Applications and Thermodynamic Analysis of Equilibrium Solution for Secondary Phases in Ti–N–C Gear Steel System with Nano-Particles
Abstract
:1. Introduction
2. Thermodynamic Analysis
3. Engineering Applications
4. Conclusions
- (1)
- Thermodynamic calculation results show that the complete dissolution temperature increases with higher levels of either C, N, or Ti, and the effects of Ti and N are more significant compared with C. At a given temperature, the amount of dissolved Ti increases naturally as the content of Ti microalloyed element increases, while it decreases obviously with more N. It will decline rapidly in the complete dissolution temperature range, until 1100 °C in steels. The coefficient k1 increases as the temperature decreases, and increases with the higher content of Ti or C, but decreases with the higher N content under the same temperature.
- (2)
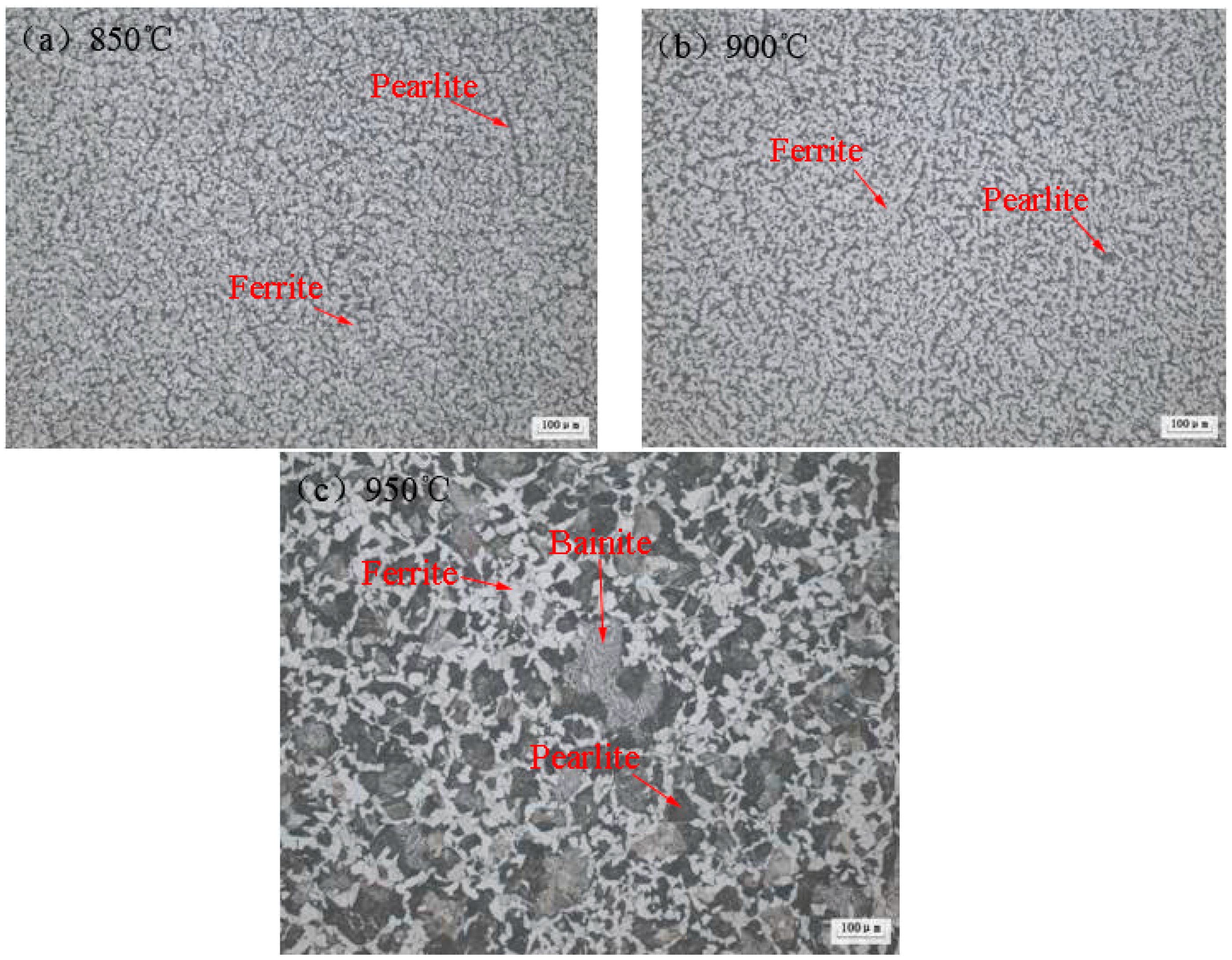
- For the 20CrMnTi gear steels produced by Fangda Special Steel Technology Co., Ltd., the heat treatment shows that when the temperature is above 950 °C, the grains begin to coarsen in this steel. When the temperature is 1000 °C, the grains coarsen sharply, the grain size is No. 5.0 grade, and the phases are ferrite and bainite, containing a small amount of pearlite.
- (3)
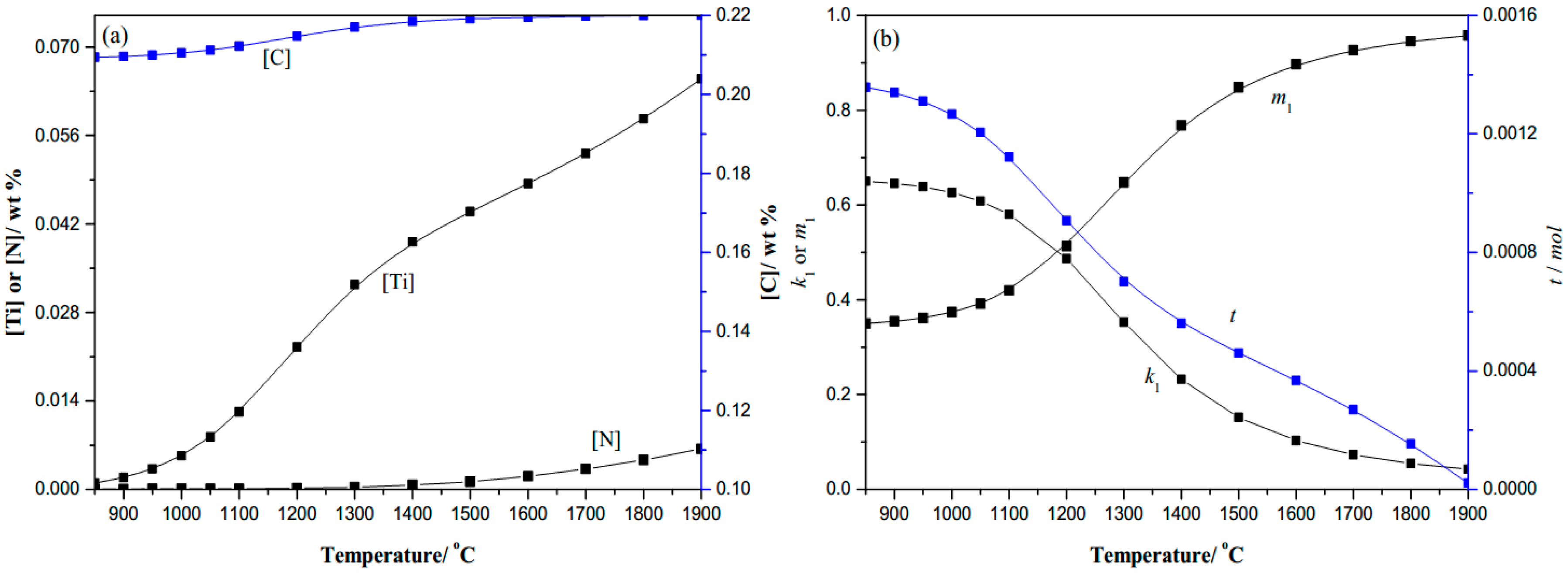
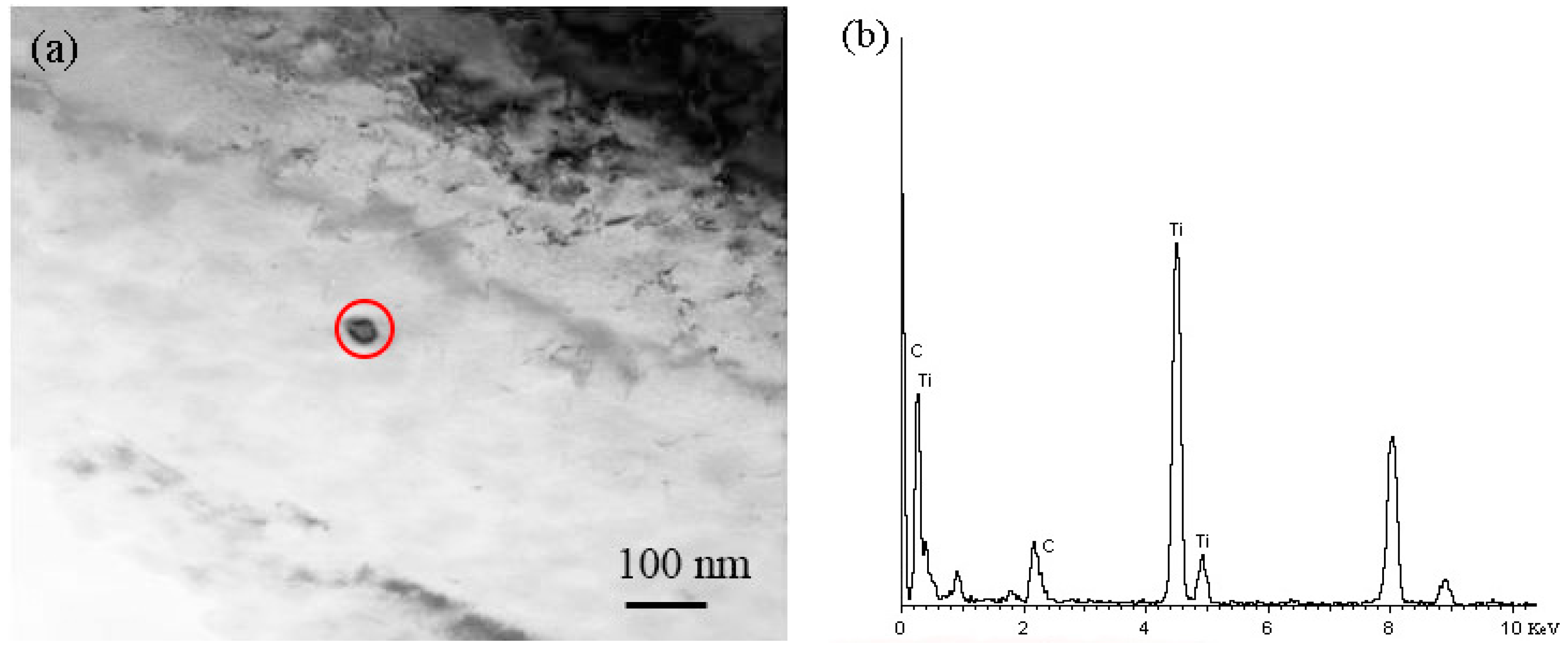
- Thermodynamic analysis shows that the nitrogen has been precipitated largely as TiN micron-particles above 1400 °C in the gear steels, and then the titanium is precipitated mainly as TiC nano-particles, so this secondary phase hinders grain growth during heat treatment, consistent with experimental results.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kimura, Y.; Inoue, T.; Yin, F.; Tsuzaki, K. Inverse temperature dependence of toughness in an ultrafine grain-structure steel. Science 2008, 320, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Adrian, H. Thermodynamic model for precipitation of carbonitrides in high strength low alloy steels containing up to three microalloying elements with or without additions of aluminium. Mater. Sci. Technol. 1992, 8, 406–420. [Google Scholar] [CrossRef]
- Gao, N.; Baker, T. Influence of AlN precipitation on thermodynamic parameters in Al-VN microalloyed steels. ISIJ Int. 1997, 37, 596–604. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, X.; Hao, B. Precipitation behaviour aged in α phase after relaxation for different times in niobium bearing microalloyed steel. Mater. Res. Innov. 2013, 17, 26–28. [Google Scholar] [CrossRef]
- Uhm, S.; Moon, J.; Lee, C.; Yoon, J.; Lee, B. Prediction model for the austenite grain size in the coarse grained heat affected zone of Fe-C-Mn steels: Considering the effect of initial grain size on isothermal growth behavior. ISIJ Int. 2004, 44, 1230–1237. [Google Scholar] [CrossRef]
- Fu, J.; Li, G.; Yu, Y.; Mao, X.; Fang, K. Comprehensive strengthening mechanism of steel based on nano-scale cementite precipitates. Eng. Sci. 2011, 13, 31–42. [Google Scholar]
- Medina, S.F. Determination of precipitation–time–temperature (PTT) diagrams for Nb, Ti or V micro-alloyed steels. J. Mater. Sci. 1997, 32, 1487–1492. [Google Scholar] [CrossRef]
- Akamatsu, S.; Hasebe, M.; Senuma, T.; Matsumura, Y.; Kisue, O. Thermodynamic Calculation of Solute Carbon and Nitrogen in Nb and Ti Added Extra-low Carbon Steels. ISIJ Int. 1994, 34, 9–16. [Google Scholar] [CrossRef]
- Hong, S.; Jun, H.; Kang, K.; Park, C. Evolution of precipitates in the Nb–Ti–V microalloyed HSLA steels during reheating. Scr. Mater. 2003, 48, 1201–1206. [Google Scholar] [CrossRef]
- Suzuki, K.-I.; Miyagawa, S.; Saito, Y.; Shiotani, K. Effect of microalloyed nitride forming elements on precipitation of carbonitride and high temperature ductility of continuously cast low carbon Nb containing steel slab. ISIJ Int. 1995, 35, 34–41. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhao, H.; Chen, Y. Influence factors on solid-solution of carbonitride of niobium in steel. J. Univ. Sci. Technol. B 2009, 31, 194–198. [Google Scholar]
- Xiang, S.; Liu, G.; Li, C.; Wang, A.; Han, Q. Thermodynamic model for carbonitride precipitation in low carbon steels. J. Univ. Sci. Technol. B 2006, 28, 818–822. [Google Scholar]
- Wang, Y.; Zhu, J. Numerical Analysis Model of Equilibrium Solution for the Multivariate Second-Phase in Steels. Patent CN201310,300,994.4, 18 July 2013. [Google Scholar]
- Wang, Y.; Zhuo, L.; Chen, M.; Wang, Z. Thermodynamic model for precipitation of carbonitrides in microalloyed steels and its application in Ti–V–C–N system. Rare Met. 2016, 35, 735–741. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuo, L.; Chen, M.; Wang, Z. Precipitation behaviour of carbonitrides in Ti-Nb-C-N microalloyed steels and an engineering application with homogenously precipitated nano-particles. Mater. Sci. 2015, 21, 527–531. [Google Scholar] [CrossRef]
- Gan, Y. Practical Manual of Modern Continuous Casting Steel; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
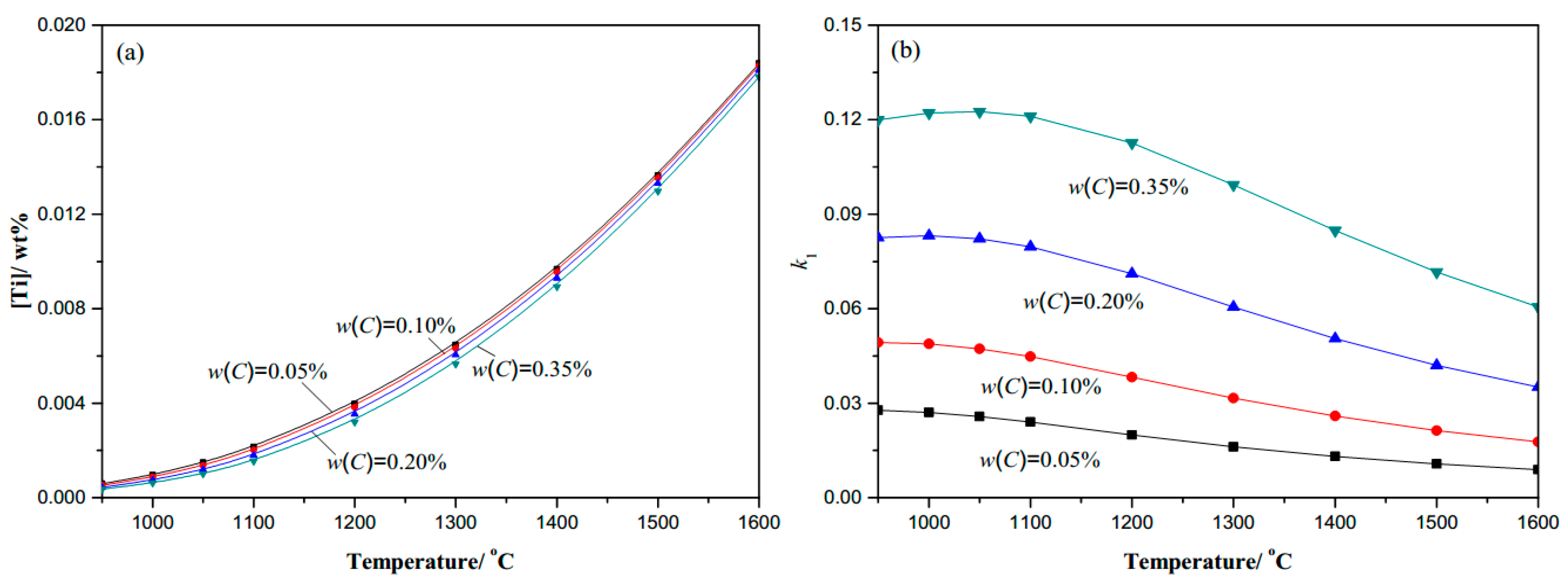
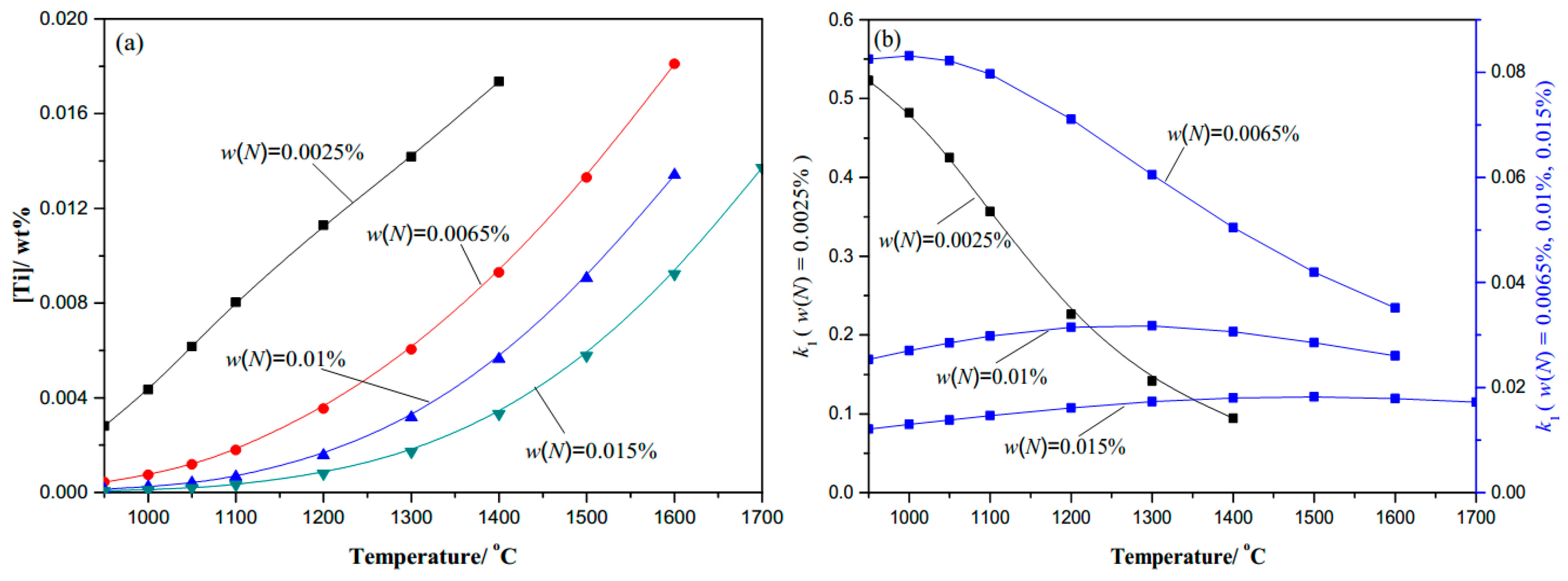
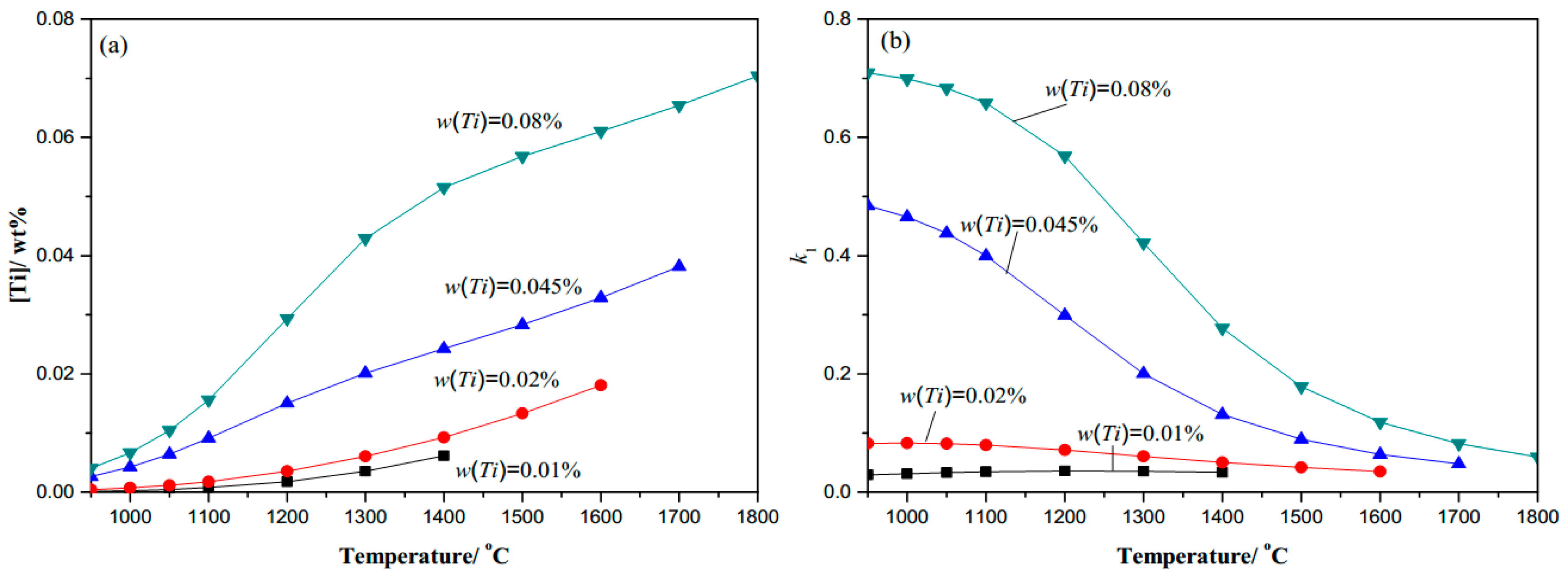

| Variation of C (0.0065N-0.02Ti) | Variation of N (0.20C-0.02Ti) | Variation of Ti (0.20C-0.0065N) | |||
|---|---|---|---|---|---|
| C (wt. %) | TAS (°C) | N (wt. %) | TAS (°C) | Ti (wt. %) | TAS (°C) |
| 0.05C | 1630.69 | 0.0025N | 1470.73 | 0.01Ti | 1507.28 |
| 0.10C | 1632.35 | 0.005N | 1586.74 | 0.03Ti | 1719.72 |
| 0.20C | 1635.65 | 0.01N | 1722.56 | 0.045Ti | 1811.57 |
| 0.35C | 1640.54 | 0.015N | 1812.61 | 0.08 Ti | 1957.49 |
| Steel | C | Si | Mn | Cr | Ti | N | P | S |
|---|---|---|---|---|---|---|---|---|
| Standard | 0.17–0.23 | 0.17–0.37 | 0.80–1.15 | 1.00–1.35 | 0.04–0.10 | ≤0.01 | ≤0.035 | ≤0.035 |
| Sample | 0.22 | 0.22 | 0.88 | 1.14 | 0.066 | 0.0067 | 0.023 | 0.003 |
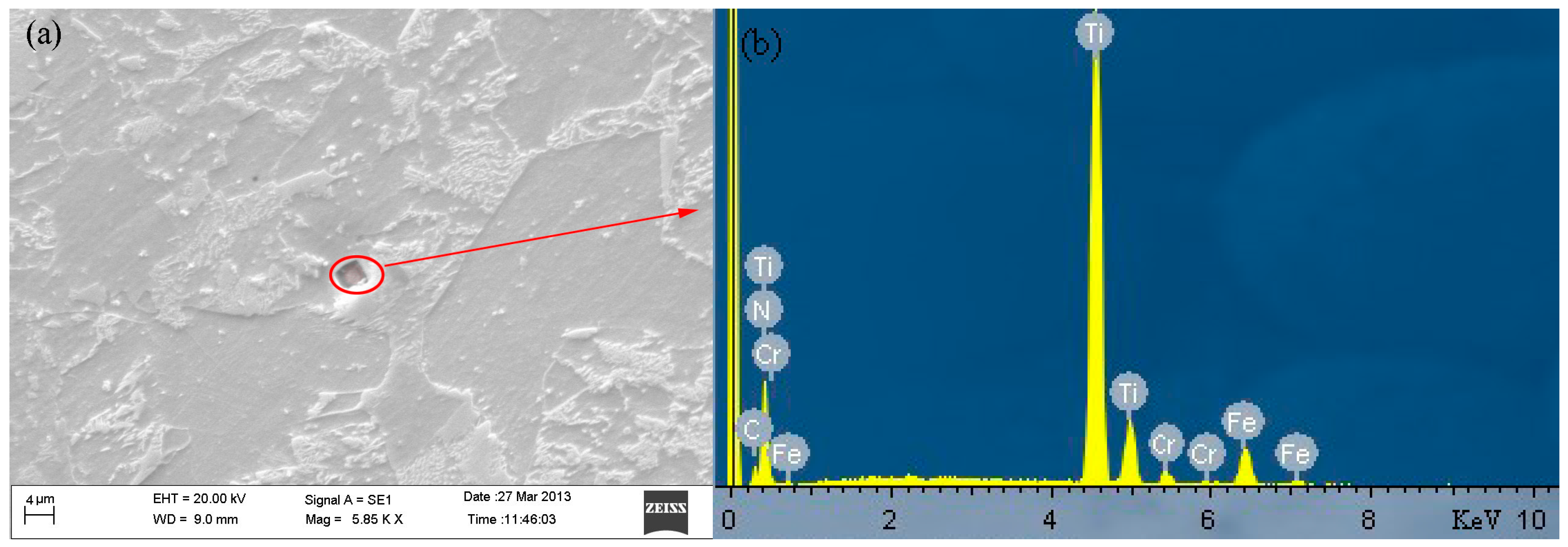
| Element | Weight % | Atomic % |
|---|---|---|
| C K1 | 0.22 | 0.60 |
| N K1 | 21.27 | 48.36 |
| Ti K1 | 65.22 | 43.35 |
| Cr K1 | 2.85 | 1.75 |
| Fe K1 | 10.43 | 5.95 |
| Material | Heat Treatment Temperature | Phases | Grain Size | Hardness, (HBW) |
|---|---|---|---|---|
| 20CrMnTi | 850 °C | Ferrite, Pearlite | No. 11.5–12.0 grade | 172 |
| 900 °C | Ferrite, Pearlite | No. 11.5–12.0 grade | 170 | |
| 950 °C | Ferrite, Pearlite and Bainite | No. 8.5–10.0 grade | 217 | |
| 1000 °C | Ferrite, Bainite and a small amount of Pearlite | No. 5.0 grade | 234 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, M.; Pang, X.; Volinsky, A.A.; Chen, M.; Gao, K. Applications and Thermodynamic Analysis of Equilibrium Solution for Secondary Phases in Ti–N–C Gear Steel System with Nano-Particles. Metals 2017, 7, 110. https://doi.org/10.3390/met7040110
Wang Y, Zhou M, Pang X, Volinsky AA, Chen M, Gao K. Applications and Thermodynamic Analysis of Equilibrium Solution for Secondary Phases in Ti–N–C Gear Steel System with Nano-Particles. Metals. 2017; 7(4):110. https://doi.org/10.3390/met7040110
Chicago/Turabian StyleWang, Yanlin, Meng Zhou, Xiaolu Pang, Alex A. Volinsky, Mingwen Chen, and Kewei Gao. 2017. "Applications and Thermodynamic Analysis of Equilibrium Solution for Secondary Phases in Ti–N–C Gear Steel System with Nano-Particles" Metals 7, no. 4: 110. https://doi.org/10.3390/met7040110





