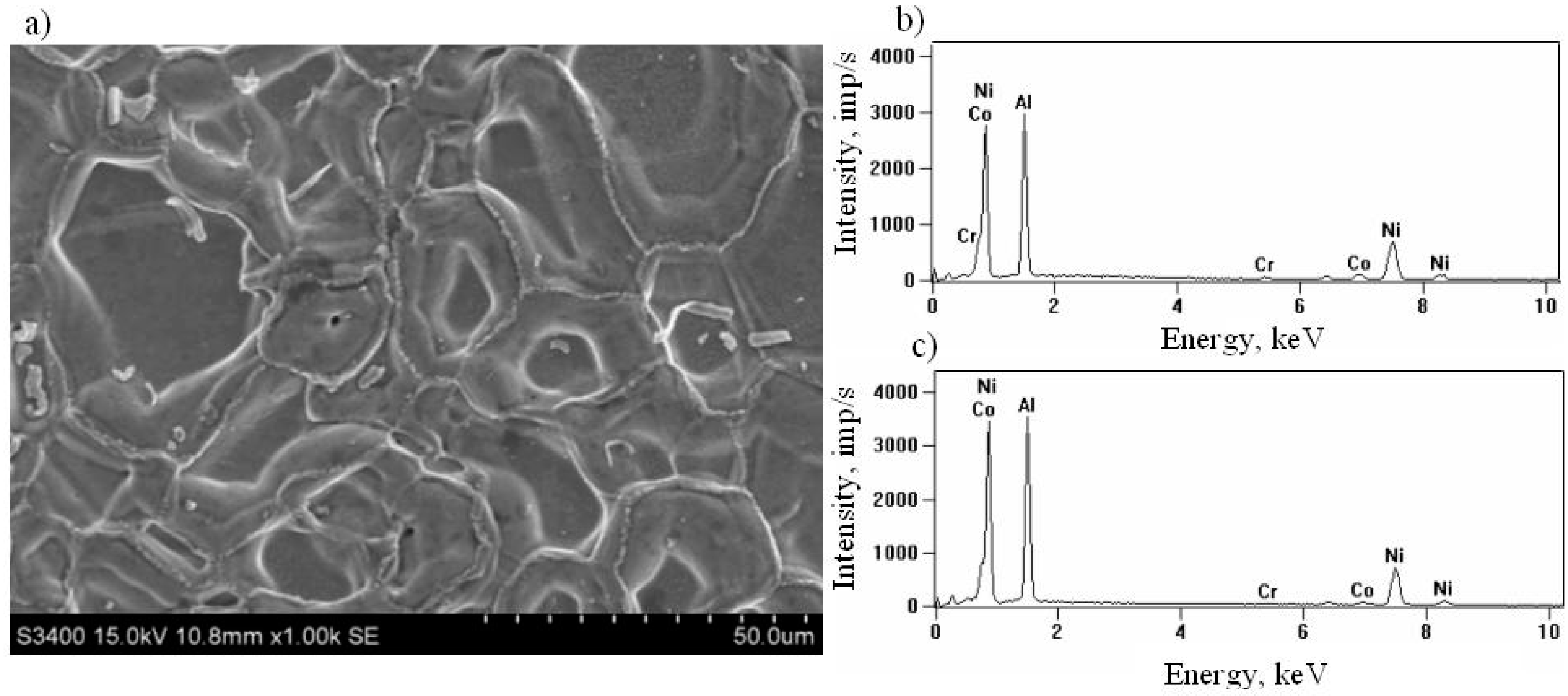
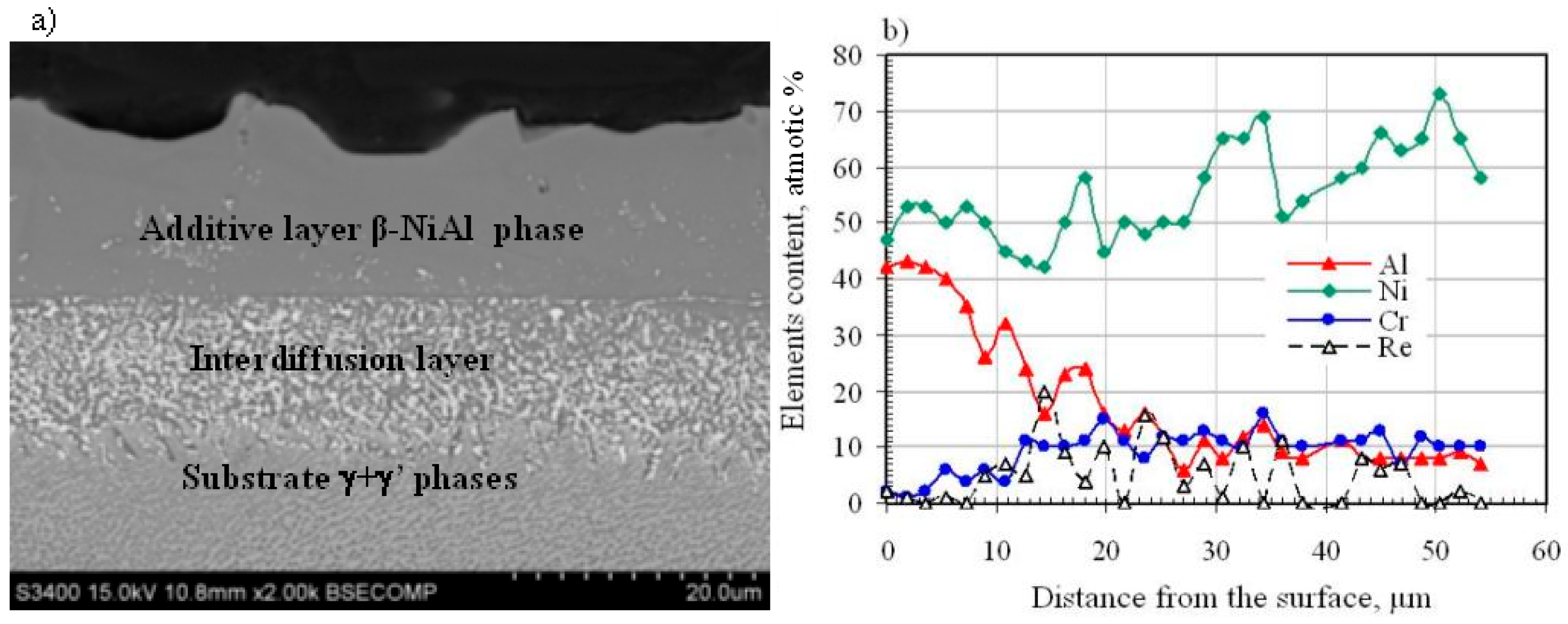
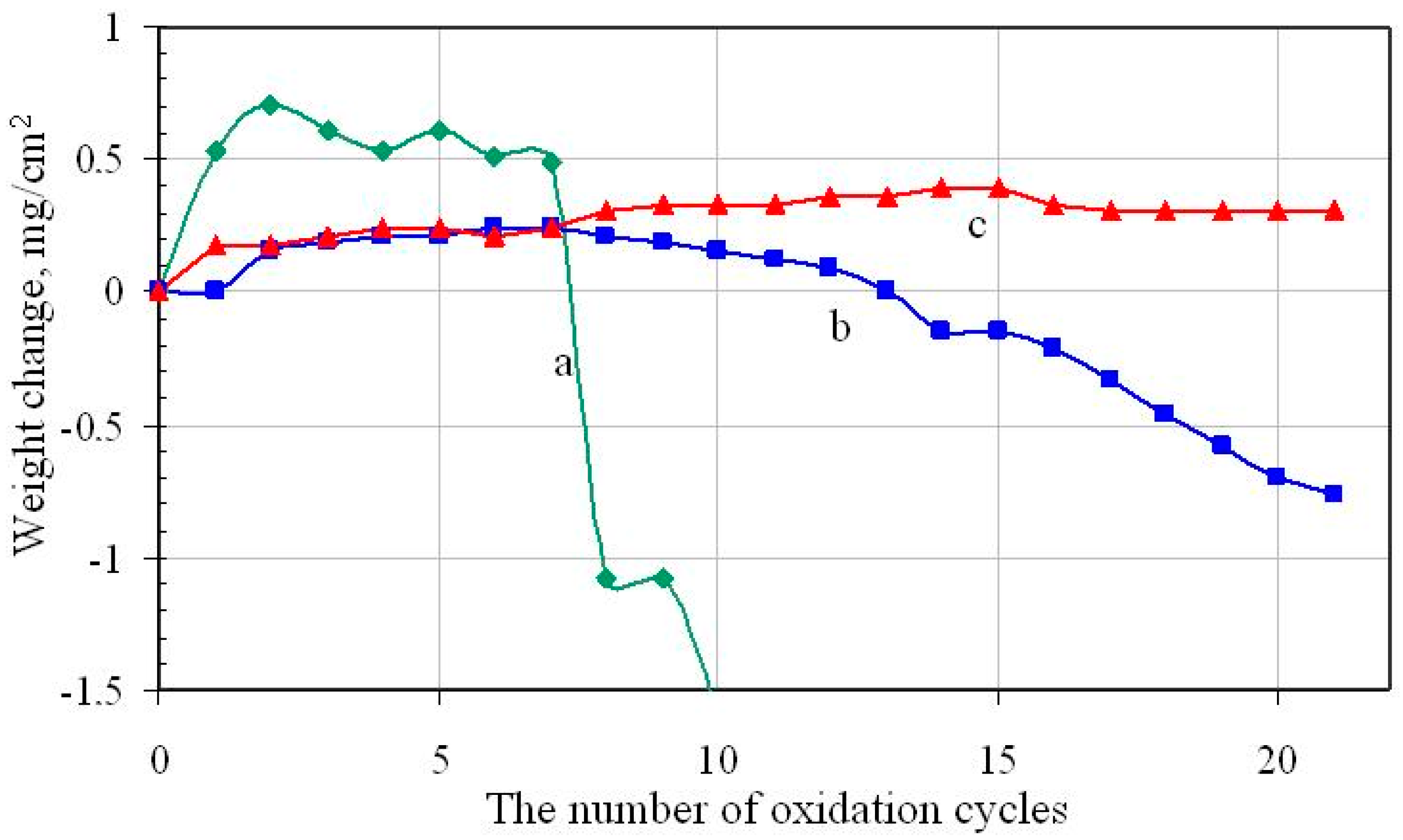
3.1. Nonmodified Aluminide Coatings (a)
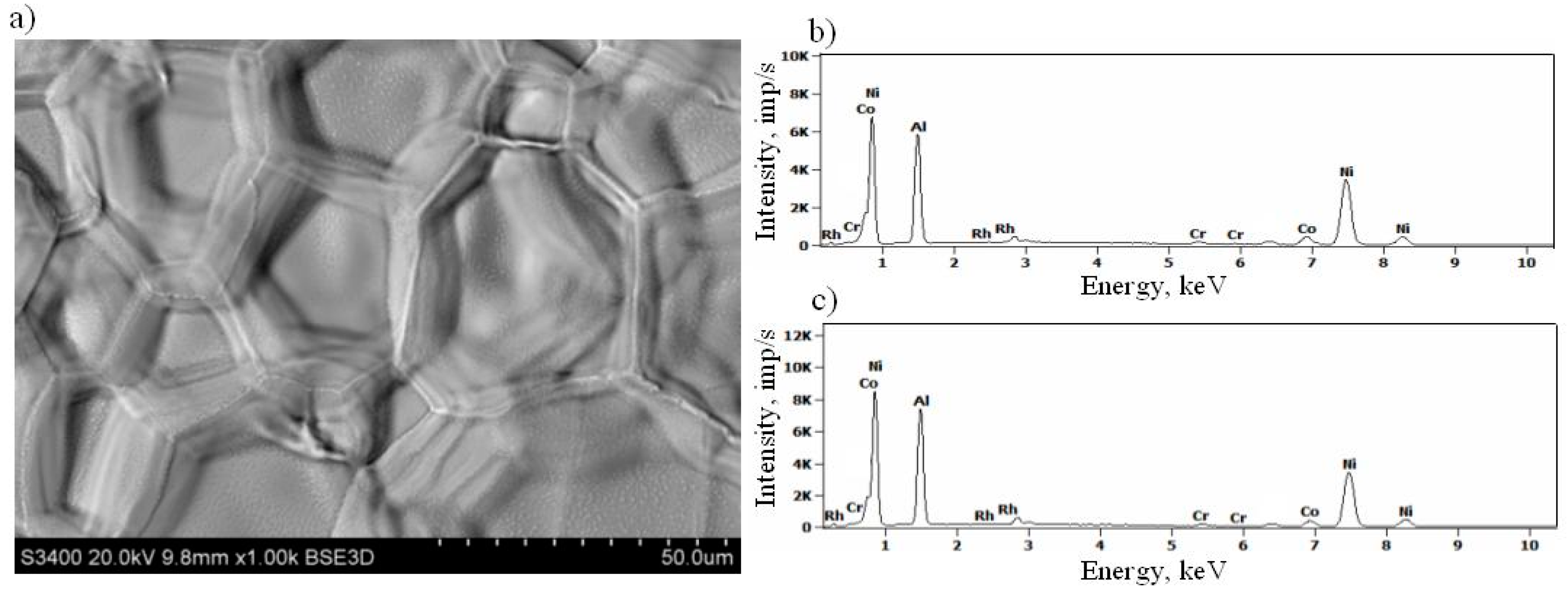
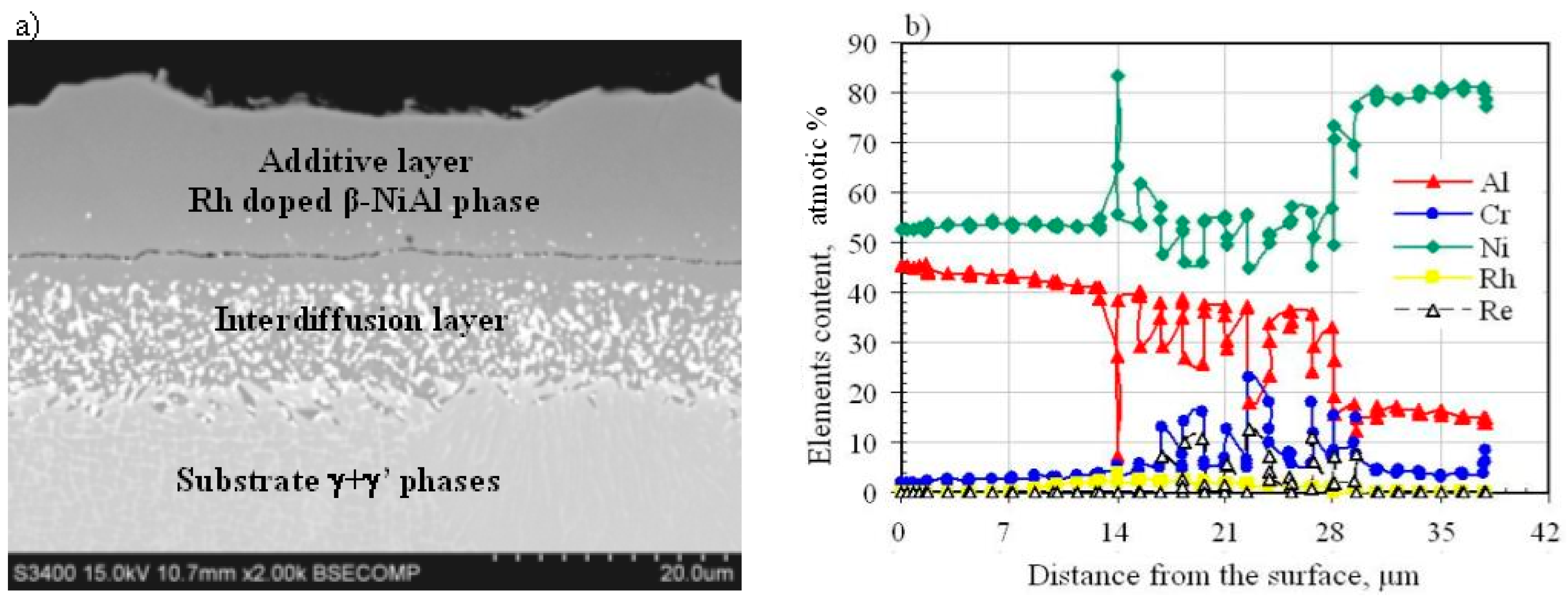
3.2. Rhodium-Modified Aluminide Coatings (b)
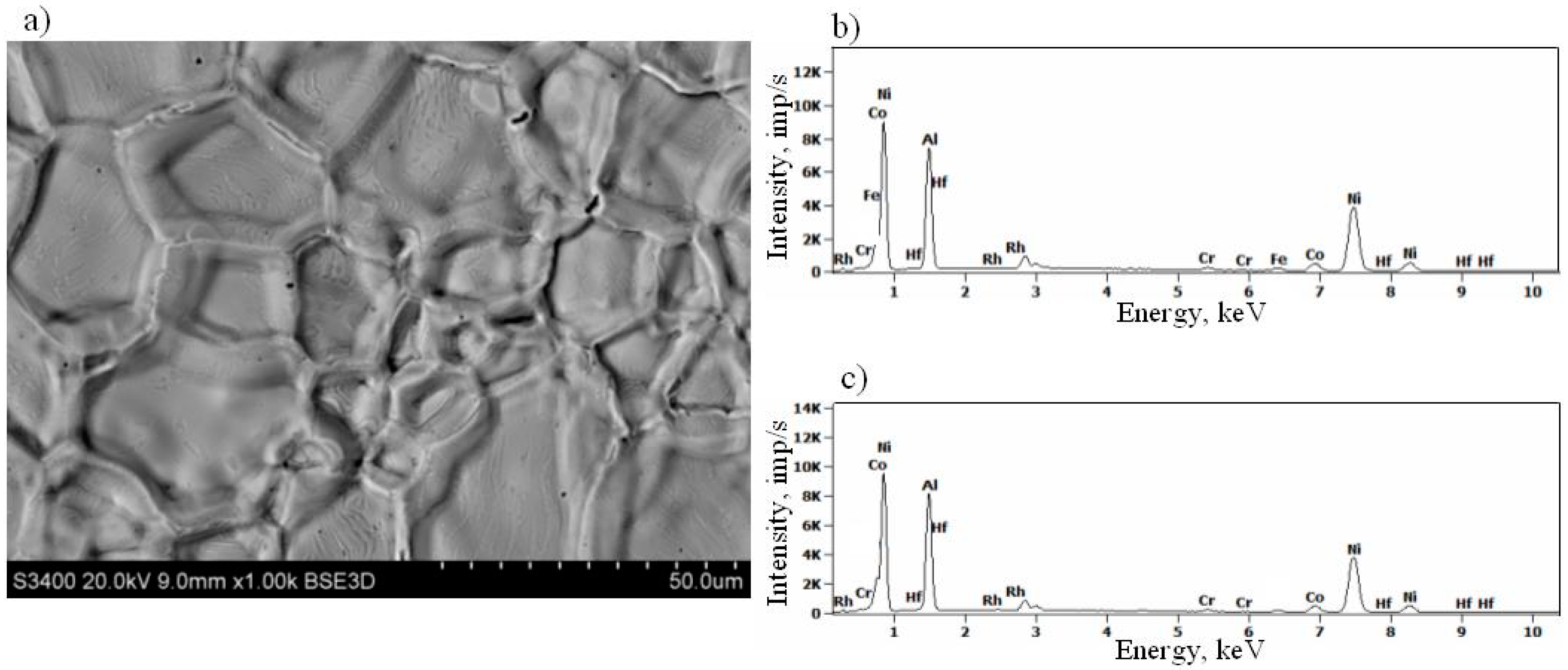
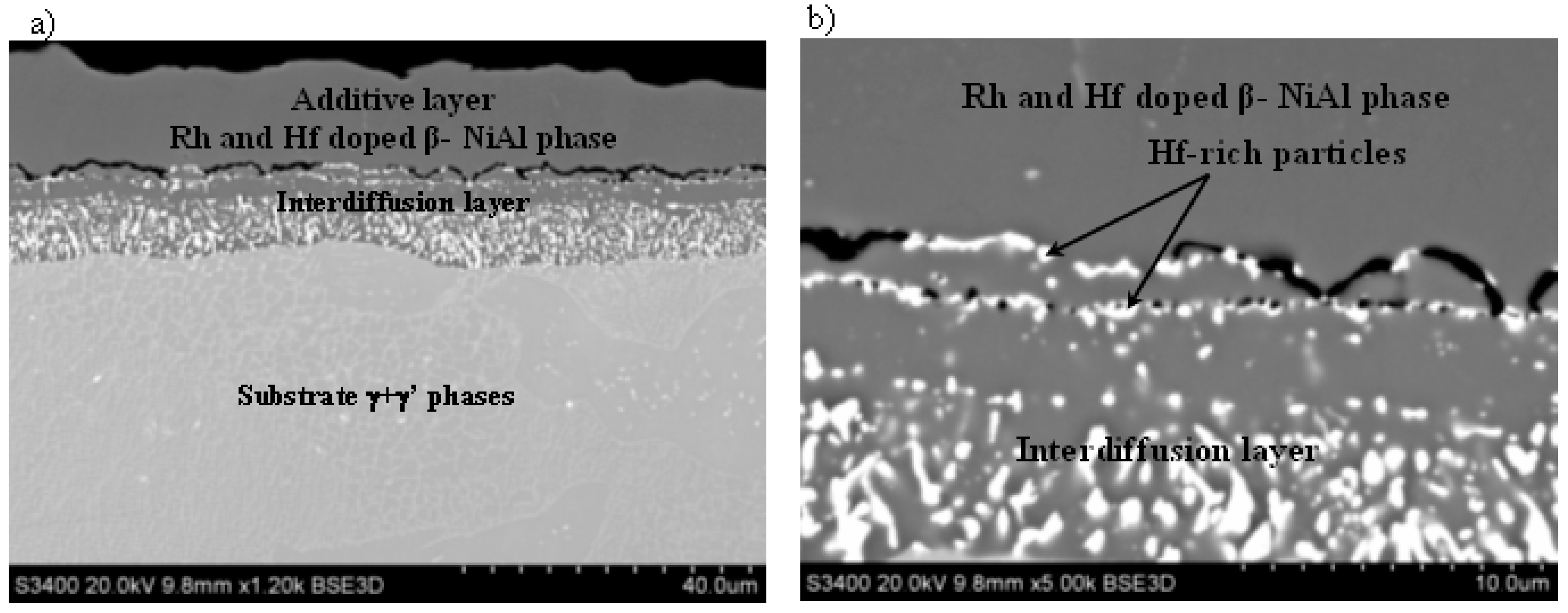
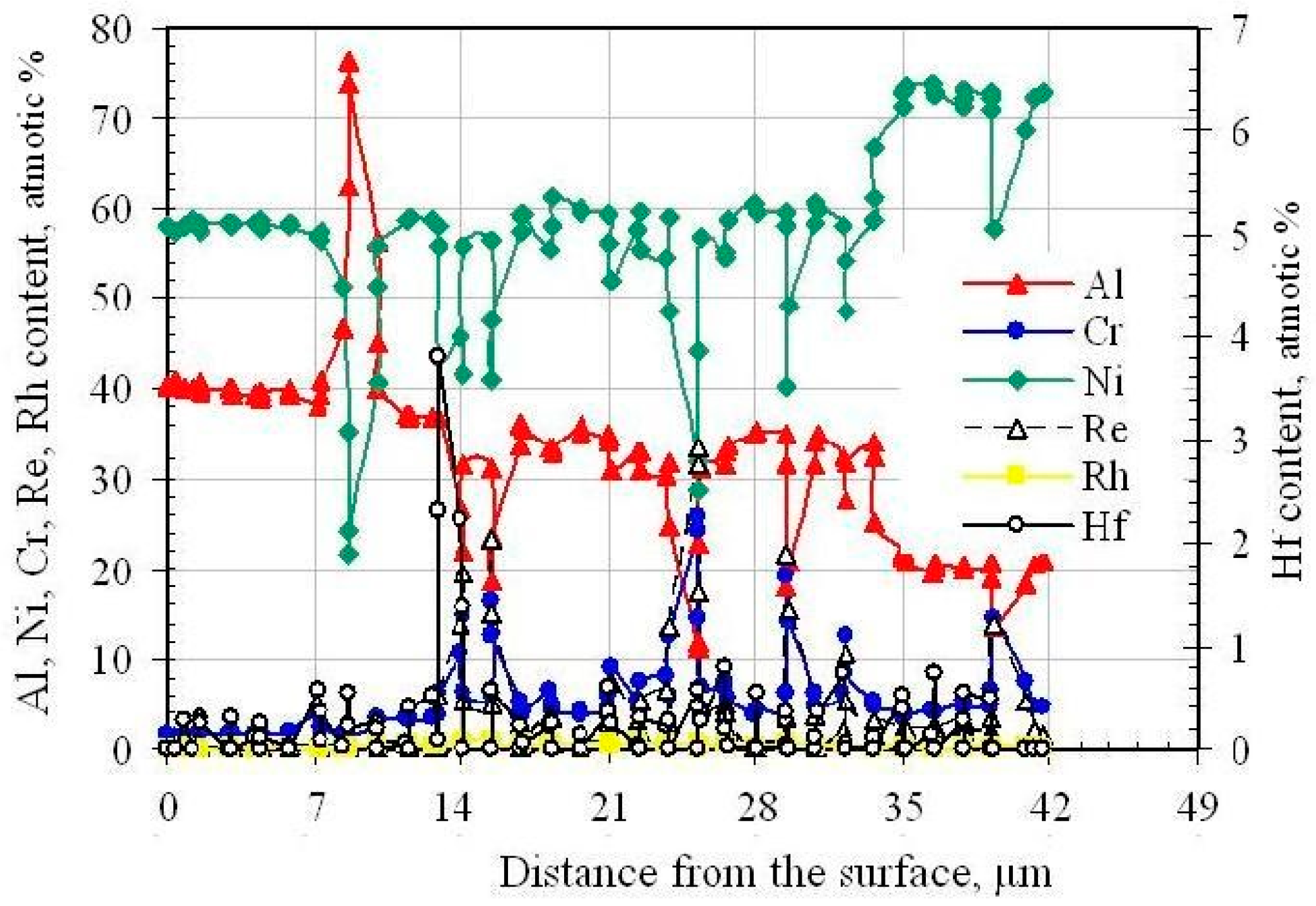
3.3. Rhodium- and Hafnium-Modified Aluminide Coatings (c)
4. Discussion
- (1)
- diffusion of nickel, cobalt, chromium, titanium and other superalloys’ elements from the substrate to the surface, leading to formation of the interdiffusion layer;
- (2)
- reaction of nickel with aluminum supplied by the gas phase in the CVD process, and formation of the additive layer.
- (1)
- rhodium probably dissolves in γ + γ′ phases near the surface of the CMSX 4 alloy during heating of the alloy with a 0.5 μm rhodium layer at 1040 °C;
- (2)
- aluminum—during aluminizing in the CVD process—arrives at the surface, where Rh is diluted by Ni. Since aluminum has more affinity to nickel than rhodium, it forms the (Ni,Rh)Al phase layer, similar to the case of the platinum-modified aluminide coating [7]. It is possible that the RhxNiy phase reacts with Al and a (Ni,Rh)Al phase is formed.
5. Conclusions
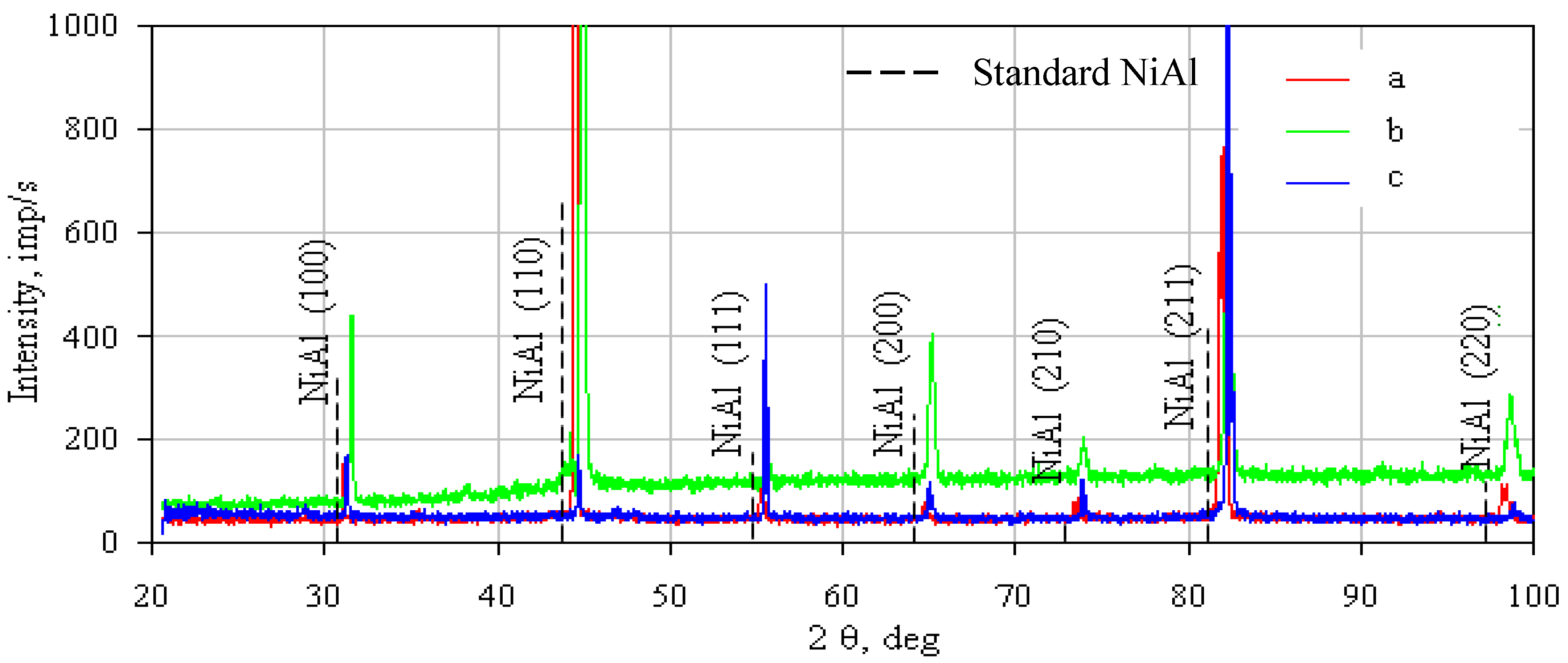
- The nonmodified (a), rhodium-modified (b), and rhodium- and hafnium-modified (c) aluminide coatings consist of two layers (additive and interdiffusion).
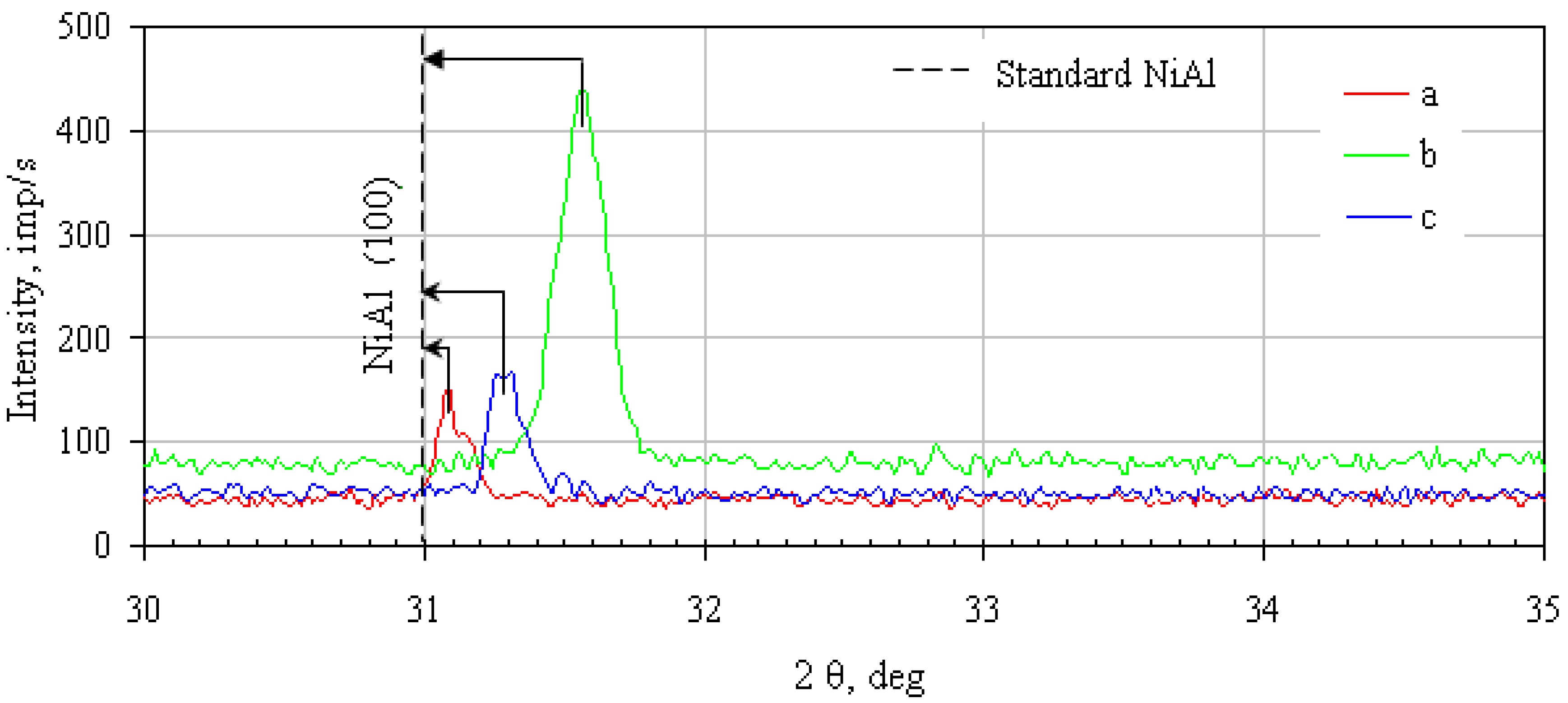
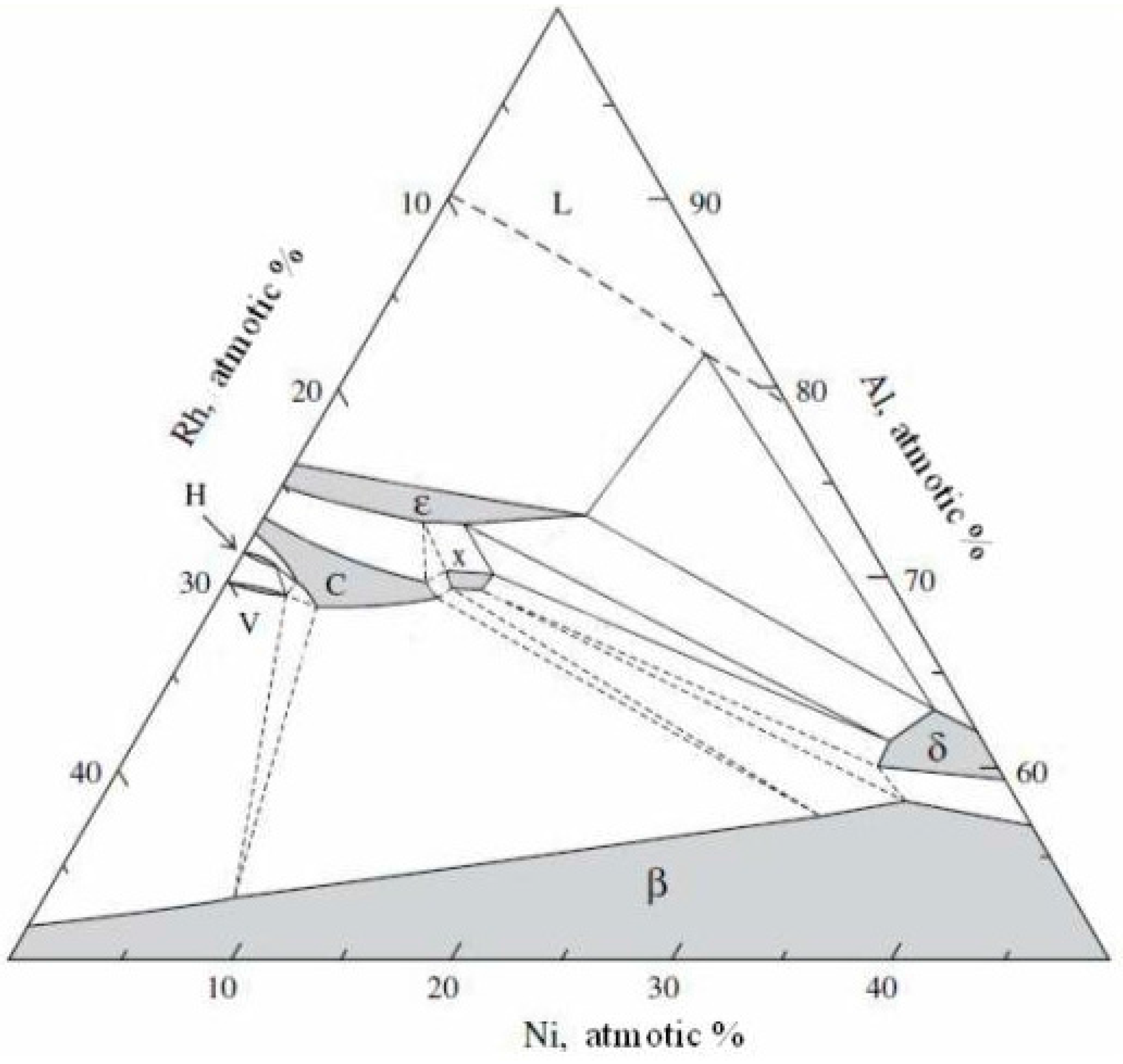
- Rhodium-doped (rhodium- and hafnium-doped) β-NiAl phase was found in the additive layer of the rhodium-modified (rhodium- and hafnium-modified) aluminide coating.
- Rhodium atoms replace nickel atoms in the lattice of the β-NiAl phase, and form the (Ni,Rh)Al phase between the additive and the interdiffusion layers.
- Hf-rich particles precipitate in the (Ni,Rh)Al phase at the additive/interdiffusion layer interface in the rhodium- and hafnium-modified aluminide coating.
- The rhodium-modified aluminide coating (b) has better oxidation resistance than the nonmodified one (a), whereas the rhodium- and hafnium-modified aluminide coating (c) has better oxidation resistance than the rhodium-modified (b) and nonmodified (a) coatings.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beranoagirre, A.; Olvera, D.; López de Lacalle, L.N. Milling of gamma titanium–aluminum alloys. Int. J. Adv. Manuf. Technol. 2012, 62, 83–88. [Google Scholar] [CrossRef]
- Shiomi, S.; Miyake, M.; Hirato, T.; Sato, A. Aluminide coatings fabricated on nickel by aluminium electrodeposition from DMSO2-based electrolyte and subsequent annealing. Mater. Trans. 2011, 52, 1216–1221. [Google Scholar] [CrossRef]
- Latief, H.; Sherif, M.; Kakehi, K. Role of aluminide coating on oxidation resistance of Ni-based single crystal superalloy at 900 °C. Int. J. Electrochem. Sci. 2015, 10, 1873–1882. [Google Scholar]
- Latief, H.; Kakehi, K.; Tashiro, Y. Oxidation behavior characteristics of an aluminized Ni-based single crystal superalloy CM186LC between 900 °C and 1100 °C in air. J. Ind. Eng. Chem. 2013, 19, 1926–1932. [Google Scholar] [CrossRef]
- Zhan, Z.; He, Y.; Li, L.; Liu, H.; Dai, Y. Low-temperature formation and oxidation resistance of ultrafine aluminide coatings on Ni-base superalloy. Surf. Coat. Technol. 2009, 203, 2337–2342. [Google Scholar] [CrossRef]
- Lee, J.; Kuo, Y. A study on the microstructure and cyclic oxidation behavior of the pack aluminized Hastelloy X at 1100 °C. Surf. Coat. Technol. 2006, 201, 3867–3871. [Google Scholar] [CrossRef]
- Benoist, J.; Badawi, K.F.; Malie, A.; Ramade, C. Microstructure of Pt-modified aluminide coatings on Ni-based superalloys. Surf. Coat. Technol. 2004, 182, 14–23. [Google Scholar] [CrossRef]
- Purvis, A.; Warnes, B. The effects of platinum concentration on the oxidation resistance of superalloys coated with single-phase platinum aluminide. Surf. Coat. Technol. 2001, 146–147, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Sayre, G. Synthesis of simple and platinum-modified aluminide coatings on cobalt (Co)-base superalloys via a vapor phase aluminizing process. Surf. Coat. Technol. 2008, 203, 256–263. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Sieniawski, J. Microstructural study on oxidation resistance of nonmodified and platinum modified aluminide coating. J. Mater. Eng. Perform. 2014, 23, 918–926. [Google Scholar] [CrossRef]
- Pedraza, F.; Kennedy, A.D.; Koperek, J.; Moretto, P. Investigation of the microstructure of platinum-modified aluminide coatings. Surf. Coat. Technol. 2006, 200, 4032–4039. [Google Scholar] [CrossRef]
- Gleeson, B.; Mu, N.; Hayashi, S. Compositional factors affecting the establishment and maintenance of Al2O3 scales on Ni-Al-Pt systems. J. Mater. Sci. 2009, 4, 1704–1710. [Google Scholar] [CrossRef]
- Koul, A.; Immarigeon, J.; Dainty, R.; Patnaik, P. Degradation of Advanced Aero Engine Turbine Blades; ASM International, Materials Park: Geauga County, OH, USA, 1994; Volume 12, p. 69. [Google Scholar]
- Haynes, J.A.; Zhang, Y.; Lee, W.Y. Elevated Temperature Coatings: Science and Technology III; TMS: Warrendale, PA, USA, 1999; pp. 185–196. [Google Scholar]
- Schneider, K.; Arnim, H.; Grünling, H.W. Influence of coatings and hot corrosion on the fatigue behaviour of nickel-based superalloys. Thin Solid Films 1981, 84, 29–36. [Google Scholar] [CrossRef]
- Mu, N.; Izumi, T.; Zhang, L.; Gleeson, B. The development and performance of novel Pt + Hf modified γ′-Ni3Al + γ-Ni bond coatings for advanced thermal barrier coatings systems. Miner. Met. Mater. Soc. 2008, 38, 629–637. [Google Scholar]
- Lee, S.H.; Jun, H.S.; Park, J.H.; Kim, W.; Oh, S.; Park, J.S. Properties of hafnium-aluminum-zinc-oxide thin films for the application of oxide-transistors. Thin Solid Films 2016, 620, 82–87. [Google Scholar] [CrossRef]
- Kim, G.Y.; He, L.M.; Meyer, J.D.; Lee, W.Y.; Quintero, A.; Haynes, J.A. Mechanisms of Hf dopant incorporation during the early stage of chemical vapor deposition aluminide coating growth under continuous doping conditions. Metall. Mater. Trans. A 2004, 35, 3581–3593. [Google Scholar] [CrossRef]
- Felten, E. Use of platinum and rhodium to improve oxide adherence on Ni-8Cr-6Al alloys. Oxid. Met. 1976, 10, 23–28. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Wierzbińska, M.; Gancarczyk, K.; Sieniawski, J. Degradation of nonmodified and rhodium modified aluminide coating deposited on CMSX 4 superalloy. J. Microsc. 2016, 23, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Romanowska, J. Aluminum diffusion in aluminide coatings deposited by the CVD method on pure nickel. Calphad 2014, 44, 114–118. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Sieniawski, J. Cyclic oxidation of palladium modified and nonmodified aluminide coatings deposited on nickel base superalloys. Arch. Civ. Mech. Eng. 2018, 18, 130–139. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Kocurek, P.; Pytel, M.; Sieniawski, J. Oxidation resistance of turbine blades made of ŻS6K superalloy after aluminizing by low-activity CVD and VPA methods. J. Mater. Eng. Perform. 2016, 25, 1964–1973. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Morgiel, J.; Romanowska, J.; Sieniawski, J. Microstructure and oxidation behaviour investigation of rhodium modified aluminide coating deposited on CMSX 4 superalloy. J. Microsc. 2016, 261, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Przepiórzyński, B.; Mi, S.; Grushko, B.; Surowiec, M. An investigation of the Al-Ni-Rh phase diagram between 50 and 100 at% Al. Intermetallics 2007, 15, 918–928. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, C.; Yao, H.; Bao, Z.; Zhu, S.; Wang, F. Preparation and enhanced oxidation performance of a Hf-dopedsingle-phase Pt-modified aluminide coating. Corros. Sci. 2016, 113, 17–25. [Google Scholar] [CrossRef]
- Wang, Y.; Suneson, M.; Sayre, G. Synthesis of hafnium modified aluminide coatings on Ni-base superalloys. Surf. Coat. Technol. 2011, 15, 1218–1228. [Google Scholar] [CrossRef]
| Ni | Cr | Mo | Nb | Ta | Al | Ti | Co | W | Hf | Re |
|---|---|---|---|---|---|---|---|---|---|---|
| 61.7 | 6.5 | 0.6 | - | 6.5 | 5.6 | 1 | 9 | 6 | 0.1 | 3 |
| Elements Content, atom % | ||||
|---|---|---|---|---|
| Al | Cr | Ni | Hf | Rh |
| 39.1 ± 0.4 | 2.5 ± 0.1 | 54.1 ± 0.7 | 3.9 ± 0.3 | 0.4 ± 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagula-Yavorska, M.; Wierzbińska, M.; Sieniawski, J. Rhodium and Hafnium Influence on the Microstructure, Phase Composition, and Oxidation Resistance of Aluminide Coatings. Metals 2017, 7, 548. https://doi.org/10.3390/met7120548
Zagula-Yavorska M, Wierzbińska M, Sieniawski J. Rhodium and Hafnium Influence on the Microstructure, Phase Composition, and Oxidation Resistance of Aluminide Coatings. Metals. 2017; 7(12):548. https://doi.org/10.3390/met7120548
Chicago/Turabian StyleZagula-Yavorska, Maryana, Małgorzata Wierzbińska, and Jan Sieniawski. 2017. "Rhodium and Hafnium Influence on the Microstructure, Phase Composition, and Oxidation Resistance of Aluminide Coatings" Metals 7, no. 12: 548. https://doi.org/10.3390/met7120548




