Corrosion Behavior of API X100 Steel Material in a Hydrogen Sulfide Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
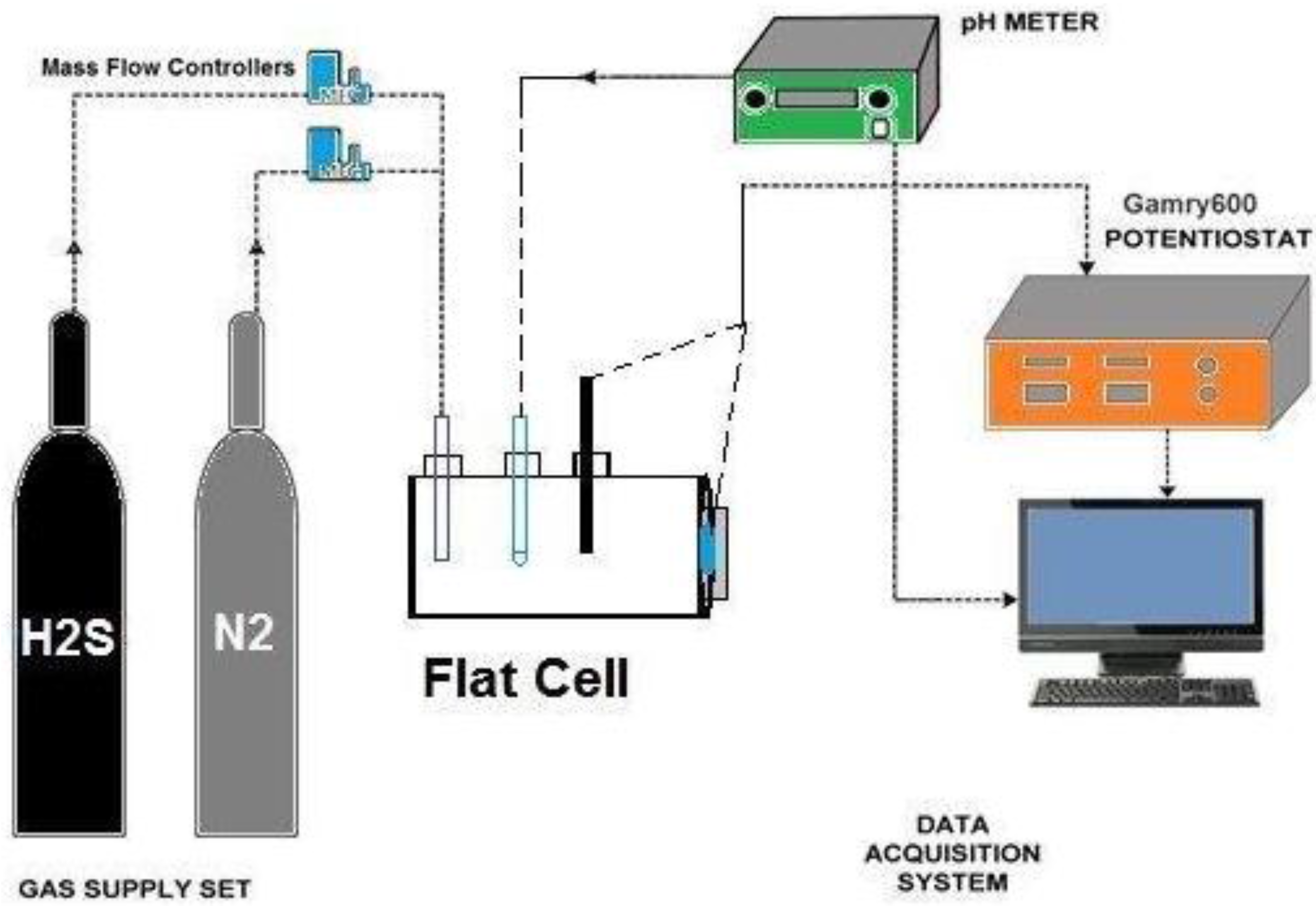
2.2. Electrochemical Measurement Setup
3. Results and Discussion
3.1. Potentiodynamic Polarization
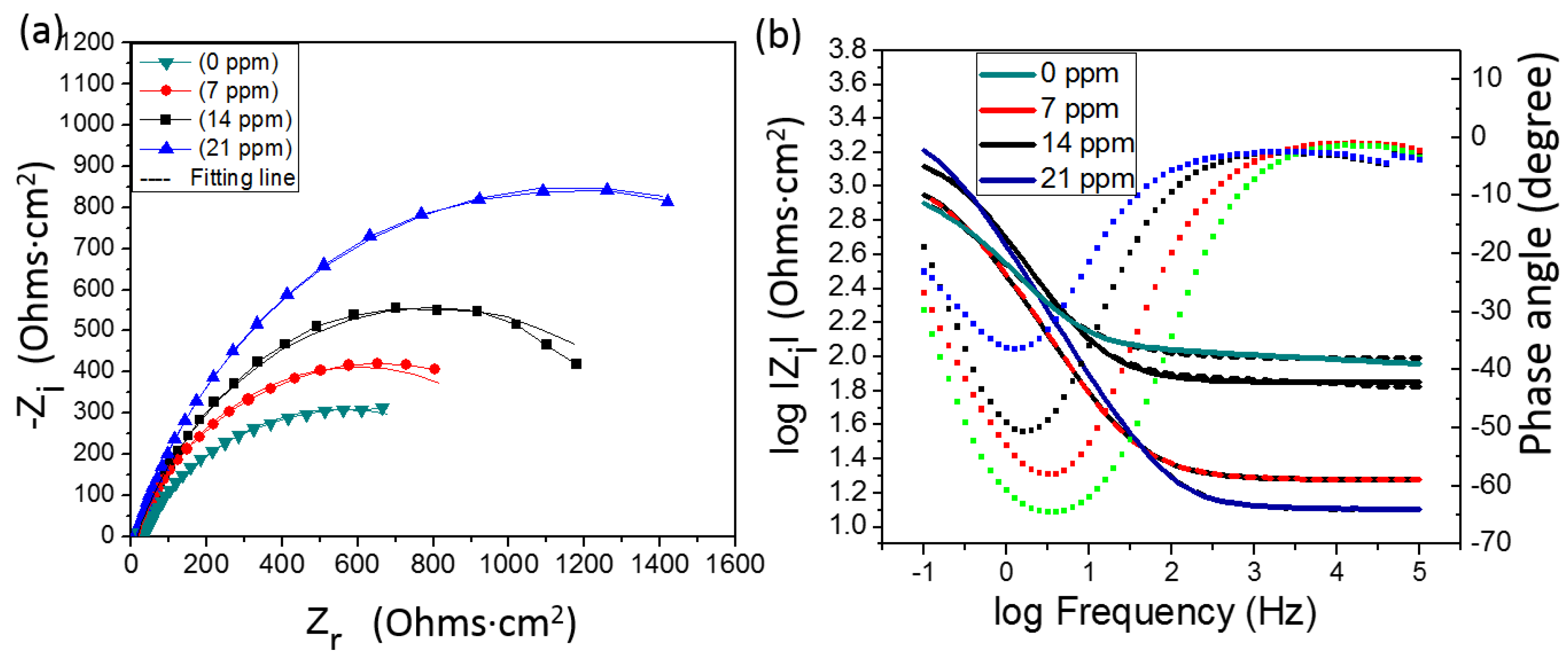
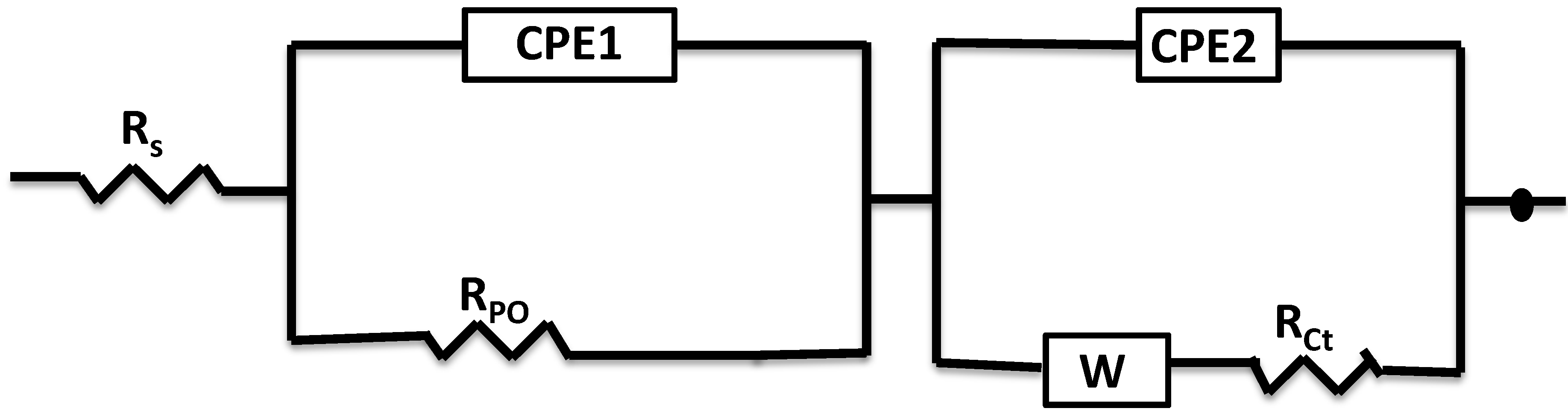
3.2. Electrochemical Impedance Spectroscopy (EIS)
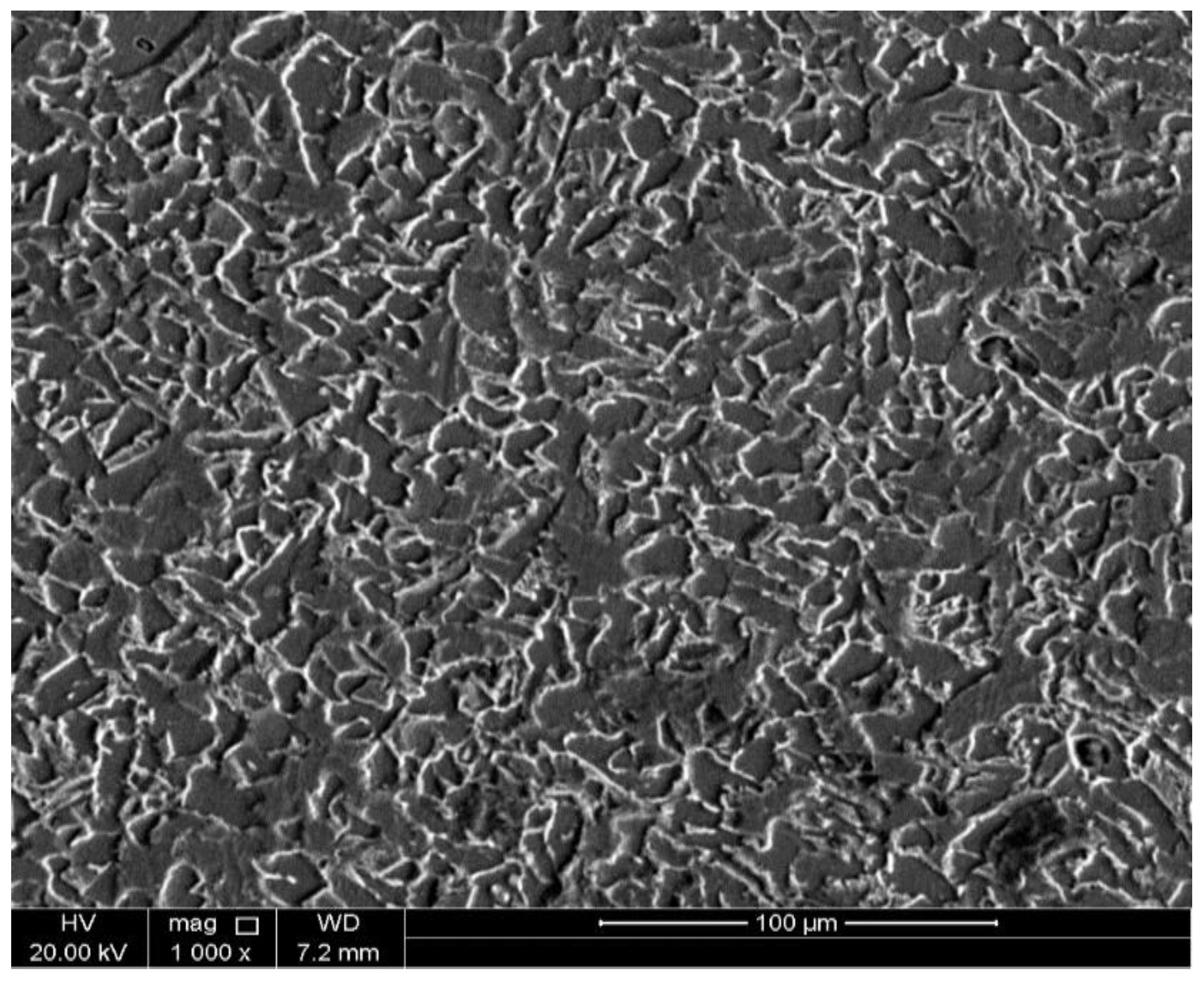
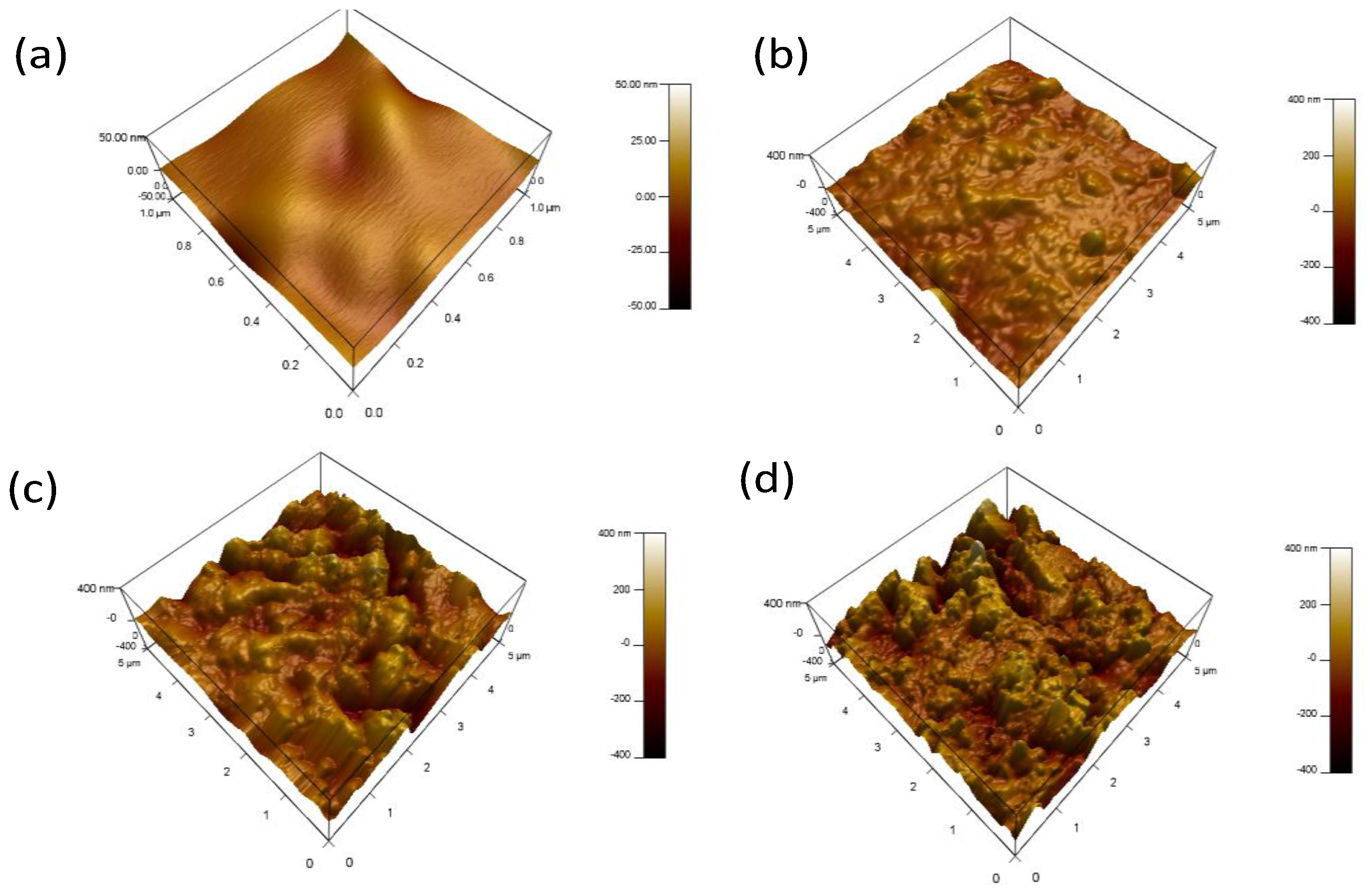
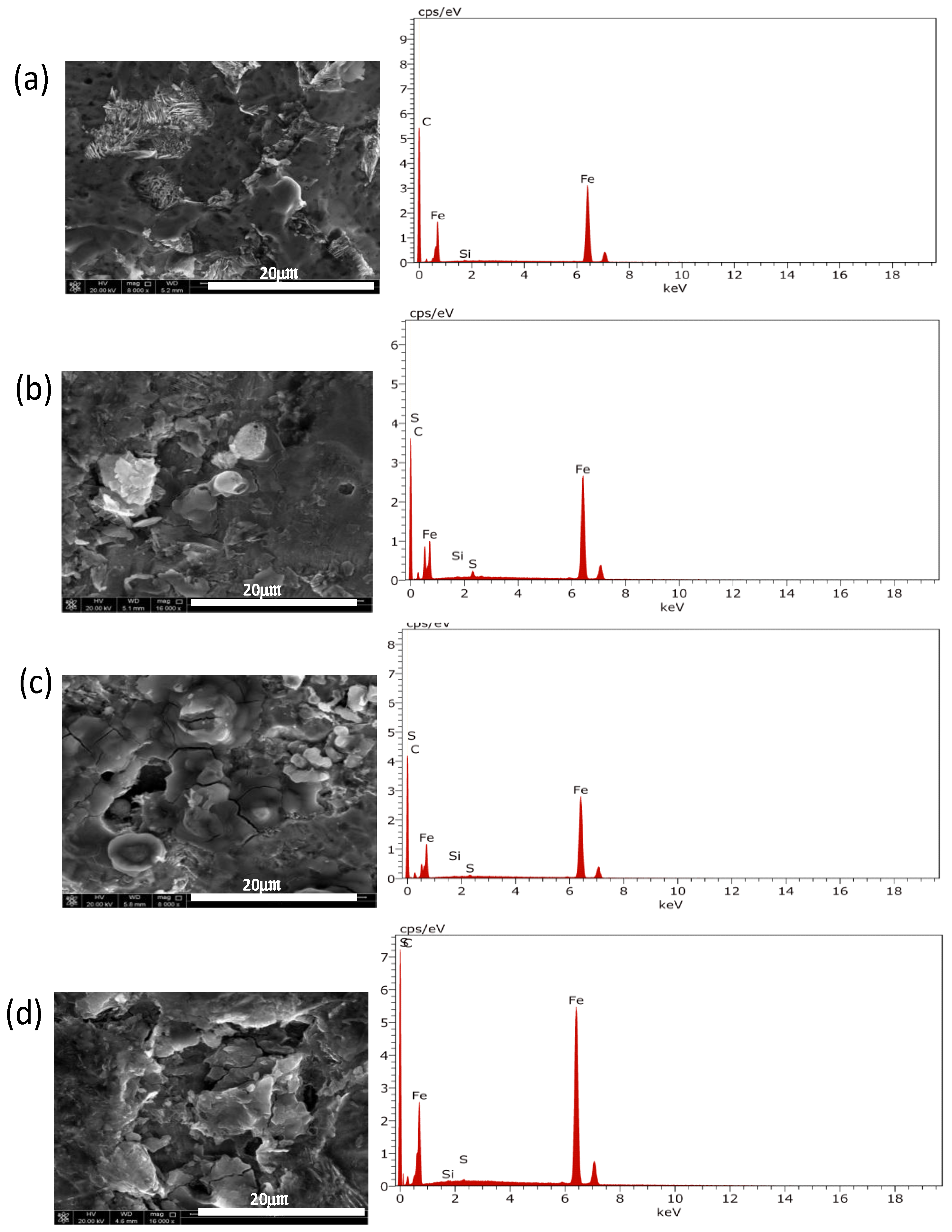
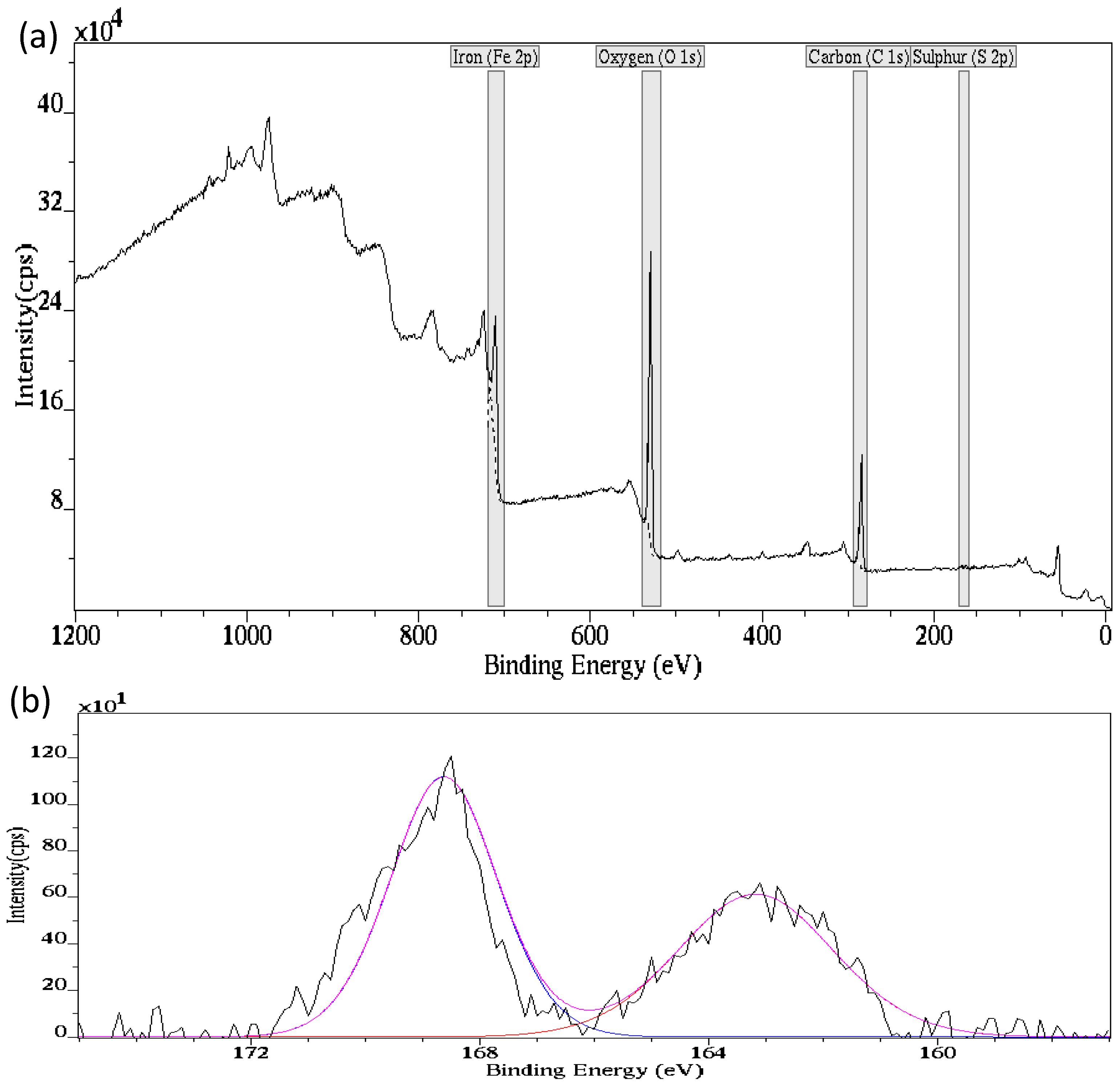
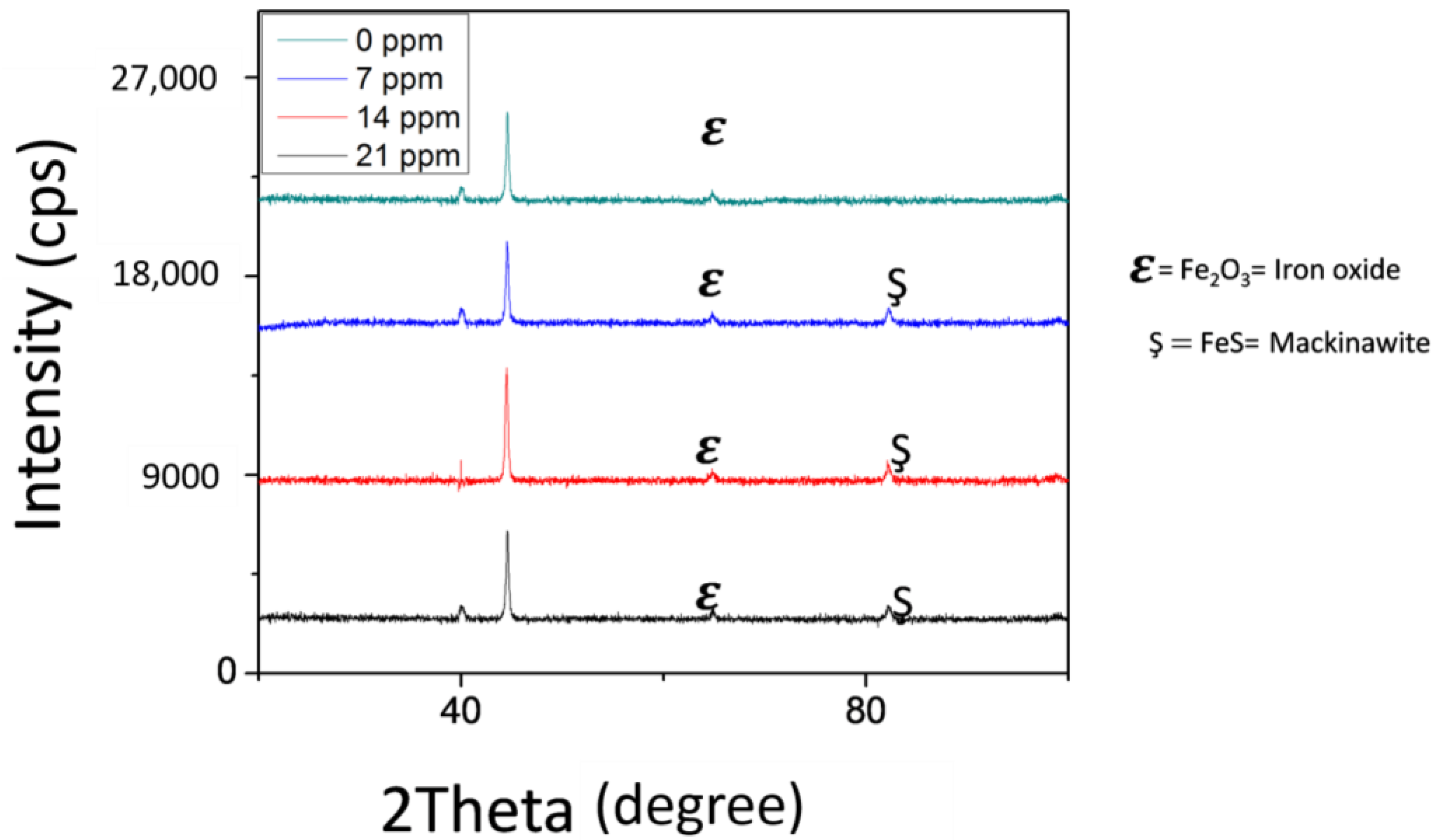
3.3. Surface Characterizations
4. Conclusions
- EDX and XPS analyses confirm the presence of sulfur on the corroded API X100 steel surfaces at different H2S concentrations.
- A significant decrease in the corrosion rate of API X100 steel in 3.5% NaCl solutions containing various concentrations of H2S is noticed which can be attributed to the formation of sulfide layer.
- SEM micrographs confirm that the formed protective sulfide layer becomes more homogenous and denser as the H2S concentration is increased.
- The formation of more homogeneous, dense and protective iron sulfide layer (mackinawite) is responsible for the decrease in the corrosion rate with increasing concentration of H2S.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Igi, S.; Sakimoto, T.; Endo, S. Effect of Internal Pressure on Tensile Strain Capacity of X80 Pipeline. Procedia Eng. 2011, 10, 1451–1456. [Google Scholar] [CrossRef]
- Jones, N.; Birch, R.S. Influence of Internal Pressure on the Impact Behavior of Steel Pipelines. J. Press. Vessel. Technol. 1996, 118, 464–471. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Tang, B.; Shan, Y.-Y.; Yang, K. Role of microstructure on sulfide stress cracking of oil and gas pipeline steels. Metall. Mater. Trans. A 2003, 34, 1089–1096. [Google Scholar]
- Hernández-Espejel, A.; Domínguez-Crespo, M.A.; Cabrera-Sierra, R.; Rodríguez-Meneses, C.; Arce-Estrada, E.M. Investigations of corrosion films formed on API-X52 pipeline steel in acid sour media. Corros. Sci. 2010, 52, 2258–2267. [Google Scholar] [CrossRef]
- López, H.F.; Raghunath, R.; Albarran, J.L.; Martinez, L. Microstructural aspects of sulfide stress cracking in an API X-80 pipeline steel. Metall. Mater. Trans. A 1996, 27, 3601–3611. [Google Scholar] [CrossRef]
- Nasirpouri, F.; Mostafaei, A.; Fathyunes, L.; Jafari, R. Assessment of localized corrosion in carbon steel tube-grade AISI 1045 used in output oil–gas separator vessel of desalination unit in oil refinery industry. Eng. Fail. Anal. 2014, 40, 75–88. [Google Scholar] [CrossRef]
- Lens, P.N.L.; Visser, A.; Janssen, A.J.H.; Hulshoff Pol, L.W.; Lettinga, G. Biotechnological Treatment of Sulfate-Rich Wastewaters. Crit. Rev. Environ. Sci. Technol. 1998, 28, 41–88. [Google Scholar] [CrossRef]
- Lucio-Garcia, M.A.; Gonzalez-Rodriguez, J.G.; Casales, M.; Martinez, L.; Chacon-Nava, J.G.; Neri-Flores, M.A.; Martinez-Villafañe, A. Effect of heat treatment on H2S corrosion of a micro-alloyed C–Mn steel. Corros. Sci. 2009, 51, 2380–2386. [Google Scholar] [CrossRef]
- Qi, Y.; Luo, H.; Zheng, S.; Chen, C.; Lv, Z.; Xiong, M. Effect of temperature on the corrosion behavior of carbon steel in hydrogen sulphide environments. Int. J. Electrochem. Sci. 2014, 9, 2101–2112. [Google Scholar]
- Liu, M.; Wang, J.Q.; Ke, W.; Han, E.H. Corrosion Behavior of X52 Anti-H2S Pipeline Steel Exposed to High H2S Concentration Solutions at 90 °C. J. Mater. Sci. Technol. 2014, 30, 504–510. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Mohamed, A.M.A. Erosion-Corrosion in Oil and Gas Industry: A Review. Int. J. Metall. Mater. Sci. Eng. 2014, 4, 7–28. [Google Scholar]
- Yin, Z.F.; Zhao, W.Z.; Bai, Z.Q.; Feng, Y.R.; Zhou, W.J. Corrosion behavior of SM 80SS tube steel in stimulant solution containing H2S and CO2. Electrochim. Acta 2008, 53, 3690–3700. [Google Scholar] [CrossRef]
- Vedage, H.; Ramanarayanan, T.A.; Mumford, J.D.; Smith, S.N. Electrochemical Growth of Iron Sulfide Films in H2S-Saturated Chloride Media. Corrosion 1993, 49, 114–121. [Google Scholar] [CrossRef]
- Bai, P.; Zheng, S.; Chen, C. Electrochemical characteristics of the early corrosion stages of API X52 steel exposed to H2S environments. Mater. Chem. Phys. 2015, 149–150, 295–301. [Google Scholar] [CrossRef]
- Schulte, M.; Schutze, M. The Role of Scale Stresses in the Sulfidation of Steels. Oxid. Met. 1999, 51, 55–77. [Google Scholar] [CrossRef]
- Carneiro, R.A.; Ratnapuli, R.C.; de Freitas Cunha Lins, V. The influence of chemical composition and microstructure of API linepipe steels on hydrogen-induced cracking and sulfide stress corrosion cracking. Mater. Sci. Eng. A 2003, 357, 104–110. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Zhou, Y.; Yang, X.; Wang, Z.; Li, Z. Effect of pressure on corrosion behavior of X60, X65, X70, and X80 carbon steels in water-unsaturated supercritical CO2 environments. Int. J. Greenh. Gas Control. 2016, 51, 357–368. [Google Scholar] [CrossRef]
- Gadala, I.M.; Alfantazi, A. A study of X100 pipeline steel passivation in mildly alkaline bicarbonate solutions using electrochemical impedance spectroscopy under potentiodynamic conditions and Mott–Schottky. Appl. Surf. Sci. 2015, 357, 356–368. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Mechanisms of Corrosion and Electrochemical Significance of Metallurgy and Environment with Corrosion of Iron and Steel in Bicarbonate and Carbonate Solutions—A Review. Corrosion 2014, 70, 880–898. [Google Scholar] [CrossRef]
- Sherar, B.W.A.; Keech, P.G.; Noël, J.J.; Worthingham, R.G.; Shoesmith, D.W. Effect of Sulfide on Carbon Steel Corrosion in Anaerobic Near-Neutral pH Saline Solutions. Corrosion 2012, 69, 67–76. [Google Scholar] [CrossRef]
- Papavinasam, S.; Doiron, A.; Revie, R.W. Model to Predict Internal Pitting Corrosion of Oil and Gas Pipelines. Corrosion 2010, 66. [Google Scholar] [CrossRef]
- Sridhar, N.; Dunn, D.S.; Anderko, A.M.; Lencka, M.M.; Schutt, H.U. Effects of Water and Gas Compositions on the Internal Corrosion of Gas Pipelines—Modeling and Experimental Studies. Corrosion 2001, 57, 221–235. [Google Scholar] [CrossRef]
- Han, J.; Carey, J.W.; Zhang, J. Effect of sodium chloride on corrosion of mild steel in CO2-saturated brines. J. Appl. Electrochem. 2011, 41, 741–749. [Google Scholar] [CrossRef]
- Shi, J.; Sun, W.; Jiang, J.; Zhang, Y. Influence of chloride concentration and pre-passivation on the pitting corrosion resistance of low-alloy reinforcing steel in simulated concrete pore solution. Constr. Build. Mater. 2016, 111, 805–813. [Google Scholar] [CrossRef]
- Abayarathna, D.; Naraghi, A.R.; Wang, S.H. The Effect of Surface Films on Corrosion of Carbon Steel in a CO2–H2S–H2O System in Corrosion/2005; NACE: Houston, TX, USA, 2005. [Google Scholar]
- Valcarce, M.B.; Vázquez, M. Carbon steel passivity examined in alkaline solutions: The effect of chloride and nitrite ions. Electrochim. Acta 2008, 53, 5007–5015. [Google Scholar] [CrossRef]
- Rao, T.S.; Sairam, T.N.; Viswanathan, B.; Nair, K.V.K. Carbon steel corrosion by iron oxidising and sulphate reducing bacteria in a freshwater cooling system. Corros. Sci. 2000, 42, 1417–1431. [Google Scholar] [CrossRef]
- Paul, C.O.; Ahmed, E.; Mohamed, A.M.A. Effect of Temperature on the Corrosion Behavior of API X80 Steel Pipeline. Int. J. Electrochem. Sci. 2015, 10, 10246–10260. [Google Scholar]
- Okonkwo, P.; Shakoor, R.; Zagho, M.; Mohamed, A. Erosion Behaviour of API X100 Pipeline Steel at Various Impact Angles and Particle Speeds. Metals 2016, 6, 232. [Google Scholar] [CrossRef]
- TM0284–2003, N.S. Evaluation of Pipeline and Pressure Vessel Steels for Resistance to Hydrogen-induced Cracking; NACE International: Houston, TX, USA, 2003. [Google Scholar]
- Saravanamoorthy, S.; Velmathi, S. Physiochemical interactions of chiral Schiff bases on high carbon steel surface: Corrosion inhibition in acidic media. Prog. Org. Coat. 2013, 76, 1527–1535. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Rahoma, A.A.B.; Mesmari, H. Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J. Mol. Struct. 2010, 969, 233–237. [Google Scholar] [CrossRef]
- Liu, H.J.; Xu, Q.; Yan, C.W.; Cao, Y.Z.; Qiao, Y.L. The Effect of Temperature on the Electrochemical Behavior of the V(IV)/V(V) Couple on a Graphite Electrode. Int. J. Electrochem. Sci. 2011, 6, 3483–3496. [Google Scholar]
- Islam, M.A.; Farhat, Z.N. Characterization of the Corrosion Layer on Pipeline Steel in Sweet Environment. J. Mater. Eng. Perform. 2015, 24, 3142–3158. [Google Scholar] [CrossRef]
- Huang, H.-H.; Tsai, W.-T.; Lee, J.-T. Electrochemical behavior of the simulated heat-affected zone of A516 carbon steel in H2S solution. Electrochim. Acta 1996, 41, 1191–1199. [Google Scholar] [CrossRef]
- Tang, J.; Shao, Y.; Guo, J.; Zhang, T.; Meng, G.; Wang, F. The effect of H2S concentration on the corrosion behavior of carbon steel at 90 °C. Corros. Sci. 2010, 52, 2050–2058. [Google Scholar] [CrossRef]
- Elboujdaini, M.; Revie, R.W. Metallurgical factors in stress corrosion cracking (SCC) and hydrogen-induced cracking (HIC). J. Solid State Electrochem. 2009, 13, 1091–1099. [Google Scholar] [CrossRef]
- Lu, B.T.; Luo, J.L. Relationship between Yield Strength and Near-Neutral pH Stress Corrosion Cracking Resistance of Pipeline Steels—An Effect of Microstructure. Corrosion 2006, 62, 129–140. [Google Scholar] [CrossRef]
- Kittel, J.; Smanio, V.; Fregonese, M.; Garnier, L.; Lefebvre, X. Hydrogen-induced cracking (HIC) testing of low alloy steel in sour environment: Impact of time of exposure on the extent of damage. Corros. Sci. 2010, 52, 1386–1392. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. The surface oxidation of pyrite. Appl. Surf. Sci. 1987, 27, 437–452. [Google Scholar] [CrossRef]
- Hamilton, I.C.; Woods, R. An investigation of surface oxidation of pyrite and pyrrhotite by linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1981, 118, 327–343. [Google Scholar] [CrossRef]
- King, R.A.; Miller, J.D.A.; Smith, J.S. Corrosion of Mild Steel by Iron Sulphides. Br. Corros. J. 1973, 8, 137–141. [Google Scholar]
- Lee, W.; Lewandowski, Z.; Nielsen, P.H.; Hamilton, W.A. Role of sulfate-reducing bacteria in corrosion of mild steel: A review. Biofouling 1995, 8, 165–194. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Nesic, S.; Ling, S. Effect of H2S on the CO2 corrosion of carbon steel in acidic solutions. Electrochimica Acta 2011, 56, 1752–1760. [Google Scholar] [CrossRef]
- Sardisco, J.B.; Pitts, R.E. Corrosion of Iron in an H2S-CO2-H2O System Mechanism of Sulfide Film Formation and Kinetics of Corrosion Reaction. Corrosion 1965, 21, 245–253. [Google Scholar] [CrossRef]
- Bonis, M.; Crolet, J.-L. Practical aspects of the influence of in situ pH on H2S-induced cracking. Corros. Sci. 1987, 27, 1059–1070. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Rickard, D. Metastable Sedimentary Iron Sulfides. Dev. Sedimentol. 2012, 65, 195–231. [Google Scholar]
- Bai, P.; Zheng, S.; Chen, C.; Zhao, H. Investigation of the iron-sulfide phase transformation in nanoscale. Cryst. Growth Des. 2014, 14, 4295–4302. [Google Scholar] [CrossRef]
- Solehudin, A.; Nurdin, I.; Suratno, W.; Agma, M. EIS Study of Temperature and H2S Concentration Effect on API 5LX65 Carbon Steel Corrosion in Chloride Solution. J. Mater. Sci. Eng. A 2011, 1, 496–505. [Google Scholar]
- Lowson, R.T. Aqueous oxidation of pyrite by molecular oxygen. Chem. Rev. 1982, 82, 461–497. [Google Scholar] [CrossRef]
- El Mendili, Y.; Abdelouas, A.; Bardeau, J.F. Impact of a sulphidogenic environment on the corrosion behavior of carbon steel at 90 °C. RSC Adv. 2013, 3, 15148–15156. [Google Scholar] [CrossRef]

| H2S Gas Bubbling Duration (h) | H2S Concentration (ppm) | PH |
|---|---|---|
| 0 | 0 | 7.13 |
| 1 | 7 | 6.58 |
| 3 | 14 | 6.02 |
| 6 | 21 | 4.91 |
| H2S Concentration (ppm) | Ecorr (V) | Icorr (µA·cm−2) | βc (V·decade−1) | βa (V·decade−1) × 10−3 |
|---|---|---|---|---|
| 0 | −0.633 ± 0.011 | 52.29 ± 0.091 | 1.011 ± 0.012 | 98.92 ± 0.007 |
| 7 | −0.667 ± 0.020 | 28.52 ± 0.054 | 1.018 ± 0.013 | 112.1 ± 0.013 |
| 14 | −0.679 ± 0.015 | 22.15 ± 0.013 | 1.012 ± 0.006 | 116.7 ± 0.008 |
| 21 | −0.748 ± 0.012 | 6.89 ± 0.017 | 535.8 ± 0.014 | 103.8 ± 0.015 |
| H2S Concentration (ppm) | Rs (ohm·cm2) | Rpo (ohm·cm2) | CPE1 (Ssncm−2) × 10−3 | n1 | Rct (ohm·cm2) | CPE2 (Ssncm−2) × 10−4 | n2 |
|---|---|---|---|---|---|---|---|
| 0 | 12.73 | 52.95 | 4.52 | 0.735 | 840 | 5.17 | 0.869 |
| 7 | 19.05 | 67.13 | 3.31 | 0.718 | 1095 | 4.82 | 0.785 |
| 14 | 70.99 | 98.22 | 2.78 | 0.632 | 1440 | 3.09 | 0.807 |
| 21 | 98.19 | 106.10 | 1.52 | 0.640 | 1747 | 2.17 | 0.676 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonkwo, P.C.; Shakoor, R.A.; Benamor, A.; Amer Mohamed, A.M.; Al-Marri, M.J.F.A. Corrosion Behavior of API X100 Steel Material in a Hydrogen Sulfide Environment. Metals 2017, 7, 109. https://doi.org/10.3390/met7040109
Okonkwo PC, Shakoor RA, Benamor A, Amer Mohamed AM, Al-Marri MJFA. Corrosion Behavior of API X100 Steel Material in a Hydrogen Sulfide Environment. Metals. 2017; 7(4):109. https://doi.org/10.3390/met7040109
Chicago/Turabian StyleOkonkwo, Paul C., Rana Abdul Shakoor, Abdelbaki Benamor, Adel Mohamed Amer Mohamed, and Mohammed Jaber F A Al-Marri. 2017. "Corrosion Behavior of API X100 Steel Material in a Hydrogen Sulfide Environment" Metals 7, no. 4: 109. https://doi.org/10.3390/met7040109








