Evolution of Anode Porosity under Air Oxidation: The Unveiling of the Active Pore Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Air Reactivity Tests
2.3. Analytical Instruments
- -
- Between 0.0014 and 0.100 µm of pore size, a gas adsorption analyzer (Tristar II 3020, Micromeritics, Norcross, GA, USA) with the Density Functional Theory (DFT) equation and the nitrogen adsorptive gas was used;
- -
- For the pores superior to 0.1 µm to about 1000 µm (ISO 15901-1), a mercury porosimeter (Auto Pore IV, Micromeritics, Norcross, GA, USA) was used.
3. Results
3.1. Thermo-Gravimetric (TG) Analysis
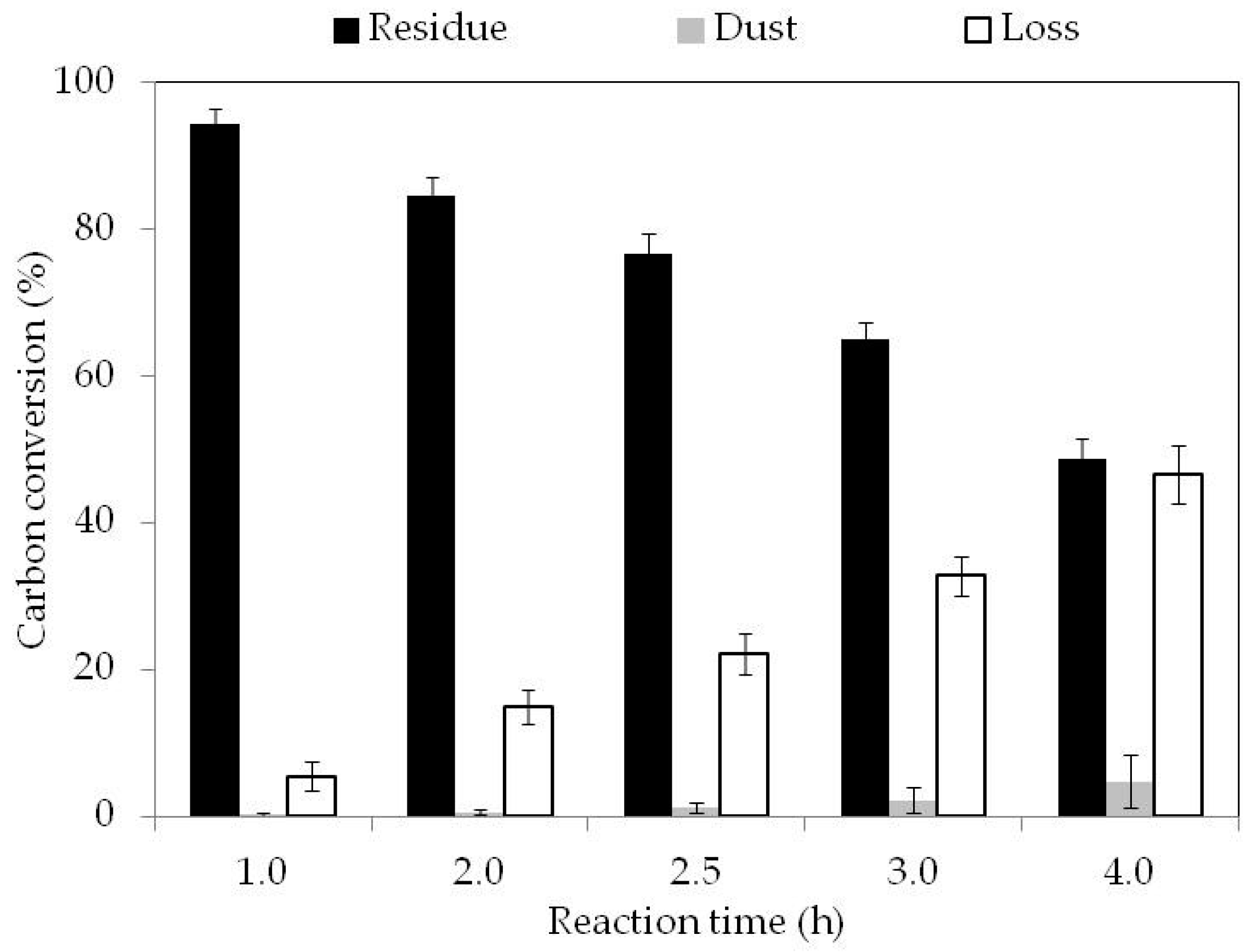
3.2. Fixed Bed Reactor Gasification
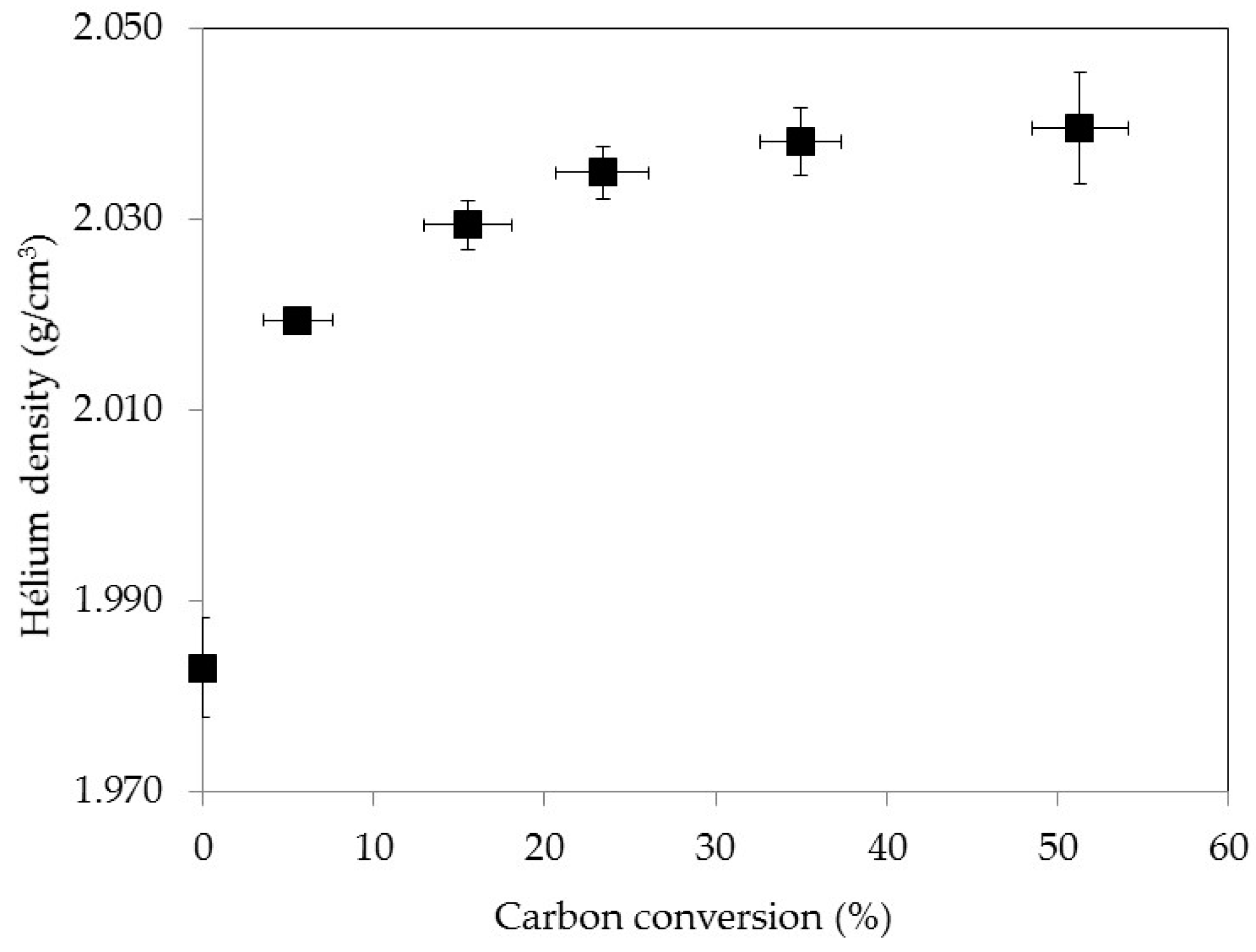
3.3. Density Evolution during Gasification
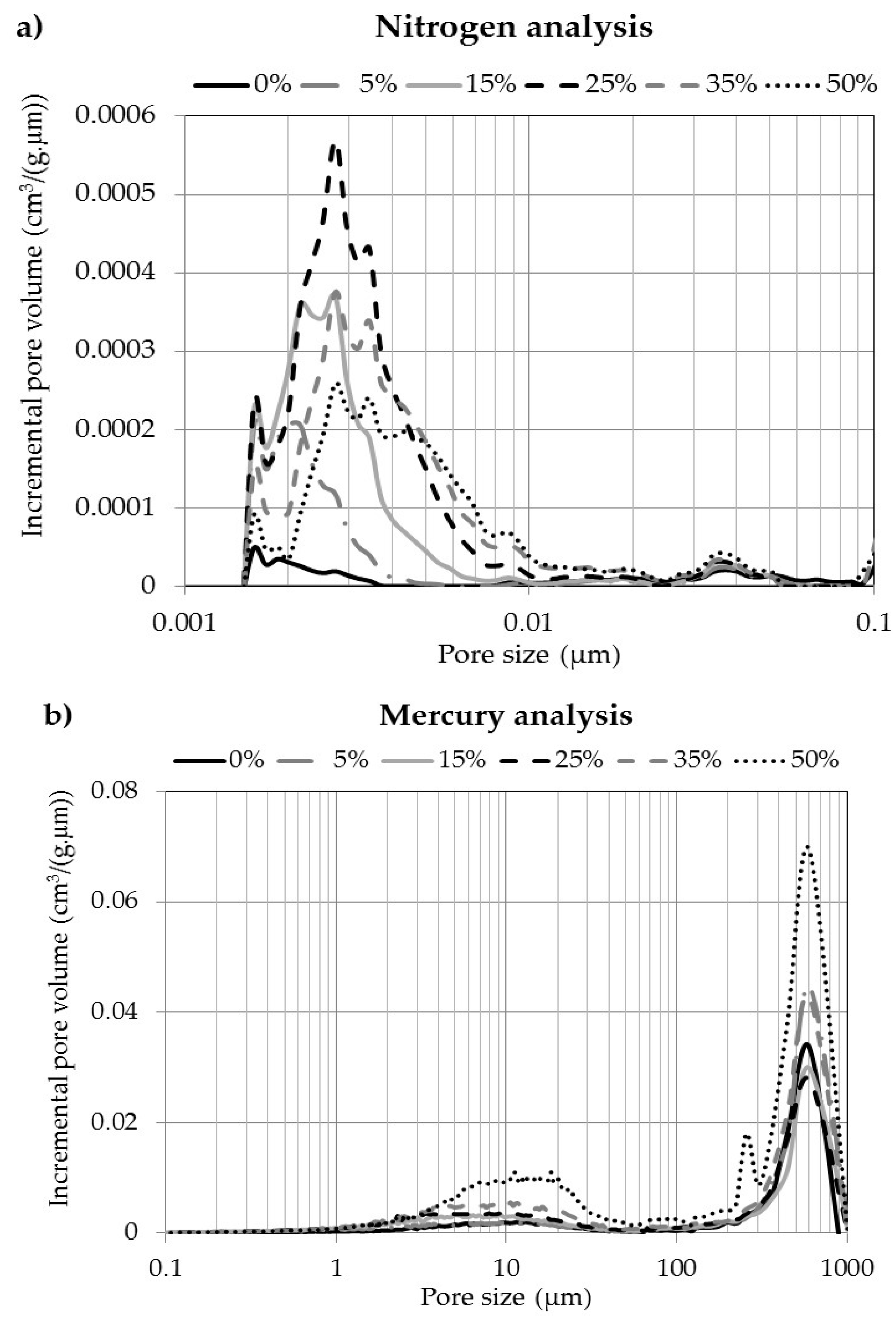
3.4. Pore Size and Volume Distribution
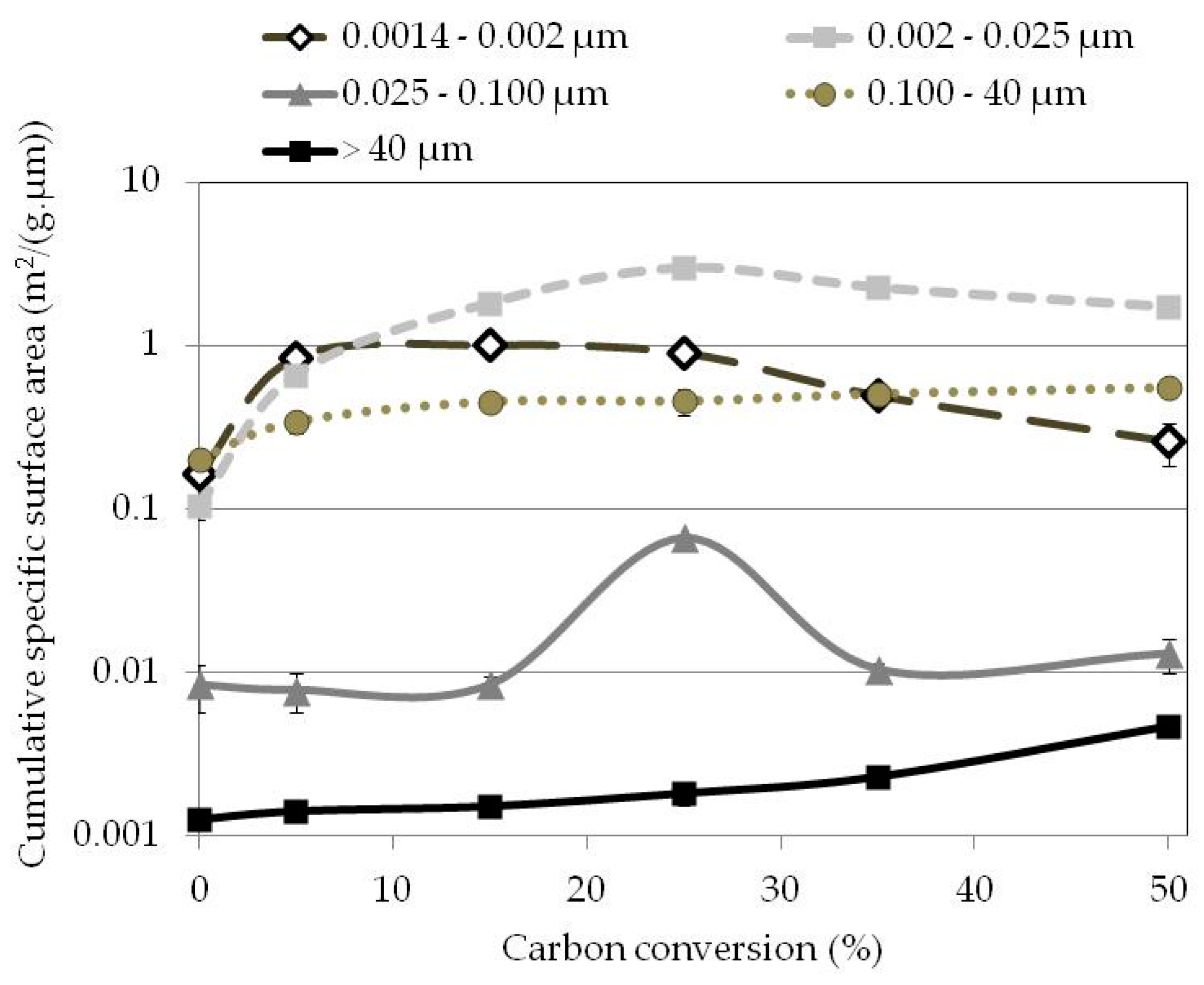
3.5. Specific Surface Area
3.6. Pore Volumes
3.7. Active Pore Size
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fischer, W.K.; Perruchoud, R.C. Factors Influencing the Carboxy- and Air-Reactivity Behavior of Prebaked Anodes in Hall—Heroult Cells. Light Met. 1986, 2, 575–580. [Google Scholar]
- Houston, G.J.; Øye, H.A. Consumption of Anode Carbon During Aluminium Electrolysis. Aluminium 1985, 61, 251–254. [Google Scholar]
- Brym, M.J.C.; Gagnon, A.; Boulanger, C.; Lepage, D.; Savard, G.; Bouchard, G.; Lagace, C.; Charette, A. Anode reactivity: Effect of coke calcination level. Light Met. 2009, 905–908. [Google Scholar]
- Lhuissier, J.; Bezamanifary, L.; Gendre, M.; Chollier, M.J. Use of under-calcined coke for the production of low reactivity anodes. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 109–113. [Google Scholar]
- Fischer, W.K.; Perruchoud, R. Influence of coke calcining parameters on petroleum coke quality. Light Met. 1985, 811–826. [Google Scholar]
- Rolle, J.G.; Czikall, R.A. Use of coke air reactivity testing for predicting anode air reactivity. Light Met. 2001, 67, 5–67. [Google Scholar]
- Engvoll, M.A.; Øye, H.A.; Sørlie, M. Influence of bath contaminations on anode reactivity. Light Met. 2001, 661–667. [Google Scholar]
- Müftüoglu, T.; Steine, B.; Fernandez, R. Anode burning behaviour and sodium sensitivity of coke from different feedstocks: A pilot scale study. Light Met. 1993, 543–548. [Google Scholar]
- Müftüoglu, T.; Øye, H. Reactivity and electrolytic consumption of anode carbon with various additives. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 667–671. [Google Scholar]
- Rolle, J.G.; Hoang, Y.K. Studies of the impact of vanadium and sodium on the air reactivity of coke and anodes. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 606–610. [Google Scholar]
- Edwards, L.; Vogt, F.; Robinette, M.; Love, R.; Ross, A.; McClung, M.; Roush, R.; Morgan, W. Use of shot coke as an anode raw material. Light Met. 2009, 985–990. [Google Scholar]
- Schmidt-Hatting, W.; Kooijman, A.; Perruchoud, R. Investigation of the quality of recycled anode butts. Light Met. 1991, 705–720. [Google Scholar]
- Buhler, U.; Perruchoud, R.C. Dynamic process optimization. Light Met. 1995, 707–714. [Google Scholar]
- Lustenberger, M. Heat treatment of anodes for the Aluminium Industry. In Institut des Matériaux; Faculté Sciences et Techniques de l’Ingénieur: Lausanne, Switzerland, 2004; p. 143. [Google Scholar]
- Coste, B.; Schneider, J.P. Influence of coke real density on anode reactivity consequence on anode baking. Light Met. 1994, 583–591. [Google Scholar]
- Foosnæs, T.; Kulset, N.; Linga, H.; Naeumann, G.; Werge-Olsen, A. Measurement and control of the calcining level in anode baking furnaces. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 418–421. [Google Scholar]
- Fischer, W.K.; Keller, F.; Perruchoud, R.; Oderbolz, S. Baking parameters and the resulting anode quality. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 427–433. [Google Scholar]
- Kuang, Z.; Thonstad, J.; Sørlie, M. Effects of additives on the electrolytic consumption of carbon anodes in aluminium electrolysis. Carbon 1995, 33, 1479–1484. [Google Scholar] [CrossRef]
- Thorne, R.J.; Sommerseth, C.; Ratvik, A.P.; Rørvik, S.; Sandnes, E.; Lossius, L.P.; Linga, H.; Svensson, A.M. Correlation between Coke Type, Microstructure and Anodic Reaction Overpotential in Aluminium Electrolysis. J. Electrochem. Soc. 2015, 162, E296–E306. [Google Scholar] [CrossRef]
- Thonstad, J.; Hove, E. On the anodic overvoltage in aluminum electrolysis. Can. J. Chem. 1964, 42, 1542–1550. [Google Scholar] [CrossRef]
- Rey Boero, J.F. Studies on anode reactivity to oxidant gases. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 580–588. [Google Scholar]
- Kuang, Z.-L.; Thonstad, J.; Rolseth, S.; Sørlie, M. Effect of baking temperature and anode current density on anode carbon consumption. Metall. Mater. Trans. B 1996, 27, 177–183. [Google Scholar] [CrossRef]
- Edwards, L. The History and Future Challenges of Calcined Petroleum Coke Production and Use in Aluminum Smelting. JOM 2015, 67, 308–321. [Google Scholar] [CrossRef]
- Tkac, M. Porosity development in composite carbon materials during heat treatment. In Department of Materials Science and Engineering; Norwegian University of Science and Technology: Trondheim, Norway, 2007; p. 189. [Google Scholar]
- Keller, F.; Fischer, W.K. Development of anode quality criteria by statistical evaluation of operational results in the electrolysis. Light Met. 1982, 729–740. [Google Scholar]
- Perruchoud, R.C.; Meier, M.W.; Fischer, W.K. Bath impregnation of carbon anodes. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 673–679. [Google Scholar]
- Beltiskus, D.; Danka, D. A comprehensive determination of effects of calcined petroleum coke properties on aluminium reduction cell anode properties. Light Met. 1988, 429–439. [Google Scholar]
- Hardin, E.E.; Beilharz, C.L.; Melvin, L.L. Comprehensive review of the effect of coke structure and properties when calcined at various temperatures. Light Met. 1993, 501–508. [Google Scholar]
- Suriyapraphadilok, U.; Halleck, P.; Grader, A.; Andresen, J.M. Physical, chemical and X-Ray Computed Tomography characterization of anode butt cores. In Proceedings of the Technical Sessions at the TMS Aluminum Committee at the 134th TMS Annual Meeting, San Francisco, CA, USA, 13–17 February 2005; pp. 617–621.
- Bird, N.; McEnaney, B.; Sadler, B.A. Some practical consequences of analyses of the carboxy and airburn reactions of anode carbons. In Essential Readings in Light Metals: Electrode Technology for Aluminum Production; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2013; Volume 4, pp. 589–593. [Google Scholar]
- Turkdogan, E.T.; Olsson, R.; Vinters, J. Pore characteristics of carbons. Carbon 1970, 8. [Google Scholar] [CrossRef]
- Tran, K.N.; Bhatia, S.K.; Tomsett, A. Air reactivity of petroleum cokes: Role of inaccessible porosity. Ind. Eng. Chem. Res. 2006, 46, 3265–3274. [Google Scholar] [CrossRef]
- Tordai, T. Anode Dusting during the Electrolytic Production of Aluminium; École Polytechnique Fédérale de Lausanne: Lausanne, Switzerland, 2007; p. 351. [Google Scholar]
- Chevarin, F.; Alamdari, H.; Lauzon-Gauthier, J.; Azari, K.; Fafard, M.; Duchesne, C.; Ziegler, D. Effects of Microstructural Characteristics on Anode Reactivity. In Proceeedings of the COM-2011 40th Annual conference of metallurgists of CIM, Montréal, QC, Canada, 2–5 October 2011.
- Azari, K.; Alamdari, H.; Ammar, H.; Fafard, M.; Adams, A.; Ziegler, D. Influence of mixing parameters on the density and compaction behavior of carbon anodes used in aluminum production. In Thermec 2012; Trans Tech Publications Ltd.: Quebec city, QC, Canada, 2012; pp. 17–22. [Google Scholar]
- Azari, K.; Alamdari, H.; Aryanpour, G.; Ziegler, D.; Picard, D.; Fafard, M. Compaction properties of carbon materials used for prebaked anodes in aluminum production plants. Powder Technol. 2013, 246, 650–657. [Google Scholar] [CrossRef]
- Azari, K. Investigation of the materials and paste relationship to improve forming process and anode quality. In Mining, Metallurgical and Materials Engineering Department; Laval University: Québec, QC, Canada, 2013; p. 148. [Google Scholar]
- Hussein, A.; Larachi, F.; Ziegler, D.; Alamdari, H. Effects of heat treatment and acid washing on properties and reactivity of charcoal. Biomass Bioenergy 2016, 90, 101–113. [Google Scholar] [CrossRef]
- Chevarin, F.; Lemieux, L.; Ziegler, D.; Fafard, M.; Alamdari, H. Air and CO2 Reactivity of Carbon Anode and Its Constituents: An Attempt to Understand Dusting Phenomenon. In Light Metals 2015; Springer: New York, NY, USA, 2015; pp. 1147–1152. [Google Scholar]
- Chevarin, F.; Azari, K.; Lemieux, L.; Ziegler, D.; Fafard, M.; Alamdari, H. Active pore sizes during the CO2 gasification of carbon anode at 960 °C. Fuel 2016, 178, 93–102. [Google Scholar] [CrossRef]
- Chevarin, F.; Lemieux, L.; Picard, D.; Ziegler, D.; Fafard, M.; Alamdari, H. Characterization of carbon anode constituents under CO2 gasification: A try to understand the dusting phenomenon. Fuel 2015, 156, 198–210. [Google Scholar] [CrossRef]
- Tran, K.N.; Berkovich, A.J.; Tomsett, A.; Bhatia, S.K. Crystalline Structure Transformation of Carbon Anodes during Gasification. Energy Fuels 2008, 22, 1902–1910. [Google Scholar] [CrossRef]
- Chevarin, F.; Azari, K.; Ziegler, D.; Gauvin, R.; Fafard, M.; Alamdari, H. Substrate effect of coke particles on the structure and reactivity of coke/pitch mixtures in carbon anodes. Fuel 2016, 183, 123–131. [Google Scholar] [CrossRef]
- D’amore, M.; Tognotti, L.; Sarofim, A. Oxidation rates of a single char particle in an electrodynamic balance. Combust. Flame 1993, 95, 374–382. [Google Scholar] [CrossRef]
- Boero, J.R. The reaction of petroleum cokes with air. Carbon 1987, 25, 477–483. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980, 26, 379–386. [Google Scholar] [CrossRef]
- Ballal, G.; Zygourakis, K. Evolution of pore surface area during noncatalytic gas-solid reactions. II: Experimental results and model validation. Ind. Eng. Chem. Res. 1987, 26, 1787–1796. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO15901–1. Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption—Part 1: Mercury Porosimetry 2005; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Hume, S.M.; Fischer, W.K.; Perruchoud, R.C.; Welch, B.J. Model for petroleum coke reactivity. Light Met. 1993, 525–531. [Google Scholar]
- Farr-Wharton, R.; Welch, B.J.; Hannah, R.C.; Dorin, R.; Gardner, H.J. Chemical and electrochemical oxidation of heterogeneous carbon anodes. Electrochim. Acta 1980, 25, 217–221. [Google Scholar] [CrossRef]
- Buzunov, V.; Mann, V.; Khramenko, S.; Johnson, J. Influence of Calcination Temperature and Sulfur Level on Coke Properties. In Light Metals 2017; Springer: New York, NY, USA, 2017; pp. 1151–1156. [Google Scholar]


| Properties | S (wt %) | Na (ppmw) | Si (ppmw) | Ca (ppmw) | V (ppmw) | Fe (ppmw) | Ni (ppmw) |
|---|---|---|---|---|---|---|---|
| Calcined coke | 2.13 ± 0.06 | 100 ± 7 | 120 ± 17 | 130 ± 7 | 360 ± 18 | 460 ± 23 | 250 ± 13 |
| Coal tar pitch | 0.55 ± 0.02 | 48 ± 3 | 254 ± 36 | 71 ± 4 | N/A | 209 ± 10 | N/A |
| Particle Sizes (US Mesh) | −4 + 8 | −8 + 16 | −16 + 30 | −30 + 50 | −50 + 100 | −100 + 200 | Fines (4000 BN *) | |
|---|---|---|---|---|---|---|---|---|
| Particle sizes (µm) | Superior limit | −4760 | −2380 | −1410 | −595 | −297 | −149 | −149 |
| Inferior limit | +2380 | +1410 | +595 | +297 | +149 | +74 | - | |
| Coke (wt %) | 22.0 | 10.0 | 11.5 | 12.7 | 8.8 | 10.8 | 24.2 | |
| Properties | S | Na | Si | Ca | V | Fe | Ni | LC | Particle Weight |
|---|---|---|---|---|---|---|---|---|---|
| Unit | (wt %) | (ppmw) | (ppmw) | (ppmw) | (ppmw) | (ppmw) | (ppmw) | (nm) | (mg) |
| Value | 1.81 | 650 | 624 | 377 | 224 | 119 | 341 | 2.9 | 73.2 |
| Standard deviation | 0.05 | 91 | 31 | 19 | 11 | 8 | 17 | 0.1 | 22.0 |
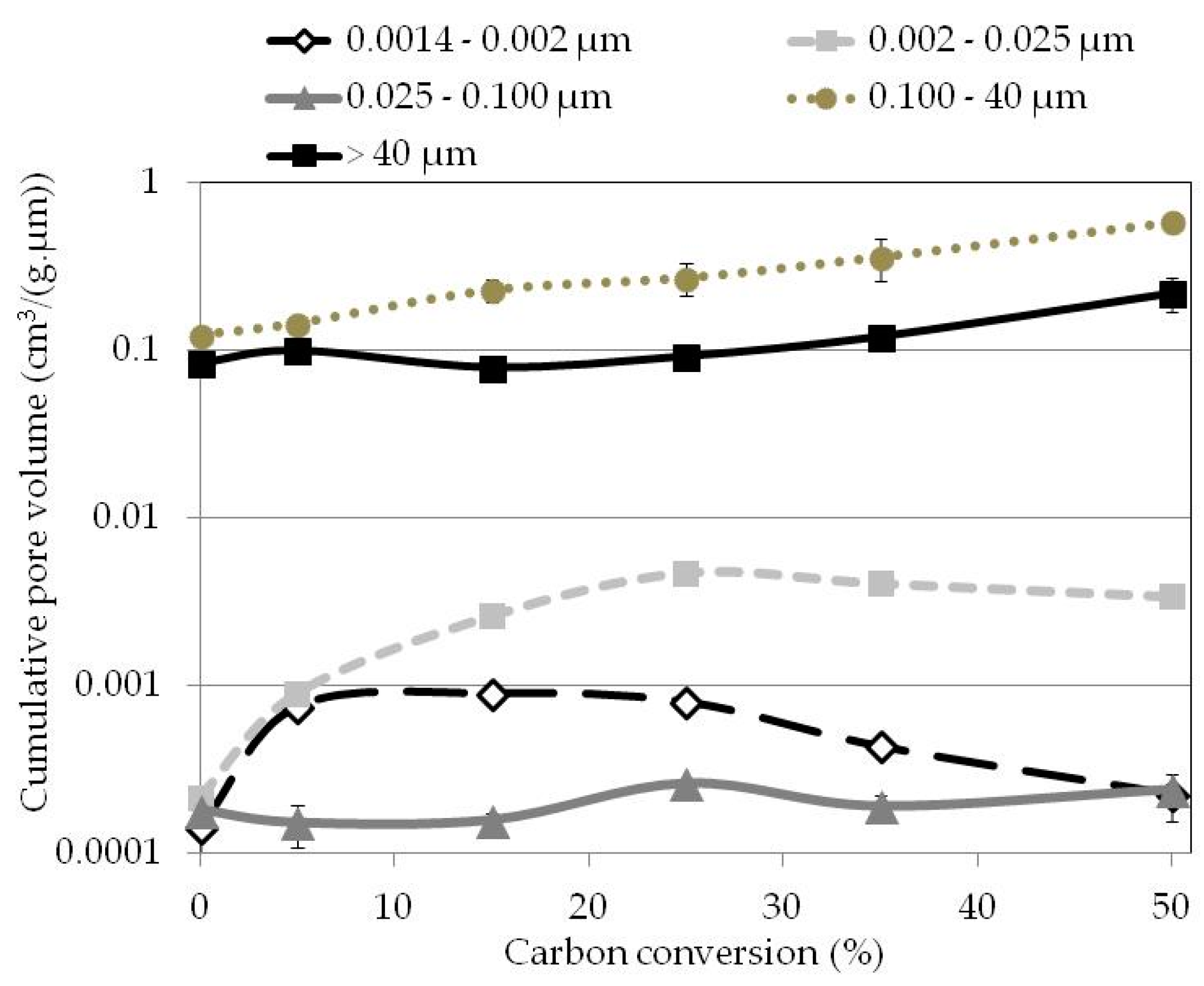
| Carbon Conversion (wt %) | Pore Size Range (µm) | ||||
|---|---|---|---|---|---|
| 0.0014–0.0020 | 0.002–0.025 | 0.025–0.100 | 0.1–40.0 | Superior at 40 | |
| Pore Volume (mm3/g) Measured by Nitrogen Adsorption | Pore Volume (cm3/g) Measured by Mercury Infiltration | ||||
| 0 | 0.142 ± 0.042 | 0.216 ± 0.019 | 0.181 ± 0.032 | 0.123 ± 0.012 | 0.084 ± 0.006 |
| 5 | 0.753 ± 0.030 | 0.900 ± 0.147 | 0.151 ± 0.044 | 0.145 ± 0.011 | 0.100 ± 0.004 |
| 15 | 0.905 ± 0.050 | 2.616 ± 0.145 | 0.157 ± 0.017 | 0.230 ± 0.034 | 0.079 ± 0.008 |
| 25 | 0.802 ± 0.002 | 4.740 ± 0.148 | 0.261 ± 0.010 | 0.272 ± 0.061 | 0.092 ± 0.013 |
| 35 | 0.434 ± 0.008 | 4.088 ± 0.318 | 0.190 ± 0.029 | 0.363 ± 0.105 | 0.121 ± 0.022 |
| 50 | 0.225 ± 0.070 | 3.400 ± 0.150 | 0.240 ± 0.021 | 0.588 ± 0.054 | 0.221 ± 0.051 |
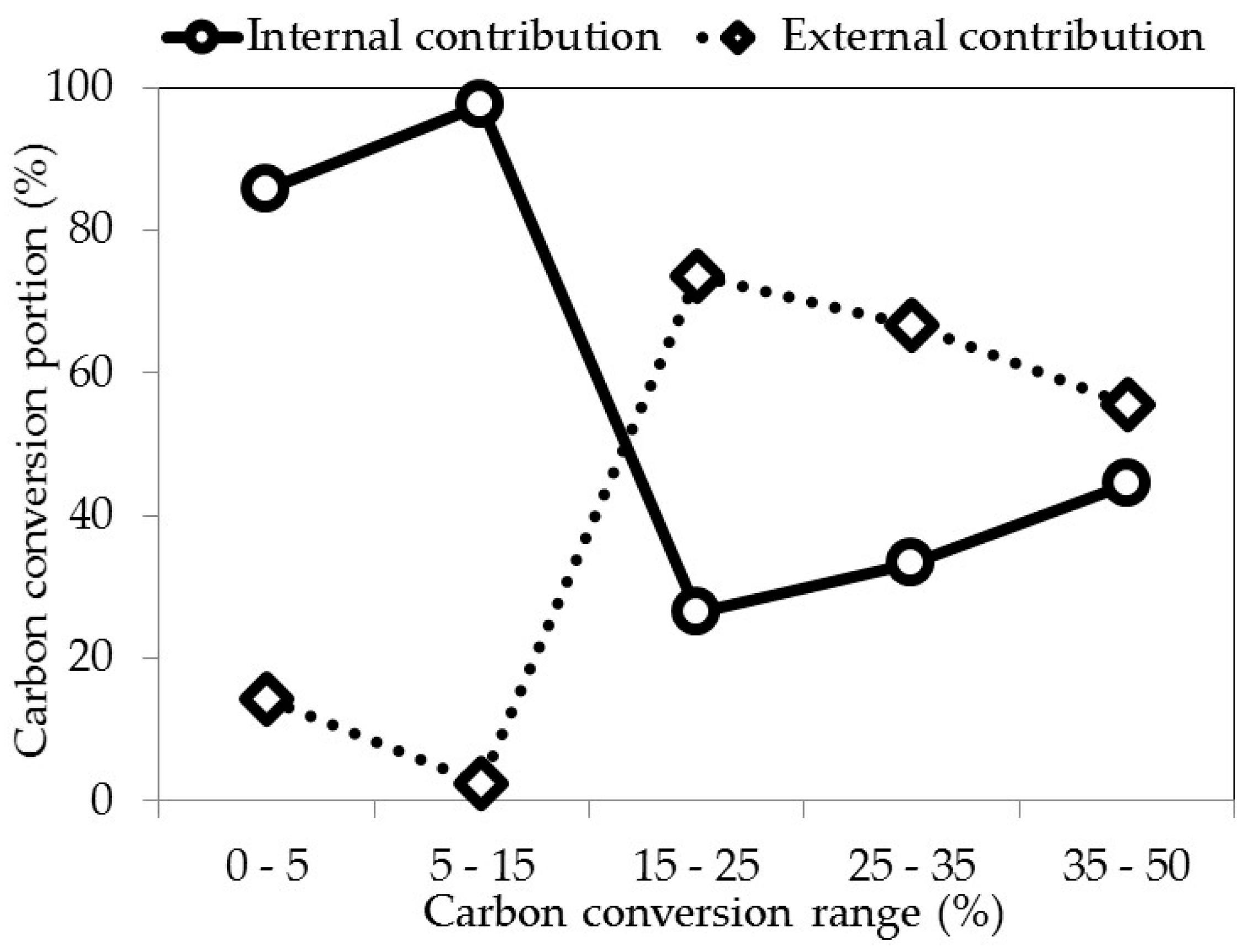
| Carbon Conversion Range (wt %) | 0–5 | 5–15 | 15–25 | 25–35 | 35–50 |
|---|---|---|---|---|---|
| Total carbon amount remaining for an initial amount of sample of 1 g | 0.95 | 0.85 | 0.75 | 0.65 | 0.5 |
| Total amount of carbon loss (g) | 0.05 | 0.15 | 0.25 | 0.35 | 0.5 |
| Amount of carbon loss associated at the pore volume variation in the pores inferior at 40 µm (g) | 0.043 | 0.146 | 0.066 | 0.117 | 0.222 |
| Amount of carbon loss associated at the pore volume variation in the pores superior at 40 µm (g) | 0.007 | 0.004 | 0.184 | 0.233 | 0.278 |
| Total carbon conversion (%) | 5 | 15 | 25 | 35 | 50 |
| Carbon conversion in the pores inferior at 40 µm (%) | 4.3 | 14.6 | 6.6 | 11.7 | 22.2 |
| Carbon conversion in the pores superior at 40 µm (%) | 0.7 | 0.4 | 18.4 | 23.3 | 27.8 |
| Internal contribution, inferior at 40 µm (%) | 86.0 | 97.3 | 26.4 | 33.4 | 44.4 |
| External contribution, superior at 40 µm (%) | 14.0 | 2.7 | 73.8 | 66.7 | 55.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevarin, F.; Ishak, R.; Ziegler, D.; Fafard, M.; Alamdari, H. Evolution of Anode Porosity under Air Oxidation: The Unveiling of the Active Pore Size. Metals 2017, 7, 101. https://doi.org/10.3390/met7030101
Chevarin F, Ishak R, Ziegler D, Fafard M, Alamdari H. Evolution of Anode Porosity under Air Oxidation: The Unveiling of the Active Pore Size. Metals. 2017; 7(3):101. https://doi.org/10.3390/met7030101
Chicago/Turabian StyleChevarin, Francois, Ramzi Ishak, Donald Ziegler, Mario Fafard, and Houshang Alamdari. 2017. "Evolution of Anode Porosity under Air Oxidation: The Unveiling of the Active Pore Size" Metals 7, no. 3: 101. https://doi.org/10.3390/met7030101





