Preparation and Properties of Microarc Oxidation Self-Lubricating Composite Coatings on Aluminum Alloy
Abstract
:1. Introduction
2. Experimental Details
2.1. Samples Preparation
2.2. Testing and Characterization
3. Results and Discussion
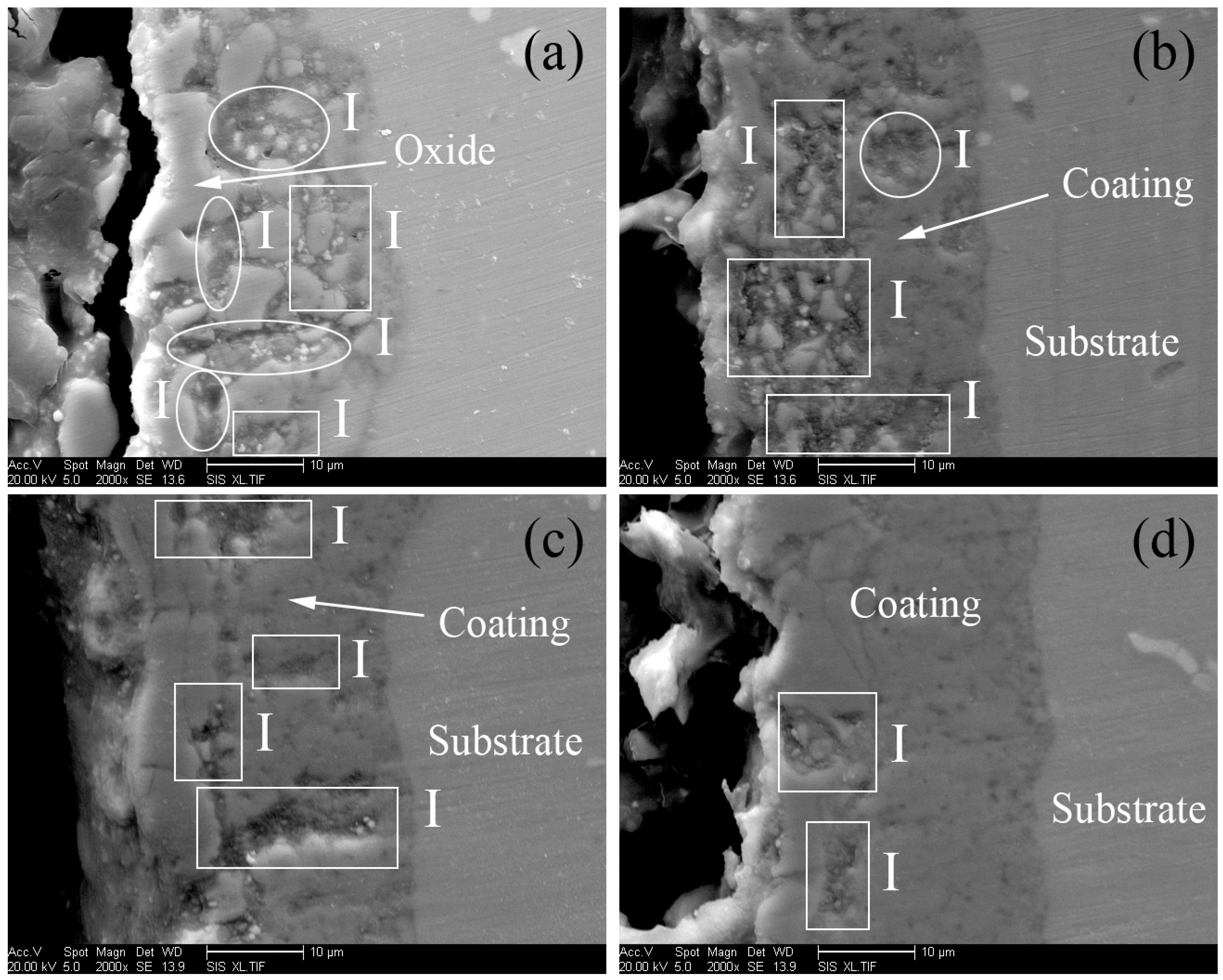
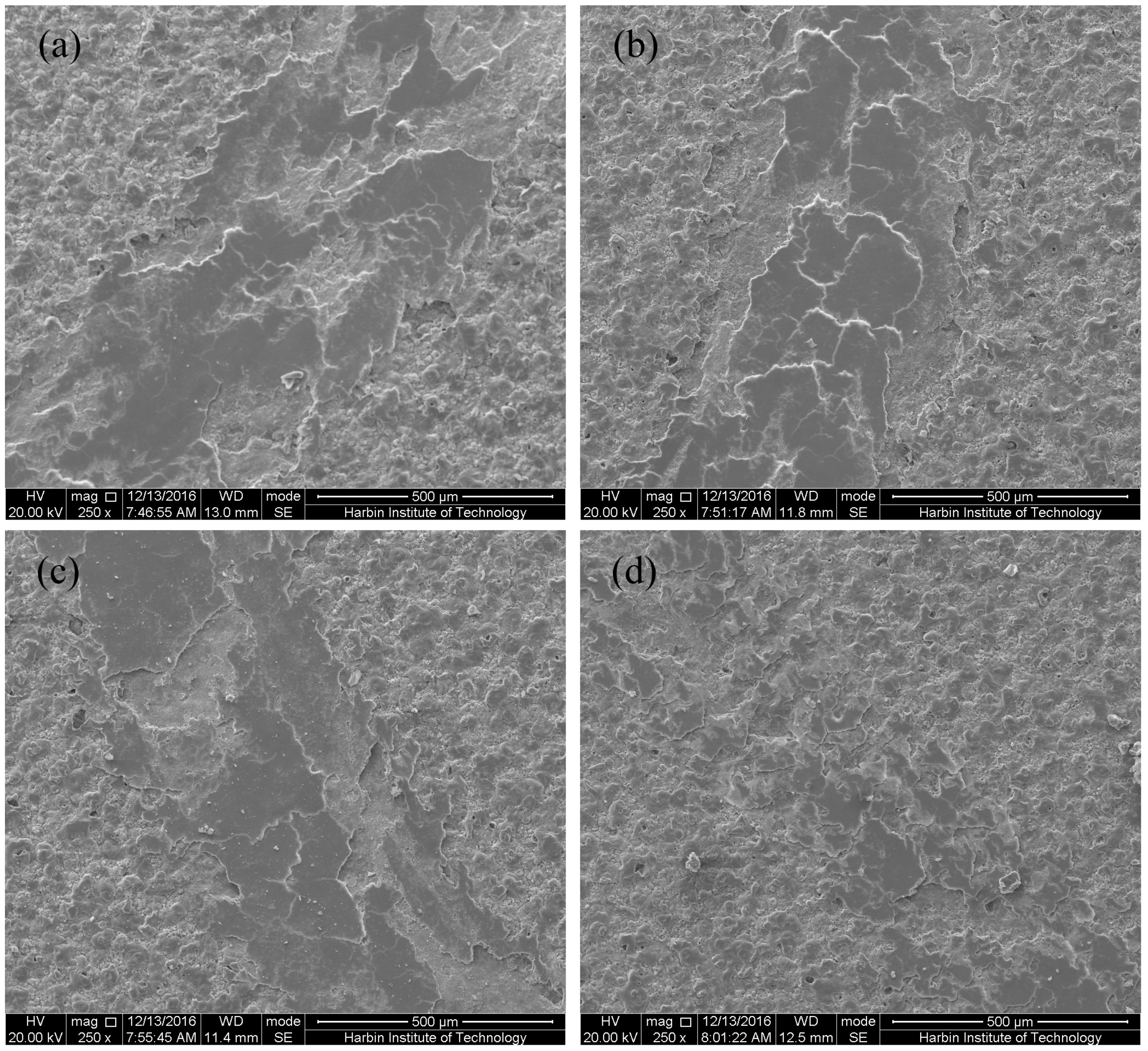
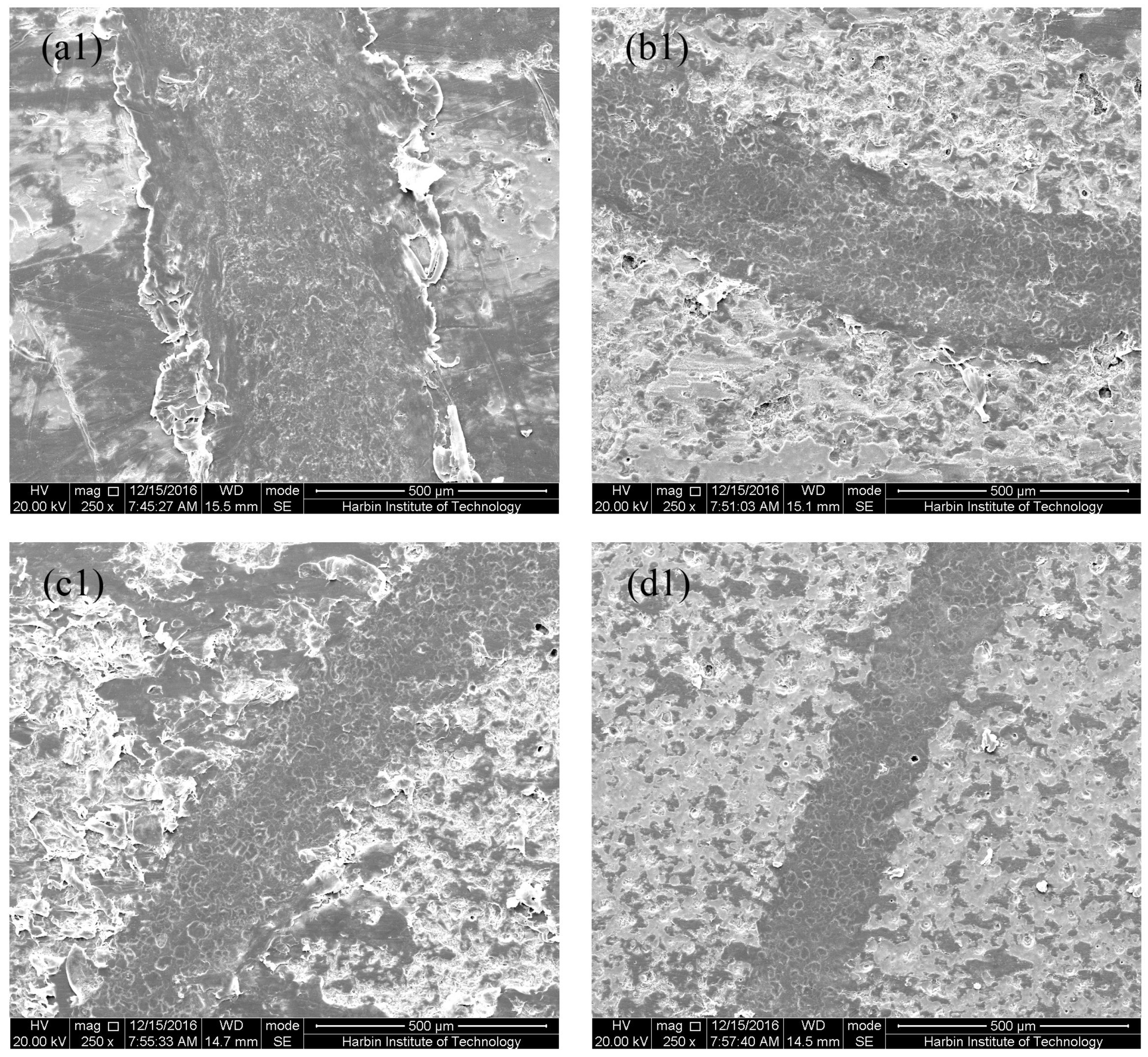
3.1. Microstructure of MAO Coatings and MAO Self-Lubricating Composite Coatings
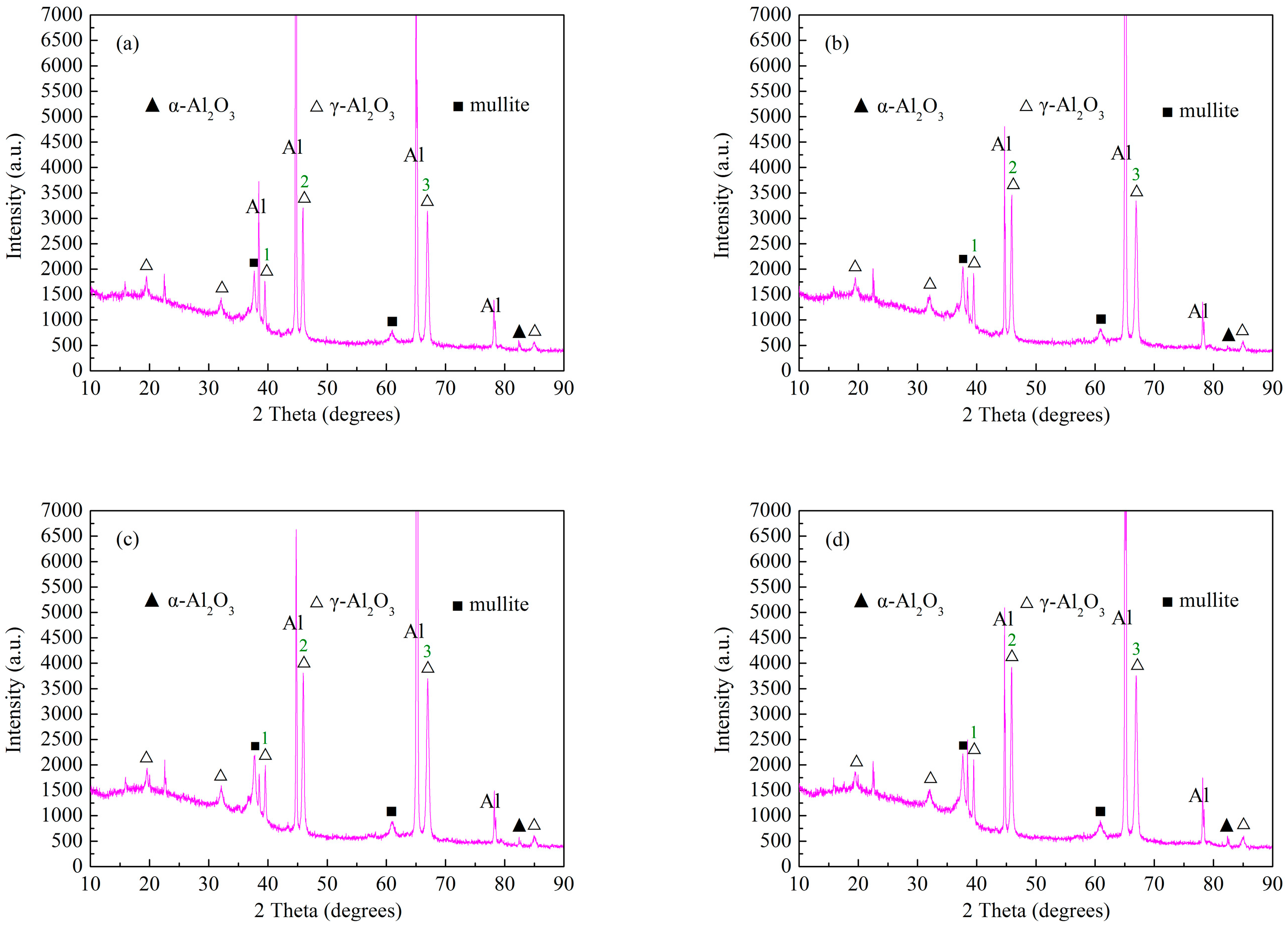
3.2. Effect of Cathode Current Density on Phase Structure of MAO Ceramic Coatings
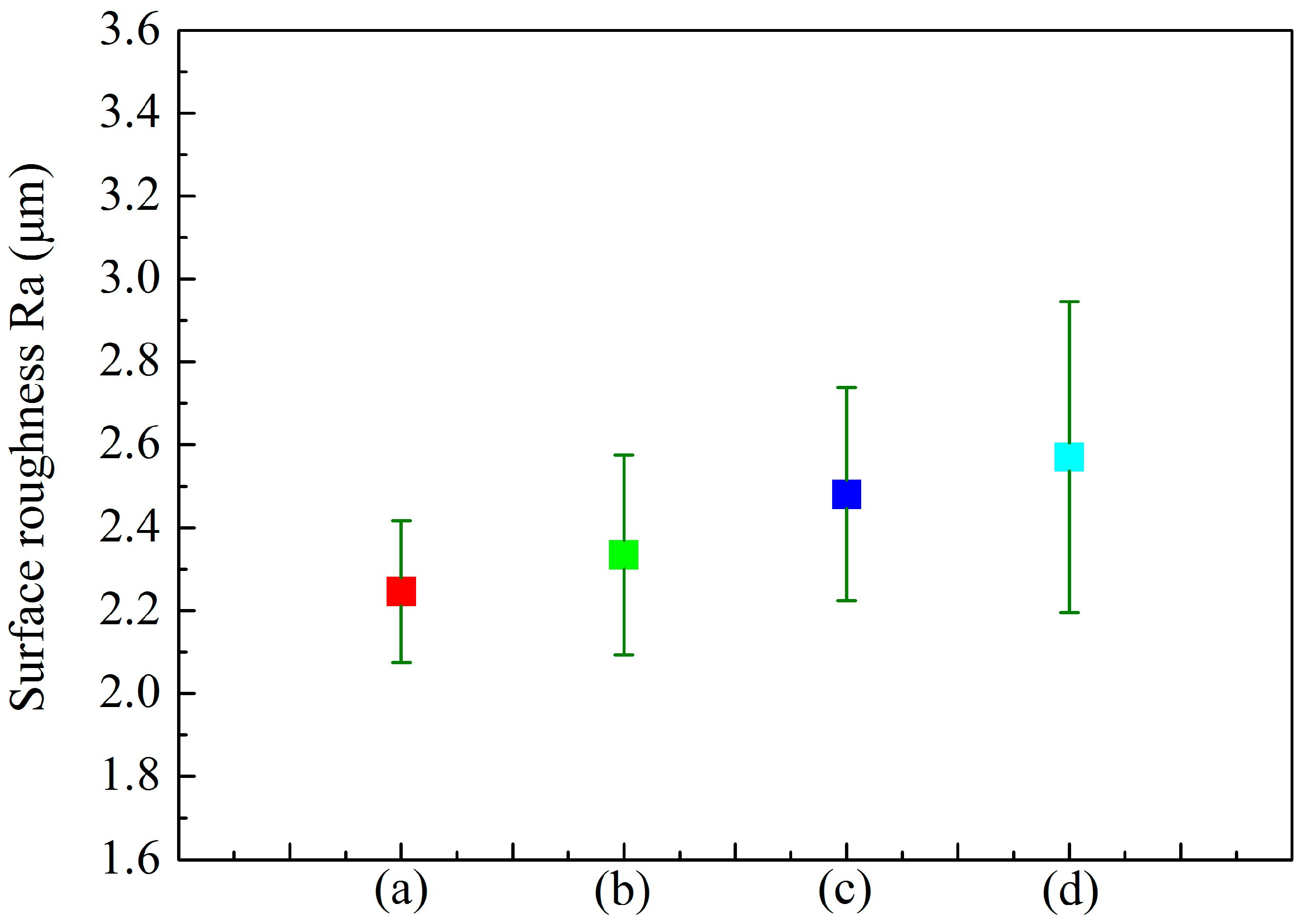
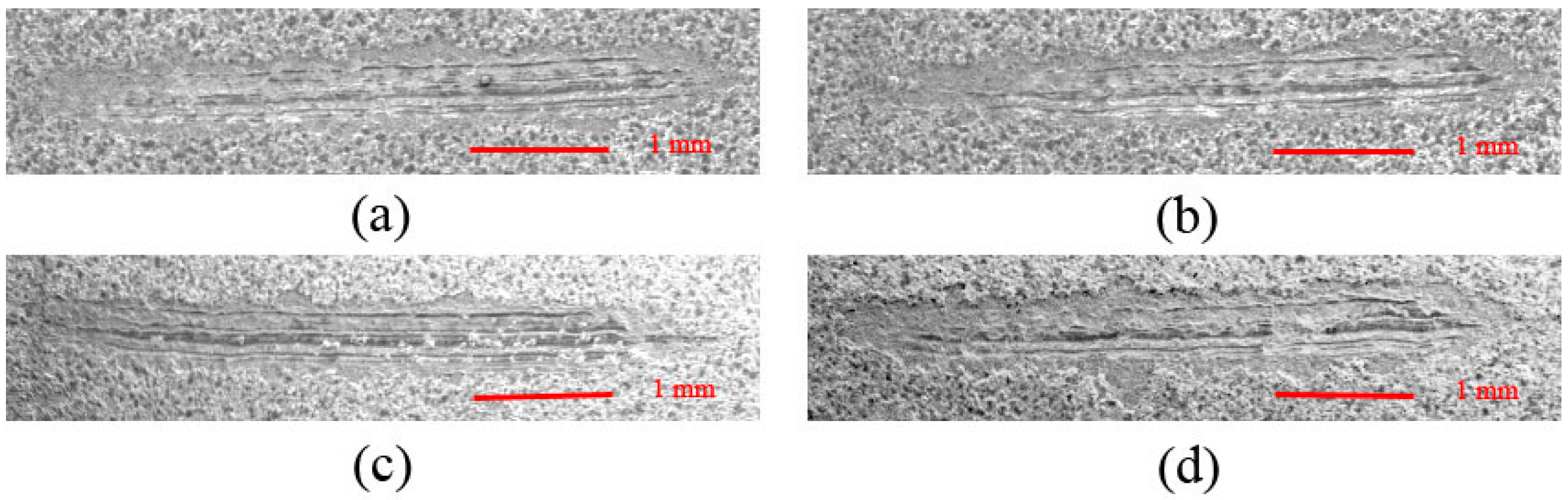
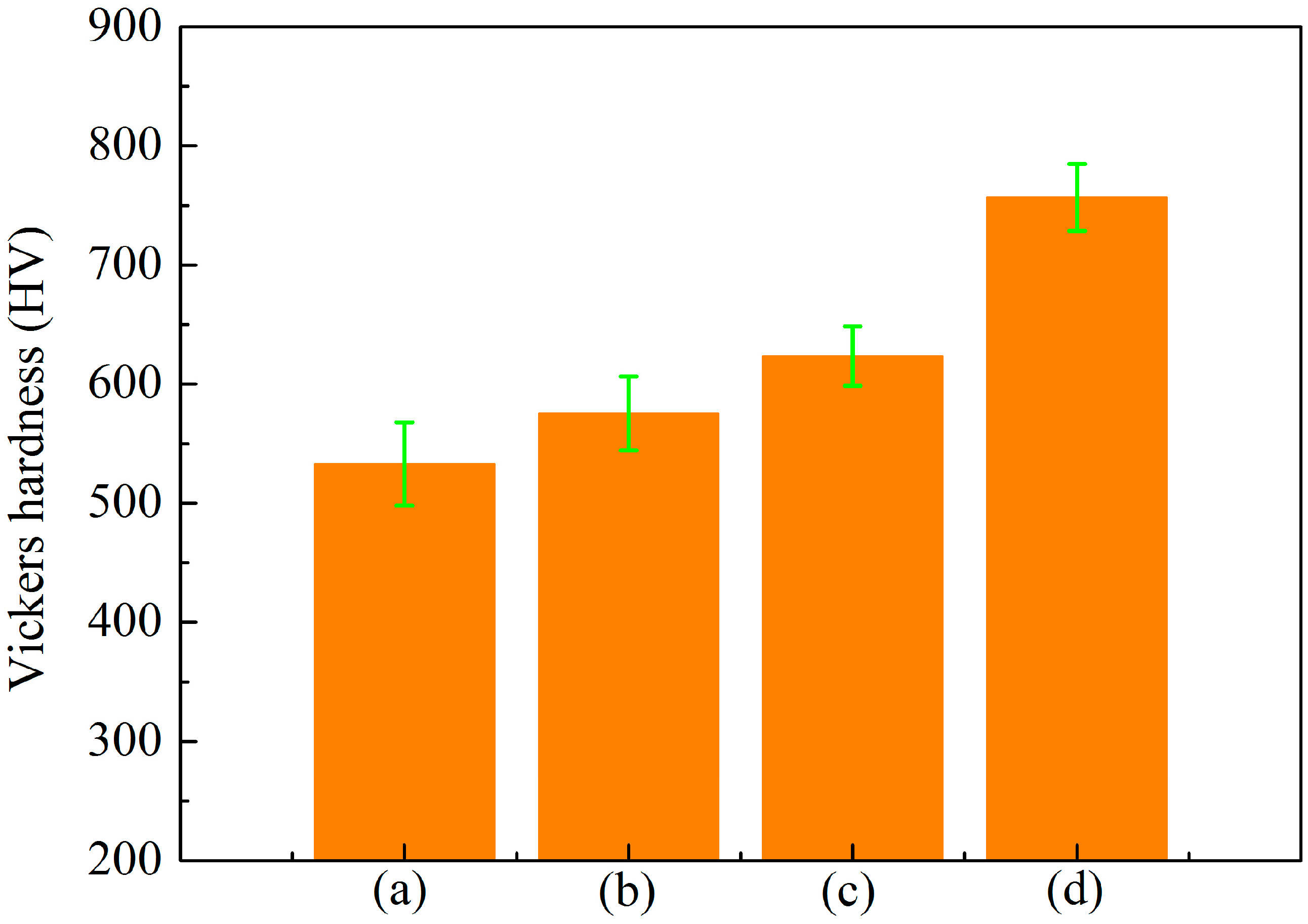
3.3. Effect of Cathode Current Density on Adhesion Strength of MAO Ceramic Coatings
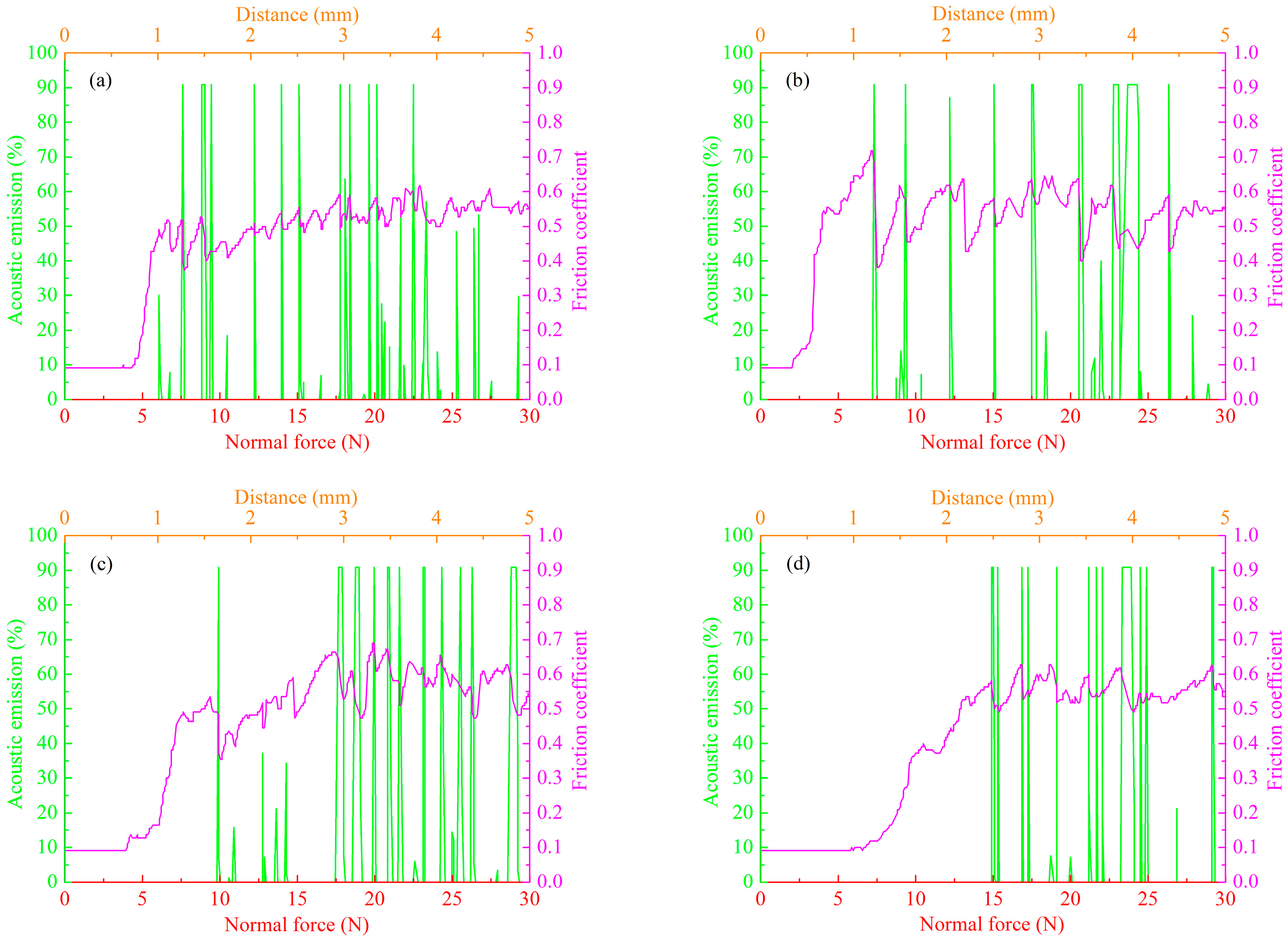
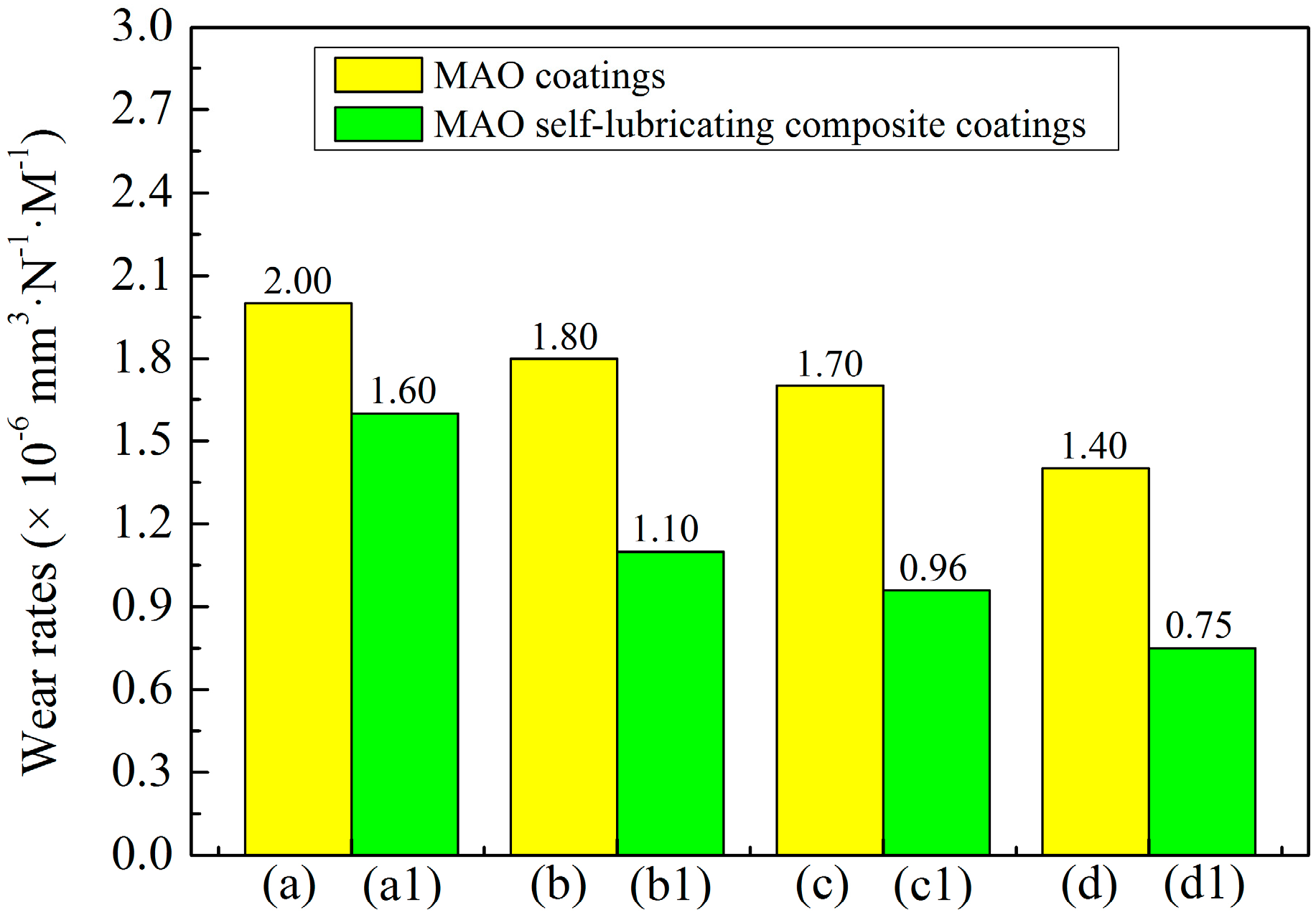
3.4. Effect of Cathode Current Density on Wear Resistance of MAO Ceramic Coatings
3.5. Proposed Wear Mechanism of MAO Ceramic Coatings
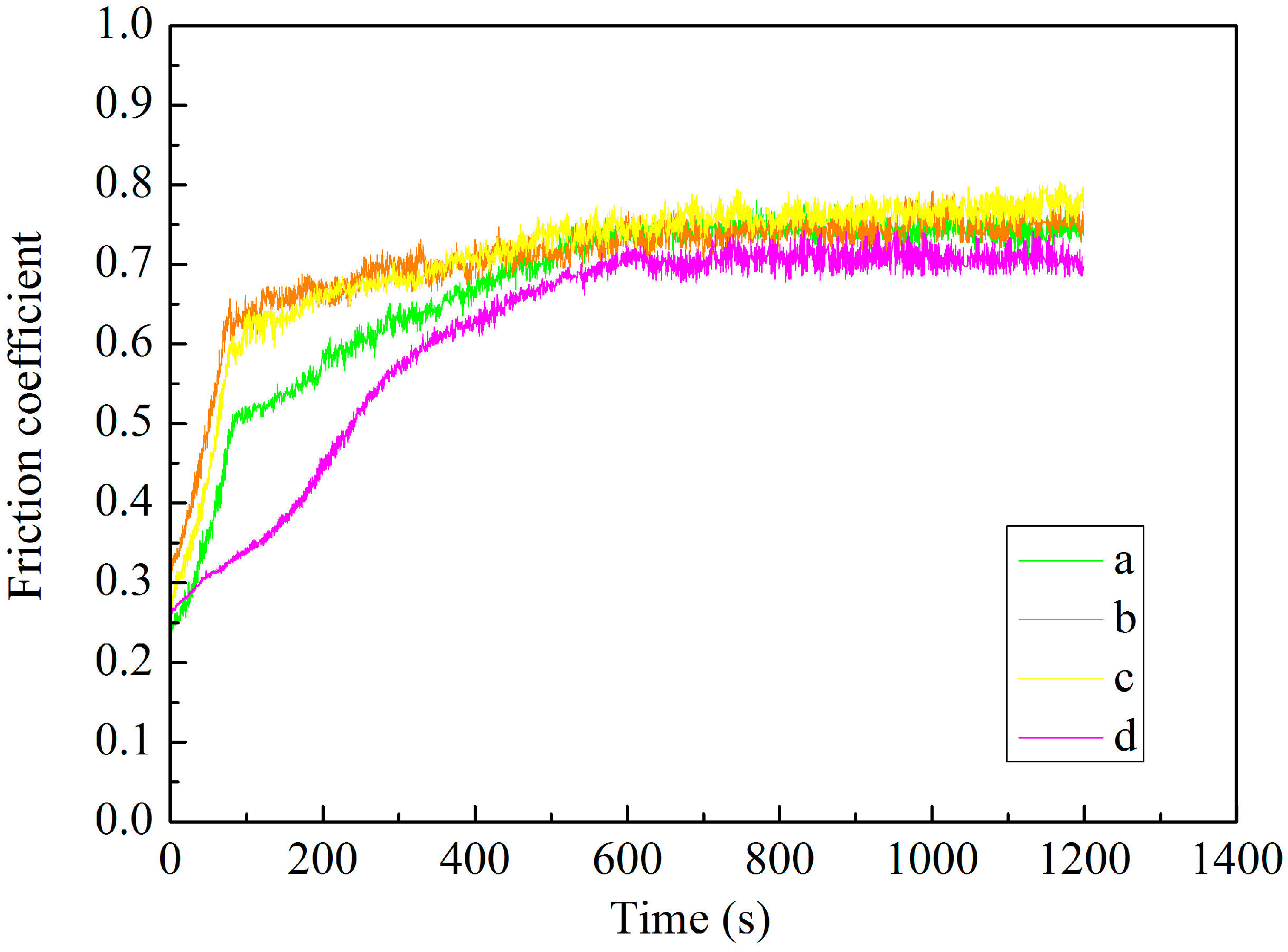
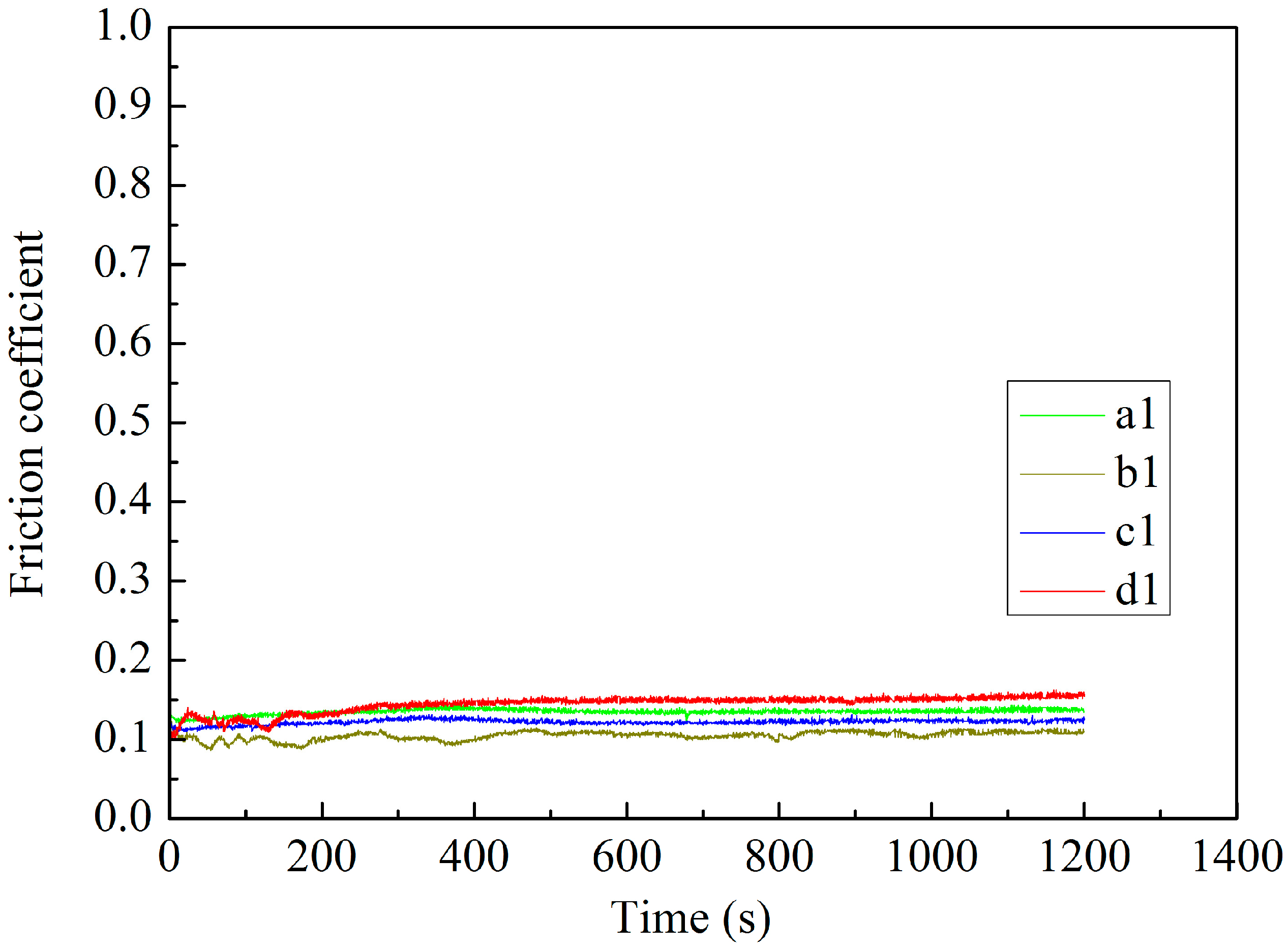
3.6. Tribological Properties of MAO Self-Lubricating Composite Coatings
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.S.; Guo, X.W.; Li, X.D.; Ge, D.Y. Improvement on the fatigue performance of 2024-T4 alloy by synergistic coating technology. Materials 2014, 7, 3533–3546. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.P.; Guo, Y.C.; Yang, Z.; Wang, J.L. Ceramic coating formation on high Si containing Al alloy by PEO process. Surf. Eng. 2016, 32, 428–434. [Google Scholar] [CrossRef]
- Rao, R.N.; Das, S.; Mondal, D.P.; Dixit, G. Effect of heat treatment on the sliding wear behavior of aluminium alloy (Al–Zn–Mg) hard particle composite. Tribol. Int. 2010, 43, 330–339. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lee, J.L.; Kuo, T.H.; Kuo, S.N.; Tseng, K.H. The influence of sodium tungstate concentration and anodizing conditions on microarc oxidation (MAO) coatings for aluminum alloy. Surf. Coat. Technol. 2012, 206, 3437–3443. [Google Scholar] [CrossRef]
- Polat, A.; Makaraci, M.; Usta, M. Influence of sodium silicate concentration on structural and tribological properties of microarc oxidation coatings on 2017A aluminum alloy substrate. J. Alloys Compd. 2010, 504, 519–526. [Google Scholar] [CrossRef]
- Nimura, K.; Sugawara, T.; Jibiki, T.; Ito, S.; Shima, M. Surface modification of aluminum alloy to improve fretting wear properties. Tribol. Int. 2016, 93, 702–708. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, L.; Qi, Y.L.; Cai, W.; Jiang, Z.H. Self-lubricating Al2O3/PTFE composite coating formation on surface of aluminium alloy. Surf. Coat. Technol. 2010, 204, 3315–3318. [Google Scholar] [CrossRef]
- Tsutumi, Y.; Niinomi, M.; Nakai, M.; Shimabukuro, M.; Ashida, M.; Chen, P.; Doi, H.; Hanawa, T. Electrochemical surface treatment of a β-titanium alloy to realize an antibacterial property and bioactivity. Metals 2016, 6, 76. [Google Scholar] [CrossRef]
- Yavari, S.A.; Necula, B.S.; Fratila-Apachitei, L.E.; Duszczyk, J.; Apachitei, I. Biofunctional surfaces by plasma electrolytic oxidation on titanium biomedical alloys. Surf. Eng. 2016, 32, 411–417. [Google Scholar] [CrossRef]
- Han, O.S.; Hwang, M.J.; Song, Y.H.; Song, H.J.; Park, Y.J. Effects of surface structure and chemical composition of binary Ti alloys on cell differentiation. Metals 2016, 6, 150. [Google Scholar] [CrossRef]
- Mioc, U.B.; Stojadinovic, S.; Nedic, Z. Characterization of bronze surface layer formed by microarc oxidation process in 12-tungstophosphoric acid. Materials 2010, 3, 110–126. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xue, Z.G.; Wang, Q.; Wu, X.Q.; Matykina, E.; Skeldon, P.; Thompson, G.E. New findings on properties of plasma electrolytic oxidation coatings from study of an Al-Cu-Li alloy. Electrochim. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Chen, M.A.; Ou, Y.C.; Yu, C.Y.; Xiao, C.; Liu, S.Y. Corrosion performance of epoxy/BTESPT/MAO coating on AZ31 alloy. Surf. Eng. 2016, 32, 38–46. [Google Scholar] [CrossRef]
- Guan, Y.J.; Xia, Y.; Xu, F.T. Interface fracture property of PEO ceramic coatings on aluminum alloy. Surf. Coat. Technol. 2008, 202, 4204–4209. [Google Scholar] [CrossRef]
- Martin, J.; Leone, P.; Nomine, A.; Renaux, D.V.; Henrion, G.; Belmonte, T. Influence of electrolyte ageing on the plasma electrolytic oxidation of aluminium. Surf. Coat. Technol. 2015, 269, 36–46. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Su, J.F.; Xie, X. A study of the interactive effects of hybrid current modes on the tribological properties of a PEO (plasma electrolytic oxidation) coated AM60B Mg-alloy. Surf. Coat. Technol. 2013, 215, 421–430. [Google Scholar] [CrossRef]
- Wu, H.H.; Lu, X.Y.; Long, B.H.; Wang, X.Q.; Wang, J.B.; Jin, Z.S. The effects of cathodic and anodic voltages on the characteristics of porous nanocrystalline titania coatings fabricated by microarc oxidation. Mater. Lett. 2005, 59, 370–375. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.L.; Yu, D.Z.; Wang, F.P.; Zhao, L.C. Effects of cathodic voltages on the structure and properties of ceramic coatings formed on NiTi alloy by microarc oxidation. Mater. Chem. Phys. 2010, 121, 172–177. [Google Scholar] [CrossRef]
- Su, P.B.; Wu, X.H.; Guo, Y.; Jiang, Z.H. Effects of cathode current density on structure and corrosion resistance of plasma electrolytic oxidation coatings formed on ZK60 Mg alloy. J. Alloys Compd. 2009, 475, 773–777. [Google Scholar] [CrossRef]
- Li, Q.B.; Liang, J.; Liu, B.X.; Peng, Z.J.; Wang, Q. Effects of cathodic voltages on structure and wear resistance of plasma electrolytic oxidation coatings formed on aluminium alloy. Appl. Surf. Sci. 2014, 297, 176–181. [Google Scholar] [CrossRef]
- Mu, M.; Zhou, X.J.; Xiao, Q.; Liang, J.; Huo, X.D. Preparation and tribological properties of self-lubricating TiO2/graphite composite coating on TI6Al4V alloy. Appl. Surf. Sci. 2012, 258, 8570–8576. [Google Scholar] [CrossRef]
- Wang, S.Y.; Si, N.C.; Xia, Y.P.; Liu, L. Influence of nano-SiC on microstructure and property of MAO coating formed on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1926–1934. [Google Scholar] [CrossRef]
- Hussein, R.O.; Xie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behavior during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203–105215. [Google Scholar] [CrossRef]
- Tekin, K.C.; Malayoglu, U.; Shrestha, S. Tribological behaviour of plasma electrolytic oxide coatings on Ti6Al4V and cp-Ti alloys. Surf. Eng. 2016, 32, 435–442. [Google Scholar] [CrossRef]
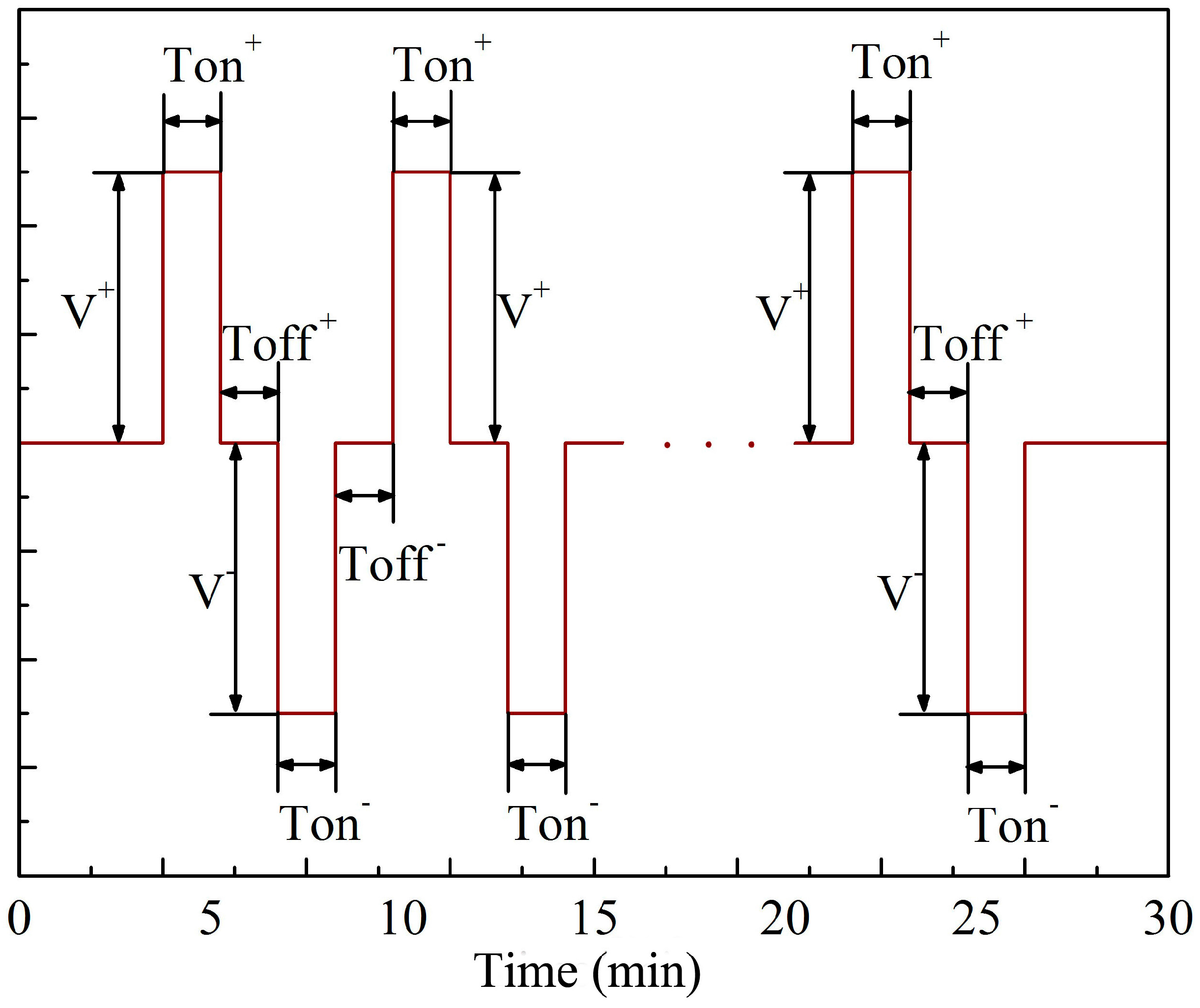
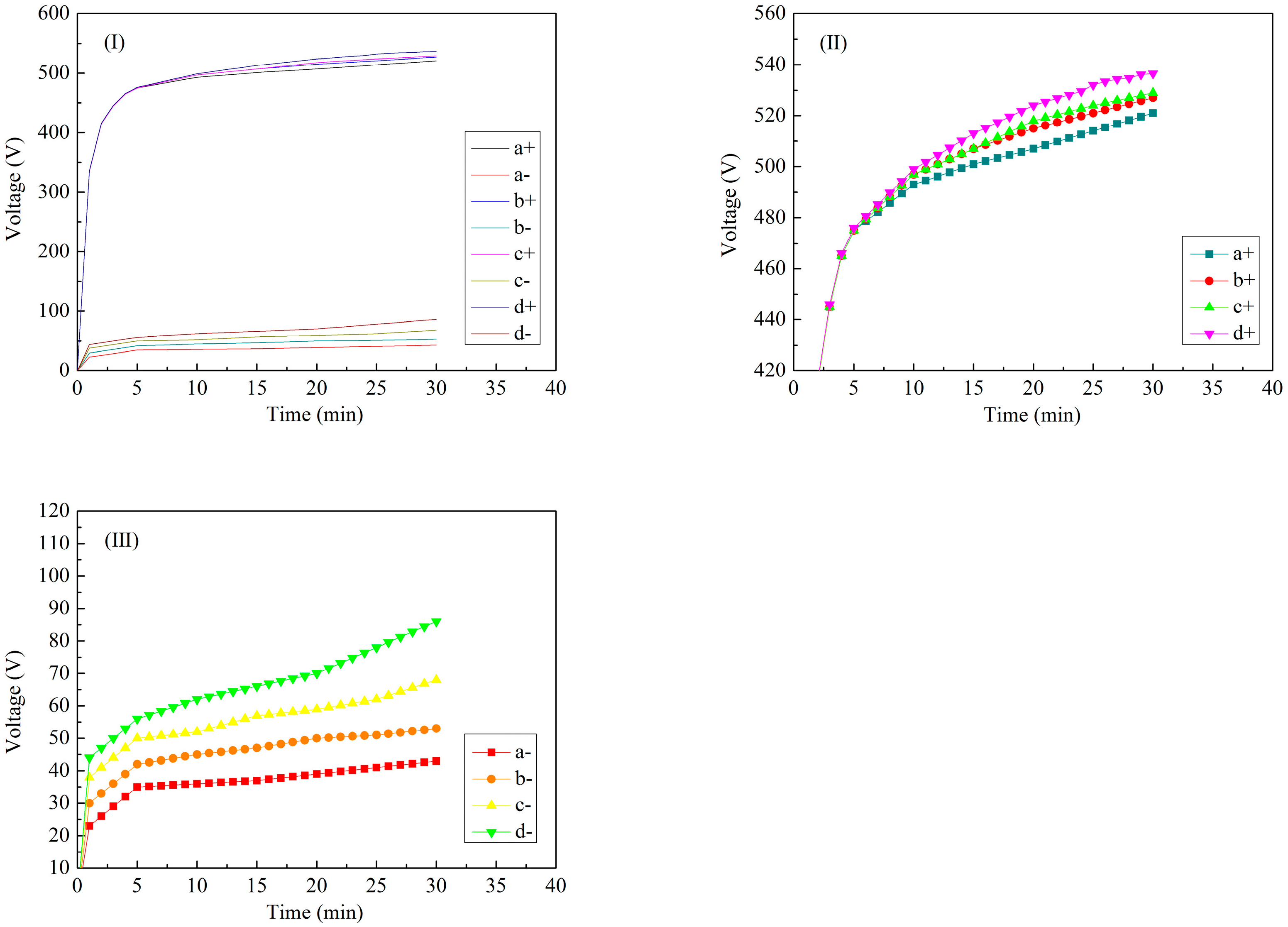

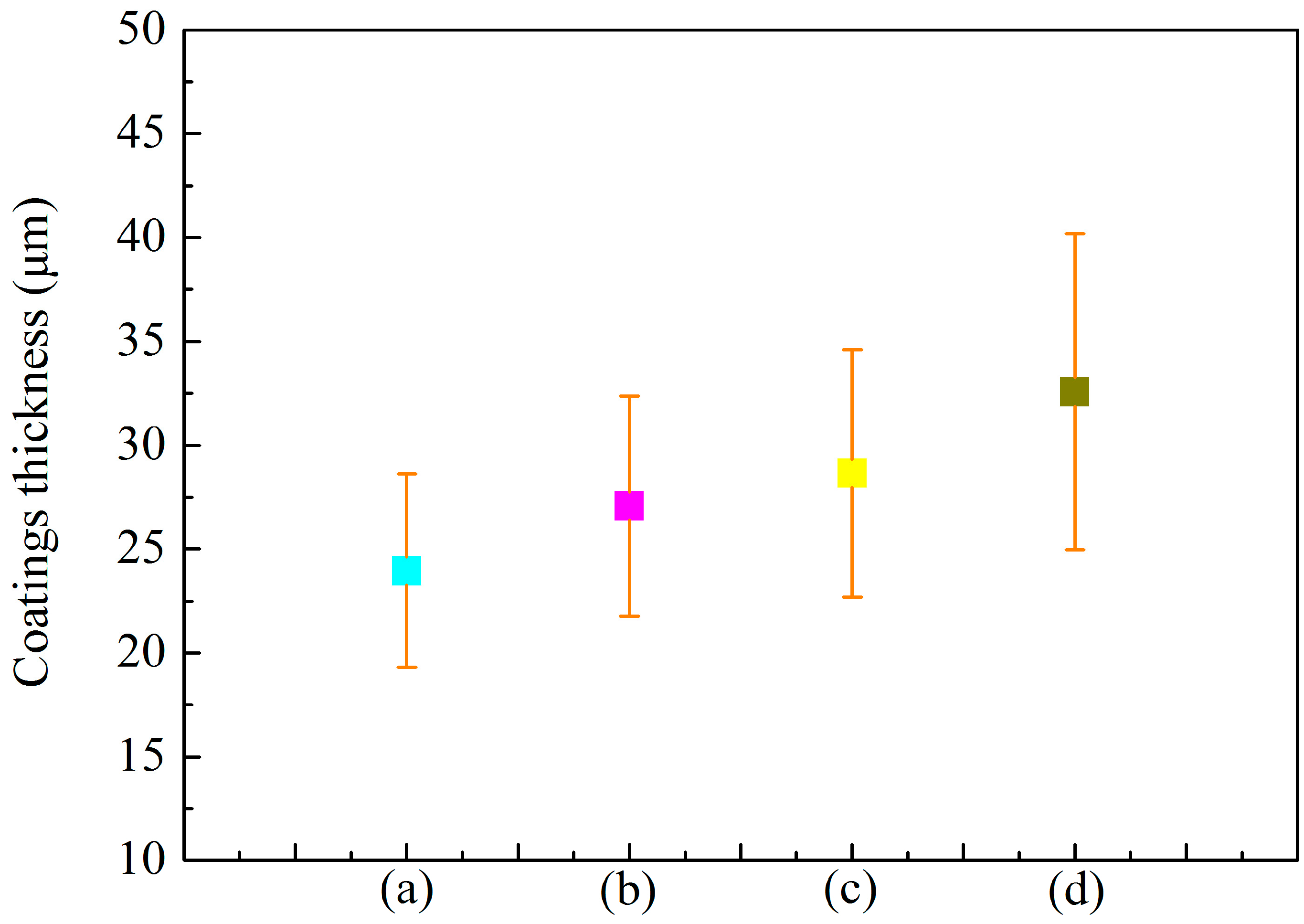
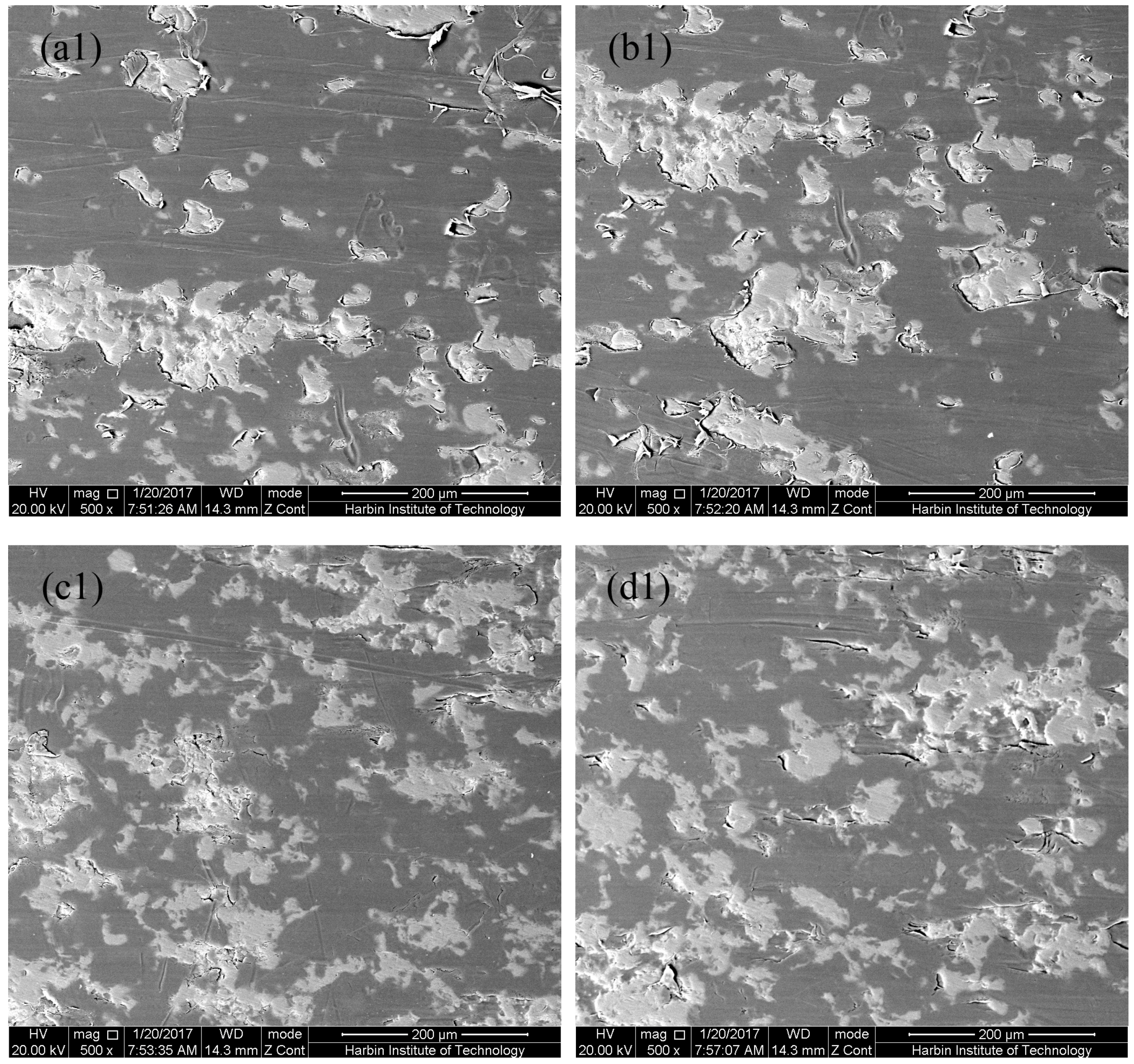
| Labels | Ton+ (μs) | Toff+ (μs) | Ton− (μs) | Toff− (μs) | V+ (V) | V− (V) | I+ (A/dm2) | I− (A/dm2) | Time (min) |
|---|---|---|---|---|---|---|---|---|---|
| a (a1) | 2000 | 800 | 2000 | 800 | Figure 2 | Figure 2 | 46 | 9 | 30 |
| b (b1) | 2000 | 800 | 2000 | 800 | Figure 2 | Figure 2 | 46 | 18 | 30 |
| c (c1) | 2000 | 800 | 2000 | 800 | Figure 2 | Figure 2 | 46 | 27 | 30 |
| d (d1) | 2000 | 800 | 2000 | 800 | Figure 2 | Figure 2 | 46 | 37 | 30 |
| Labels | Intensity (a.u.) | |||
|---|---|---|---|---|
| a | b | c | d | |
| 1 | 1765 | 1917 | 1992 | 2101 |
| 2 | 3206 | 3458 | 3809 | 3921 |
| 3 | 3143 | 3345 | 3698 | 3759 |
| Coatings | Lc (N) |
|---|---|
| A (Figure 6a) | 6.17 |
| B (Figure 6b) | 7.33 |
| C (Figure 6c) | 9.83 |
| D (Figure 6d) | 15.00 |
| Coatings | Wear Time (s) | Wear Track Width (μm) | Wear Track Depth (μm) | Wear Rate (mm3·N−1·m−1) |
|---|---|---|---|---|
| a | 1200 | 630.84 | 4.79 | 0.20 × 10−5 |
| b | 1200 | 584.11 | 4.43 | 0.18 × 10−5 |
| c | 1200 | 537.38 | 4.08 | 0.17 × 10−5 |
| d | 1200 | 443.93 | 3.37 | 0.14 × 10−5 |
| Coatings | Wear Time/s | Wear Track Width (μm) | Wear Track Depth (μm) | Wear Rate (mm3·N−1·m−1) |
|---|---|---|---|---|
| a1 | 1200 | 518.69 | 3.94 | 0.16 × 10−5 |
| b1 | 1200 | 364.48 | 2.77 | 0.11 × 10−5 |
| c1 | 1200 | 303.74 | 2.31 | 0.96 × 10−6 |
| d1 | 1200 | 238.32 | 1.81 | 0.75 × 10−6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Di, S. Preparation and Properties of Microarc Oxidation Self-Lubricating Composite Coatings on Aluminum Alloy. Metals 2017, 7, 127. https://doi.org/10.3390/met7040127
Li Z, Di S. Preparation and Properties of Microarc Oxidation Self-Lubricating Composite Coatings on Aluminum Alloy. Metals. 2017; 7(4):127. https://doi.org/10.3390/met7040127
Chicago/Turabian StyleLi, Zhenwei, and Shichun Di. 2017. "Preparation and Properties of Microarc Oxidation Self-Lubricating Composite Coatings on Aluminum Alloy" Metals 7, no. 4: 127. https://doi.org/10.3390/met7040127





