Impact of the Solidification Rate on the Chemical Composition of Frozen Cryolite Bath
Abstract
:1. Introduction
2. Methodology
2.1. Solidified Bath Samples Produced Using Cold Finger Technique
2.2. Mathematical Model of Transient Freeze Formation
2.3. XRD Analysis
2.4. Chemical Element Mapping
3. Results and Discussion
3.1. Identification of Zones with Different Structures
3.1.1. Sample Obtained with the Transient Probe
- 1)
- Compact, low porosity and relatively hard layer formed with very fast homogeneous nucleation at very high heat flux (110–2200 kW/m2); a large number of microcrystals without any visible orientation (micro-aphanitic structure).
- 2)
- Transition zone between zones 1 and 3 with structure similar to zone 3 but containing even more porosity.
- 3)
- Fragile and porous layer, formed at medium heat flux (60–100 kW/m2) except a very thin layer being longer in direct contact with the bath at the right side; characterized by rounded shaped crystals and exsolution (process through which an initially homogeneous solid solution separates into at least two different crystalline minerals without the addition or removal of any materials). The exsolution is indicated by very small, acne-like outgrowths on the crystal surface [12]. Both morphological features could be the result of long exposure to strongly acidic entrapped liquid pockets (high AlF3 excess). In fact, in the presence of large amounts of AlF3, the cryolite can be recrystallized into chiolite around 750 °C [14]: 5Na3AlF6 + 4AlF3 = 3Na5Al3F14.
3.1.2. Sample Obtained with Steady-State Probe
- 4)
- Thin layer with strong crystal orientation due to the heterogeneous nucleation at the probe surface. It is formed mainly at medium heat flux (50–100 kW/m2). Similarly to zone 3, pores, rounded shaped crystals and exsolution can be observed at a certain distance from the probe surface (beyond the above mentioned very first layers).
- 5)
- Relatively low porosity layer with well-defined straight crystal boundaries, formed at low heat flux (28–50 kW/m2). Such straight boundaries can be formed when the growing crystal is exposed for long time to low acidity bulk liquid.
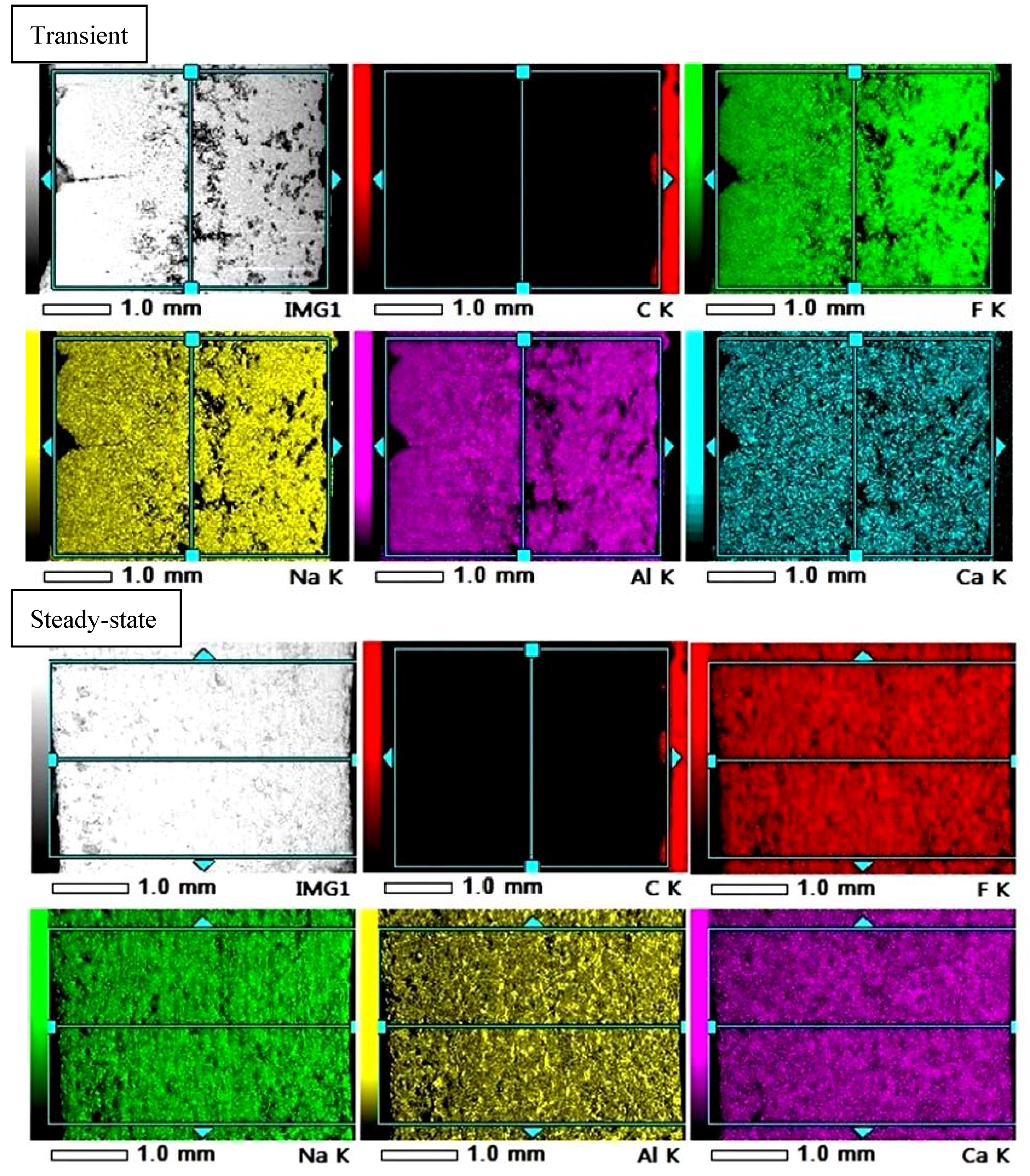
- The probe side of the transient sample (TP) contained zone 1
- The bath side of the transient sample (TB) contained zones 2 and 3
- The probe side of the steady-state sample (SP) contained zone 4 and a smaller portion of the zone 5 close to probe
- The bath side of the steady-state sample (SB) contained the bigger portion of the zone 5 which is close to the bath
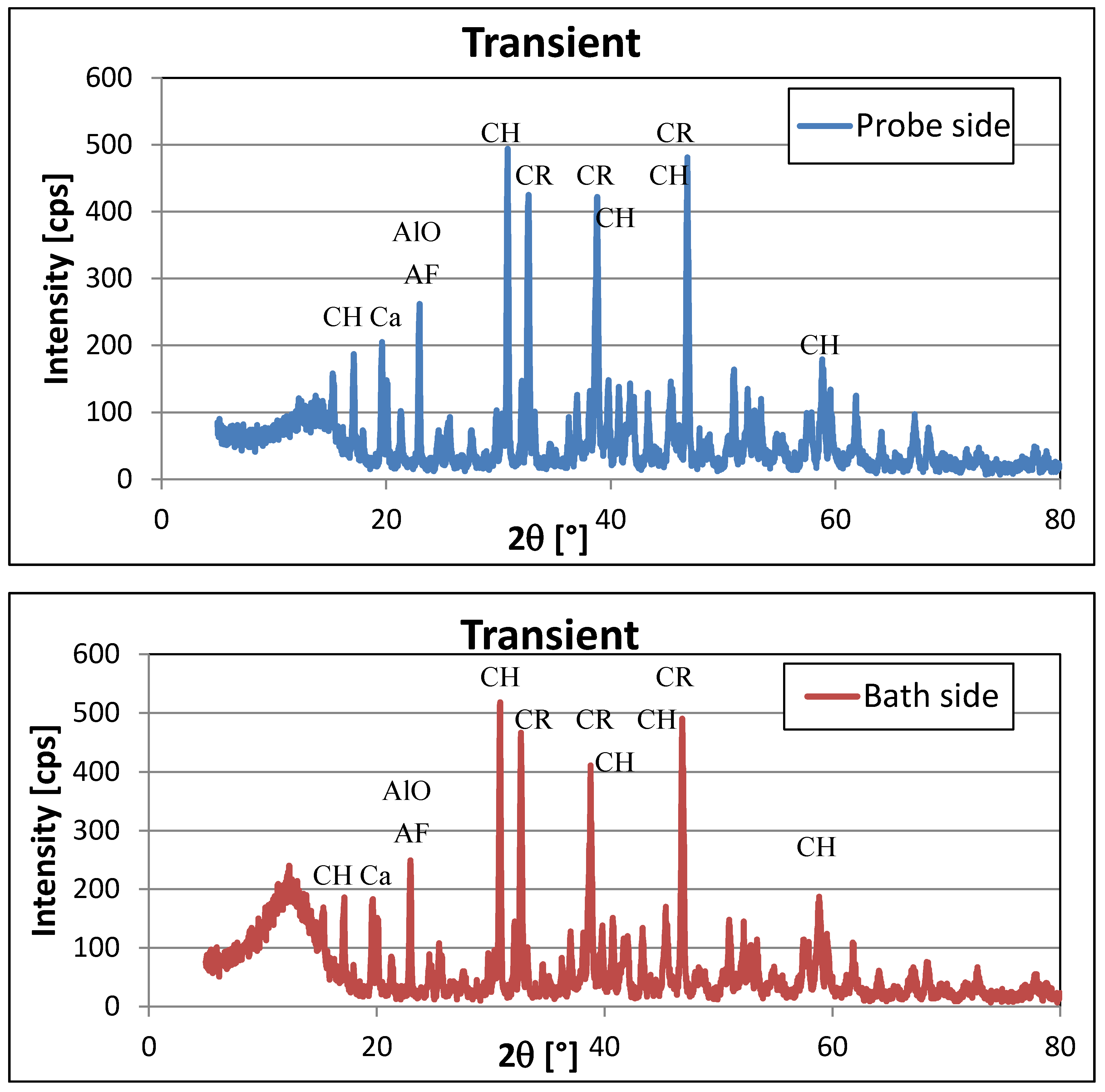
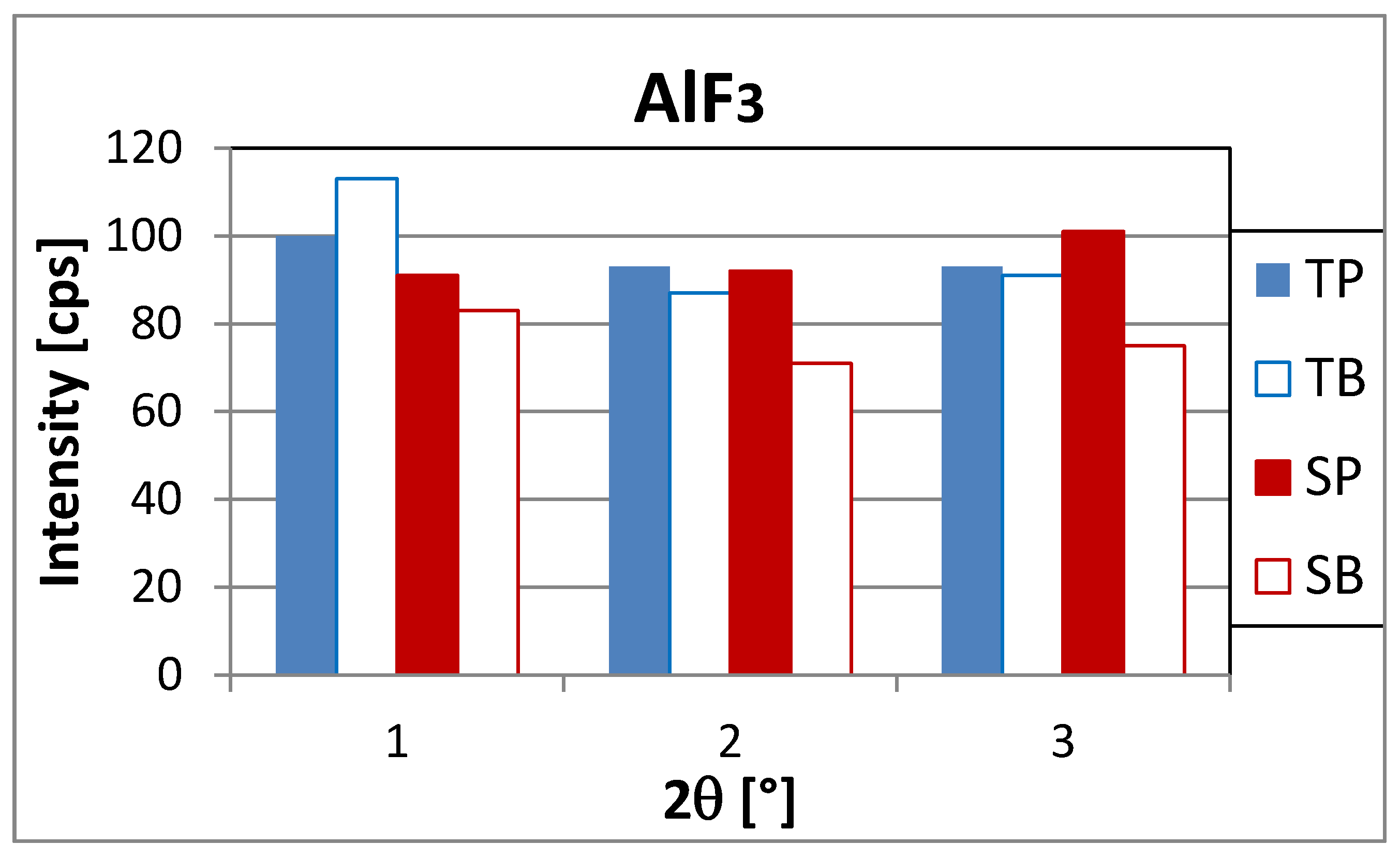
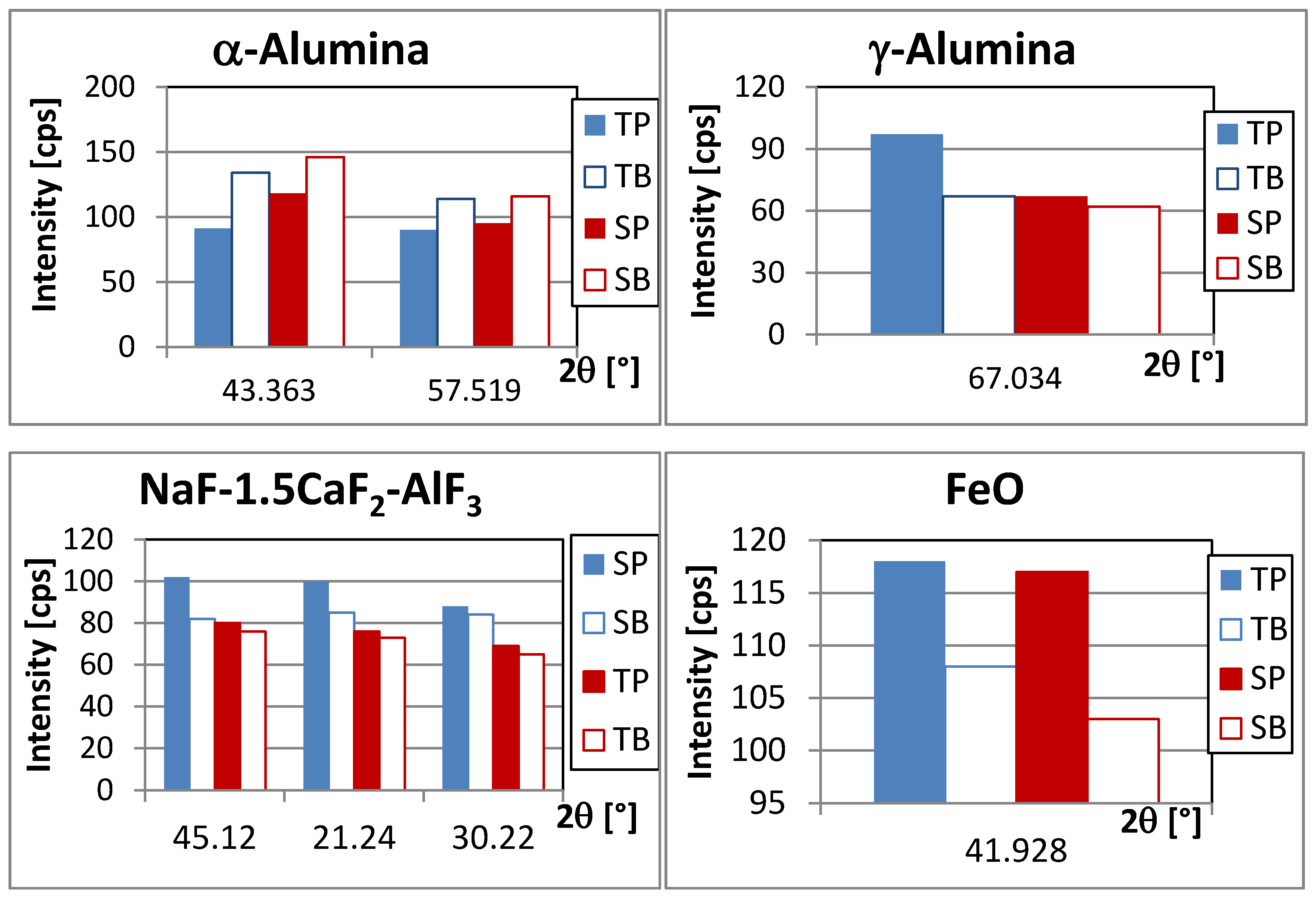
3.2. XRD Analysis
3.3. Analysis of Elementary Composition Using Electron Microscope with BSE Mode
4. Conclusions
- In accordance with the expectations, the cryolite and chiolite are the two main components of the frozen bath.
- The distribution of the chemical elements is more inhomogeneous in the transient sample similarly to the structure and in accordance with the expectation. In fact, the heat flux and solidification rate change substantially in the successively formed layers at different distance from the cold finger probe.
- Very fast solidification of acidic bath with AlF3 excess at very high cooling rate blocks the migration of ions that decreases the Na to Al ratio. It seems to slightly increase the local concentration of chiolite and calcium containing compounds and reduces γ–α conversion as well.
- The earlier presented hypothesis that medium solidification rate promotes the recrystallization of cryolite to chiolite (exsolution), due to the presence of acidic liquid pockets entrapped by growing crystals, was confirmed by XRD.
- The contact between the molten bath and the steel probe forms some FeO that is entrapped mainly in a thin, generally darker layer, close to the probe.
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Zhang, Q.; Taylor, M.; Chen, J. Composition and Thermal Analysis of Crust Formed from Industrial Anode Cover. In Light Metals 2013; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 675–680. [Google Scholar]
- Solheim, A. Some Aspects of Heat Transfer between Bath and Sideledge in Aluminium Reduction Cells. In Light Metals 2011; Springer: Berlin, Germany, 2011; pp. 381–386. [Google Scholar]
- Thonstad, J.; Fellner, P.; Haarberg, G.M.; Hives, J.; Kvande, H.; Sterten, A. Aluminium Electrolysis−Fundamentals of the Hall-Héroult Process, 3rd ed.; Aluminium-Verlag: Dusseldorf, Germany, 2001. [Google Scholar]
- Solheim, A.; Støen, L.I.R. On the Composition of Solid Deposits Frozen out from the Cryolitic Melts. In Light Metals; Metallurgical Society of AIME: Warrendale, PA, USA, 1997; pp. 325–332. [Google Scholar]
- Chartrand, P.; Pelton, A.D. A Predictive Thermodynamic Model for the Al-NaF-AlF3-CaF2-Al2O3 System. In Light Metals; Metallurgical Society of AIME: Warrendale, PA, USA, 2002; pp. 245–252. [Google Scholar]
- Solheim, A.; Rolseth, S.; Skybakmoen, E.; Støen, L.; Sterten, Å.; Støre, T. Liquidus Temperatures for Primary Crystallisation of Cryolite in Molten Salt Systems of Interest for the Aluminium Electrolysis. Met. Trans. B 1996, 27, 739–744. [Google Scholar] [CrossRef]
- Grandfield, J.; Poncsák, S.; Kiss, L.; St-Pierre, R.; Guérard, S.; Bilodeau, J.F. Structural Characterisation and Thermophysical Properties of the Side Ledge in Hall-Héroult Cells. In Light Metals 2014; Springer: Berlin, Germany, 2014. [Google Scholar]
- Thonstad, J.; Rolseth, S. Equilibrium between Bath and Sideledge. In TMS Light Metals; Metallurgical Society of AIME: Warrendale, PA, USA, 1983; pp. 415–424. [Google Scholar]
- Taylor, M.P.; Zhang, W.D.; Wills, V.; Schmid, S. A dynamic model for the energy balance of an electrolysis cell. Chem. Eng. Res. Des. 1996, 8, 913–933. [Google Scholar] [CrossRef]
- Taylor, M.P.; Welch, B.J. Melt/freeze heat transfer measurements in cryolite-based electrolytes. Metall. Trans. B 1987, 2, 391–398. [Google Scholar] [CrossRef]
- Hyland, M.; Allard, F.; Désilets, M.; LeBreux, M.; Biais, A. Chemical Characterization and Thermodynamic Investigation of Anode Crust Used in Aluminum Electrolysis Cells. In Light Metals 2015; Springer: Berlin, Germany, 2015; pp. 656–670. [Google Scholar]
- Williams, E.; Poncsák, S.; Kiss, L.; Raymond, V.D.; Kaszás, C.; Guérard, S.; Bilodeau, J. Impact of the heat flux on solidification of cryolite based bath. In Light Metals 2016; Springer: Berlin, Germany, 2016; pp. 359–364. [Google Scholar]
- Dassylva-Raymond, V.; Istvan Kiss, L.; Poncsak, S.; Guérard, S. Modelling the behavior of alumina agglomerate in Hall-Héroult process. In Light Metals 2014; Springer: Berlin, Germany, 2014; pp. 603–608. [Google Scholar]
- Lindsay, S.J.; Wang, X.; Tarcy, G.; Batista, E.; Wood, G. Active Pot Control using Alcoa STARprobe™. In Light Metals 2011; Springer: Berlin, Germany, 2011; pp. 483–489. [Google Scholar]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef]



| Parameters | Transient | Steady-State |
|---|---|---|
| Probe | solid (full) block 12.5 × 11 × 2.54 cm | hollow with inner channels 12.5 × 11 × 2.54 cm with a wall thickness of 6.35 mm |
| Initial temperature | preheated to ~150 °C in order to remove humidity | preheated to ~150 °C in order to remove humidity and then preheated to the bath temperature in the molten bath |
| Sample producing process | insertion of cold probe to the bath in order to obtain the thickest possible crust | insertion of cold probe into the molten bath, then waiting until the transient crust is completely re-melted and the temperature of the probe stabilizes close to the bulk temperature; near steady-state crust is formed by the circulation of dried and compressed cold air in the channels of the probe with constant flow rate |
| Duration of insertion | 3 min this time was estimated by our mathematical model [13] | ~30 min probes were removed when the temperature became constant inside the probe |
| Compound | Lattice | Characteristic Peaks | |
|---|---|---|---|
| 2θ [°] | Intensity | ||
| Cryolite Na3AlF6 | Monoclinic | 46.789 * | 350–490 |
| 32.533 * | 410–421 | ||
| 38.61 * | 203–275 | ||
| Chiolite Na5Al3F14 | Tetragonal | 30.699 | 309–518 |
| 17.104 | 101–186 | ||
| 38.784 | 300–420 | ||
| 45.546 * | 84–140 | ||
| 50.978 * | 105–148 | ||
| 59.6 | 96–134 | ||
| Aluminium-fluoride AlF3 | Hexagonal | 14.747 | 91–144 |
| 24.971 * | very weak peak | ||
| 25.689 * | 71–93 | ||
| 29.746 | 75–101 | ||
| α-alumina | Rhombohedral | 43.363 | 118–151 |
| 35.136 | very weak peak | ||
| 57.519 | 90–116 | ||
| 25.584 * | 81–92 | ||
| γ–alumina | Face-centered cubic | 45.864 * | overlapping with strong peak of cryolite |
| 67.034 | 62–97 | ||
| α-NaCaAlF6 | Monoclinic | 24.5 * | not evident due to overlapping |
| 50.182 * | |||
| NaF-1.5CaF2-AlF3 | Body-centered cubic | 45.12 * | 76–102 |
| 21.24 | 73–100 | ||
| 30.22 | 74–98 | ||
| FeO | Face-centered cubic | 41.928 | 103–118 |
| 36.041 | too weak | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poncsák, S.; Kiss, L.I.; Guérard, S.; Bilodeau, J.-F. Impact of the Solidification Rate on the Chemical Composition of Frozen Cryolite Bath. Metals 2017, 7, 97. https://doi.org/10.3390/met7030097
Poncsák S, Kiss LI, Guérard S, Bilodeau J-F. Impact of the Solidification Rate on the Chemical Composition of Frozen Cryolite Bath. Metals. 2017; 7(3):97. https://doi.org/10.3390/met7030097
Chicago/Turabian StylePoncsák, Sándor, László I. Kiss, Sébastien Guérard, and Jean-François Bilodeau. 2017. "Impact of the Solidification Rate on the Chemical Composition of Frozen Cryolite Bath" Metals 7, no. 3: 97. https://doi.org/10.3390/met7030097






