Magnesium–Gold Alloy Formation by Underpotential Deposition of Magnesium onto Gold from Nitrate Melts
Abstract
:1. Introduction
2. Materials and Methods
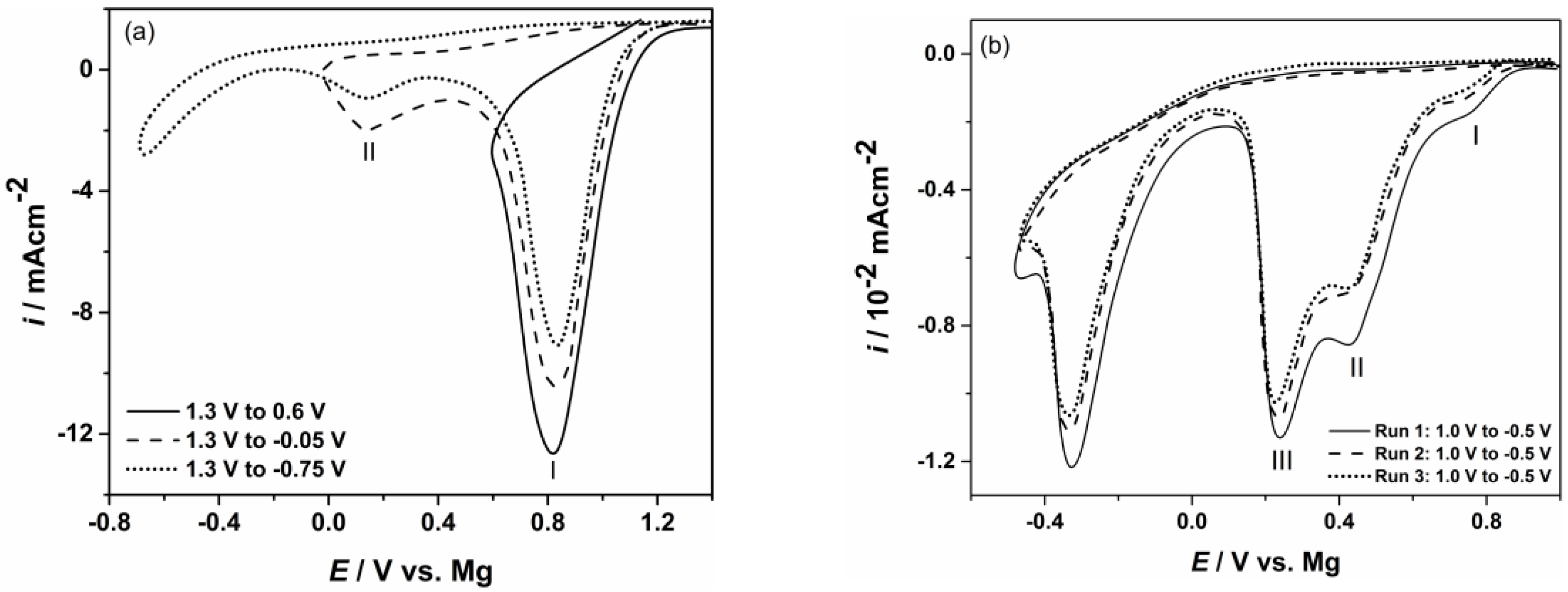
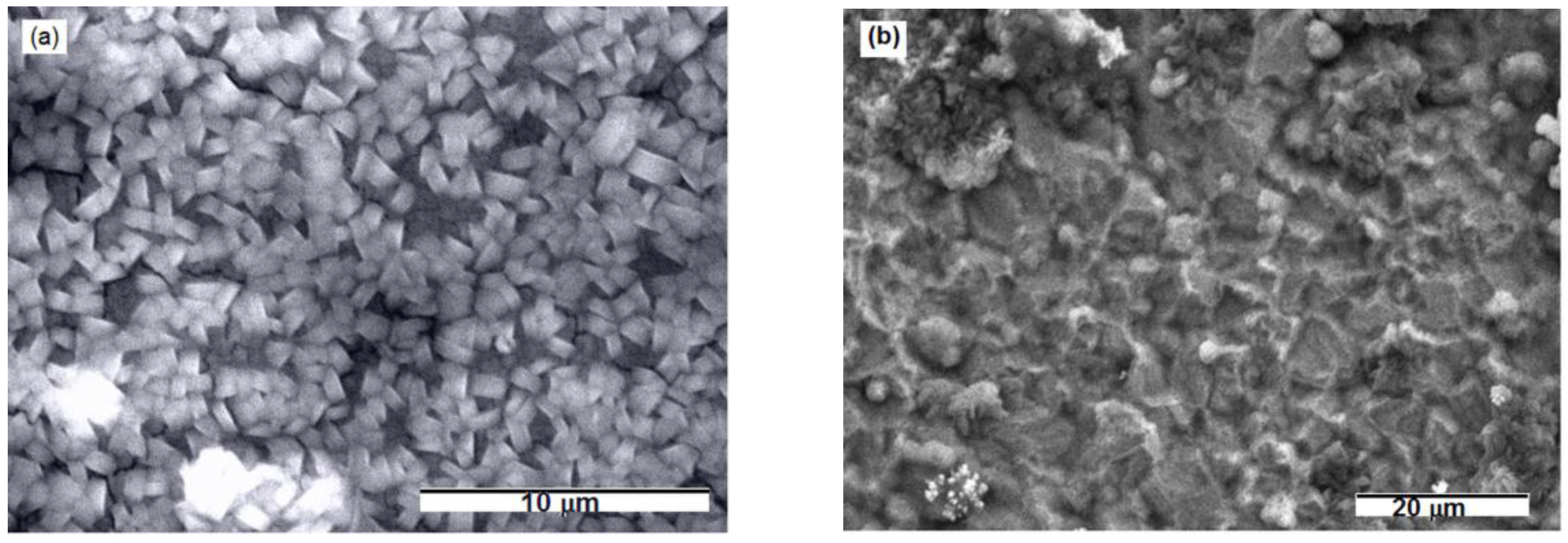
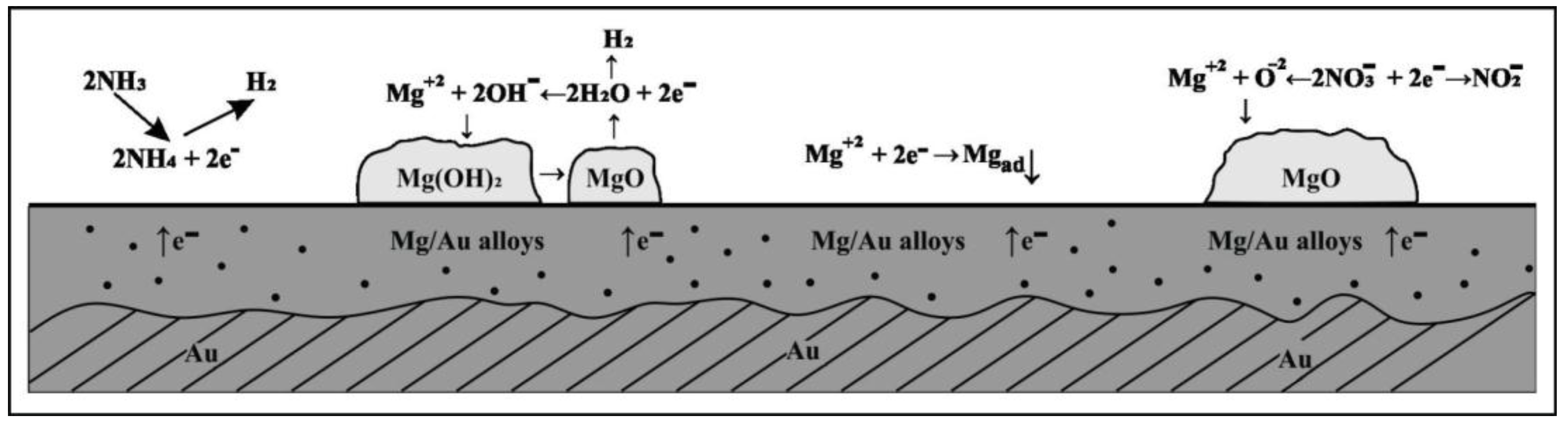
3. Results and Discussion
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takeda, Y.; Kuriiwa, T.; Kamegawa, A.; Okada, M. Grain size refinements of Au-Mg alloy by hydrogen absorption/desorption treatments. Mater. Trans. 2009, 50, 494–498. [Google Scholar] [CrossRef]
- Oyamada, T.; Sasabe, H.; Adachi, C. Formation of MgAu alloy cathode by photolithography and its application to organic light-emitting diodes and organic field effect transistors. Electr. Eng. Jpn. 2005, 152, 37–42. [Google Scholar] [CrossRef]
- Li, Y.F.; Kuang, X.Y.; Wang, S.J.; Li, Y.; Zhao, Y.R. Geometries, stabilities, and electronic properties of gold-magnesium (Aun Mg) bimetallic clusters. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2011, 375, 1877–1882. [Google Scholar]
- Dubecký, M.; Dubecký, F. The work functions of Au/Mg decorated Au(100), Mg(001), and AuMg alloy surfaces: A theoretical study. J. Chem. Phys. 2014, 141, 94705. [Google Scholar] [CrossRef] [PubMed]
- Burch, R. Gold catalysts for pure hydrogen production in the water-gas shift reaction: Activity, structure and reaction mechanism. Phys. Chem. Chem. Phys. 2006, 8, 5483–5500. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.H.; Curtarolo, S.; Hart, G.L.W. Guiding the experimental discovery of magnesium alloys. Phys. Rev. B 2011, 84, 84101. [Google Scholar] [CrossRef]
- Takenaka, T.; Ono, T.; Narazaki, Y.; Naka, Y.; Kawakami, M. Improvement of corrosion resistance of magnesium metal by rare earth elements. Electrochim. Acta 2007, 53, 117–121. [Google Scholar] [CrossRef]
- Viestfrid, Y.; Levi, M.D.; Gofer, Y.; Aurbach, D. Microelectrode studies of reversible Mg deposition in THF solutions containing complexes of alkylaluminum chlorides and dialkylmagnesium. J. Electroanal. Chem. 2005, 576, 183–195. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Mishra, B.K.; Deka, R.C. A DFT study on structure, stabilities and electronic properties of double Magnesium doped Gold clusters. RSC Adv. 2014, 4, 56571–56581. [Google Scholar] [CrossRef]
- Majumder, C.; Anil K., K.; Puru, J. Structure and bonding of Au5M (M = Na, Mg, Al, Si, P, and S) clusters. Phys. Rev. B 2006, 74, 1–6. [Google Scholar] [CrossRef]
- Koyasu, K.; Naono, Y.; Akutsu, M. Photoelectron spectroscopy of binary Au cluster anions with a doped metal atom: AunM- (n = 2–7), M = Pd, Ni, Zn, Cu, and Mg. Chem. Phys. Lett. 2006, 422, 62–66. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Xie, L.; Zheng, J.; Liu, Y.; Li, X. Hydrogen absorption-desorption, optical transmission properties and annealing effect of Mg thin films prepared by magnetron sputtering. Int. J. Hydrog. Energy 2009, 34, 1910–1915. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Strom-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloy. Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Rud, A.D.; Lakhnik, A.M.; Ivanchenko, V.G.; Uvarov, V.N.; Shkola, A.A.; Dekhtyarenko, V.A.; Ivaschuk, L.I.; Kuskova, N.I. Hydrogen storage of the Mg-C composites. Int. J. Hydrog. Energy 2008, 33, 1310–1316. [Google Scholar] [CrossRef]
- Gremaud, R.; Broedersz, C.P.; Borsa, D.M.; Borgschulte, A.; Mauron, P.; Schreuders, H.; Rector, J.H.; Dam, B.; Griessen, R. Hydrogenography: An optical combinatorial method to find new light-weight hydrogen-storage materials. Adv. Mater. 2007, 19, 2813–2817. [Google Scholar] [CrossRef]
- Noh, Y.Y.; Kim, J.J.; Yase, K.; Nagamatsu, S. Organic field-effect transistors by a wet-transferring method. Appl. Phys. Lett. 2003, 83, 1243–1245. [Google Scholar] [CrossRef]
- Chadwick, A.F.; Vardar, G.; DeWitt, S.; Sleightholme, A.E.S.; Monroe, C.W.; Siegel, D.J.; Thornton, K. Computational Model of Magnesium Deposition and Dissolution for Property Determination via Cyclic Voltammetry. J. Electrochem. Soc. 2016, 163, A1813–A1821. [Google Scholar] [CrossRef]
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg rechargeable batteries: An on-going challenge. Energy Environ. Sci. 2013, 6, 2265–2279. [Google Scholar] [CrossRef]
- DeWitt, S.; Hahn, N.; Zavadil, K.; Thornton, K. Computational Examination of Orientation-Dependent Morphological Evolution during the Electrodeposition and Electrodissolution of Magnesium. J. Electrochem. Soc. 2016, 163, A513–A521. [Google Scholar] [CrossRef]
- Liebenow, C. Reversibility of electrochemical magnesium deposition from Grignard solutions. J. Appl. Electrochem. 1997, 27, 221–225. [Google Scholar] [CrossRef]
- Mohtadi, R.; Matsui, M.; Arthur, T.S.; Hwang, S.J. Magnesium borohydride: From hydrogen storage to magnesium battery. Angew. Chem. Int. Ed. 2012, 51, 9780–9783. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Siegel, D.J. Interface-Induced Renormalization of Electrolyte Energy Levels in Magnesium Batteries. J. Phys. Chem. Lett. 2016, 7, 874–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, G.R.; Hussey, C.L. Electrodeposition of transition metal-aluminium alloys from chloroaluminate molten salts. In Advances in Electrochemical Science and Engineering; Alkire, R.C., Kolb, D.M., Eds.; Wiley-VCH Verlag GmbH: New York, NY, USA, 2001; Volume 7, pp. 275–348. [Google Scholar]
- Kolb, D.M. The initial stages of metal deposition as viewed by scanning tunneling microscopy. In Advances in Electrochemical Science and Engineering; Alkire, R.C., Kolb, D.M., Eds.; Wiley-VCH Verlag GmbH: New York, NY, USA, 2001; Volume 7, pp. 107–150. [Google Scholar]
- Martı́nez, A.M.; Børresen, B.; Haarberg, G.M.; Castrillejo, Y.; Tunold, R. Electrodeposition of Magnesium from CaCl2-NaCl-KCl-MgCl2 Melts. J. Electrochem. Soc. 2004, 151, C508–C513. [Google Scholar] [CrossRef]
- Martı́nez, A.M.; Børresen, B.; Haarberg, G.M.; Castrillejo, Y.; Tunold, R. Electrodeposition of Magnesium from the eutectic LiCl-KCl melt. J. Appl. Electrochem. 2004, 34, 1271–1278. [Google Scholar] [CrossRef]
- Lu, Z.; Schechter, A.; Moshkovich, M.; Aurbach, D. On the electrochemical behavior of magnesium electrodes in polar aprotic electrolyte solutions. J. Electroanal. Chem. 1999, 466, 203–217. [Google Scholar] [CrossRef]
- Wang, P.; NuLi, Y.; Yang, J.; Feng, Z. Mixed Ionic Liquids as Electrolyte for Reversible Deposition and Dissolution of Magnesium. Surf. Coat. Technol. 2006, 201, 3783–3787. [Google Scholar] [CrossRef]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef] [PubMed]
- Watkins, T.; Kumar, A.; Buttry, D.A. Designer Ionic Liquids for Reversible Electrochemical Deposition/Dissolution of Magnesium. J. Am. Chem. Soc. 2016, 138, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.; Sharma, R.C.; Gaur, H.C. Conductivity of molten hydrated salts: Mg(NO3)2·6H2O + NH4NO3 system. Electrochim. Acta 1978, 23, 1367–1369. [Google Scholar] [CrossRef]
- Tkalenko, D.A. Elektrokhimiya Nitratnykh Rasplavov; Naukova Dumka: Kiev, Ukraine, 1983. (In Russian) [Google Scholar]
- Tkalenko, D.A. Makrokinetika Katodnykh Protsessov v Gidroksidnykh i Nitratnykh Rasplavakh; Naukova Dumka: Kiev, Ukraine, 1993. (In Russian) [Google Scholar]
- Ramana, K.V.; Sharma, R.C.; Gaur, H.C. Conductivity and viscosity of molten mixtures of ferric nitrate nonahydrate with hydrates of calcium, cadmium, magnesium, and zinc nitrates. J. Chem. Eng. Data 1990, 35, 293–297. [Google Scholar] [CrossRef]
- Ramana, K.V.; Sharma, R.C.; Gaur, H.C. Conductivity and viscosity of molten mixtures of aluminum nitrate decahydrate with hydrates of calcium, cadmium, magnesium, and zinc nitrates. J. Chem. Eng. Data 1990, 35, 418–420. [Google Scholar] [CrossRef]
- Vidu, R.; Hirai, N.; Hara, S. Comparative kinetic study of Cd diffusion into Au(100) and Ag(100) during electrodeposition. Phys. Chem. Chem. Phys. 2001, 3, 3320–3324. [Google Scholar] [CrossRef]
- Radovic, B.S.; Edwards, R.A.H.; Jovicevic, J.N. Aluminium underpotential deposition from AlCl3 + NaCl melts on gold electrodes. J. Electroanal. Chem. 1997, 428, 113–121. [Google Scholar] [CrossRef]
- Radović, B.S.; Cvetković, V.S.; Edwards, R.A.H.; Jovićević, J.N. Al-Cu alloy formation by aluminium underpotential deposition from AlCl3 + NaCl melts on copper substrate. Kov. Mater. 2010, 48, 159–171. [Google Scholar]
- Radovic, B.; Edwards, R.A.H.; Cvetković, V.S.; Jovicevic, J.N. Al-Ag alloy formation by aluminium underpotential deposition from AlCl3+NaCl melts on silver substrate. Met. Mater. 2010, 48, 55–71. [Google Scholar] [CrossRef]
- Cvetković, V.S.; Bjelica, L.J.; Vukićević, N.M.; Jovićević, J.N. Alloy formation by Mg underpotential deposition on Al from nitrate melts. Chem. Ind. Chem. Eng. Q. 2015, 21, 527–536. [Google Scholar] [CrossRef]
- Cvetković, V.S.; Jovićevic, J.N.; Bjelica, L.J. Magnesium underpotential deposition from nitrate melts and alloy formation with platinum substrate. Kov. Mater. 2016, 54, 321–330. [Google Scholar] [CrossRef]
- Cvetković, V.S. Underpotential Deposition of Magnesium from Nitrate Melts (in Serbian); Zadužbina Andrejević: Beograd, Serbia, 2012. [Google Scholar]
- Amir, N.; Vestfrid, Y.; Chusid, O.; Gofer, Y.; Aurbach, D. Progress in nonaqueous magnesium electrochemistry. J. Power Sources 2007, 174, 1234–1240. [Google Scholar] [CrossRef]
- Radović, B.S.; Cvetković, V.S.; Edwards, R.A.H.; Jovićević, J.N. Al-Fe alloy formation by aluminium underpotential deposition from AlCl3 + NaCl melts on iron substrate. Int. J. Mater. Res. 2011, 102, 59–68. [Google Scholar] [CrossRef]
- Jovićević, N.; Cvetković, V.S.; Kamberović, Ž. J.; Jovićević, J.N. Al-Cd Alloy Formation by Aluminum Underpotential Deposition from AlCl3+NaCl Melts on Cadmium Substrate. Metall. Mater. Trans. B 2013, 44, 106–114. [Google Scholar]
- Cvetković, V.S.; Jovićević, N.; Jovićević, J.N. Mg-Au Surface Alloy Formation by Magnesium Underpotential Deposition on Au from Nitrate Melts. In Proceedings of the 62nd Annual Meeting of the International Society of Electrochemistry, International Society of Electrochemistry, Niigata, Japan, 11–16 Sepetmber 2011; p. poster s03-P-012.
- Burkhardt, V.K.; Schubert, K.; Toth, R.S.; Sato, H. Die Struktur der Phase Au77Mg23. Acta Crystallogr. 1968, B24, 137–142. [Google Scholar] [CrossRef]
- Brauer, G.; Haucke, W. Kristallstruktur der intermetallischen Phasen MgAu und MgHg. Z. Phys. Chem. B 1936, 33, 304–310. [Google Scholar]
- Range, K.-J.; Hafner, P. Structure refinement of AuMg3, IrMg3 and IrMg2.8. J. Alloy. Compd. 1993, 191, L5–L7. [Google Scholar] [CrossRef]
- Daams, J.L.C.; Van Vucht, J.H. Contribution on the system Mg-Au-Hg. Philips J. Res. 1984, 39, 275–292. [Google Scholar]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- Smith, D.W. Inorganic Substances. A Prelude to the Study od Descriptive Inorganic Chemistry; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Hume-Rothery, W.; Smallman, R.E.; Haworth, C.W. The Structure of Metals and Alloys, 5th ed.; The Metals and Metallurgy trust of the Institute of metals and Institution of Metallurgists: London, UK, 1969. [Google Scholar]
- Cottrell, A.H. Introduction to Modern Theory of Metals; Institute of Materials: London, UK, 1988. [Google Scholar]
- Balducci, G.; Ciccioli, A.; Gigli, G. A mass spectrometric and density functional study of the intermetallic molecules AuBe, AuMg, and AuCa. J. Chem. Phys. 2004, 121, 7748–7755. [Google Scholar] [CrossRef] [PubMed]
- Predel, B. Au-Mg (Gold-Magnesium). In Ac-Au–Au-Zr; Springer: Berlin/Heidelberg, Germany, 1992; pp. 1–2. [Google Scholar]
- Nayeb-Hashemi, A.A.; Clark, J.B. Phase Diagram of Binary Magnesium Alloys. In Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1986; p. 198. [Google Scholar]
- Hansen, M.; Anderko, K. Constitution of Binary Alloys; McGraw-Hill: New York, NY, USA, 1958. [Google Scholar]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM: Metals Park, OH, USA, 1990. [Google Scholar]
- Hashaikeh, R.; Szpunar, J.A. Electrolytic processing of MgO coatings. J. Phys. Conf. Ser. 2009, 165, 12008. [Google Scholar] [CrossRef]
- Rao, K.V.; Sunandana, C.S. Structure and microstructure of combustion synthesized MgO nanoparticles and nanocrystalline MgO thin films synthesized by solution growth route. J. Mater. Sci. 2008, 43, 146–154. [Google Scholar] [CrossRef]
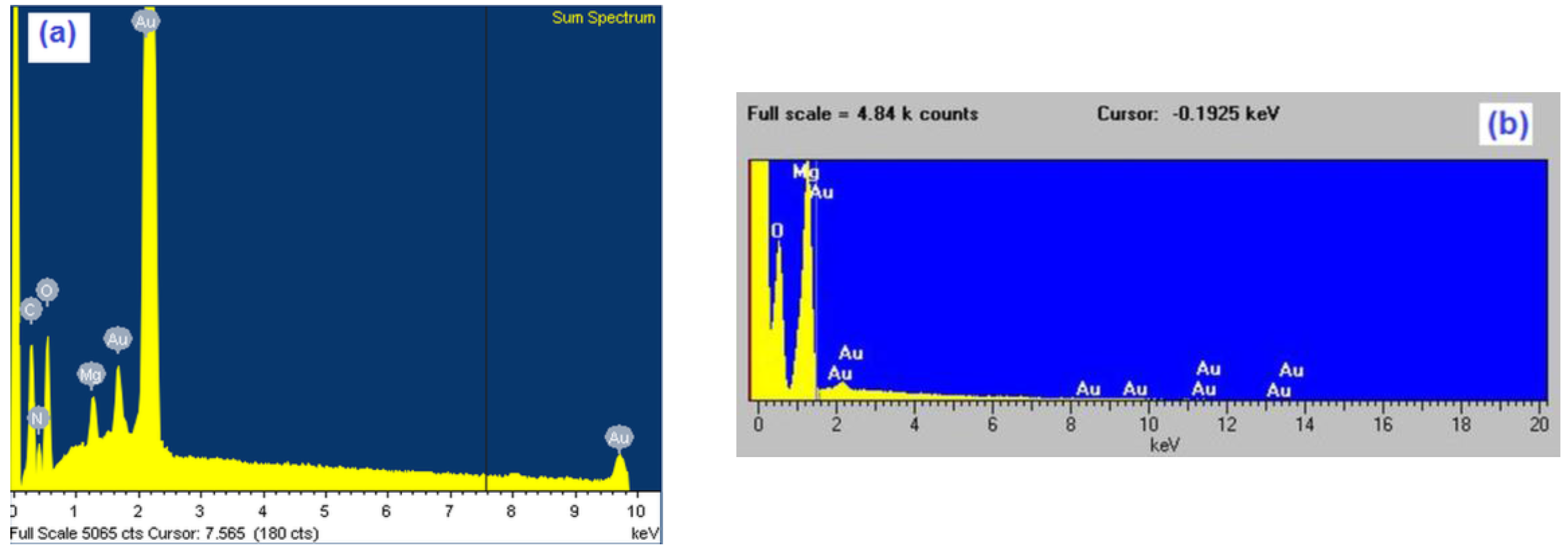
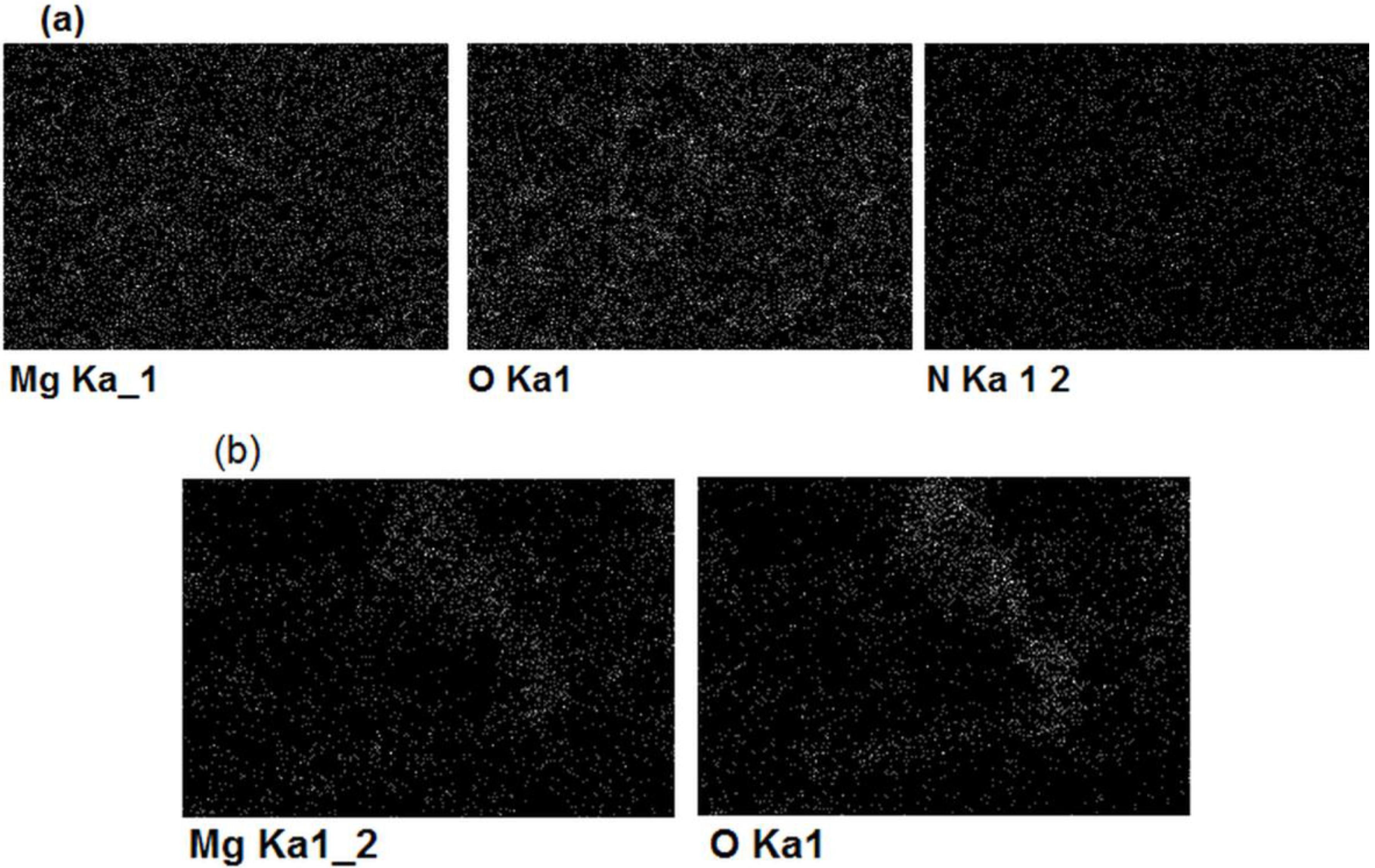
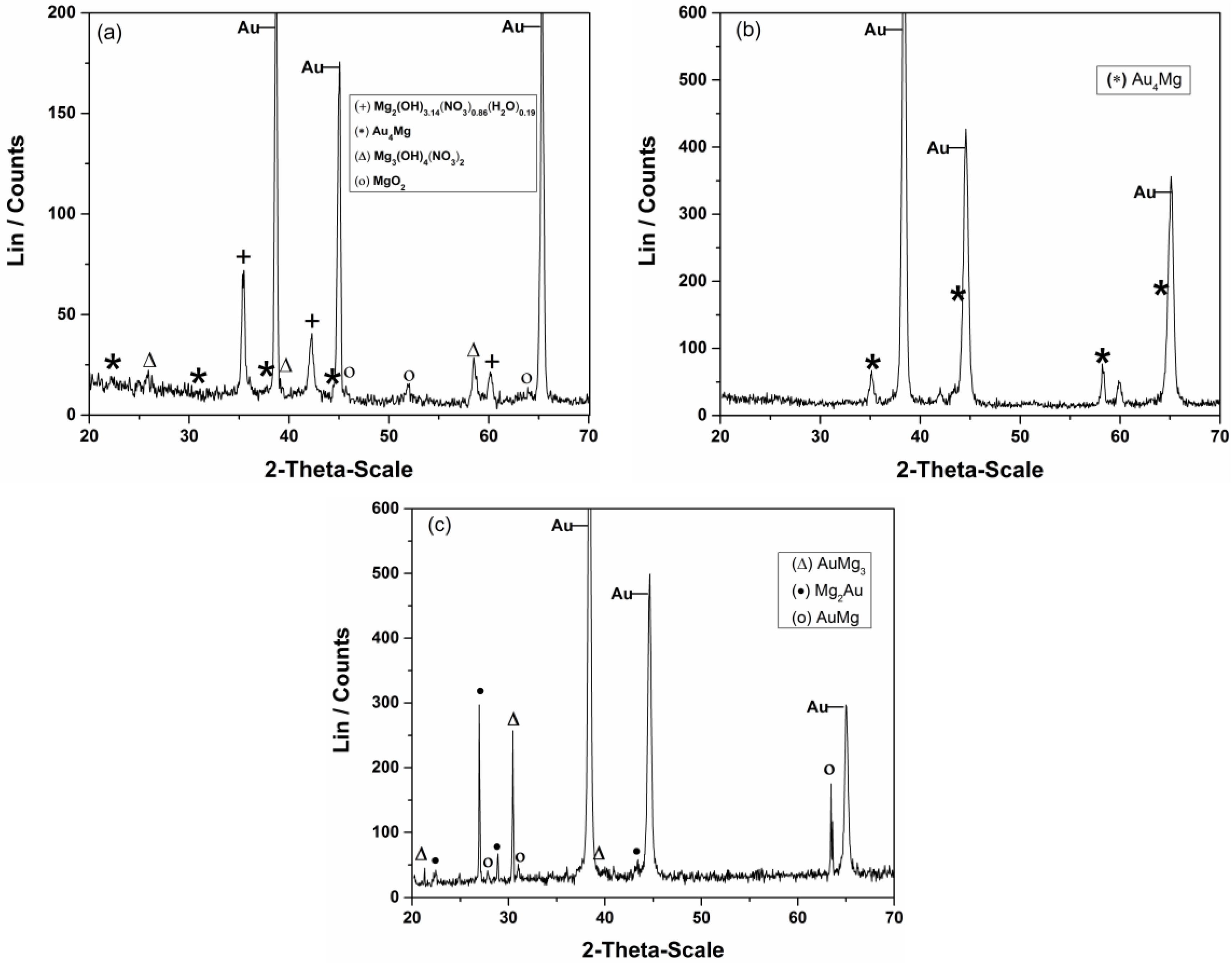
| Element | Magnesium/Ammonium Nitrate Eutectic Mixture at 400 K | Nonaqueous Magnesium/Ammonium Eutectic Mixture at 460 K | ||
|---|---|---|---|---|
| Element (%) | Atomic (%) | Element (%) | Atomic (%) | |
| O K | 71.52 | 85.26 | 65.06 | 75.52 |
| Mg K | 17.42 | 13.67 | 24.96 | 23.5 |
| Au M | 11.06 | 1.07 | 9.98 | 0.98 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Tem. (K) | Time, τ/h | Identified Phase | Structure | Approximate Composition wt. % Au | References |
|---|---|---|---|---|---|
| 460 K | 2 | Au4Mg | Orthorhombic | 94–97 | [48] |
| 5 | AuMg AuMg3 Mg2Au | Cubic Hexagonal Orthorhombic | 85–95 73–80 73–80 | [49] [50] [51] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvetković, V.S.; Jovićević, N.; Stevanović, J.S.; Pavlović, M.G.; Vukićević, N.M.; Stevanović, Z.; Jovićević, J.N. Magnesium–Gold Alloy Formation by Underpotential Deposition of Magnesium onto Gold from Nitrate Melts. Metals 2017, 7, 95. https://doi.org/10.3390/met7030095
Cvetković VS, Jovićević N, Stevanović JS, Pavlović MG, Vukićević NM, Stevanović Z, Jovićević JN. Magnesium–Gold Alloy Formation by Underpotential Deposition of Magnesium onto Gold from Nitrate Melts. Metals. 2017; 7(3):95. https://doi.org/10.3390/met7030095
Chicago/Turabian StyleCvetković, Vesna S., Niko Jovićević, Jasmina S. Stevanović, Miomir G. Pavlović, Nataša M. Vukićević, Zoran Stevanović, and Jovan N. Jovićević. 2017. "Magnesium–Gold Alloy Formation by Underpotential Deposition of Magnesium onto Gold from Nitrate Melts" Metals 7, no. 3: 95. https://doi.org/10.3390/met7030095








