Pyroelectrically Induced Pyro-Electro-Chemical Catalytic Activity of BaTiO3 Nanofibers under Room-Temperature Cold–Hot Cycle Excitations
Abstract
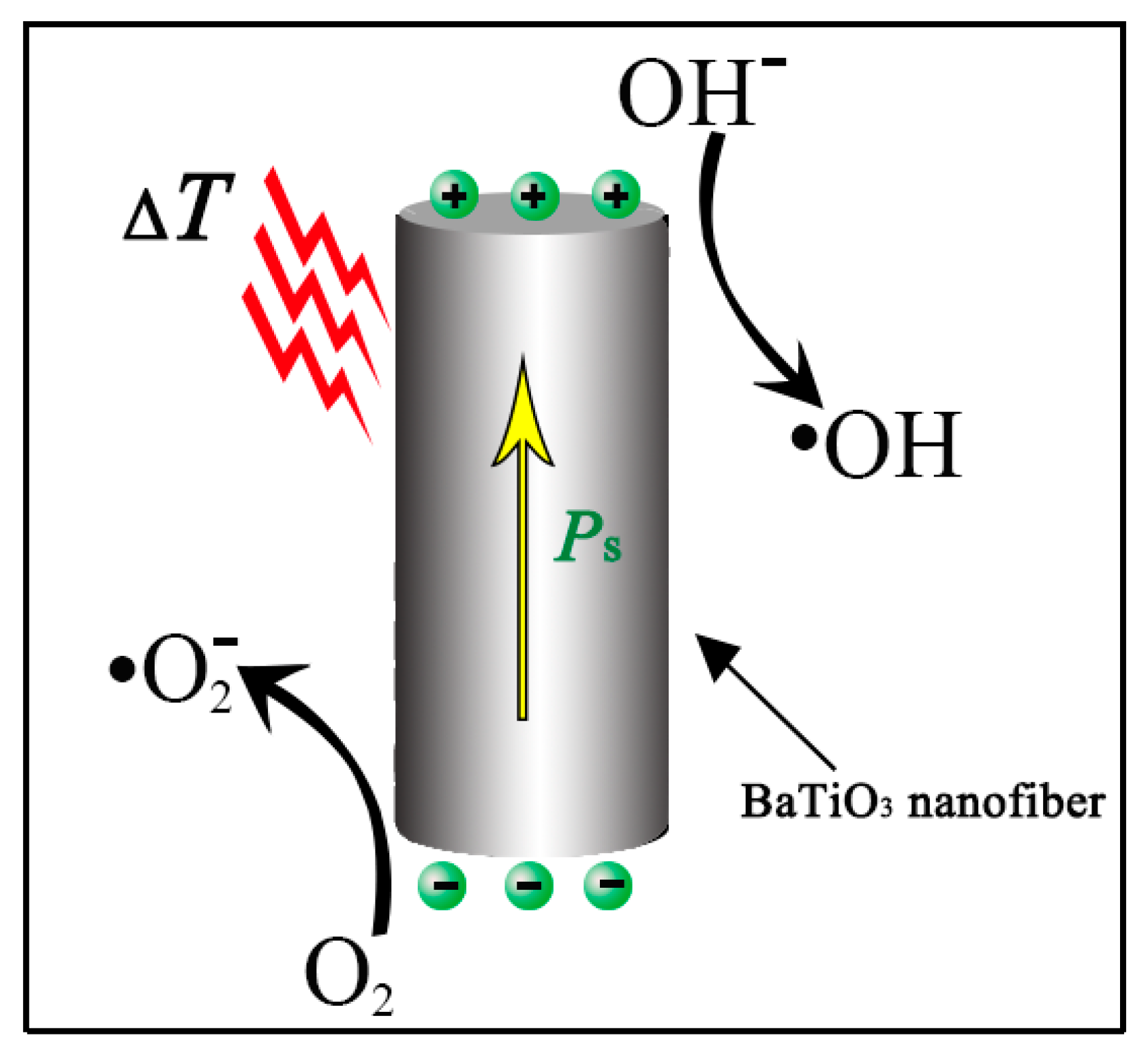
:1. Introduction
2. Materials and Methods
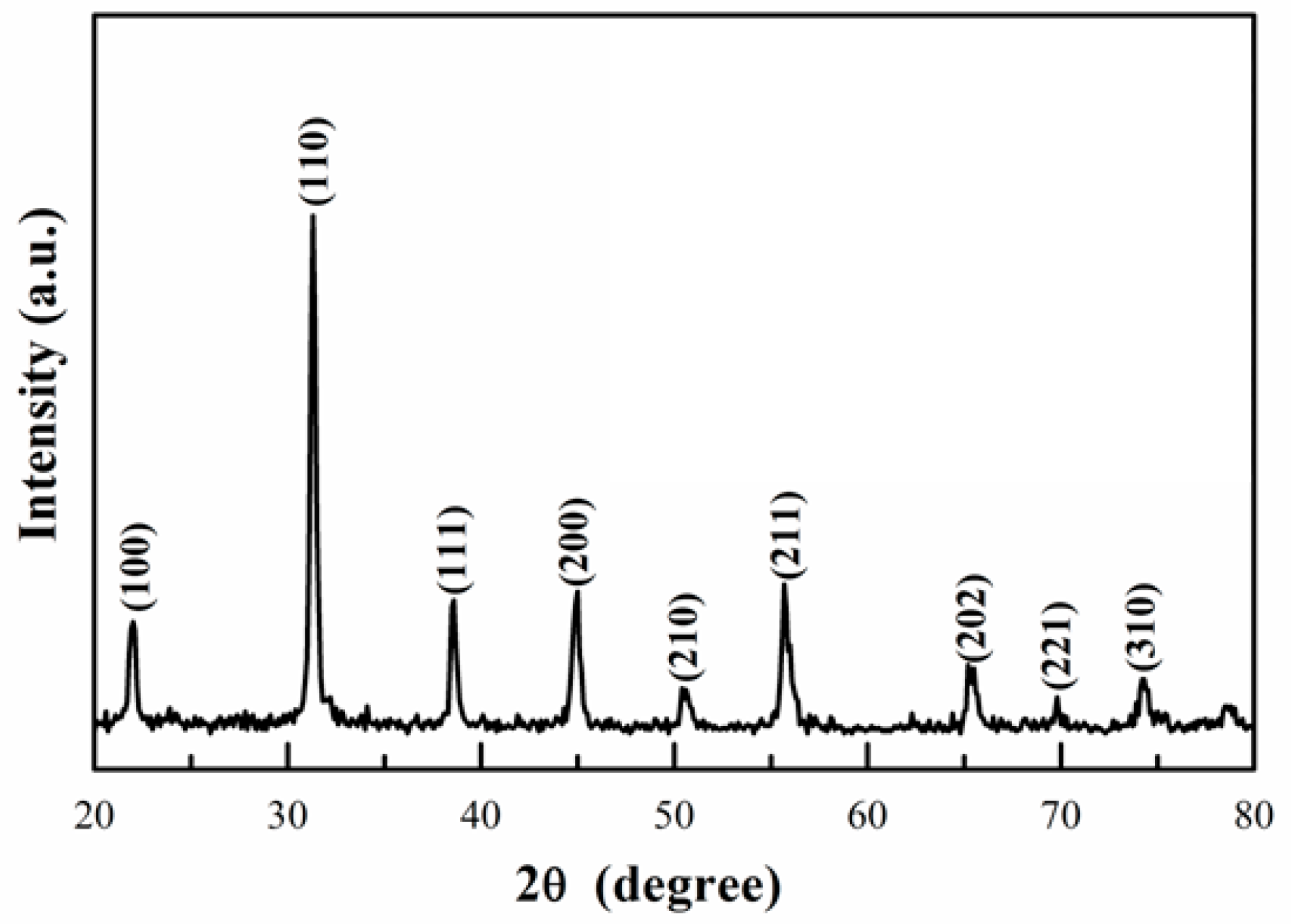
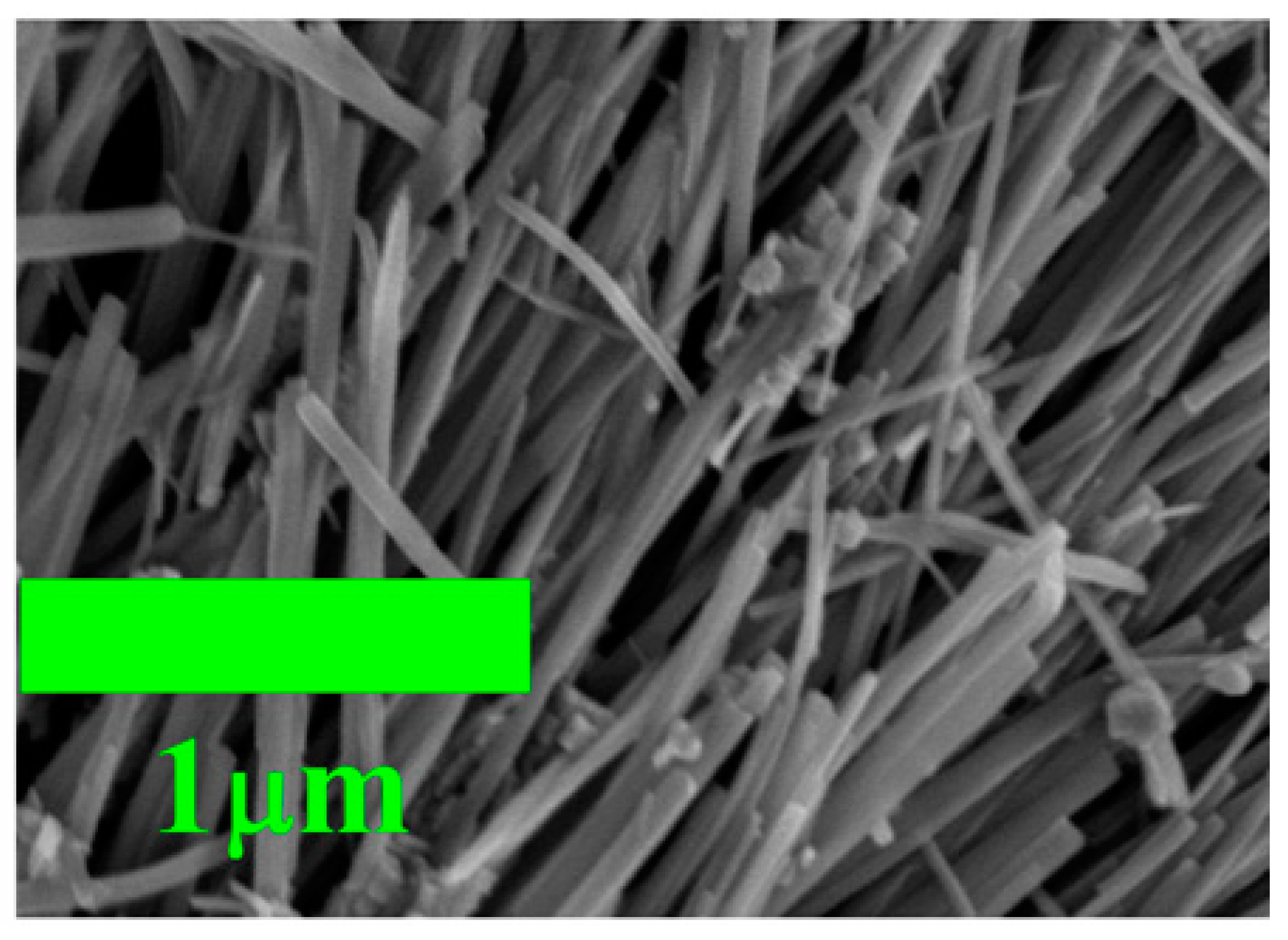
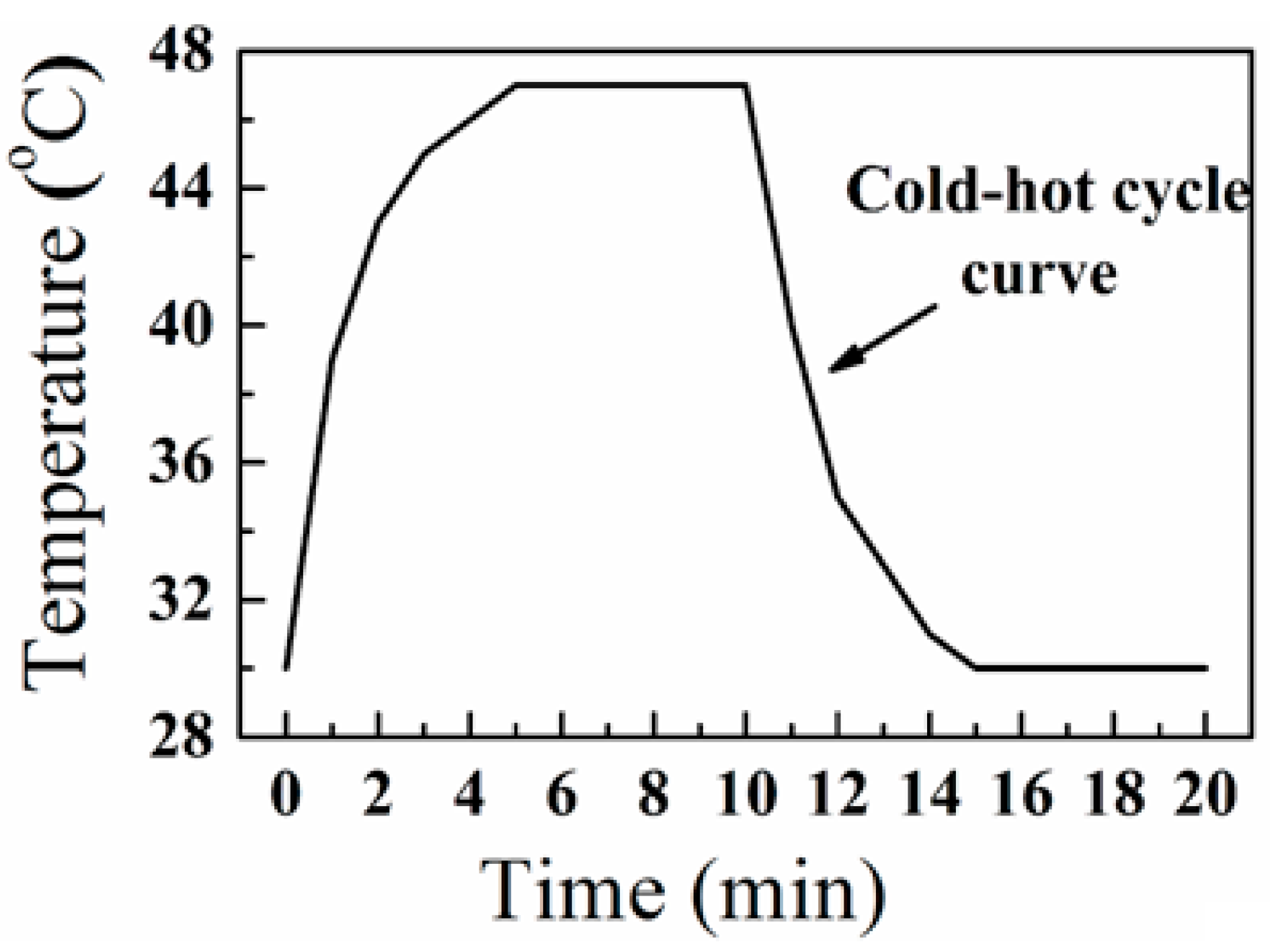
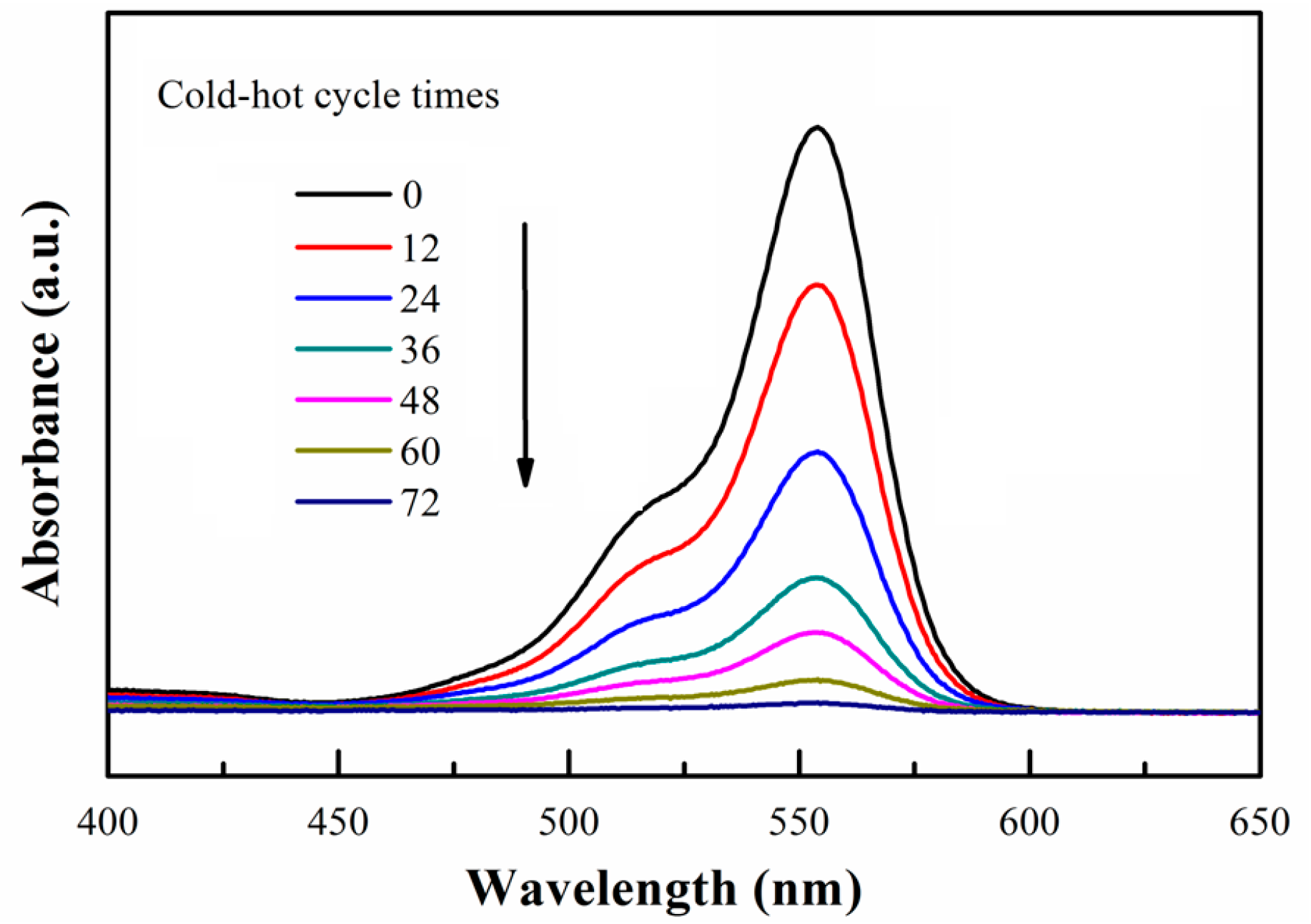
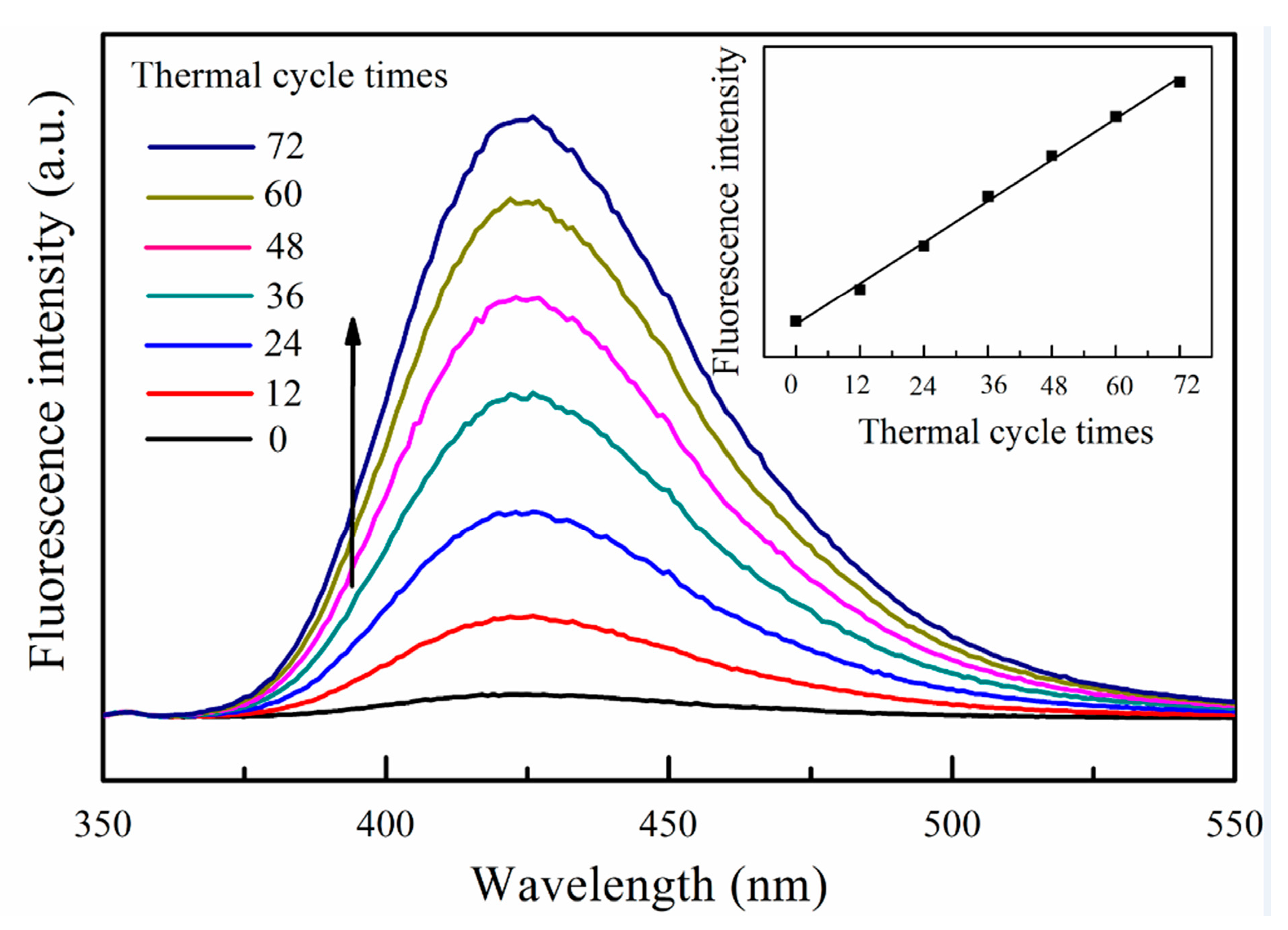
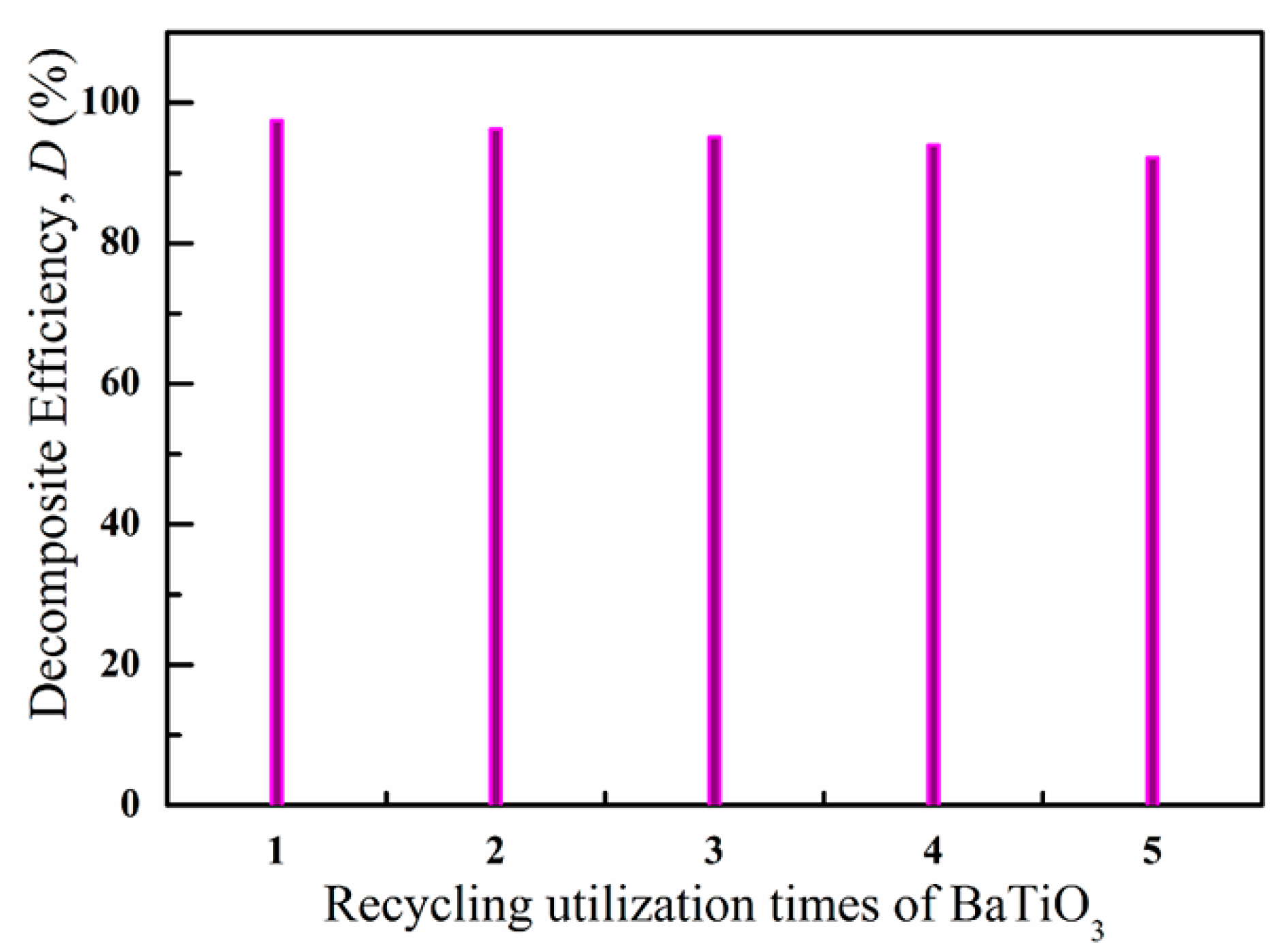
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, K.V.; Pathania, D.; Agarwal, S.; Singh, P. Adsorptional photocatalytic degradation of methylene blue onto pectin–CuS nanocomposite under solar light. J. Hazard. Mater. 2012, 243, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaoui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Z.; Jia, Y.M.; Kan, J.W.; Cheng, G.M. Piezoelectric bimorph cantilever for vibration-producing-hydrogen. Sensors 2013, 13, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.S.; Moghaddam, M.R.A.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, Z.; Chen, Z.; Yu, S.; Gao, C. Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 2011, 281, 372–378. [Google Scholar] [CrossRef]
- Patil, S.S.; Shinde, V.M. Biodegradation studies of aniline and nitrobenzene in aniline plant waste water by gas chromatography. Environ. Sci. Technol. 1988, 22, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.T.; Vira, A.; Fogel, S. Biodegradation of trans-1,2-dichloroethylene by methane-utilizing bacteria in an aquifer simulator. Environ. Sci. Technol. 1989, 23, 403–406. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Klissurski, D.K. Surface chemistry of titania (anatase) and titania-supported catalysts. Chem. Soc. Rev. 1996, 25, 61–69. [Google Scholar] [CrossRef]
- Heller, A. Chemistry and applications of photocatalytic oxidation of thin organic films. Acc. Chem. Res. 1995, 28, 503–508. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Sebald, G.; Guyomar, D.; Agbossou, A. On thermoelectric and pyroelectric energy harvesting. Smart Mater. Struct. 2009, 18, 125006. [Google Scholar] [CrossRef]
- Tang, Y.X.; Luo, L.L.; Jia, Y.M.; Luo, H.S.; Zhao, X.Y.; Xu, H.Q.; Lin, D.; Sun, J.L.; Meng, X.J.; Zhu, J.H.; et al. Mn-doped 0.71Pb(Mg1/3Nb2/3O3)–0.29PbTiO3pyroelectric crystals for uncooled infrared focal plane arrays applications. Appl. Phys. Lett. 2006, 89, 162906. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Pechy, P.; Renouard, T.; Zakeeruddin, S.M.; Robin, H.-B.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.M.; Luo, H.; Zhao, X.; Wang, F. Giant magnetoelectric response from a piezoelectric/magnetostrictive laminated composite combined with a piezoelectric transformer. Adv. Mater. 2008, 20, 4776–4779. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Bowen, C. Piezoelectric effects and electromechanical theories at the nanoscale. Nanoscale 2014, 6, 13314–13327. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, H.; Sodano, H.A. Vertically aligned arrays of BaTiO3 nanowires. Appl. Mater. Inter. 2013, 5, 11894–11899. [Google Scholar] [CrossRef] [PubMed]
- Im, B.; Jun, H.; Lee, K.H.; Lee, S.H.; Yang, I.K.; Jeong, Y.H.; Lee, J.S. Fabrication of a vertically aligned ferroelectric perovskite nanowire array on conducting substrate. Chem. Mater. 2010, 22, 4806–4813. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, X.; Lai, C.W.; Wang, J.; Tang, X.G.; Dai, J.Y. Synthesis and piezoresponse of highly ordered Pb (Zr0.53Ti0.47) O3 nanowire arrays. Appl. Phys. Lett. 2004, 85, 4190–4192. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Yu, M.F. One-dimensional ferroelectric monodomain formation in single crystalline BaTiO3 nanowire. Appl. Phys. Lett. 2006, 89, 263119. [Google Scholar] [CrossRef]
- Koka, A.; Sodano, H.A. High-sensitivity accelerometer composed of ultra-long vertically aligned barium titanate nanowire arrays. Nat. Commun. 2013, 4, 2682. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hansen, B.J.; Wang, Z.L. Piezoelectric-nanowire-enabled power source for driving wireless microelectronics. Nat. Commun. 2010, 1, 93. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, M.; Wang, C.; Wang, L.; Su, W.; Gai, Z.; Wang, C.; Li, J.; Zhang, J. Enhanced piezoelectricity in plastically deformed nearly amorphous Bi12TiO20-BaTiO3 nanocomposites. Appl. Phys. Lett. 2016, 109, 032904. [Google Scholar] [CrossRef]
- Wang, F.; Mai, Y.W.; Wang, D.; Ding, R.; Shi, W. High quality barium titanate nanofibers for flexible piezoelectric device applications. Sens. Actuators A Phys. 2015, 233, 195–201. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.L.; Wang, W.N.; Cong, H.P.; Qian, H.S. Titanium dioxide/upconversion nanoparticles/cadmium sulfide nanofibers enable enhanced full-spectrum absorption for superior solar light driven photocatalysis. ChemSusChem 2016, 9, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, C.L.; Peng, H.Y.; Cong, H.P.; Qian, H.S. Near-infrared photocatalytic upconversion nanoparticles/TiO2 nanofibers assembled in large scale by electrospinning. Part. Part. Syst. Charact. 2016, 33, 248–253. [Google Scholar] [CrossRef]
- Xie, M.; Dunn, S.; Boulbar, E.L.; Bowen, C.R. Pyroelectric energy harvesting for water splitting. Int. J. Hydrog. Energy 2017. [Google Scholar] [CrossRef]
- Li, Y.F.; Liu, Z.P. Particle size, shape and activity for photocatalysis on titania anatase nanoparticles in aqueous surroundings. J. Am. Chem. Soc. 2011, 133, 15743–15752. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wu, Z.; Jia, Y.M.; Li, W.J.; Zheng, R.K.; Luo, H.S. Piezoelectrically induced mechano-catalytic effect for degradation of dye wastewater through vibrating Pb(Zr0.52Ti0.48)O3 fibers. Appl. Phys. Lett. 2014, 104, 162907. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Bonnel, D.A. Screening phenomena on oxide surfaces and its implications for local electrostatic and transport measurements. Nano Lett. 2004, 4, 555–560. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, Y.R. Ultraviolet-light-assisted formation of ZnO nanowires in ambient air: Comparison of photoresponsive and photocatalytic activities in zinc hydroxide. J. Phys. Chem. C 2011, 115, 2235–2243. [Google Scholar] [CrossRef]
- Konieczny, A.; Mondal, K.; Wiltowski, T.; Dydo, P. Catalyst development for thermocatalytic decomposition of methane to hydrogen. J. Hydrog. Energy 2008, 33, 264–272. [Google Scholar] [CrossRef]
- Wu, J.; Mao, W.; Wu, Z.; Xu, X.; You, H.; Jia, Y. Strong pyro-catalysis of pyroelectric BiFeO3 nanoparticles under a room-temperature cold–hot alternation. Nanoscale 2016, 8, 7343–7350. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, Y.; Zhao, F.; Gao, H.; Chen, X. Facile microwave synthesis and photocatalytic activity of monodispersed BaTiO3 nanocuboids. Mater. Charact. 2016, 114, 243–253. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Jia, Y.; Qian, W.; Xu, X.; Wu, Z.; Han, Z.; Hong, Y.; You, H.; Ismail, M.; Bai, G.; et al. Pyroelectrically Induced Pyro-Electro-Chemical Catalytic Activity of BaTiO3 Nanofibers under Room-Temperature Cold–Hot Cycle Excitations. Metals 2017, 7, 122. https://doi.org/10.3390/met7040122
Xia Y, Jia Y, Qian W, Xu X, Wu Z, Han Z, Hong Y, You H, Ismail M, Bai G, et al. Pyroelectrically Induced Pyro-Electro-Chemical Catalytic Activity of BaTiO3 Nanofibers under Room-Temperature Cold–Hot Cycle Excitations. Metals. 2017; 7(4):122. https://doi.org/10.3390/met7040122
Chicago/Turabian StyleXia, Yuntao, Yanmin Jia, Weiqi Qian, Xiaoli Xu, Zheng Wu, Zichen Han, Yuanting Hong, Huilin You, Muhammad Ismail, Ge Bai, and et al. 2017. "Pyroelectrically Induced Pyro-Electro-Chemical Catalytic Activity of BaTiO3 Nanofibers under Room-Temperature Cold–Hot Cycle Excitations" Metals 7, no. 4: 122. https://doi.org/10.3390/met7040122





