Antibacterial Properties and Corrosion Resistance of the Newly Developed Biomaterial, Ti–12Nb–1Ag Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Conditions for Heat Treatment
2.3. Preparation of Experimental Samples
2.4. Analysis of Antibacterial Properties
2.4.1. Antibacterial Assay of Experimental Samples
2.4.2. Bacterial Count
2.5. Electrochemical Analysis
2.6. Analysis of Microstructure
3. Results
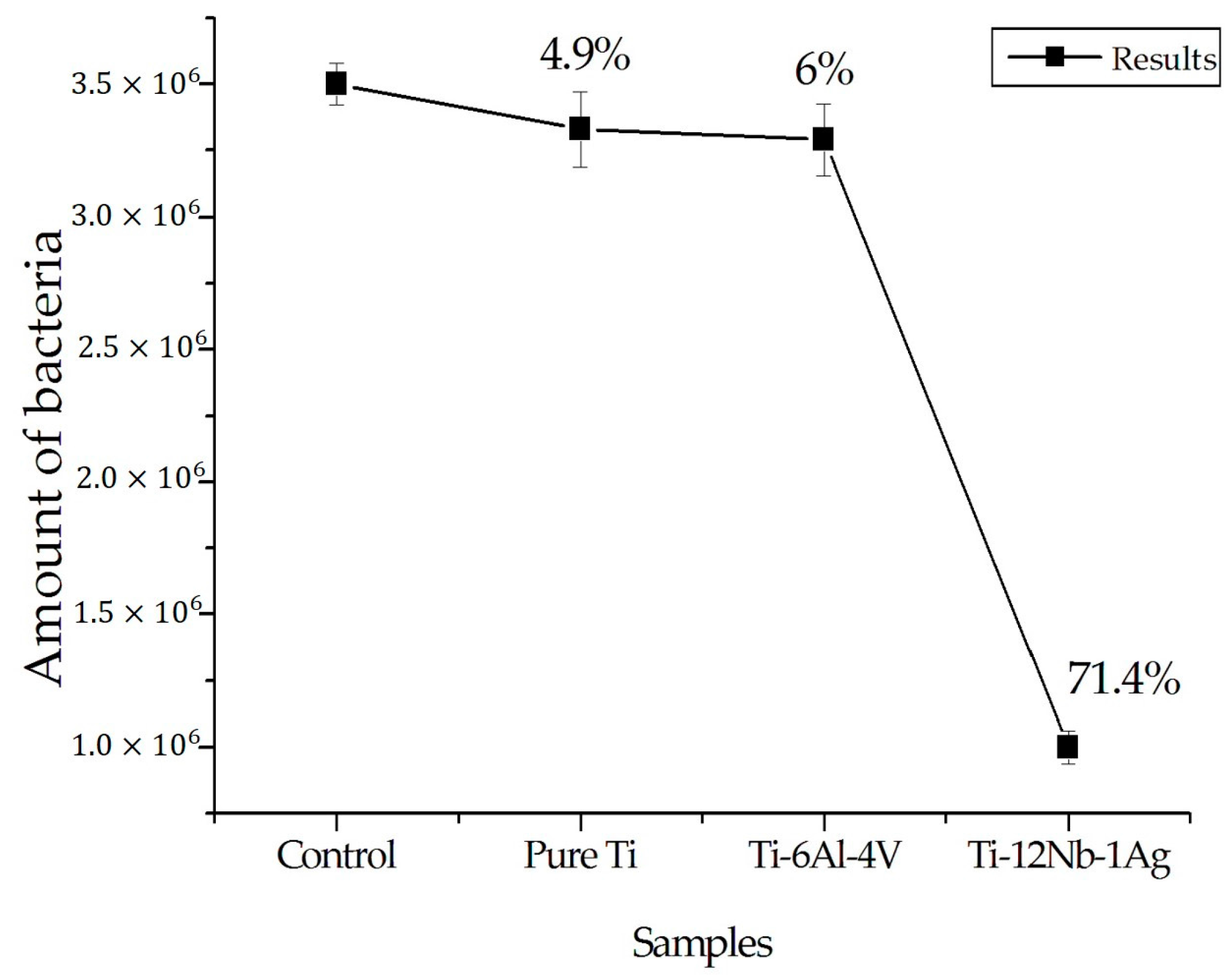
3.1. E. coli Antibacterial Testing of Ti–12Nb–1Ag
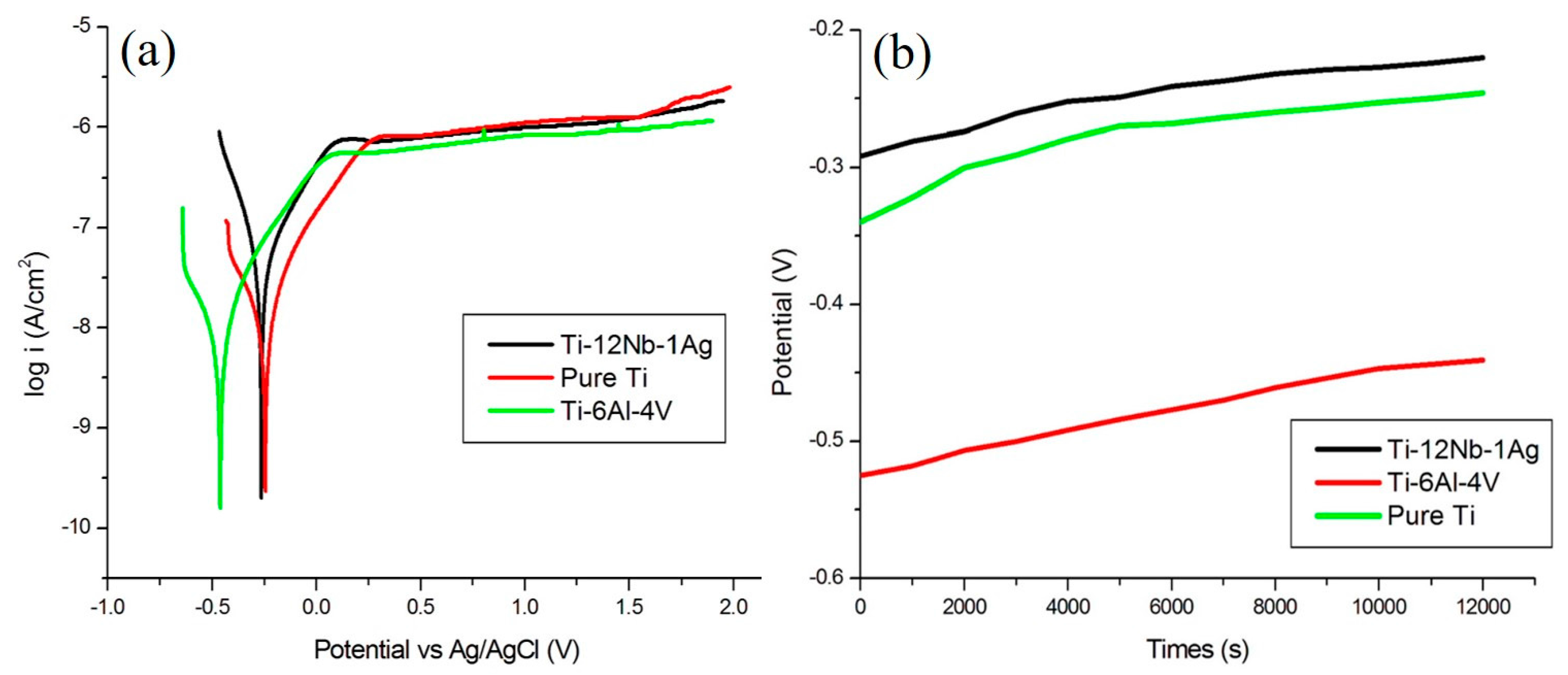
3.2. Electrochemical Testing of Ti–12Nb–1Ag
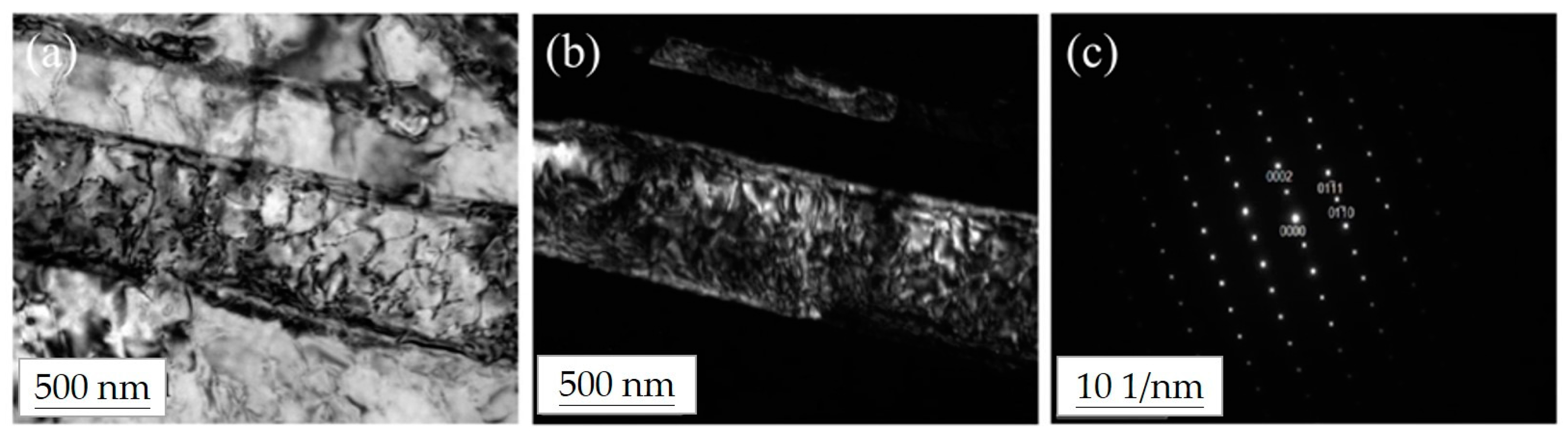
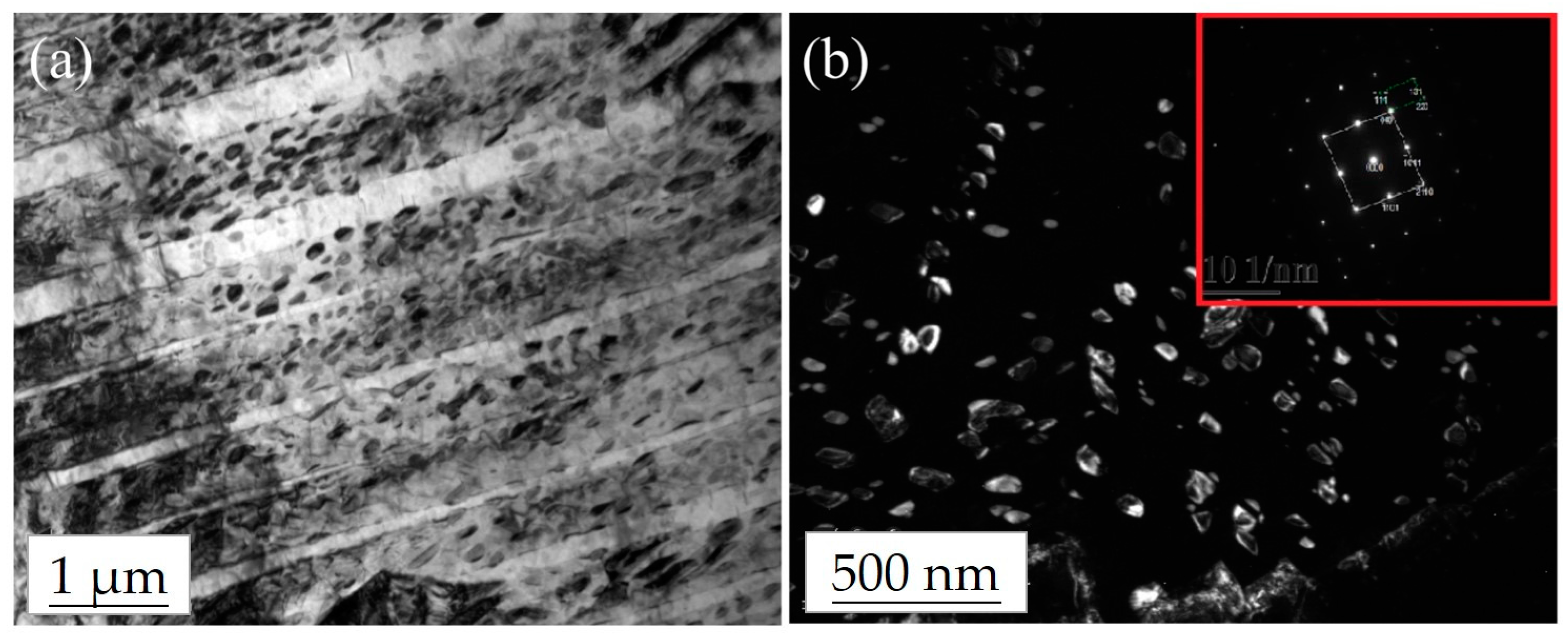
3.3. Microstructural Analysis of the Ti–12Nb–1Ag Alloy
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsu, H.-C.; Wu, S.-C.; Hsu, S.-K.; Syu, J.-Y.; Ho, W.-F. The structure and mechanical properties of as-cast Ti–25Nb–xSn alloys for biomedical applications. Mater. Sci. Eng. A 2013, 568, 1–7. [Google Scholar] [CrossRef]
- Lopes, E.S.N.; Cremasco, A.; Afonso, C.R.M.; Caram, R. Effects of double aging heat treatment on the microstructure, vickers hardness and elastic modulus of Ti–Nb alloys. Mater. Charact. 2011, 62, 673–680. [Google Scholar] [CrossRef]
- Mantani, Y.; Tajima, M. Phase transformation of quenched α″ martensite by aging in Ti–Nb alloys. Mater. Sci. Eng. A 2006, 438–440, 315–319. [Google Scholar] [CrossRef]
- Liu, H.; Niinomi, M.; Nakai, M.; Hieda, J.; Cho, K. Deformation-induced changeable Young’s modulus with high strength in β-type Ti–Cr–O alloys for spinal fixture. J. Mech. Behav. Biomed. Mater. 2014, 30, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.J.; Wang, Y.B.; Cheng, Y.; Deng, F.; Zheng, Y.F.; Wei, S.C. Comparative study on the corrosion behavior of Ti–Nb and TMA alloys for dental application in various artificial solutions. Mater. Sci. Eng. C 2011, 31, 702–711. [Google Scholar] [CrossRef]
- Latifi, A.; Imani, M.; Khorasani, M.T.; Joupari, M.D. Electrochemical and chemical methods for improving surface characteristics of 316L stainless steel for biomedical applications. Surf. Coat. Technol. 2013, 221, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Ding, M.H.; Zheng, H.; Zhang, H.S.; Zhang, B.; Li, X.Q.; Wu, G.Y. Surface modification of biomedical AISI 316L stainless steel with zirconium carbonitride coatings. Appl. Surf. Sci. 2015, 340, 113–119. [Google Scholar] [CrossRef]
- Zalnezhad, E.; Hamouda, A.M.S.; Faraji, G.; Shamshirband, S. TiO2 nanotube coating on stainless steel 304 for biomedical applications. Ceram. Int. 2015, 41, 2785–2793. [Google Scholar] [CrossRef]
- Huang, H.-H.; Wu, C.-P.; Sun, Y.-S.; Lee, T.-H. Improvements in the corrosion resistance and biocompatibility of biomedical Ti–6Al–7Nb alloy using an electrochemical anodization treatment. Thin Solid Films 2013, 528, 157–162. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Mozetic, P.; Rainer, A.; Trombetta, M. Biological response of human mesenchymal stromal cells to titanium grade 4 implants coated with PCL/ZrO2 hybrid materials synthesized by sol–gel route: In vitro evaluation. Mater. Sci. Eng. C 2014, 45, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-S.; Lee, K.-J.; Kwon, E.-P.; Tsuchiyama, T.; Takaki, S. Variation of work hardening rate by oxygen contents in pure titanium alloy. Mater. Sci. Eng. A 2015, 632, 120–126. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Xu, L.; Liu, Z.; Woo, K.-D. Effects of Sn content on the microstructure, mechanical properties and biocompatibility of Ti–Nb–Sn/hydroxyapatite biocomposites synthesized by powder metallurgy. Mater. Des. 2013, 49, 511–519. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Gordin, D.-M.; Ungureanu, G.; Gloriant, T. Comparative corrosion study of Ti–Ta alloys for dental applications. Acta Biomater. 2009, 5, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Taddei, E.B.; Henriques, V.A.R.; Silva, C.R.M.; Cairo, C.A.A. Production of new titanium alloy for orthopedic implants. Mater. Sci. Eng. C 2004, 24, 683–687. [Google Scholar] [CrossRef]
- Yu, S.; Yu, Z.; Wang, G.; Han, J.; Ma, X.; Dargusch, M.S. Biocompatibility and osteoconduction of active porous calcium–phosphate films on a novel Ti–3Zr–2Sn–3Mo–25Nb biomedical alloy. Colloids Surf. B Biointerfaces 2011, 85, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.M.G.; Ramos, W.S.; de Blas, J.C.G.; Lopes, E.S.N.; Caram, R.; Batista, W.W.; Souza, S.A. Influence of Si addition on the microstructure and mechanical properties of Ti–35Nb alloy for applications in orthopedic implants. J. Mech. Behav. Biomed. Mater. 2015, 51, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Tobe, H.; Kim, H.Y.; Inamura, T.; Hosoda, H.; Nam, T.H.; Miyazaki, S. Effect of Nb content on deformation behavior and shape memory properties of Ti–Nb alloys. J. Alloys Compd. 2013, 577, S435–S438. [Google Scholar] [CrossRef]
- Elias, L.M.; Schneider, S.G.; Schneider, S.; Silva, H.M.; Malvisi, F. Microstructural and mechanical characterization of biomedical Ti–Nb–Zr(–Ta) alloys. Mater. Sci. Eng. A 2006, 432, 108–112. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Cheng, X.; Zhao, X. A metastable β-type Ti–Nb binary alloy with low modulus and high strength. J. Alloys Compd. 2015, 644, 411–415. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Wu, W.T.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Liu, Y.; Wu, H.; Song, M.; Zhang, T.-Y.; Lan, X.-D.; Yao, T.-H. Elastic modulus of phases in Ti–Mo alloys. Mater. Charact. 2015, 106, 302–307. [Google Scholar] [CrossRef]
- Xie, F.; He, X.; Cao, S.; Mei, M.; Qu, X. Influence of pore characteristics on microstructure, mechanical properties and corrosion resistance of selective laser sintered porous Ti–Mo alloys for biomedical applications. Electrochim. Acta 2013, 105, 121–129. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Wu, H.; Song, M.; Wang, W.; Li, N.; Tang, H. Synthesis of Ti–Ta alloys with dual structure by incomplete diffusion between elemental powders. J. Mech. Behav. Biomed. Mater. 2015, 51, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Kesteven, J.; Kannan, M.B.; Walter, R.; Khakbaz, H.; Choe, H.-C. Low elastic modulus Ti–Ta alloys for load-bearing permanent implants: Enhancing the biodegradation resistance by electrochemical surface engineering. Mater. Sci. Eng. C 2015, 46, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Khalid, F.A.; Zaigham, H.; Abidi, I.H. Superelastic behaviour of Ti–Nb–Al ternary shape memory alloys for biomedical applications. Mater. Lett. 2014, 121, 58–61. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ikehara, Y.; Kim, J.I.; Hosoda, H.; Miyazaki, S. Martensitic transformation, shape memory effect and superelasticity of Ti–Nb binary alloys. Acta Mater. 2006, 54, 2419–2429. [Google Scholar] [CrossRef]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-doped nanocomposite carbon coatings (Ag-DLC) for biomedical applications—Physiochemical and biological evaluation. Appl. Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- Gimeno, M.; Pinczowski, P.; Pérez, M.; Giorello, A.; Martínez, M.Á.; Santamaría, J.; Arruebo, M.; Luján, L. A controlled antibiotic release system to prevent orthopedic-implant associated infections: An in vitro study. Eur. J. Pharm. Biopharm. 2015, 96, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.R.; Ponmozhi, J.; Campos, J.B.L.M.; Miranda, J.M.; Mergulhão, F.J. Micro- and macro-flow systems to study Escherichia coli adhesion to biomedical materials. Chem. Eng. Sci. 2015, 126, 440–445. [Google Scholar] [CrossRef]
- Fordham, W.R.; Redmond, S.; Westerland, A.; Cortes, E.G.; Walker, C.; Gallagher, C.; Medina, C.J.; Waecther, F.; Lunk, C.; Ostrum, R.F.; et al. Silver as a bactericidal coating for biomedical implants. Surf. Coat. Technol. 2014, 253, 52–57. [Google Scholar] [CrossRef]
- Li, M.; Nan, L.; Xu, D.; Ren, G.; Yang, K. Antibacterial performance of a Cu-bearing stainless steel against microorganisms in tap water. J. Mater. Sci. Technol. 2015, 31, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Pang, L.Q.; Zhong, L.J.; Zhou, H.F.; Wu, X.E.; Chen, X.D. Grafting of ionic liquids on stainless steel surface for antibacterial application. Colloids Surf. B Biointerfaces 2015, 126, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Gao, L.; Liu, E.; Yu, F.; Shu, X.; Wang, H. Microstructure, corrosion and tribological and antibacterial properties of Ti–Cu coated stainless steel. J. Mech. Behav. Biomed. Mater. 2015, 50, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Fojt, J.; Joska, L.; Malek, J.; Sefl, V. Corrosion behavior of Ti–39Nb alloy for dentistry. Mater. Sci. Eng. C 2015, 56, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.C.; Stan, G.E.; Enculescu, M.; Tanase, C.; Tulyaganov, D.U.; Ferreira, J.M.F. Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering. J. Mech. Behav. Biomed. Mater. 2015, 51, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Chen, C.; Zhao, Z. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ren, L.; Liu, R.; Yang, K.; Zhang, Y.; Liao, Z.; Liu, W.; Qi, M.; Misra, R.D.K. Effect of heat treatment on Cu distribution, antibacterial performance and cytotoxicity of Ti–6Al–4V–5Cu alloy. J. Mater. Sci. Technol. 2015, 31, 723–732. [Google Scholar] [CrossRef]
- Yang, S.-M.; Chen, Y.-C.; Chen, C.-H.; Huang, W.-P.; Lin, D.-Y. Microstructural characterization of δ/γ/σ/γ2/χ phases in silver-doped 2205 duplex stainless steel under 800 °C aging. J. Alloys Compd. 2015, 633, 48–53. [Google Scholar] [CrossRef]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Feng, Q.-L.; Kim, T.-N.; Wu, J.; Park, E.-S.; Kim, J.-O.; Lim, D.-Y.; Cui, F.-Z. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Films 1998, 335, 214–219. [Google Scholar] [CrossRef]
- Ou, S.-F.; Chung, R.-J.; Lin, L.-H.; Chiang, Y.-C.; Huang, C.-F.; Ou, K.-L. A mechanistic study on the antibacterial behavior of silver doped bioceramic. J. Alloys Compd. 2015, 629, 362–367. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Dubey, P.; Uday Kumar, S.; Bhushan, B.; Gopinath, P. Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces 2014, 115, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Koizhaiganova, M.; Yaşa, I.; Gülümser, G. Assessment of antibacterial activity of lining leather treated with silver doped hydroxyapatite. Int. Biodeterior. Biodegrad. 2015, 105, 262–267. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, C.; Wang, F.; Zhang, W. Experimental study and thermodynamic assessment of the Ag–Ti system. Calphad 2005, 29, 269–275. [Google Scholar] [CrossRef]
- Kang, M.-K.; Moon, S.-K.; Kwon, J.-S.; Kim, K.-M.; Kim, K.-N. Antibacterial effect of sand blasted, large-grit, acid-etched treated Ti–Ag alloys. Mater. Res. Bull. 2012, 47, 2952–2955. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, E.; Zhang, L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti–Ag sintered alloys. Mater. Sci. Eng. C 2016, 62, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C 2017, 75, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Somsanith, N.; Narayanan, T.S.N.S.; Kim, Y.-K.; Park, I.-S.; Bae, T.-S.; Lee, M.-H. Surface medication of Ti–15Mo alloy by thermal oxidation: Evaluation of surface characteristics and corrosion resistance in Ringer’s solution. Appl. Surf. Sci. 2015, 356, 1117–1126. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; Xu, Z.; Cai, F.; Zhang, Z.; Fu, P. Microstructure and corrosion behavior of Ti nanoparticles reinforced Ni–Ti composite coatings by electrodeposition. Mater. Des. 2015, 85, 39–46. [Google Scholar] [CrossRef]
- Gad El-Rab, S.M.F.; Fadl-allah, S.A.; Montser, A.A. Improvement in antibacterial properties of Ti by electrodeposition of biomimetic Ca–P apatite coat on anodized titania. Appl. Surf. Sci. 2012, 261, 1–7. [Google Scholar] [CrossRef]
- Niu, P.; Liu, B.; Li, Y.; Wang, Q.; Dong, A.; Hou, H.; Zhang, L.; Gao, Y.; Zhang, J. CdTe@SiO2/Ag nanocomposites as antibacterial fluorescent markers for enhanced latent fingerprint detection. Dyes Pigments 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Ou, K.-L.; Weng, C.-C.; Lin, Y.-H.; Huang, M.-S. A promising of alloying modified beta-type Titanium-Niobium implant for biomedical applications: Microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloys Compd. 2017, 697, 231–238. [Google Scholar] [CrossRef]
- Liao, K.-H.; Ou, K.-L.; Cheng, H.-C.; Lin, C.-T.; Peng, P.-W. Effect of silver on antibacterial properties of stainless steel. App. Surf. Sci. 2010, 256, 3642–3646. [Google Scholar] [CrossRef]
- Liu, J.; Hu, M.; Song, Y.; Wang, F.; Ji, J.; Li, Z. A novel strategy to prepare silver nanoparticles by ethanol-induced shape conversion of silver dendrites from modified galvanic replacement. Synth. Met. 2014, 187, 185–192. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Estakhri, S.; Nezhad, M.R.H.; Eshghi, S. Controlling the geometry of silver nanostructures for biological applications. Phys. Procedia 2013, 40, 76–83. [Google Scholar] [CrossRef]
- Lu, W.; Yao, K.; Wang, J.; Yuan, J. Ionic liquids–water interfacial preparation of triangular Ag nanoplates and their shape-dependent antibacterial activity. J. Colloid Interface Sci. 2015, 437, 35–41. [Google Scholar] [CrossRef] [PubMed]
| Element | Ti | Nb | Ag | O | C | Si | N | Fe | Cr |
|---|---|---|---|---|---|---|---|---|---|
| Mass % | Bal. | 12.12 | 1.05 | 0.12 | 0.04 | 0.12 | 0.03 | 0.12 | 0.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.-K.; Chao, C.-Y.; Chiu, K.-Y.; Chang, Y.-H.; Chen, K.-K.; Wu, J.-H.; Wu, J.-N. Antibacterial Properties and Corrosion Resistance of the Newly Developed Biomaterial, Ti–12Nb–1Ag Alloy. Metals 2017, 7, 566. https://doi.org/10.3390/met7120566
Du J-K, Chao C-Y, Chiu K-Y, Chang Y-H, Chen K-K, Wu J-H, Wu J-N. Antibacterial Properties and Corrosion Resistance of the Newly Developed Biomaterial, Ti–12Nb–1Ag Alloy. Metals. 2017; 7(12):566. https://doi.org/10.3390/met7120566
Chicago/Turabian StyleDu, Je-Kang, Chih-Yeh Chao, Kuan-Yu Chiu, Yen-Hao Chang, Ker-Kong Chen, Ju-Hui Wu, and Juyn-Nan Wu. 2017. "Antibacterial Properties and Corrosion Resistance of the Newly Developed Biomaterial, Ti–12Nb–1Ag Alloy" Metals 7, no. 12: 566. https://doi.org/10.3390/met7120566




