Improved Dehydrogenation Properties of 2LiNH2-MgH2 by Doping with Li3AlH6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structural Characterization and Property Evaluation
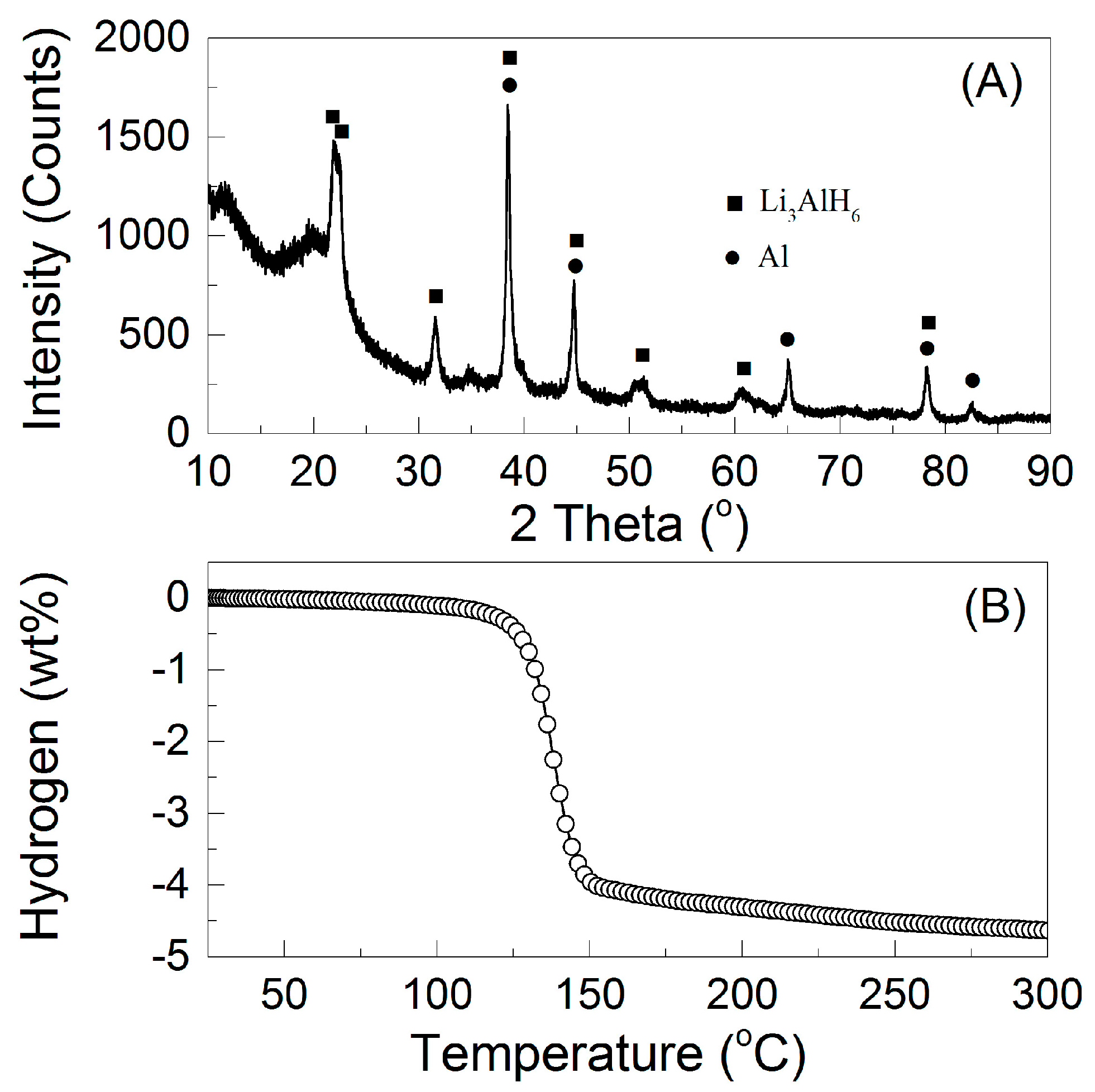
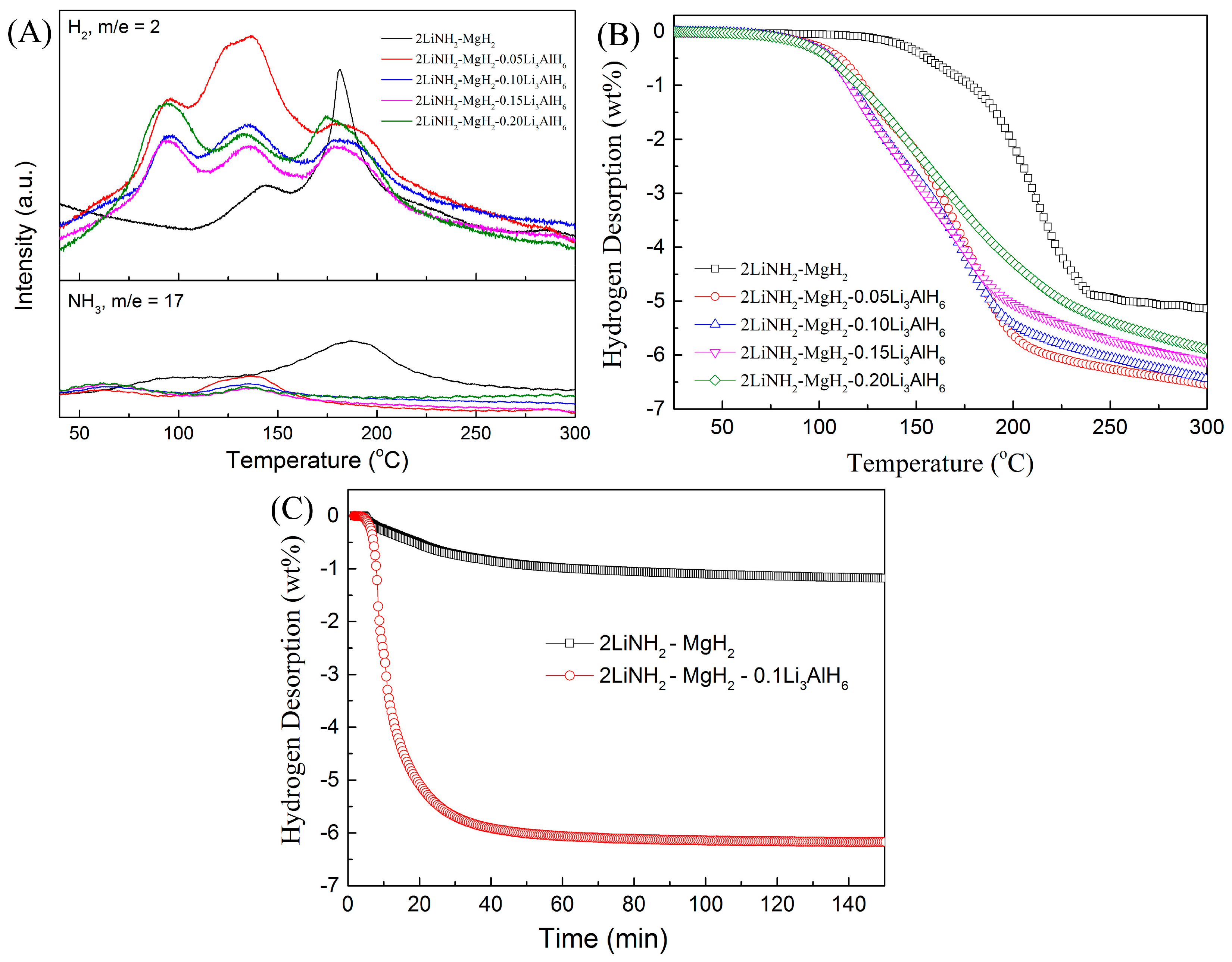
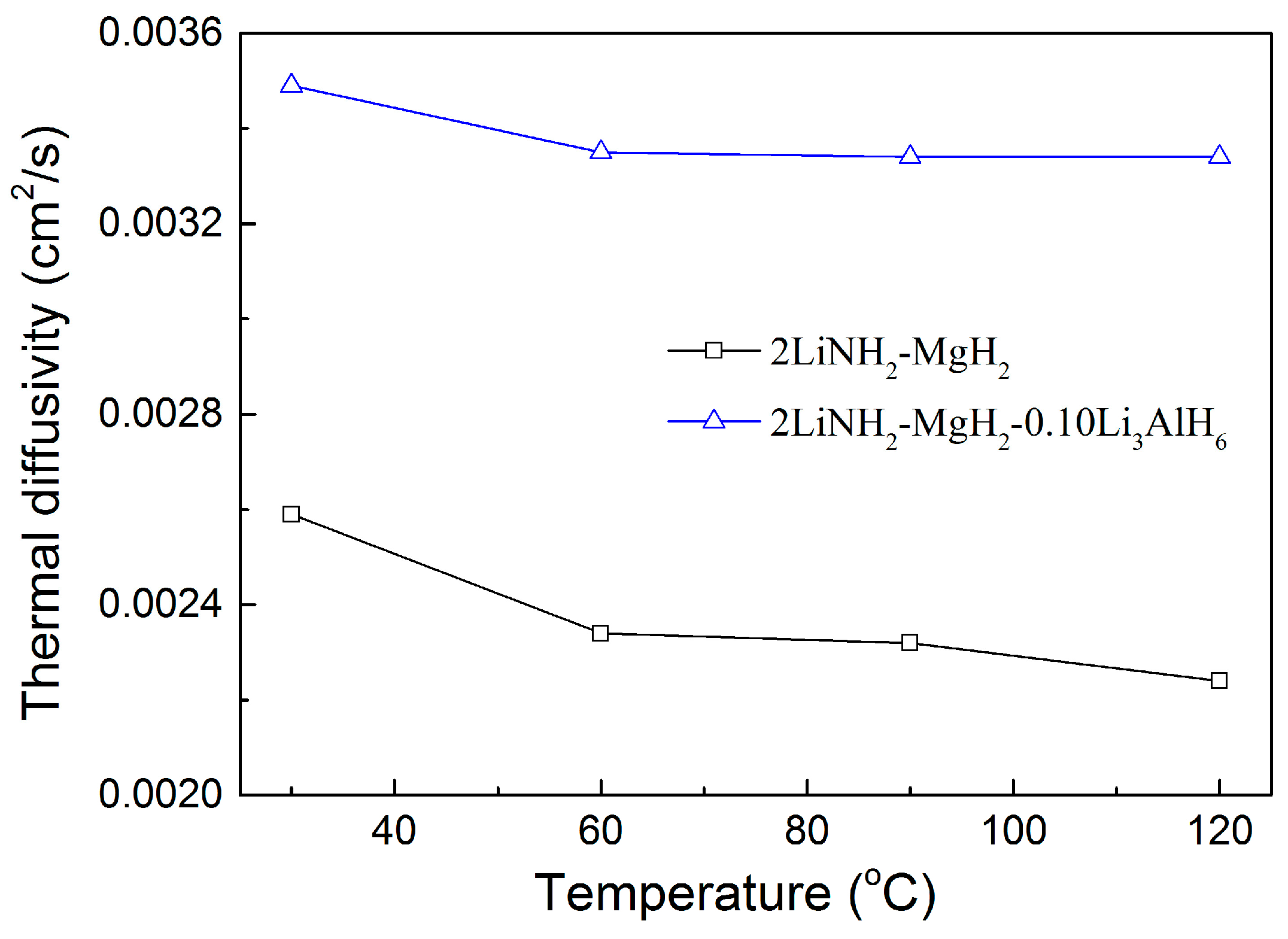
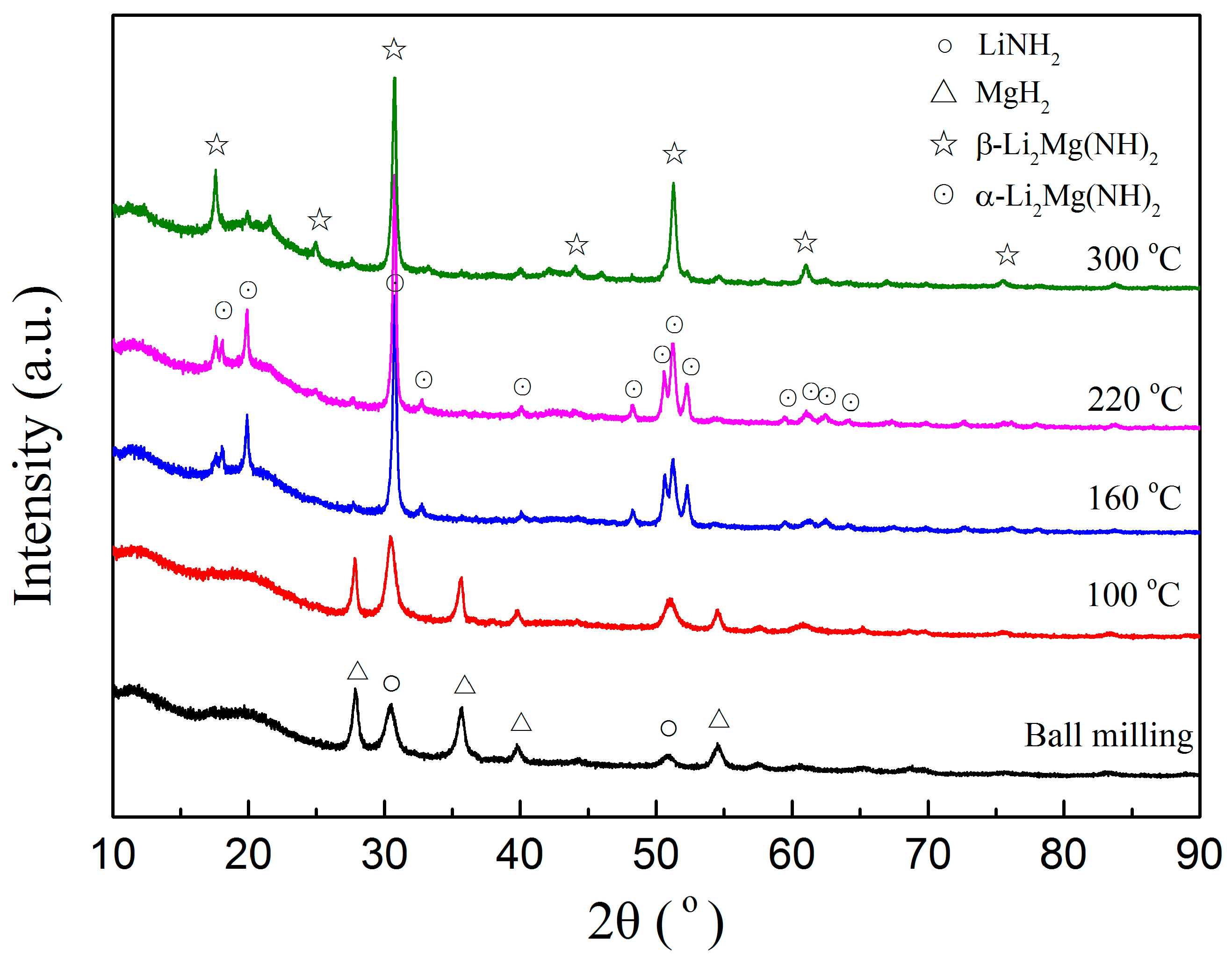
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meeks, N.D.; Baxley, S. Fuel cells and the hydrogen economy. Chem. Eng. Prog. 2016, 112, 34–38. [Google Scholar]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.M. Hydrogen adsorption and storage on porous materials. Catal. Today 2007, 120, 389–398. [Google Scholar] [CrossRef]
- Schuth, F.; Bogdanovic, B.; Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2004, 20, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Zuttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.T.; Wu, G.T.; Hu, J.J.; Chen, P. Ternary imides for hydrogen strogen. Adv. Mater. 2004, 16, 1522–1525. [Google Scholar] [CrossRef]
- Xiong, Z.T.; Wu, G.T.; Hu, J.J.; Chen, P. Investigation on chemical reaction between LiAlH4 and LiNH2. J. Power Sources 2006, 159, 167–170. [Google Scholar] [CrossRef]
- Wang, H.; Cao, H.J.; Wu, G.T.; He, T.; Chen, P. The improved hydrogen storage performances of the multi-component composite: 2Mg(NH2)2–3LiH-LiBH4. Energies 2015, 8, 6898–6909. [Google Scholar] [CrossRef]
- Lu, J.; Fang, Z.Z. Dehydrogenation of a Combined LiAlH4/LiNH2 System. J. Phys. Chem. B 2005, 109, 20830–20834. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Leng, H.Y.; Li, Q.; Chou, K.C. Improved hydrogen storage properties of LiBH4 doped Li-N-H system. Int. J. Hydrog. Energy 2014, 39, 13609–13615. [Google Scholar] [CrossRef]
- Cao, H.J.; Chua, Y.S.; Zhang, Y.; Xiong, Z.T.; Wu, G.T.; Qiu, J.S.; Chen, P. Releasing 9.6 wt % of H2 from Mg(NH2)2–3LiH-NH3BH3 through mechanochemical reaction. Int. J. Hydrog. Energy 2013, 38, 10446–10452. [Google Scholar] [CrossRef]
- Kwak, Y.J.; Kwon, S.N.; Song, M.Y. Preparation of Zn(BH4)2 and diborane and hydrogen release properties of Zn(BH4)2 + xMgH2 (x = 1, 5, 10, and 15). Met. Mater. Int. 2015, 21, 971–976. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible hydrogen storage via titanium-catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Chu, H.L.; Xiong, Z.T.; Wu, G.T.; He, T.; Wu, C.Z.; Chen, P. Hydrogen storage properties of Li–Ca–N–H system with different molar ratios of LiNH2/CaH2. Int. J. Hydrog. Energy 2010, 35, 8317–8321. [Google Scholar] [CrossRef]
- Chua, Y.S.; Chen, P.; Wu, G.T.; Xiong, Z.T. Development of amidoboranes for hydrogen storage. Chem. Commun. 2011, 47, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- David, W.I.F. Effective hydrogen storage: A strategic chemistry challenge. Faraday Discuss. 2011, 151, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhu, M. Recent progress in hydrogen storage. Mater. Today 2008, 11, 36–43. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.T.; Luo, J.Z.; Lin, J.Y.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.F. (LiNH2-MgH2): A viable hydrogen storage system. J. Alloys Compd. 2004, 381, 284–287. [Google Scholar] [CrossRef]
- Amica, G.; Larochette, P.A.; Gennari, F.C. Hydrogen storage properties of LiNH2-LiH system with MgH2, CaH2 and TiH2 added. Int. J. Hydrog. Energy 2015, 40, 9335–9346. [Google Scholar] [CrossRef]
- Li, Y.T.; Ding, X.L.; Wu, F.L.; Sun, D.L.; Zhang, Q.A.; Fang, F. Enhancement of hydrogen storage in destabilized LiNH2 with KMgH3 by quick conveyance of N-containing species. J. Phys. Chem. C 2016, 120, 1415–1420. [Google Scholar] [CrossRef]
- Shukla, V.; Bhatnagar, A.; Pandey, S.K.; Shahi, R.R.; Yadav, T.P.; Shaz, M.A.; Srivastava, O.N. On the synthesis, characterization and hydrogen storage behavior of ZrFe2 catalyzed Li-Mg-N-H hydrogen storage material. Int. J. Hydrog. Energy 2015, 40, 12294–12302. [Google Scholar] [CrossRef]
- Principi, G.; Agresti, F.; Maddalena, A.; Russo, S. The problem of solid state hydrogen storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Nakamori, Y.; Ninomiya, A.; Kitahara, G.; Aoki, M.; Noritake, T.; Miwa, K.; Kojima, Y.; Orimo, S. Dehydriding reactions of mixed complex hydrides. J. Power Sources 2006, 155, 447–455. [Google Scholar] [CrossRef]
- Ono, T.; Shimoda, K.; Tsubota, M.; Kohara, S.; Ichikawa, T.; Kojima, K.; Tansho, M.; Shimizu, T.; Kojima, Y. Ammonia desorption property and structural changes of LiAl(NH2)4 on thermal decomposition. J. Phys. Chem. C 2011, 115, 10284–10291. [Google Scholar] [CrossRef]
- Siangsai, A.; Suttisawat, Y.; Sridechprasat, P.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S. Effect of Ti compounds on hydrogen desorption/absorption of LiNH2/LiAlH2/MgH2. J. Chem. Eng. Jpn. 2008, 43, 95–98. [Google Scholar] [CrossRef]
- Andreasen, A.; Vegge, T.; Pedersen, A.S. Dehydrogenation kinetics of as-received and ball-milled LiAlH4. J. Solid State Chem. 2005, 178, 3672–3678. [Google Scholar] [CrossRef]
- Andreasen, A. Effect of Ti-doping on the dehydrogenation kinetic parameters of lithium aluminum hydride. J. Alloys Compd. 2006, 419, 40–44. [Google Scholar] [CrossRef]
- Lu, J.; Fang, Z.Z.; Sohn, H.Y.; Bowman, R.C.; Hwang, S.J. Potential and reaction mechanism of Li-Mg-Al-N-H system for reversible hydrogen storage. J. Phys. Chem. C 2007, 111, 16686–16692. [Google Scholar] [CrossRef]
- Xiong, Z.T.; Wu, G.T.; Hu, J.J.; Liu, Y.F.; Chen, P.; Luo, W.F.; Wang, J. Reversible hydrogen storage by a Li-Al-N-H complex. Adv. Funct. Mater. 2007, 17, 1137–1142. [Google Scholar] [CrossRef]
- Lu, J.; Fang, Z.Z.; Sohn, H.Y. A new Li-Al-N-H system for reversible hydrogen storage. J. Phys. Chem. B 2006, 110, 14236–14239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Liu, T.; Wu, G.T.; Li, W.; Liu, Y.F.; Araujo, C.M.; Scheicher, R.H.; Blomqvist, A.; Ahuja, R.; Xiong, Z.T.; et al. Potassium-modified Mg(NH2)2/2LiH system for hydrogen storage. Angew. Chem. Int. Ed. 2009, 48, 5828–5832. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xiong, Z.T.; Yang, L.F.; Wu, G.T.; Luo, W.F. Mechanistic investigations on the heterogeneous solid-state reaction of magnesium amides and lithium hydrides. J. Phys. Chem. B 2006, 110, 14221–14225. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Gao, M.X.; Pan, H.G.; Liu, Y.F. Structural transitions of ternary imide Li2Mg(NH)2 for hydrogen storage. Appl. Phys. Lett. 2014, 105, 083909. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Ma, X.; Wang, E.; Chu, H.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Improved Dehydrogenation Properties of 2LiNH2-MgH2 by Doping with Li3AlH6. Metals 2017, 7, 34. https://doi.org/10.3390/met7020034
Qiu S, Ma X, Wang E, Chu H, Zou Y, Xiang C, Xu F, Sun L. Improved Dehydrogenation Properties of 2LiNH2-MgH2 by Doping with Li3AlH6. Metals. 2017; 7(2):34. https://doi.org/10.3390/met7020034
Chicago/Turabian StyleQiu, Shujun, Xingyu Ma, Errui Wang, Hailiang Chu, Yongjin Zou, Cuili Xiang, Fen Xu, and Lixian Sun. 2017. "Improved Dehydrogenation Properties of 2LiNH2-MgH2 by Doping with Li3AlH6" Metals 7, no. 2: 34. https://doi.org/10.3390/met7020034





