Microstructure and Oxidation Behavior of CrAl Laser-Coated Zircaloy-4 Alloy
Abstract
:1. Introduction
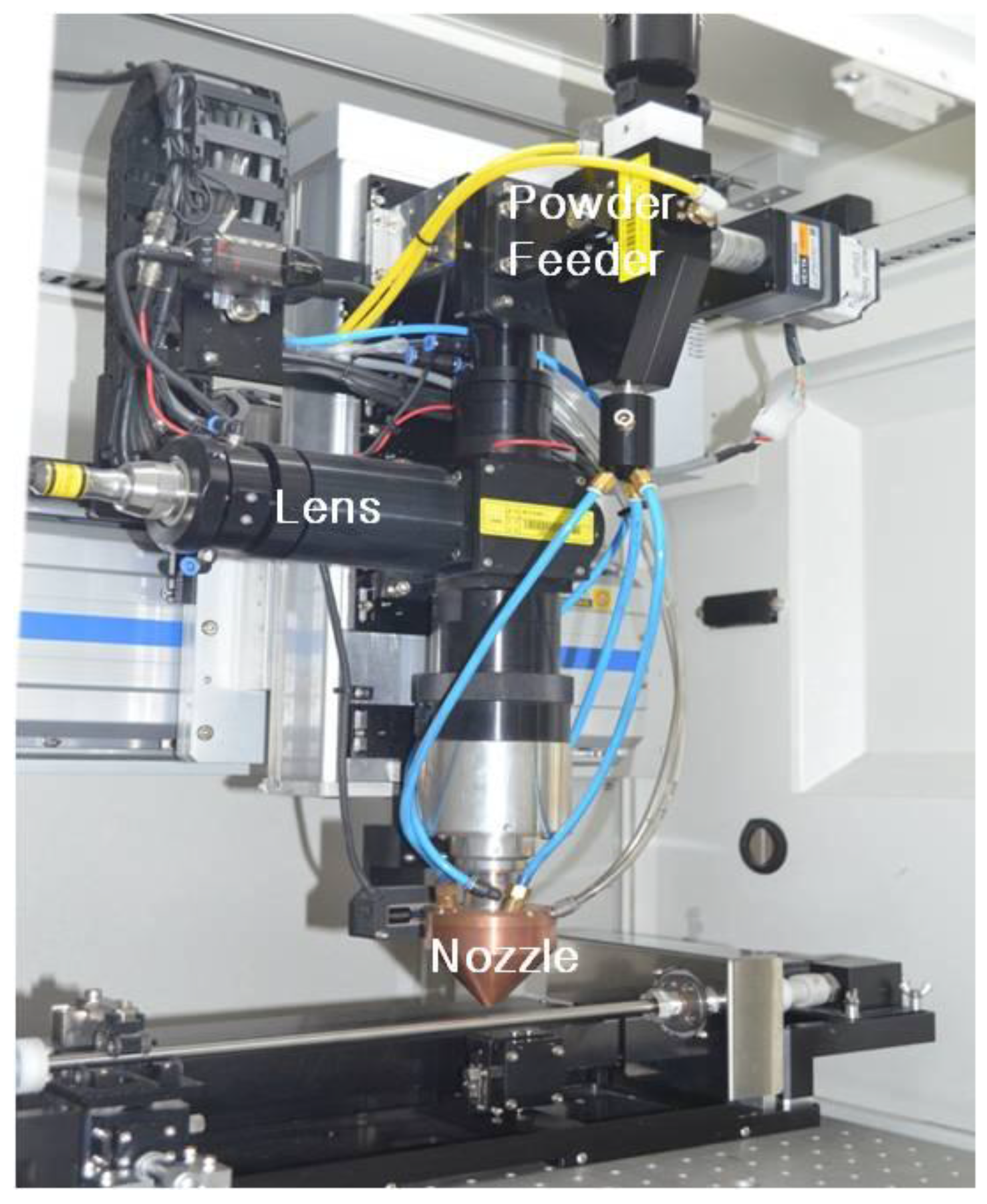
2. Materials and Methods
3. Results and Discussion
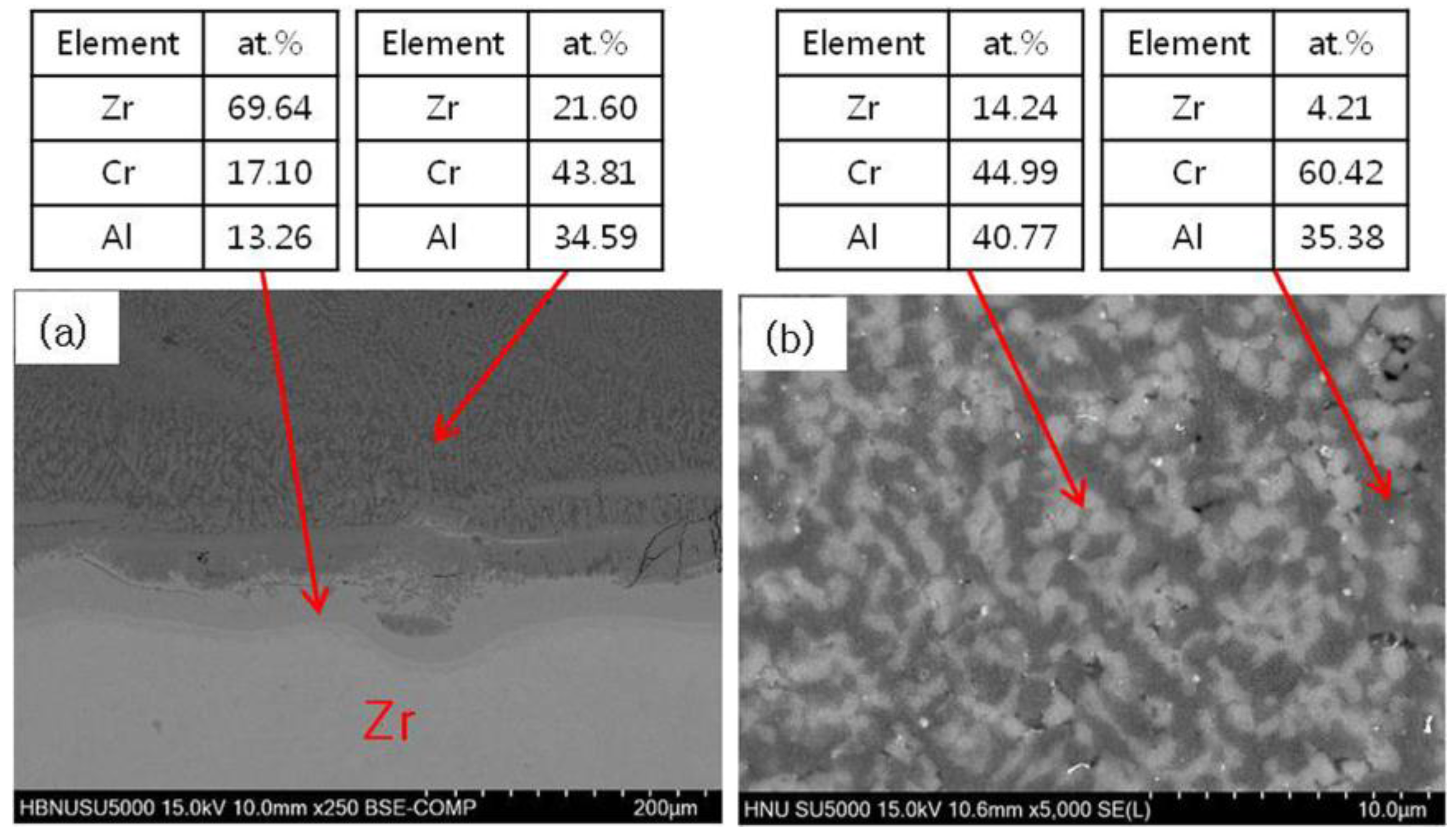
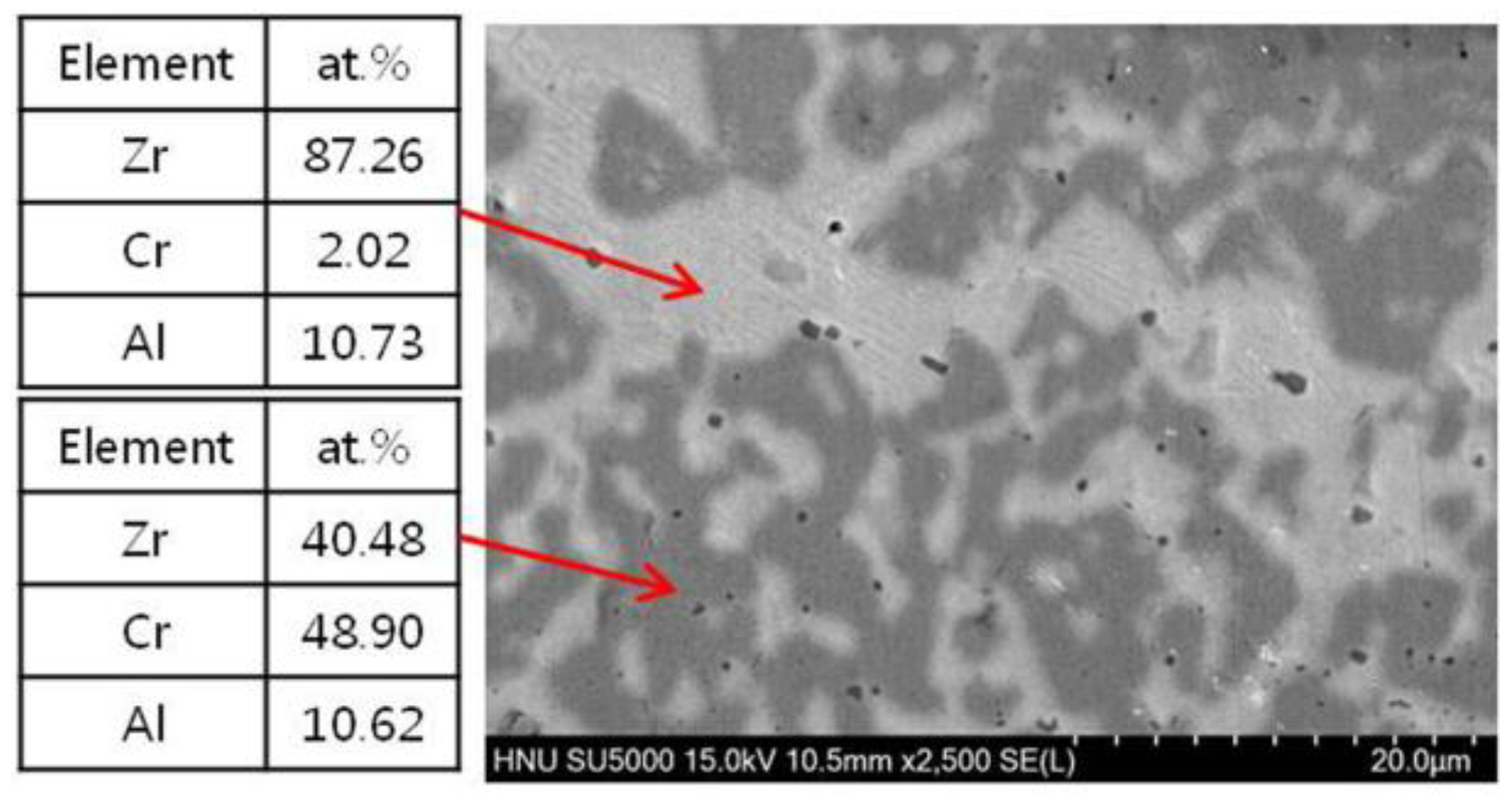
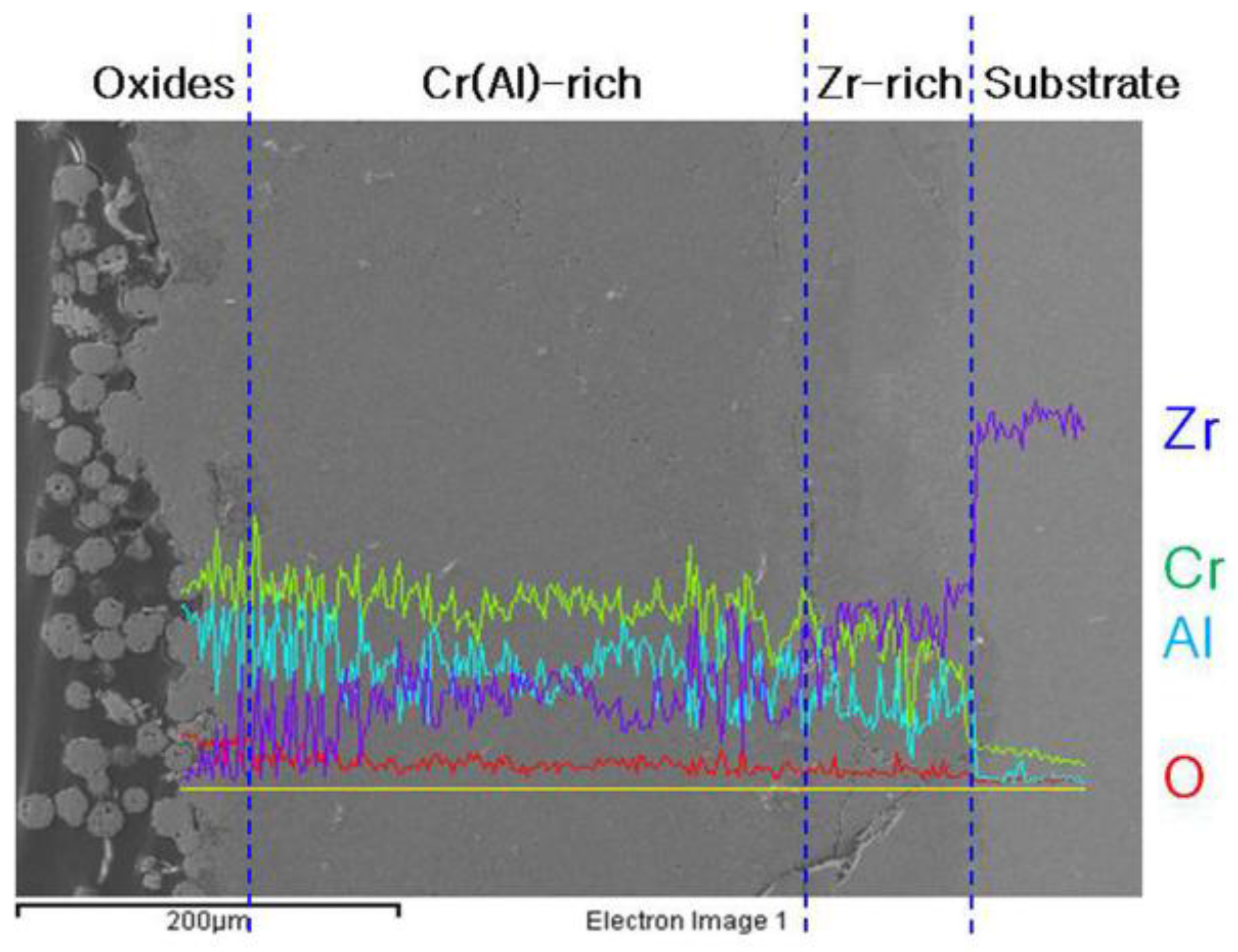
3.1. Micorstucture of CrAl Laser-Coated Zr Alloy
- Liquid → Cr at 1492–1283 °C
- Liquid → Cr + Al2Zr at 1283–1255 °C
- Liquid + Al2Zr → Cr + Al3Zr at 1255 °C
- Liquid → Cr + Al3Zr at 1255–1238 °C
- Liquid → Cr + Al3Zr + Al8Cr5 at 1238 °C
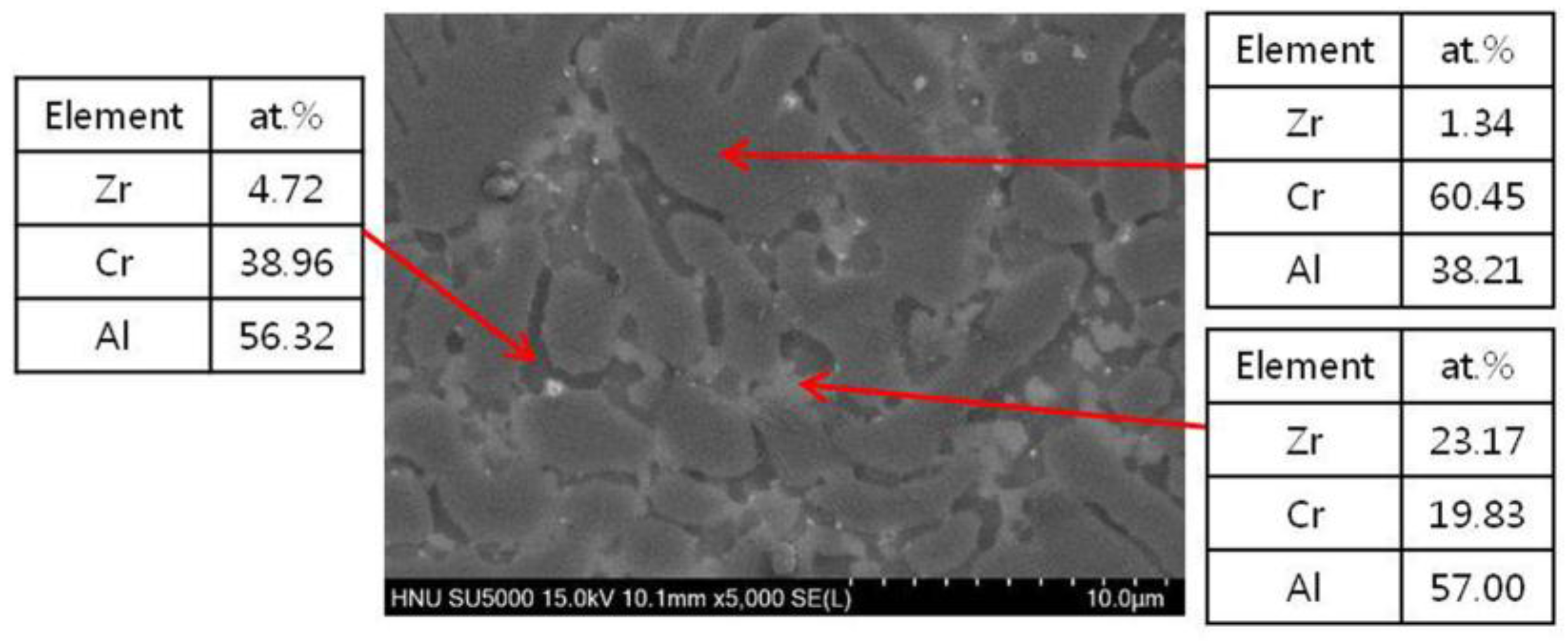
3.2. CrAl Laser-Coated Zr Alloy that Exposed to a High Temperature
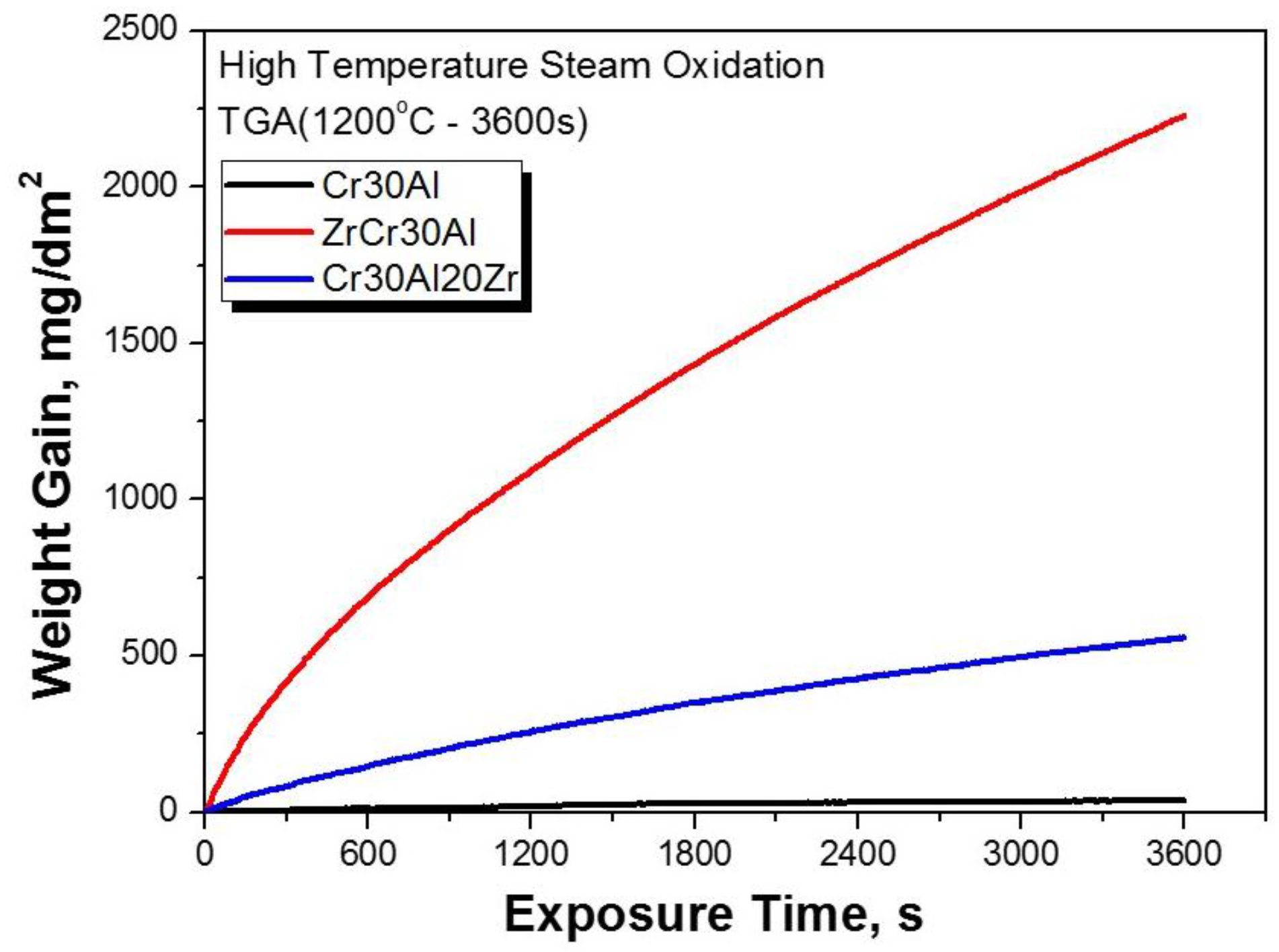
3.3. Oxidation Behavior of CrAl Laser-Coated Zr Alloy at High Temperature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Bryk, V.V.; Vasilenko, R.L.; Ovcharenko, V.D.; Reshetnyak, E.N.; Tolmachova, G.N.; Vyugov, P.N. Vacuum-arc chromium-based coatings for protection of zirconium alloys from the high temperature oxidation in air. J. Nucl. Mater. 2015, 465, 400–406. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Terrani, K.A.; Parish, C.M.; Shin, D.; Pint, B.A. Protection of zirconium by alumina- and chromia-forming iron alloys under high-temperature steam exposure. J. Nucl. Mater. 2013, 438, 64–71. [Google Scholar] [CrossRef]
- Jin, D.; Yang, F.; Zou, Z.; Gu, L.; Zhao, X.; Guo, F.; Xiao, P. A study of the zirconium alloy protection by Cr2C2-NiCr coating for nuclear reactor application. Surf. Coat. Technol. 2016, 287, 55–60. [Google Scholar] [CrossRef]
- Kim, J.M.; Ha, T.H.; Park, J.S.; Kim, H.G. Effect of laser surface treatment on the corrosion behavior of FeCrAl-coated TZM alloy. Metals 2016, 6, 29. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Duan, C.; Feng, X.; Shen, Y. Investigation of Cr-Al composite coatings fabricated on pure Ti substrate via mechanical alloying method: Effects of Cr-Al ratio and milling time on coating, and oxidation behavior of coating. J. Alloy. Compd. 2016, 660, 208–219. [Google Scholar] [CrossRef]
- Gonzalez, R.O.; Gribaudo, L.M. Analysis of controversial zones of the Zr-Cr equilibrium diagram. J. Nucl. Mater. 2005, 342, 14–19. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. High temperature oxidation behavior of Cr-coated zirconium. In Proceedings of the LWR fuel performance meeting, Charlotte, NC, USA, 15–19 September 2013; p. 840.
- PANDAT. CompuTherm, LLC, Madison, WI, USA. Available online: http://www.computherm.com (accessed on 13 February 2017).
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.O. Phase evolution and dendritic growth in laser cladding of aluminium on zirconium. J. Alloy. Compd. 2011, 509, 3705–3710. [Google Scholar] [CrossRef]
- Okamoto, H. Phase diagrams for binary alloys. In ASM Desk Handbook; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Zhang, M.; Xu, B.; Ling, G. Preparation and characterization of α-Al2O3 film by low temperature thermal oxidation of Al8Cr5 coating. Appl. Surf. Sci. 2015, 331, 1–7. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006; pp. 101–162. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-M.; Ha, T.-H.; Kim, I.-H.; Kim, H.-G. Microstructure and Oxidation Behavior of CrAl Laser-Coated Zircaloy-4 Alloy. Metals 2017, 7, 59. https://doi.org/10.3390/met7020059
Kim J-M, Ha T-H, Kim I-H, Kim H-G. Microstructure and Oxidation Behavior of CrAl Laser-Coated Zircaloy-4 Alloy. Metals. 2017; 7(2):59. https://doi.org/10.3390/met7020059
Chicago/Turabian StyleKim, Jeong-Min, Tae-Hyung Ha, Il-Hyun Kim, and Hyun-Gil Kim. 2017. "Microstructure and Oxidation Behavior of CrAl Laser-Coated Zircaloy-4 Alloy" Metals 7, no. 2: 59. https://doi.org/10.3390/met7020059



