Fracture Resistance of 14Cr ODS Steel Exposed to a High Temperature Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Exposure in Gas Environments
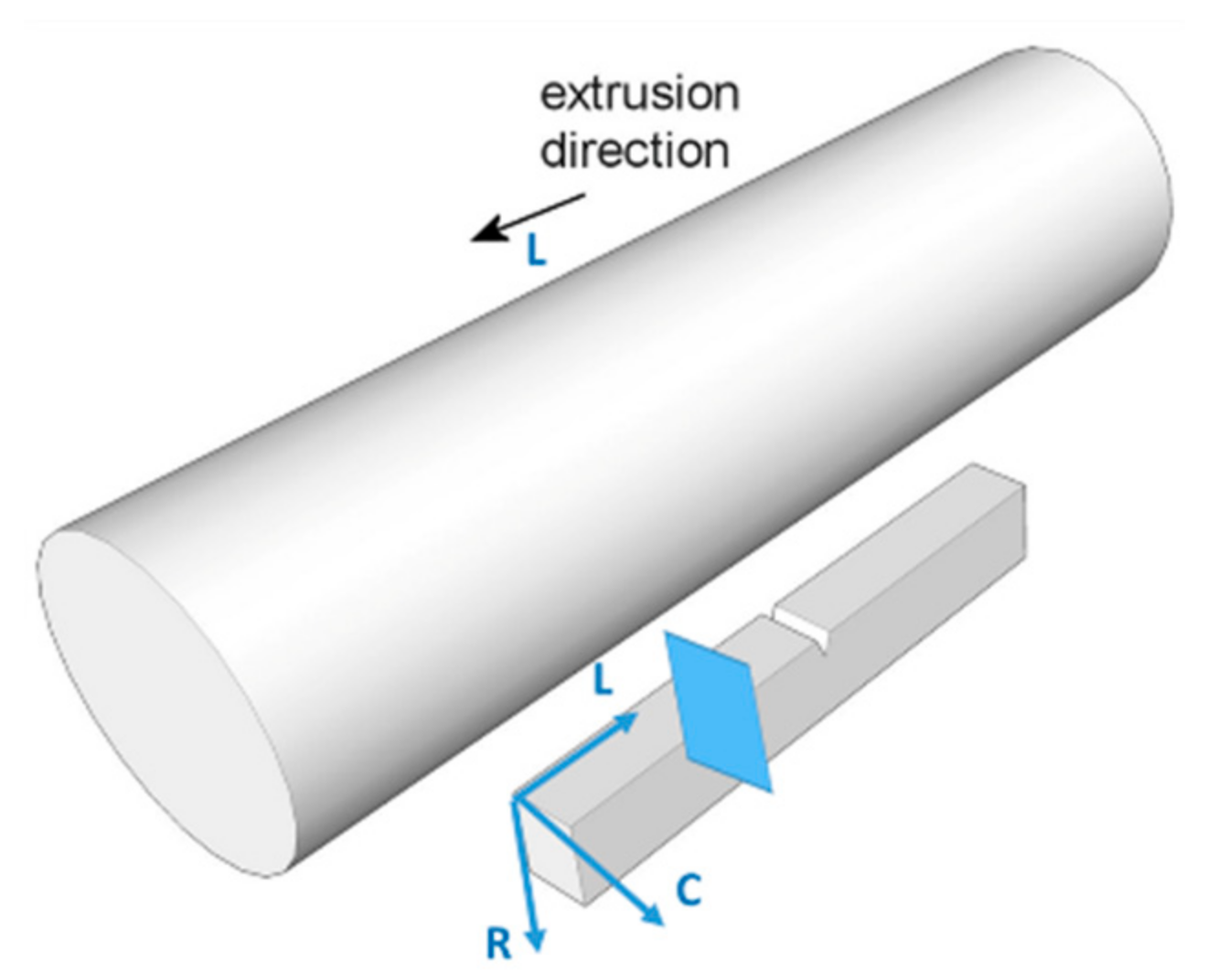
2.3. Specimens
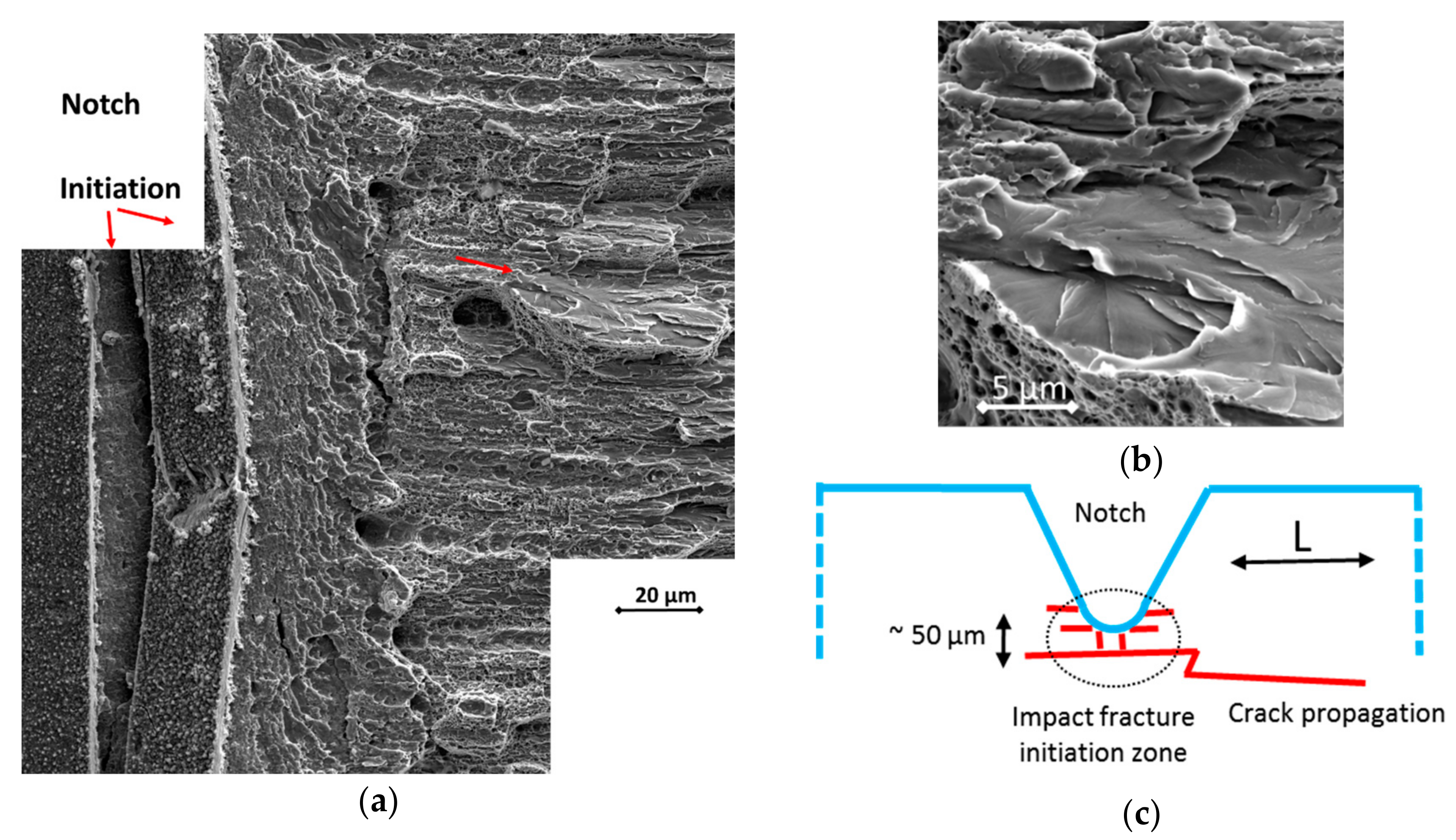
2.4. Mechanical Testing
2.5. Microscopy
3. Results
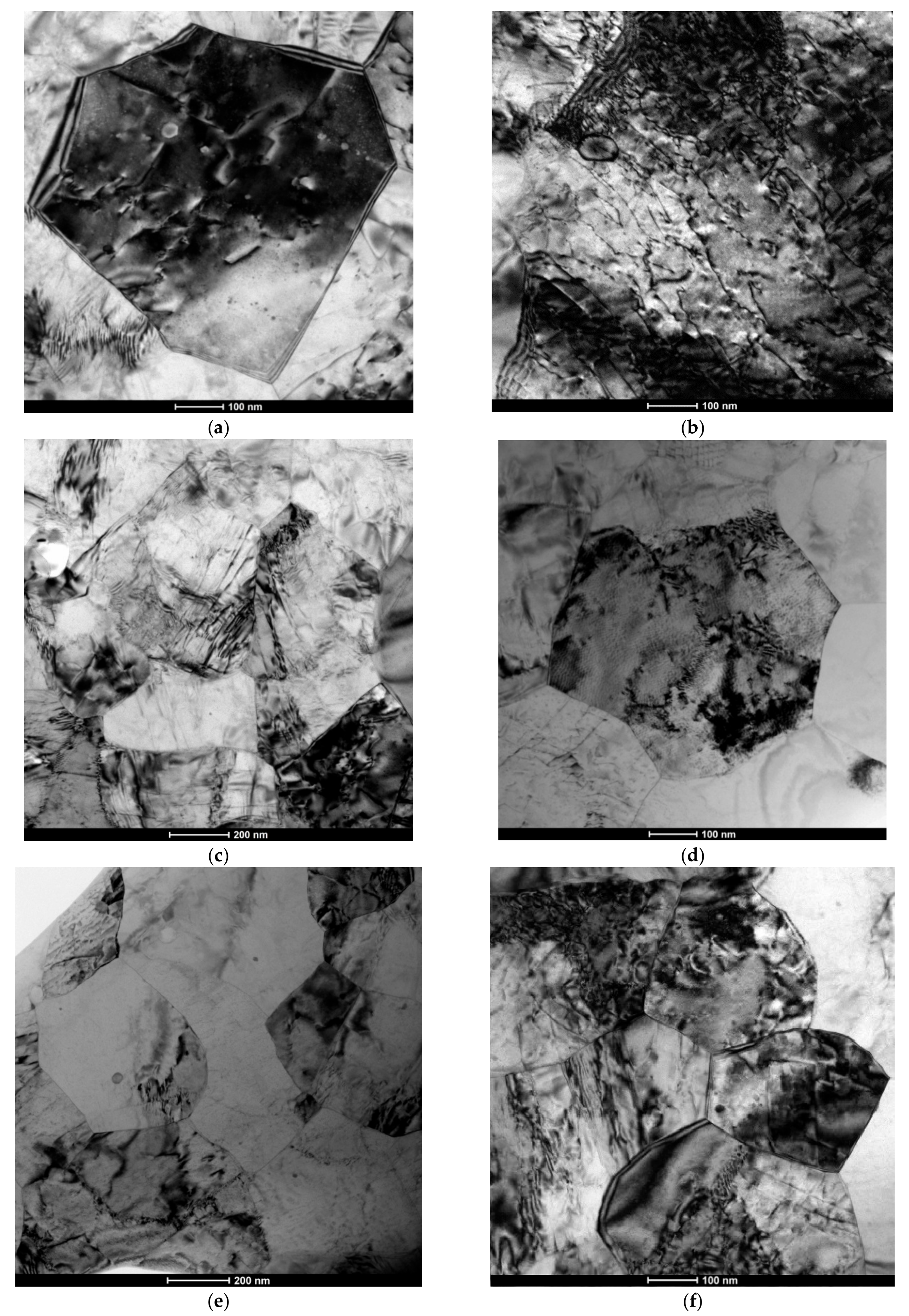
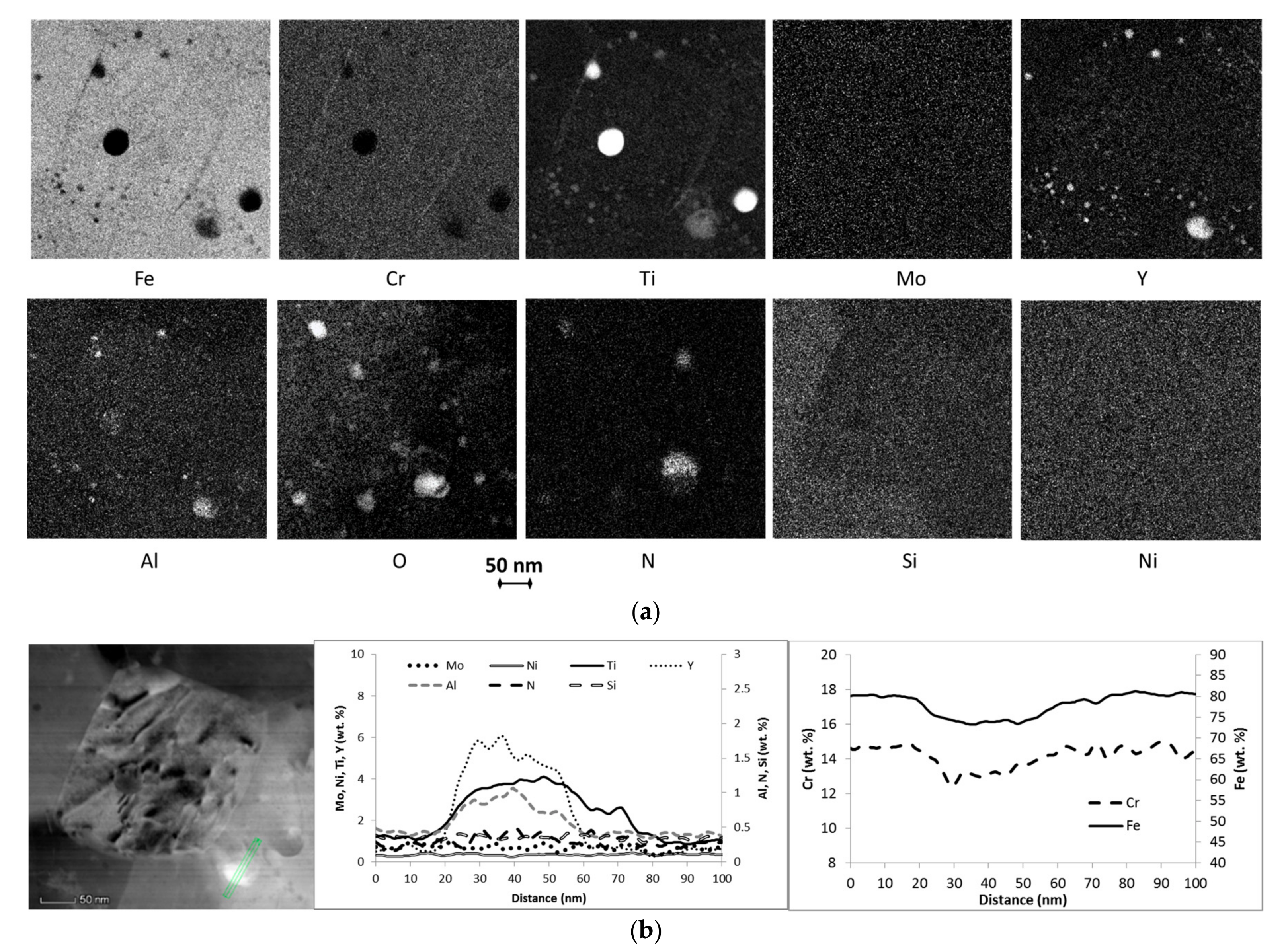
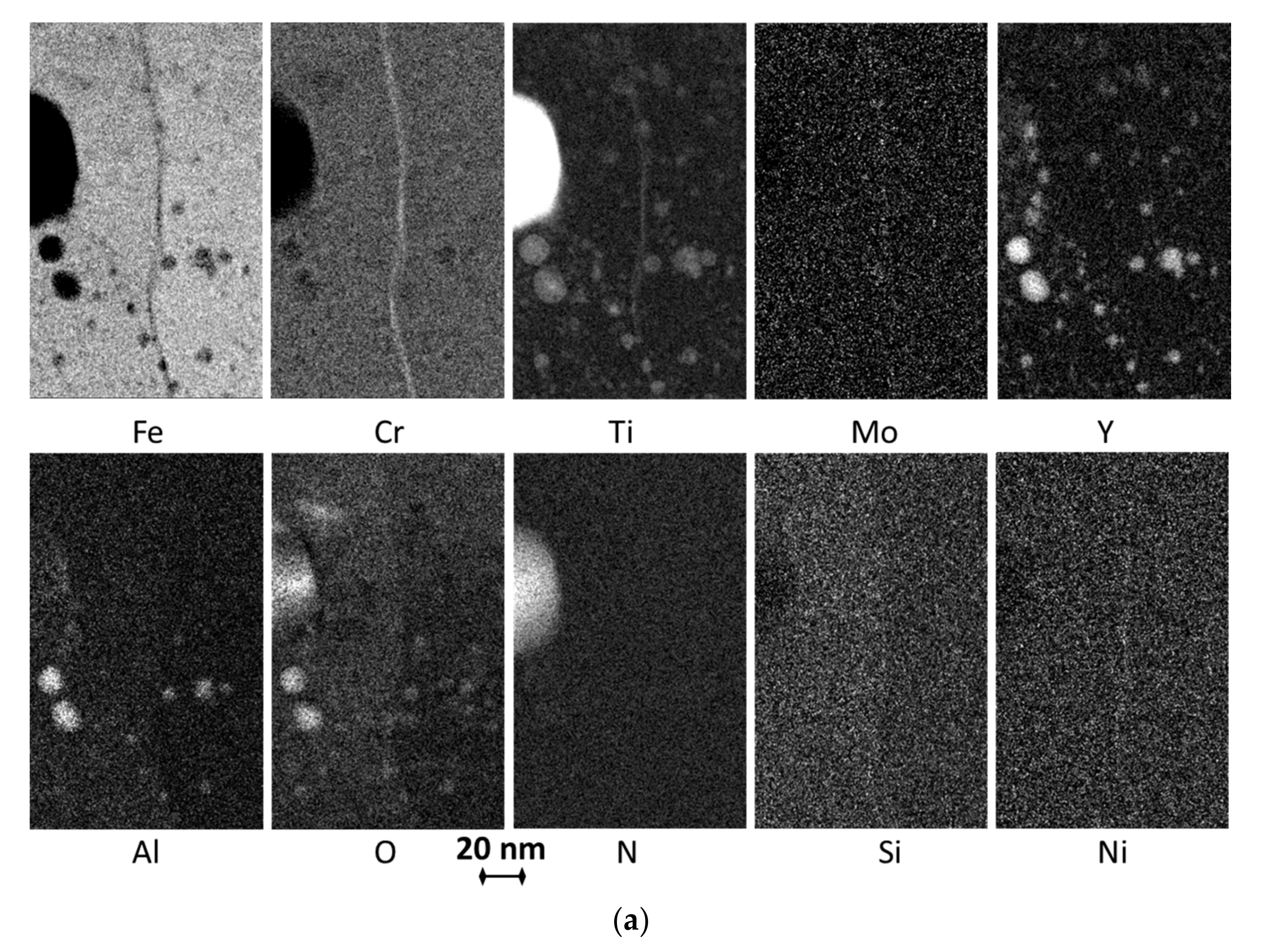
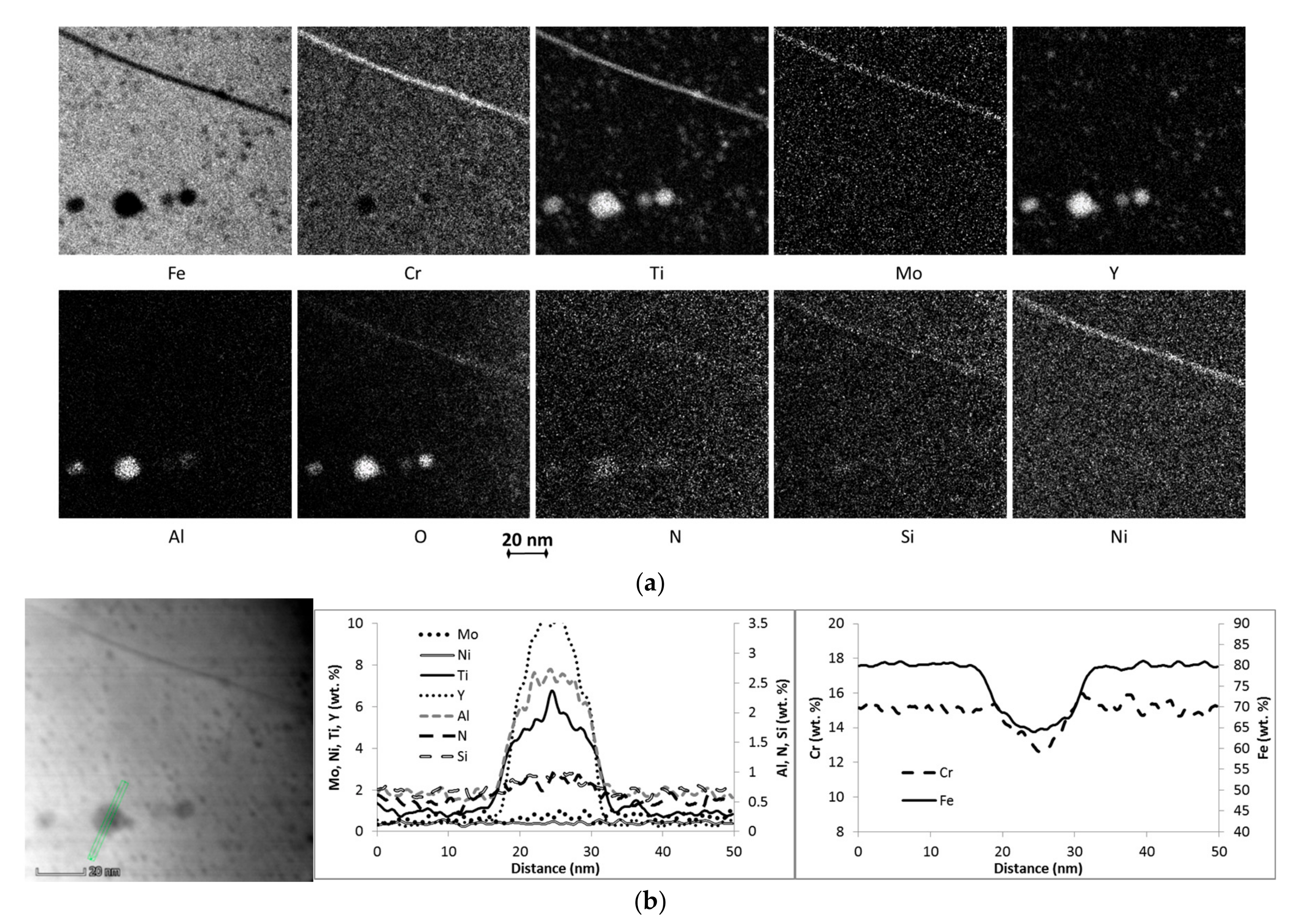
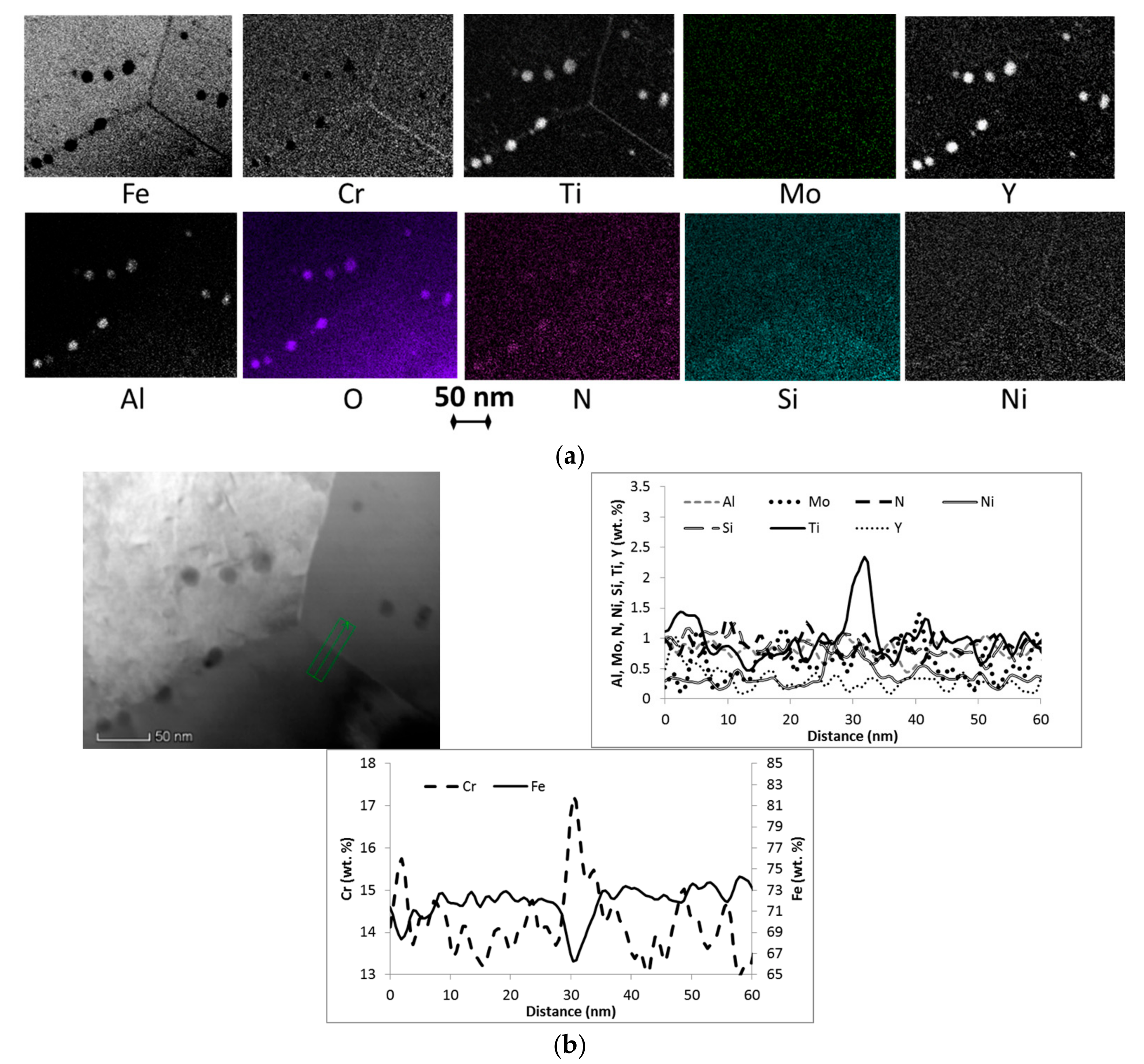
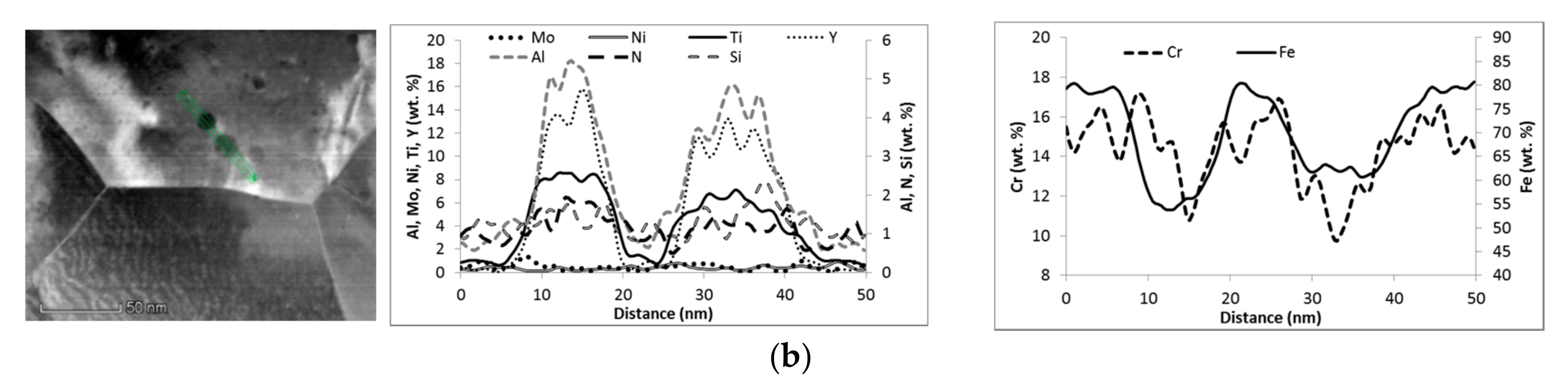
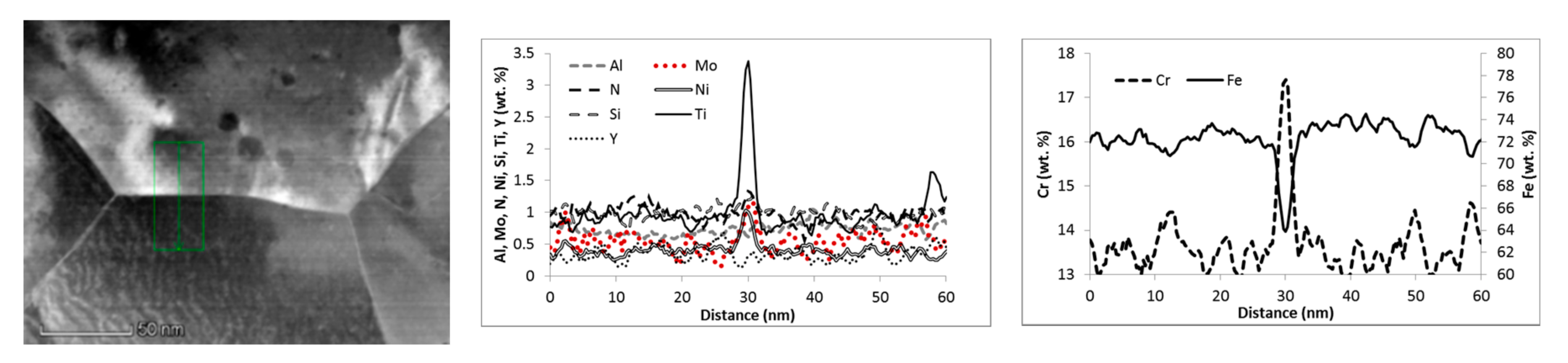
3.1. Microstructure
3.2. Effect of High-Temperature Environment on the Surface
3.3. Fracture Behavior
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| List of Acronyms | |
| AR | As-Received |
| BSE | Back Scattered Electrons |
| DBTT | Ductile-to-Brittle Transition Temperature |
| DCLL | Dual Coolant Lead Lithium |
| EDX | Energy Dispersive X-ray Spectroscopy |
| FM | Ferritic-Martensitic |
| GFR | Gas Fast Reactor |
| KLST | Kleinst-Proben Specimen Type (Mini Charpy) |
| LSE | Lower Shelf Energy |
| NFA | Nanostructured Ferritic Alloys |
| ODS | Oxide Dispersion Strengthened |
| SEM | Scanning Electron Microscope |
| STEM | Scanning Transmission Electron Microscope |
| TEM | Transmission Electron Microscope |
| USE | Upper Shelf Energy |
References
- Alinger, M.J.; Odette, G.R.; Lucas, G.E. Tensile and fracture toughness properties of MA957: Implications to the development of nanocomposited ferritic alloys. J. Nucl. Mater. 2002, 307–311, 484–489. [Google Scholar] [CrossRef]
- Alamo, A.; Lambard, V.; Averty, X.; Mathon, M.H. Assessment of ODS–14%Cr ferritic alloy for high temperature applications. J. Nucl. Mater. 2004, 329–333, 333–337. [Google Scholar] [CrossRef]
- Kurtz, R.J.; Alamo, A.; Lucon, E.; Huang, Q.; Jitsukawa, S.; Kimura, A.; Klueh, R.L.; Odette, G.R.; Petersen, C.; Sokolov, M.A.; et al. Recent progress toward development of reduced activation ferritic/martensitic steels for fusion structural applications. J. Nucl. Mater. 2009, 386–388, 411–417. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Byun, T.S.; Hoelzer, D.T.; Kim, J.H.; Maloy, S.A. A comparative assessment of the fracture toughness behavior of ferritic-martensitic steels and nanostructured ferritic alloys. J. Nucl. Mater. 2017, 484, 157–167. [Google Scholar] [CrossRef]
- Hadraba, H.; Kazimierzak, B.; Stratil, L.; Dlouhy, I. Microstructure and impact properties of ferritic ODS ODM401 (14%Cr-ODS of MA957 type). J. Nucl. Mater. 2011, 417, 241–244. [Google Scholar] [CrossRef]
- Rouffié, A.L.; Crépin, J.; Sennour, M.; Tanguy, B.; Pineau, A.; Hamon, D.; Wident, P.; Vincent, S.; Garat, V.; Fournier, B. Influences of process parameters and microstructure on the fracture mechanisms of ODS steels. J. Nucl. Mater. 2013, 433, 108–115. [Google Scholar] [CrossRef]
- Hadraba, H.; Fournier, B.; Stratil, L.; Malaplate, J.; Rouffié, A.-L.; Wident, P.; Ziolek, L.; Béchade, J.-L. Influence of microstructure on impact properties of 9–18%Cr ODS steels for fusion/fission applications. J. Nucl. Mater. 2011, 411, 112–118. [Google Scholar] [CrossRef]
- Hojna, A.; Di Gabriele, F.; Hadraba, H.; Husak, R.; Kubena, I.; Rozumova, L.; Bublikova, P.; Kalivodova, J.; Matejicek, J. Fracture behaviour of the 14Cr ODS steel exposed to helium and liquid lead. J. Nucl. Mater. 2017, 490, 143–154. [Google Scholar] [CrossRef]
- Lejček, P. Grain Boundary Segregation in Metals, 1st ed.; Springer Series in Material Science 136; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-12504-1. [Google Scholar]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. The development and stability of Y-Ti-O nanoclusters in mechanically alloyed Fe-Cr based ferritic alloys. J. Nucl. Mater. 2004, 329–333, 382–386. [Google Scholar] [CrossRef]
- Li, S.F.; Zhou, Z.J.; Wang, P.H.; Sun, H.Y.; Wang, M.; Zhang, G.M. Long-term thermal-aging stability of a 16Cr-oxide dispersion strengthened ferritic steel at 973 K. Mater. Des. 2016, 90, 318–329. [Google Scholar] [CrossRef]
- Dawson, K.; Tatlock, G.J. Characterisation of nanosized oxides in ODM401 oxide dispersion strengthened steel. J. Nucl. Mater. 2014, 444, 252–260. [Google Scholar] [CrossRef]
- Wong, C.P.C.; Abdou, M.; Dagher, M.; Katoh, Y.; Kurtz, R.J.; Malang, S.; Marriott, E.P.; Merrill, B.J.; Messadek, K.; Morley, N.B.; et al. An overview of the US DCLL ITER-TBM program. Fusion Eng. Des. 2010, 85, 1129–1132. [Google Scholar] [CrossRef]
- Stainsby, R.; Peers, K.; Mitchell, C.; Poette, C.; Mikityuk, K.; Somers, J. Gas cooled fast reactor research in Europe. Nucl. Eng. Des. 2011, 241, 3481–3489. [Google Scholar] [CrossRef]
- Lejček, P.; Šob, M.; Paidar, V. Interfacial segregation and grain boundary embrittlement: An overview and critical assessment of experimental data and calculated results. Prog. Mater. Sci. 2017, 87, 83–139. [Google Scholar] [CrossRef]
- Anderson, T.L. Fracture Mechanics: Fundamentals and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA; Ann Arbor, MI, USA; London, UK; Tokyo, Japan, 1995. [Google Scholar]
- Chauhan, A.; Bergner, F.; Etienne, A.; Aktaa, J.; de Carlan, Y.; Heintze, C.; Litvinov, D.; Hernandez-Mayoral, M.; Onorbe, E.; Radiguet, B.; et al. Microstructure characterization and strengthening mechanisms of oxide dispersion strengthened (ODS) Fe–9%Cr and Fe–14%Cr extruded bars. J. Nucl. Mater. 2017, 495, 6–19. [Google Scholar] [CrossRef]
- Fournier, B.; Steckmeyer, A.; Rouffie, A.-L.; Malaplate, J.; Garnier, J.; Ratti, M.; Wident, P.; Ziolek, L.; Tournie, I.; Rabeau, V.; et al. Mechanical behaviour of ferritic ODS steels—Temperature dependance and anisotropy. J. Nucl. Mater. 2012, 430, 142–149. [Google Scholar] [CrossRef]
- Pineau, A.; Benzerga, A.A.; Pardoen, T. Failure of metals I: Brittle and ductile fracture. Acta Mater. 2016, 107, 424–483. [Google Scholar] [CrossRef]
- Pineau, A.; Benzerga, A.A.; Pardoen, T. Failure of metals III: Fracture and fatigue of nanostructured metallic materials. Acta Mater. 2016, 107, 508–544. [Google Scholar] [CrossRef]



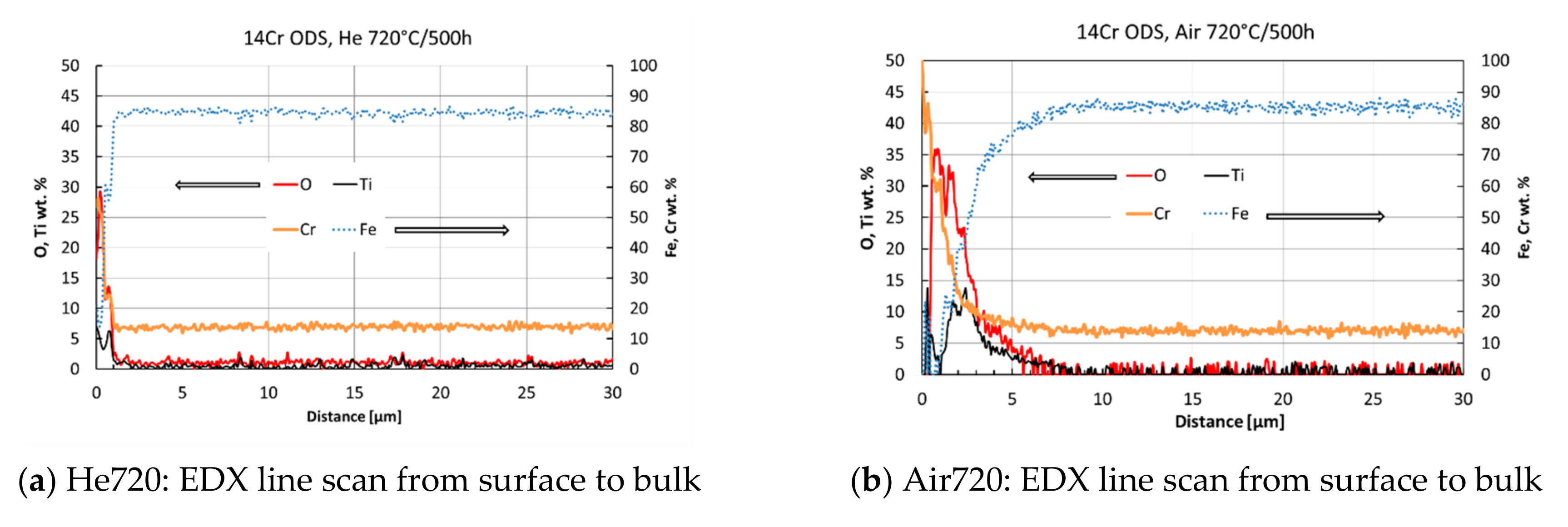
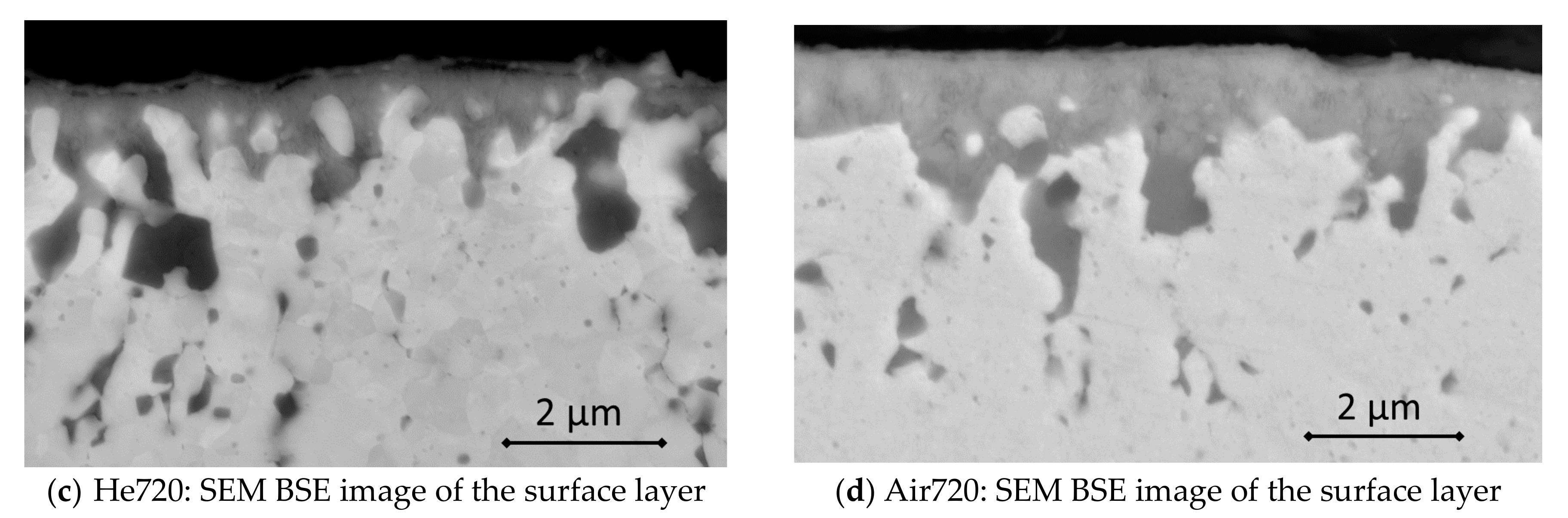
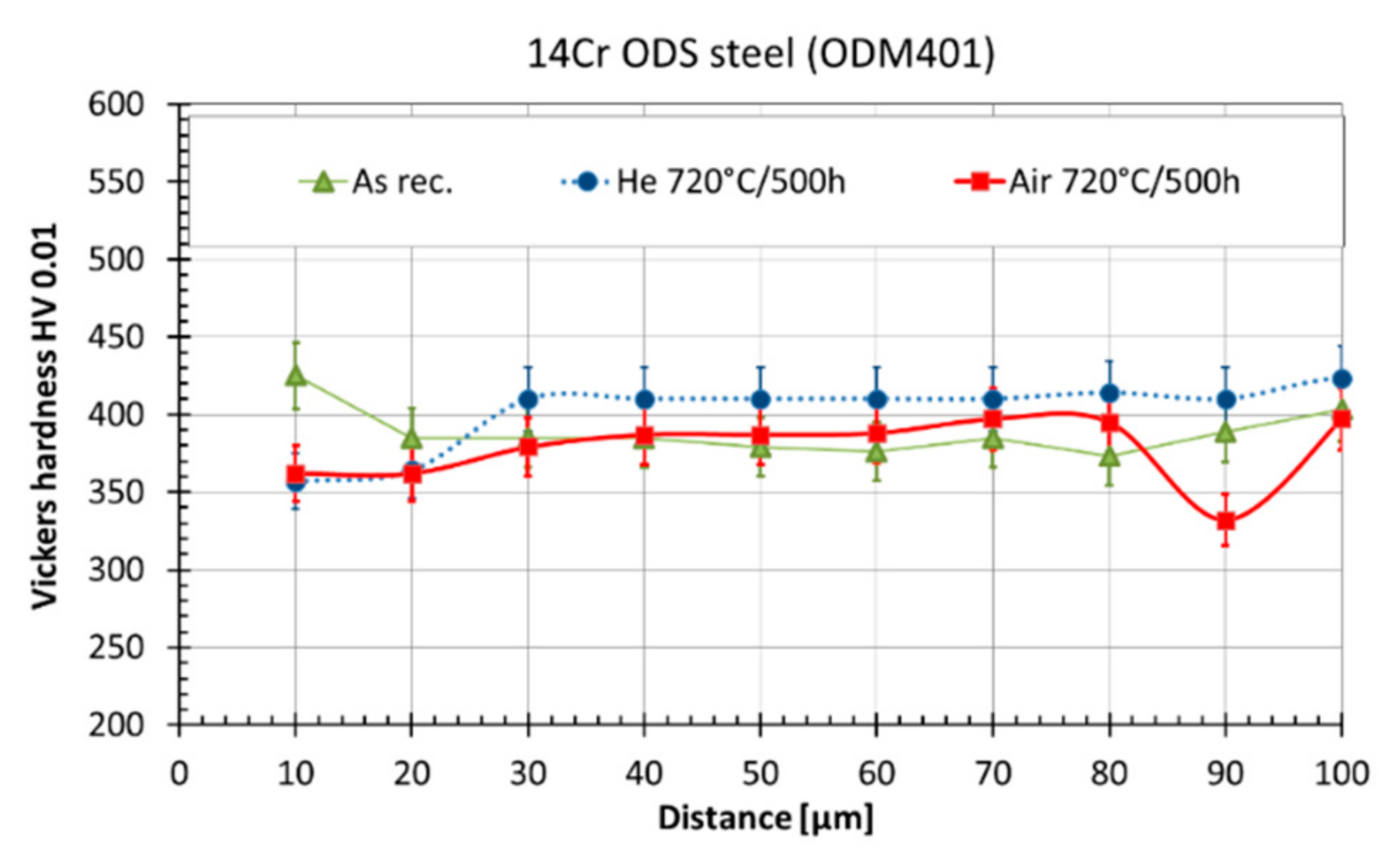
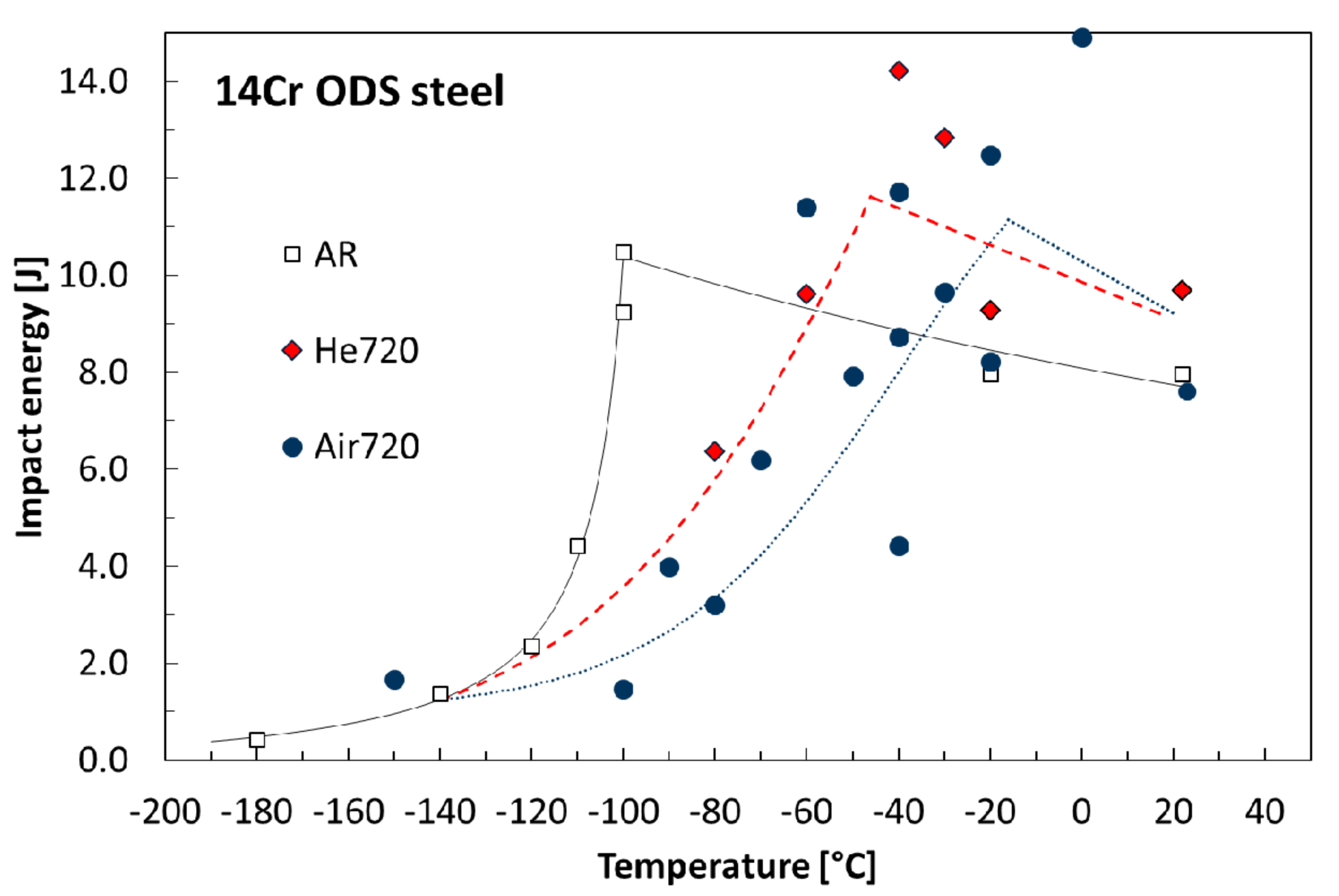

| Element | Fe | C | Cr | Ti | Mo | Y2O3 | Y | Al | O | N | Si | Ar | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14Cr ODS bar 1 | bal. | 0.024 | 13.6 | 0.85 | 0.29 | 0.25 | - | 0.06 | 0.182 | 0.048 | - | - | - |
| AR TEM-EDX 2 | 74.8 | n.a. | 14.15 | 0.93 | 0.55 | n.a. | 0.35 | 0.37 | n.a. 3 | 0.43 | 0.47 | 0.37 | 0.32 |
| Material Condition | Average Grain Size, nm | Cavity Density/Average Size, %/µm | Composition 1 of Particles >10 nm, wt. % | Grain Boundary Local Chemistry 1, wt. % |
|---|---|---|---|---|
| AR | 380 ± 140 | 0.3%/1.2 ± 0.3 | ~75Fe, 6-27Y 2, 8-13Cr, 3-14Ti, 1.5-2.5Al, 0.3Si, 0.5-1N, 0.4Ni | ~71Fe, 16Cr, 1.5Ti, 1Mo, 0.4Al, 0.4N, 0.4Si,0.4Y, 0.4Ni |
| Air720 | n.a. | 0.2%/1.5 ± 0.4 | ~55Fe, 8-16Y 2, 10-12Cr, 4-8Ti, 1-5Al, 1.2-2.3Si, 1-2N, 0.4-0.6Ni | ~64Fe, 17.5Cr, 3.4Ti, 1.2Mo, 0.8Al, 1.3N, 1.2Si, 0.3-0.4Y, 1Ni |
| He720 | 360 ± 150 | 0.2%/1.7 ± 0.4 | ~65Fe, 7-21Y, 8-12Cr, 5-15Ti, 1.5-5Al, 1Si, 1-2N, 0.4Ni | ~66Fe, 17Cr, 2.3Ti, 0.8Mo, 0.8-1Al, 0.9N, 1Si, 0.3Y, 0.6-0.8Ni |
| Material Condition | Rp0.2 (20 °C), MPa | Rm (20 °C), MPa | Rfr (20 °C), MPa | HV 0.01 50 µm below Surface | DBTT 1, °C |
|---|---|---|---|---|---|
| AR | 1005 | 1136 | 760 | 380 ± 20 | −106 ± 5 |
| Air720 | n.a. | n.a. | n.a. | 390 ± 20 | −50 ± 10 |
| He720 | 1031 | 1147 | 749 | 410 ± 20 | −70 ± 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojna, A.; Michalicka, J.; Hadraba, H.; Di Gabriele, F.; Duchon, J.; Rozumova, L.; Husak, R. Fracture Resistance of 14Cr ODS Steel Exposed to a High Temperature Gas. Metals 2017, 7, 560. https://doi.org/10.3390/met7120560
Hojna A, Michalicka J, Hadraba H, Di Gabriele F, Duchon J, Rozumova L, Husak R. Fracture Resistance of 14Cr ODS Steel Exposed to a High Temperature Gas. Metals. 2017; 7(12):560. https://doi.org/10.3390/met7120560
Chicago/Turabian StyleHojna, Anna, Jan Michalicka, Hynek Hadraba, Fosca Di Gabriele, Jan Duchon, Lucia Rozumova, and Roman Husak. 2017. "Fracture Resistance of 14Cr ODS Steel Exposed to a High Temperature Gas" Metals 7, no. 12: 560. https://doi.org/10.3390/met7120560





