The Influence of FeO on the Reaction between Fe–Al–Ca Alloy and Al2O3–CaO–FeO Oxide during Heat Treatment at 1473 K
Abstract
:1. Introduction
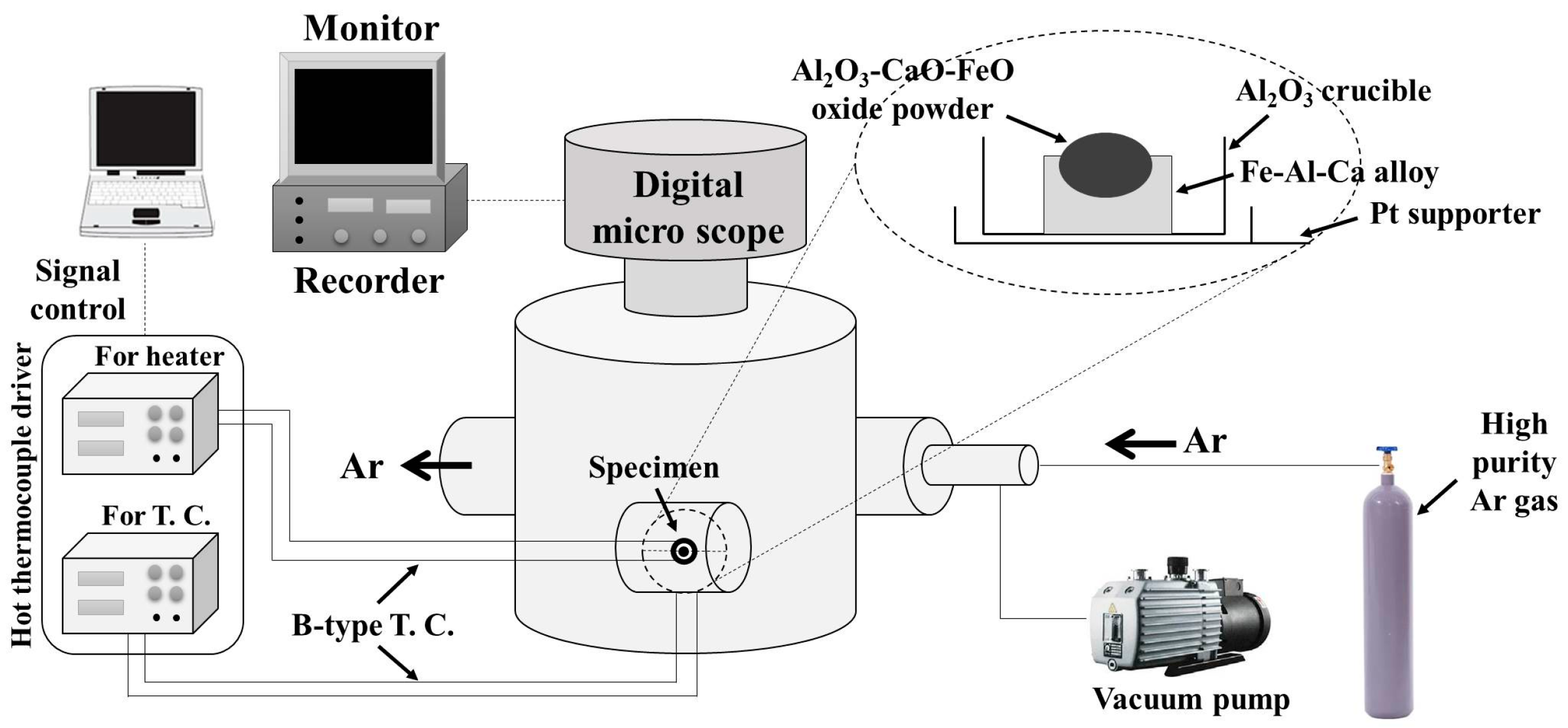
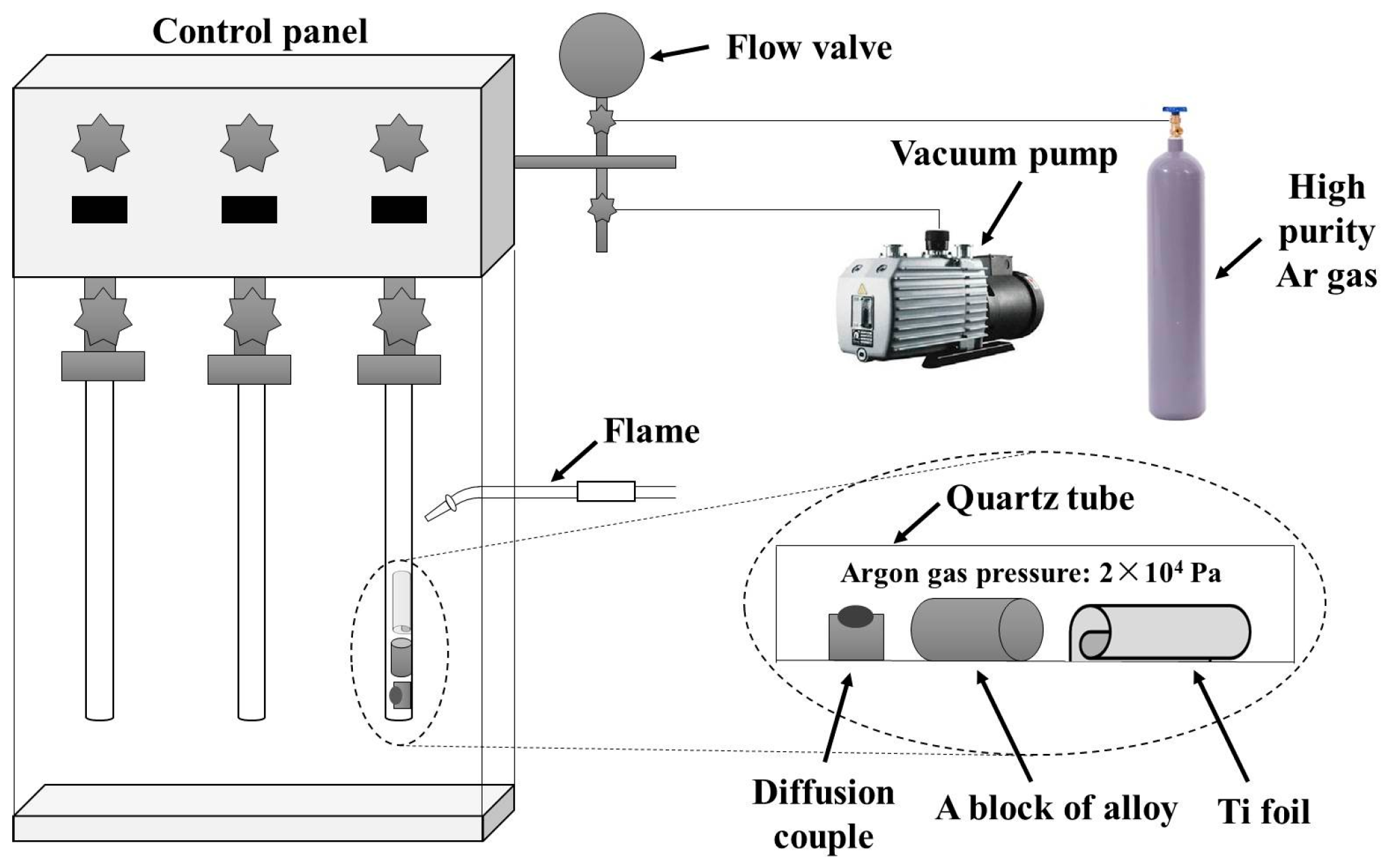
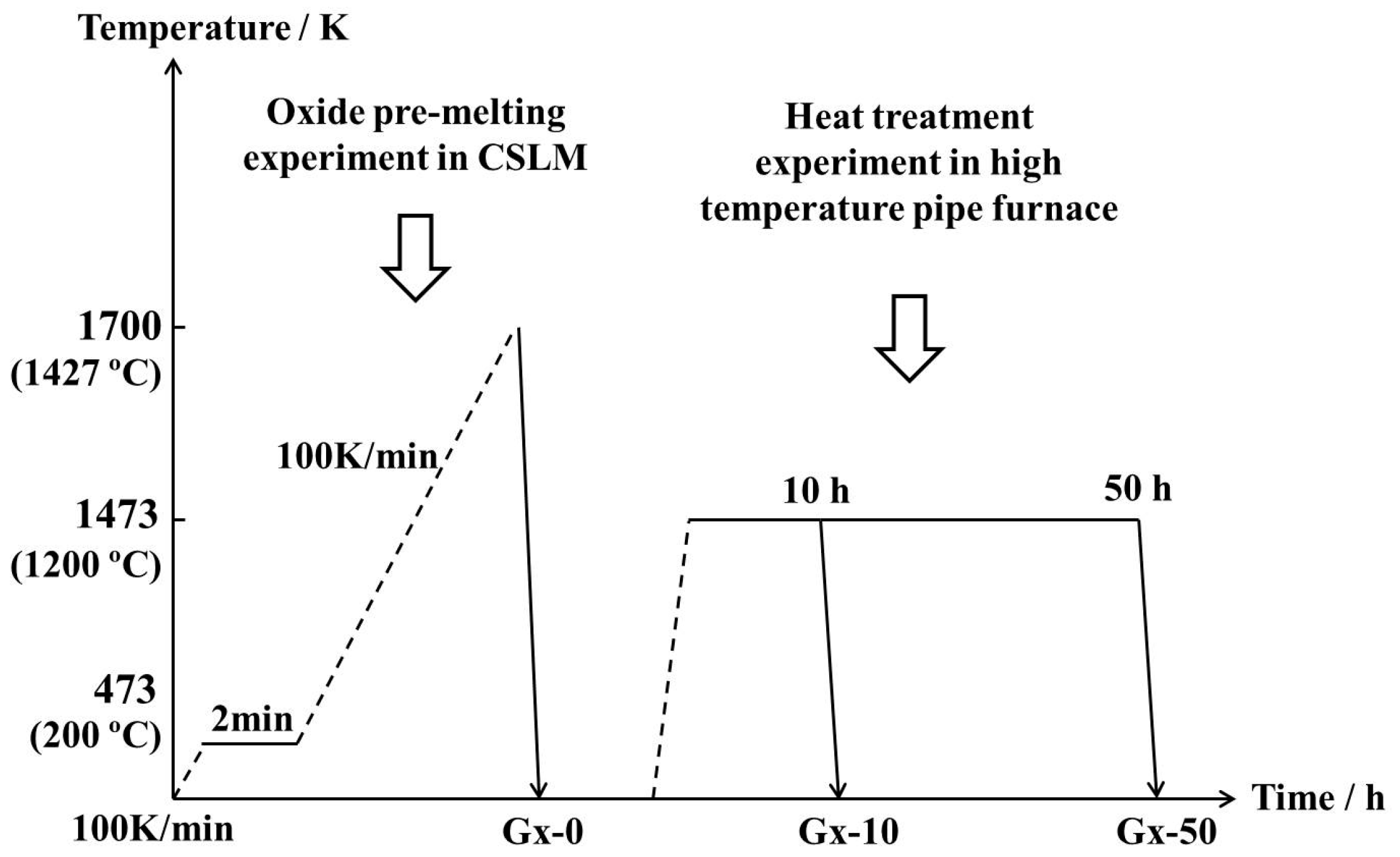
2. Experimental Methods
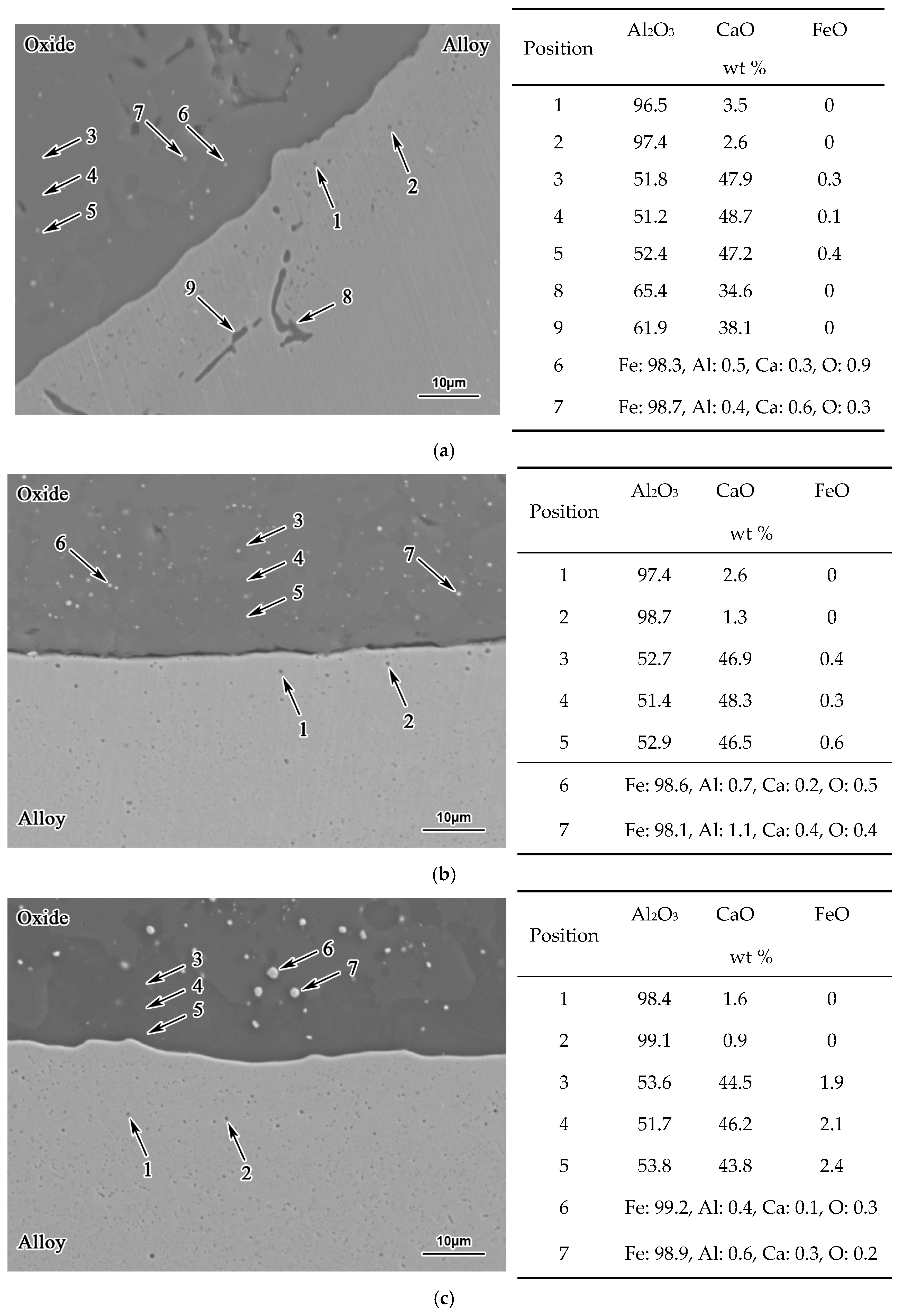
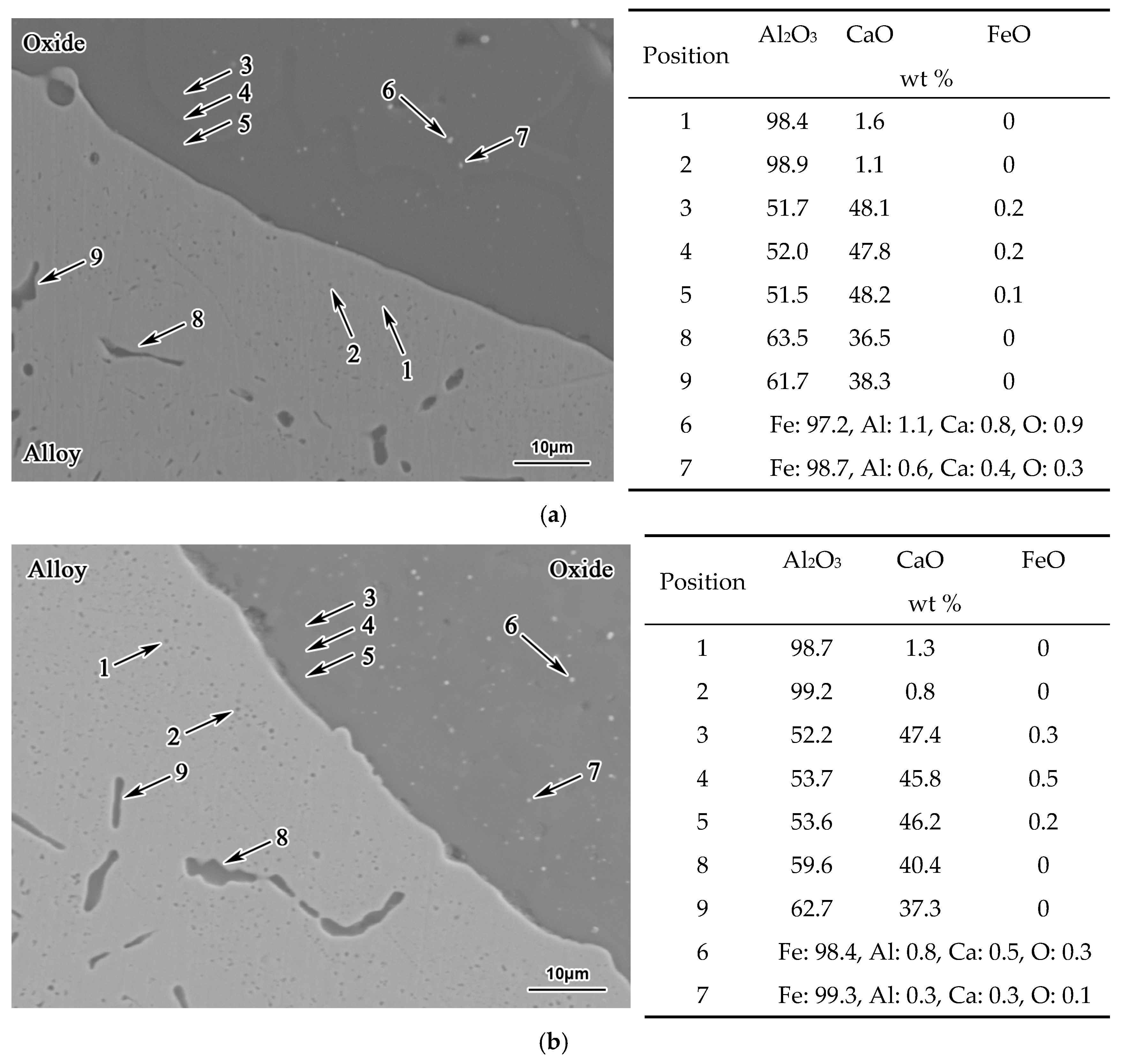
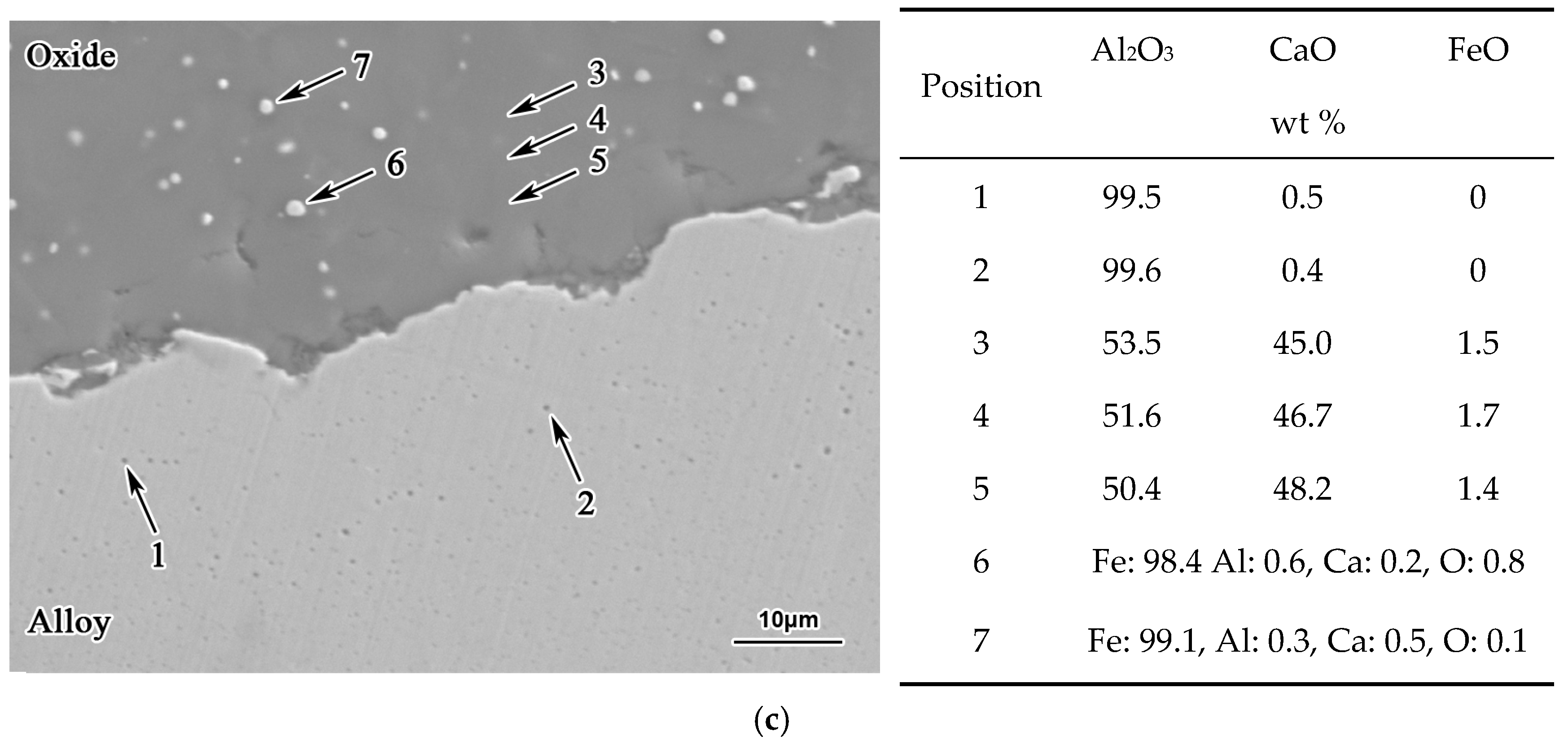
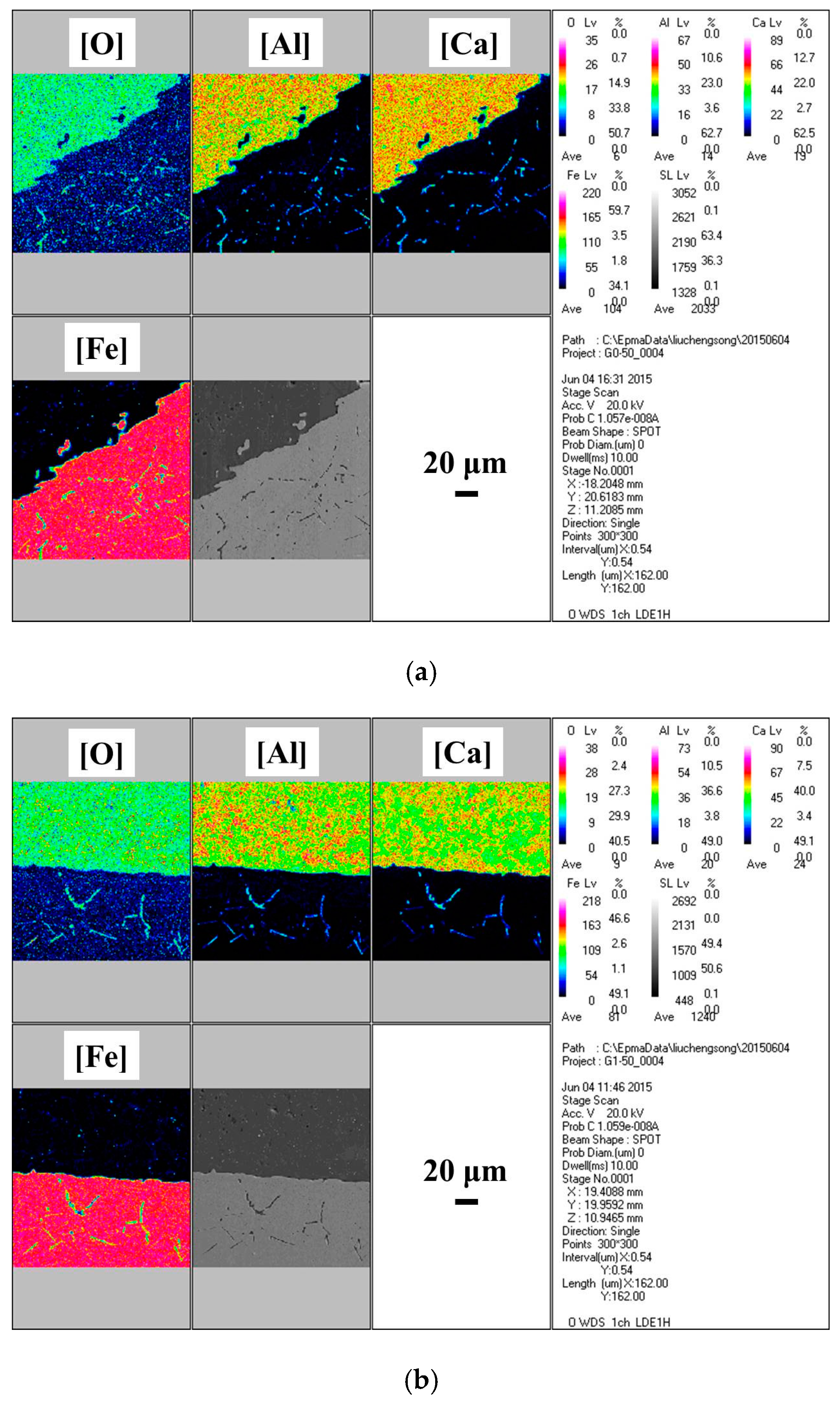
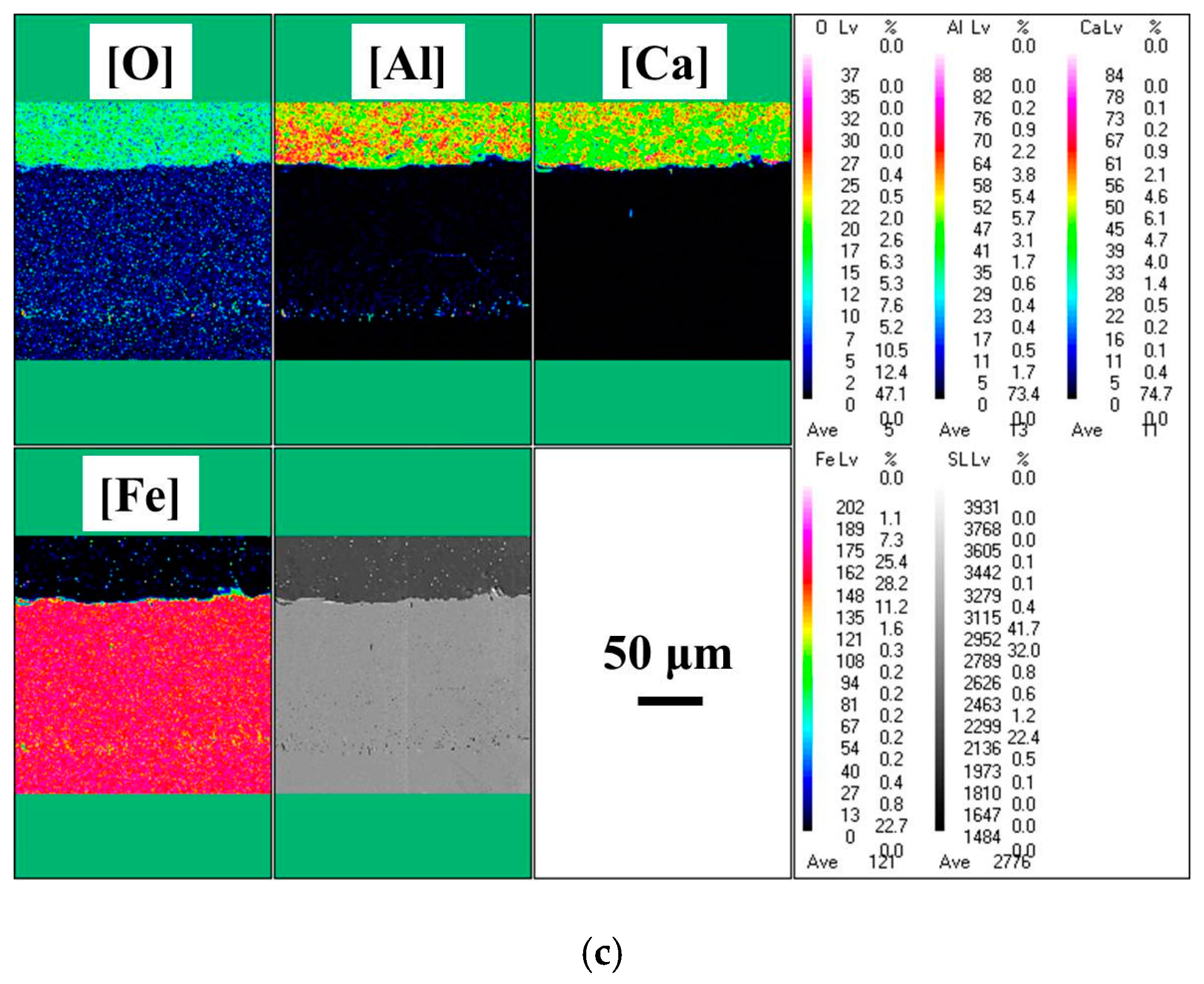
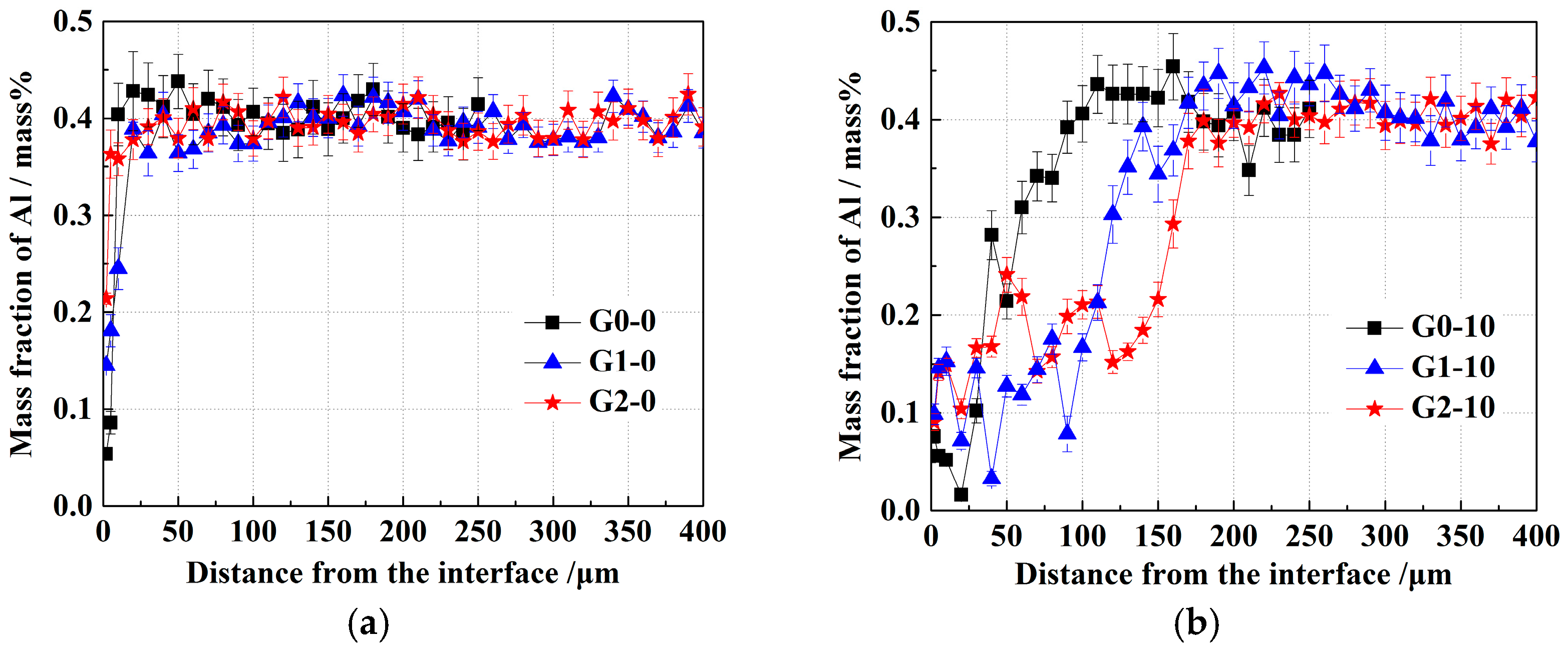
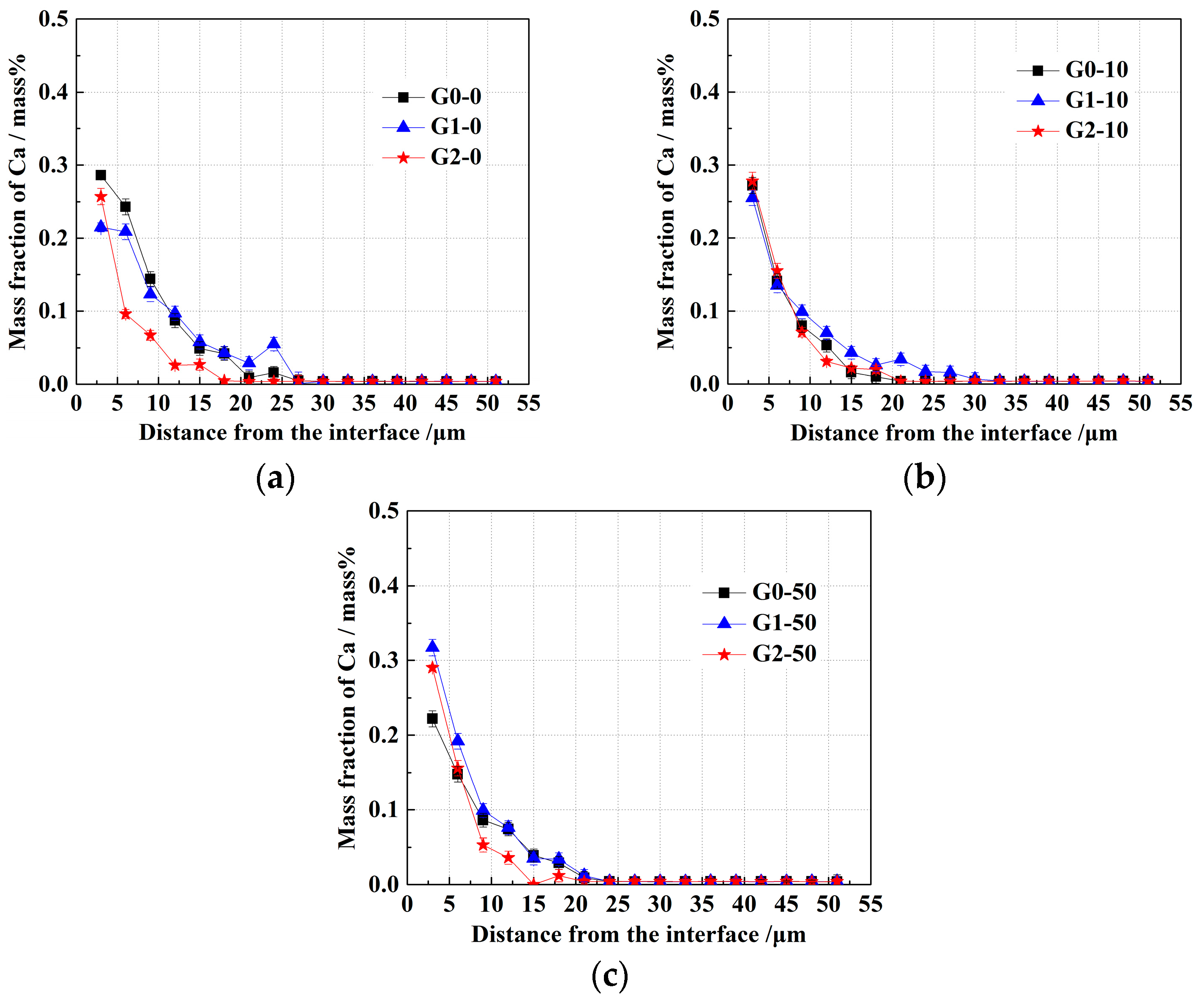
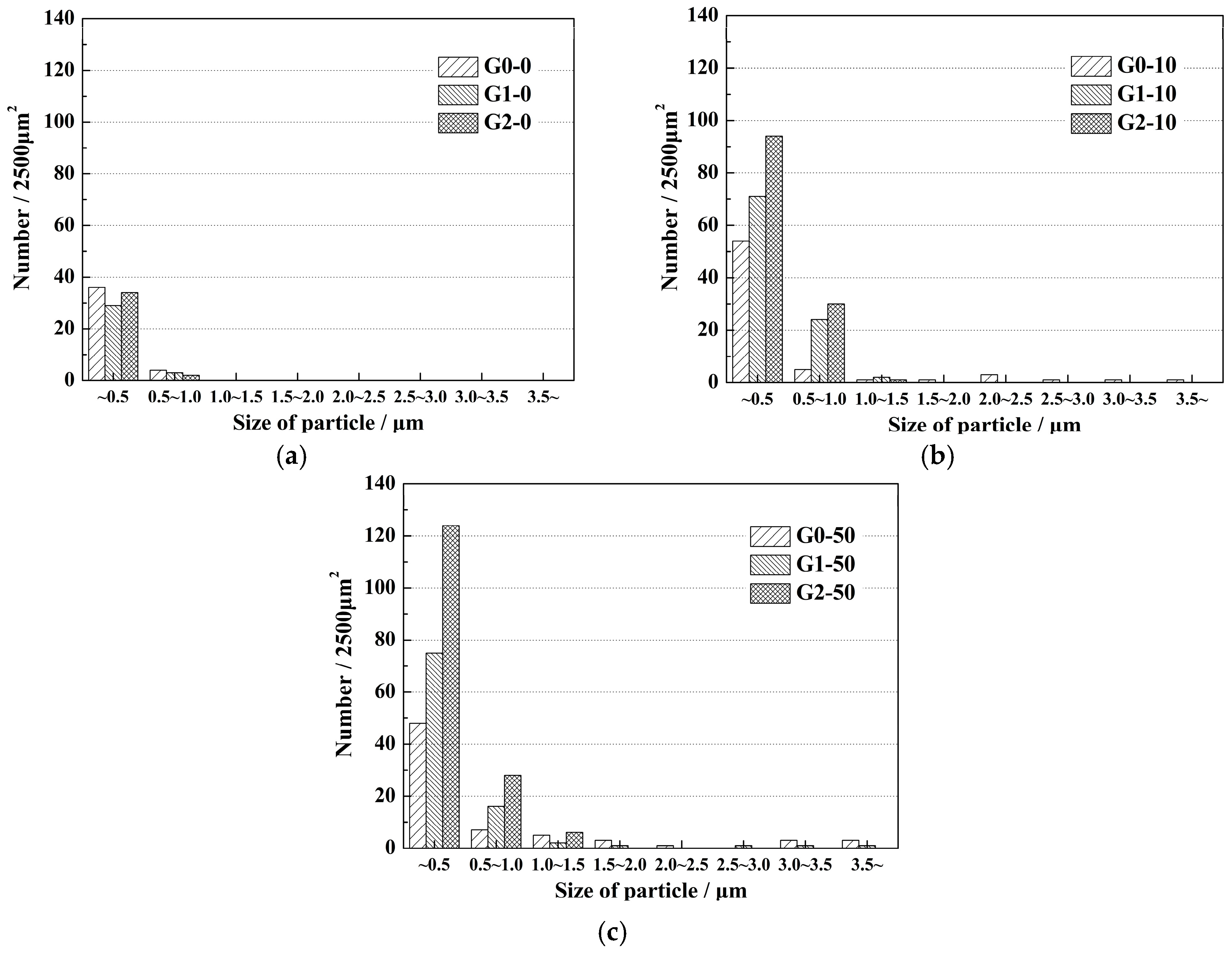
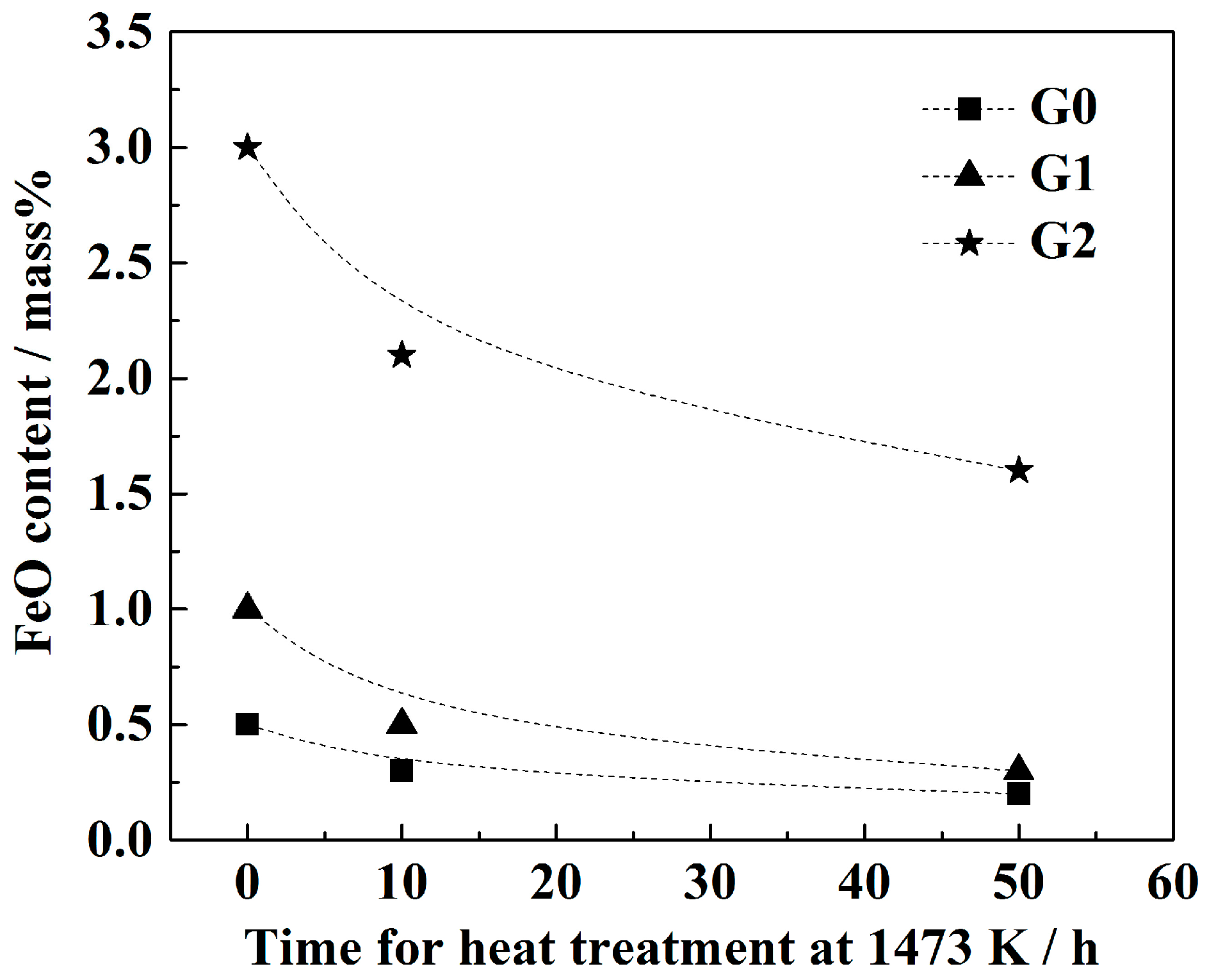
3. Results
4. Discussion
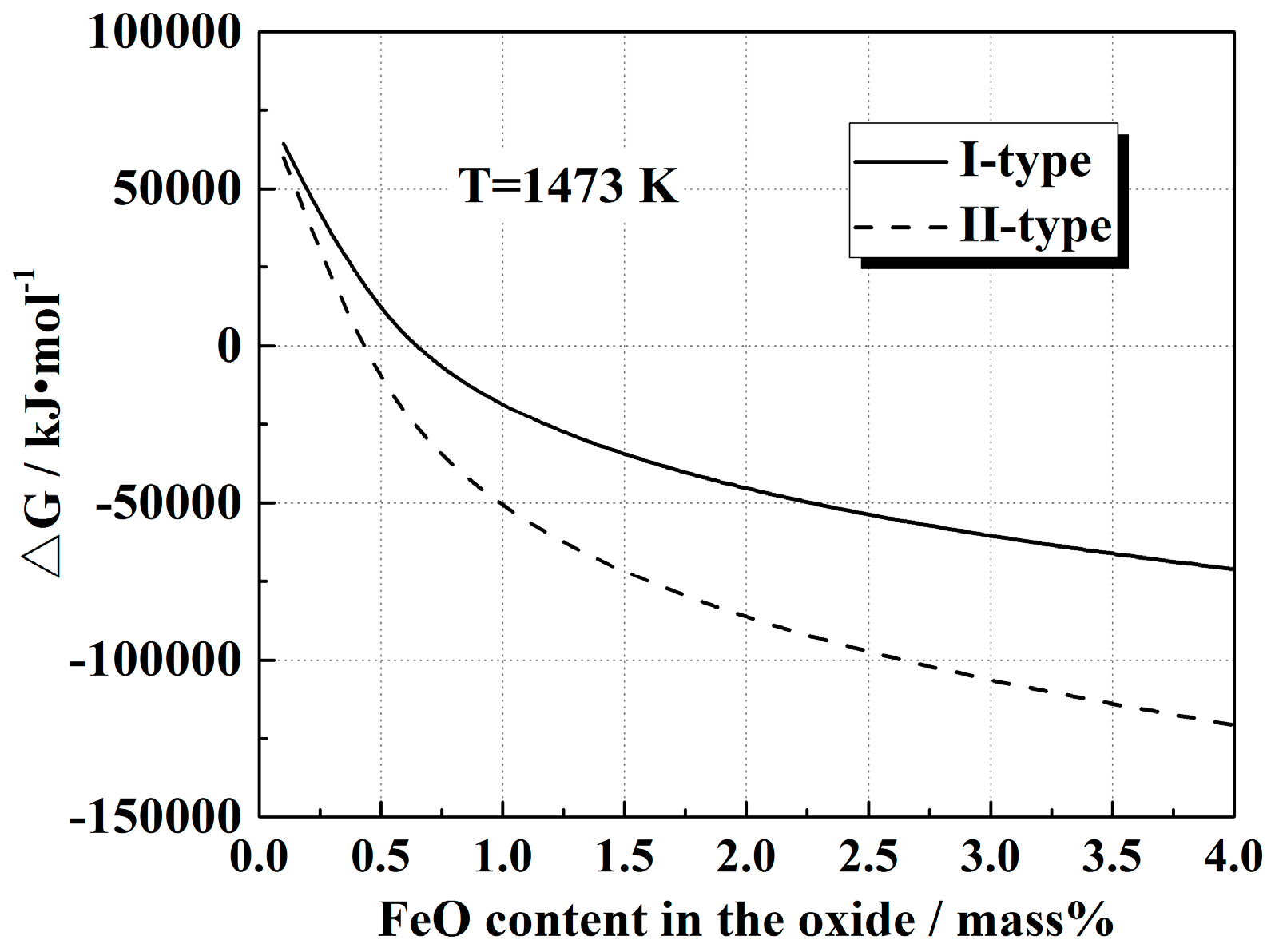
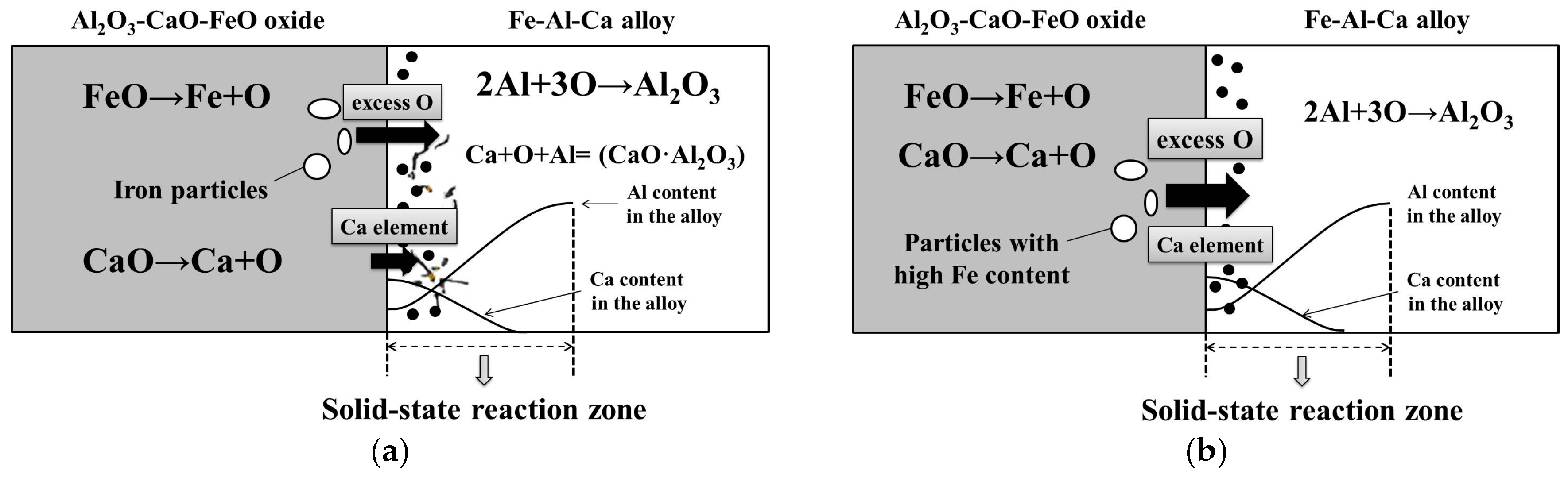
4.1. Influence Mechanism of FeO on the Solid-State Reaction
4.2. Modified Dynamic Calculation Model
5. Conclusions
- With the same heat treatment time at 1473 K, when the initial FeO content in the oxide was relatively low, fine Al2O3 particles and CaO·Al2O3 branch inclusions precipitated in the alloy near the alloy–oxide interface. With increasing initial FeO content in the oxide, in the PPZ where Al2O3 particles formed, CaO·Al2O3 branch inclusions gradually disappeared.
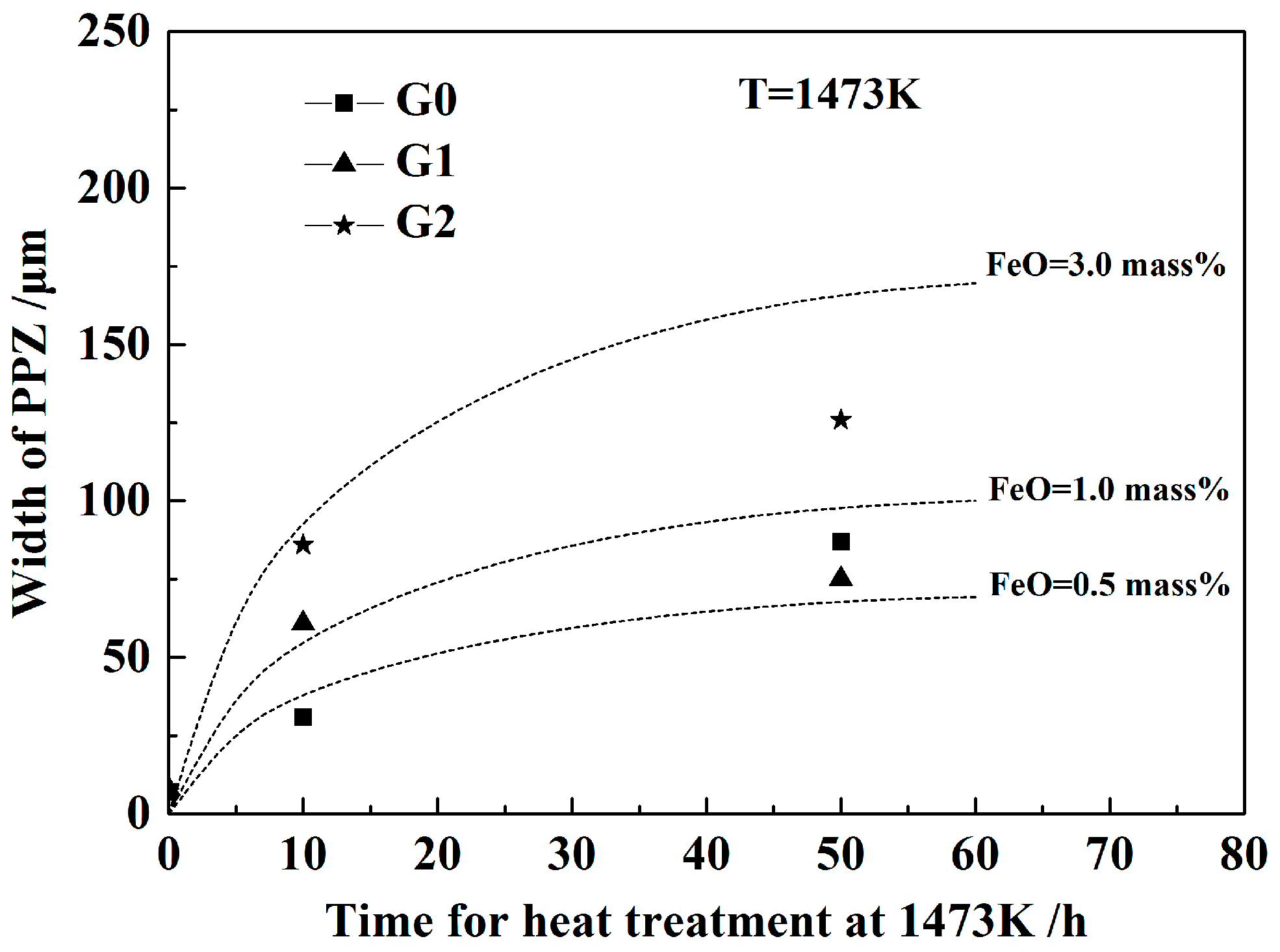
- Due to the two types of solid-state reactions and element diffusion between the alloy and oxide, Al and Ca concentrations in the alloy near the alloy–oxide interface tended to decrease and increase, respectively. An approximately linear positive correlation was confirmed between the PPZ and ADZ widths, both of which increased with the initial FeO content in the oxide.
- The I-type reaction between elemental Al in the alloy and oxygen generated by the decomposition of FeO in the oxide played a dominant role in the early stage of heat treatment in all cases. As the heat treatment time lengthened and initial FeO content in the oxide decreased, the II-type reaction between the 12CaO·7Al2O3 phase and elemental Al gradually became important.
- When the heat treatment time was relatively short, good agreement was obtained between the measured results of the PPZ width and the results calculated using a modified dynamic calculation model established based on the Wagner equation, which contributed to understanding the influence of FeO on the solid-state reactions between Fe–Al–Ca alloy and Al2O3–CaO–FeO oxide.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, Z.; Zheng, W.; Li, G.; Matsuura, H.; Tsukihashi, F. Effect of Inclusions’ Behavior on the Microstructure in Al–Ti Deoxidized and Magnesium-Treated Steel with Different Aluminum Contents. Metall. Mater. Trans. B 2015, 46, 1226–1241. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, G.; Hu, H.; Yuan, Q.; Tian, J. The Morphologies of Different Types of Fe2SiO4–FeO in Si-Containing Steel. Metals 2017, 7, 8. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, Z.; Li, G.; Zhang, Z.; Zhu, C. Effect of Al Content on the Characteristics of Inclusions in Al–Ti Complex Deoxidized Steel with Calcium Treatment. ISIJ Int. 2014, 54, 1755–1764. [Google Scholar] [CrossRef]
- Sasaki, R.; Ueda, S.; Kim, S.; Gao, X.; Kitamura, S. Reaction Behavior between B4C, 304 Grade of Stainless Steel and Zircaloy at 1473 K. J. Nucl. Mater. 2016, 477, 205–214. [Google Scholar] [CrossRef]
- Taniguchi, T.; Satoh, N.; Saito, Y.; Kubota, K.; Kumagai, A.; Tamura, Y.; Miki, T. Investigation of Compositional Change of Inclusions in Martensitic Stainless Steel during Heat Treatment by Newly Developed Analysis Method. ISIJ Int. 2011, 51, 1957–1966. [Google Scholar] [CrossRef]
- Shao, X.; Wang, X.; Ji, C.; Li, H.; Yang, C.; Zhu, G. Effect of Heat Treatment Conditions on Shape Control of Large-sized Elongated MnS Inclusions in Resulfurized Free-cutting Steels. Int. J. Min. Met. Mater. 2015, 22, 483–491. [Google Scholar] [CrossRef]
- Yuan, Q.; Xu, G.; Zhou, M.; He, B.; Hu, H. The Effect of P on the Microstructure and Melting Temperature of Fe2SiO4 in Silicon-Containing Steels Investigated by In Situ Observation. Metals 2017, 7, 37. [Google Scholar] [CrossRef]
- Takahashi, I.; Sakae, T.; Yoshida, T. Changes of the Nonmetallic Inclusion by Heating. Tetsu Hagané 1967, 53, 168–173. [Google Scholar]
- Shibata, H.; Kimura, K.; Tanaka, T.; Kitamura, S. Mechanism of Change in Chemical Composition of Oxide Inclusions in Fe–Cr Alloys Deoxidized with Mn and Si by Heat Treatment at 1473 K. ISIJ Int. 2011, 51, 1944–1950. [Google Scholar] [CrossRef]
- Choi, W.; Matsuura, H.; Tsukihashi, F. Changing Behavior of Non-metallic Inclusions in Solid Iron Deoxidized by Al–Ti Addition during Heating at 1473 K. ISIJ Int. 2011, 51, 1951–1956. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Shibata, H.; Kitamura, S. Reaction between MnO–SiO2–FeO Oxide and Fe–Mn–Si Solid Alloy during Heat Treatment. ISIJ Int. 2014, 54, 2144–2153. [Google Scholar] [CrossRef]
- Liu, C.; Kim, K.; Kim, S.; Li, J.; Ueda, S.; Gao, X.; Shibata, H.; Kitamura, S. Reaction between MnO–SiO2–FeO Solid Oxide with Solid Steel Deoxidized by Si and Mn during Heat Treatment at 1473K (1200 °C). Metall. Mater. Trans. B 2015, 46, 1875–1884. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Kim, K.; Li, J.; Shibata, H.; Kitamura, S. Influence of FeO and Sulfur on the Reaction between MnO–SiO2–FeO Oxides with Fe–Mn-Si Solid Alloy during Heat Treatment at 1473K. Int. J. Min. Met. Mater. 2015, 22, 811–819. [Google Scholar] [CrossRef]
- Zhang, L. Several Important Scientific Research Points of Non-metallic Inclusions in Steel. Steelmaking 2016, 32, 1–16. (In Chinese) [Google Scholar]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking, 1st ed.; Tohoku University Press: Sendai, Japan, 2010; pp. 10–16. [Google Scholar]
- Nagata, K.; Tanabe, J.; Goto, K.S. Standard Free Energies of Formation of CaO–Al2O3 Intermediate Compounds by Means of EMF Measurement of Galvanic Cells. Tetsu Hagané 1989, 75, 2023–2030. [Google Scholar]
- Ohba, Y.; Yamashita, Y.; Ohno, K.; Maeda, T.; Nishioka, K.; Shimizu, M. Formation Mechanism of Oxide Particles in Subscale Layer around Surface Cracks of Steel. Tetsu Hagané 2009, 95, 531–540. [Google Scholar] [CrossRef]
- Takada, J.; Yamamoto, S.; Kikuchi, S.; Adachi, M. Determination of Diffusion Coefficient of Oxygen in γ-iron from Measurements of Internal Oxidation in Fe–Al Alloys. Metall. Mater. Trans. A 1986, 17, 221–229. [Google Scholar] [CrossRef]
- Swisher, J.; Turkdogan, E. Soubility, Permeability, and Diffusivity of Oxygen in Solid Iron. Trans. Met. Soc. AIME 1967, 239, 426–431. [Google Scholar]
- Ban-Ya, S. Mathematical Expression of Slag-Metal Reactions in Steelmaking Process by Quadratic Formalism Based on the Regular Solution Model. ISIJ Int. 1993, 33, 2–11. [Google Scholar] [CrossRef]
- Madelung, O. Diffusion in Solid Metals, 1st ed.; Springer: Berlin, Germany, 1990; pp. 124–130. [Google Scholar]





| Diffusion Couple | Fe–Al–Ca Alloy | Al2O3–CaO–FeO Oxide | PO2/Pa | ||||
|---|---|---|---|---|---|---|---|
| Fe | Al | Ca | Al2O3 | CaO | FeO | ||
| G0 (Equilibrium) | Bal. | 0.4 | 0.004 | 51.26 | 48.24 | 0.5 | 1.4 × 10−13 |
| G1 | 51 | 48 | 1.0 | ||||
| G2 | 50 | 47 | 3.0 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yang, S.; Li, J.; Ni, H.; Zhang, X. The Influence of FeO on the Reaction between Fe–Al–Ca Alloy and Al2O3–CaO–FeO Oxide during Heat Treatment at 1473 K. Metals 2017, 7, 129. https://doi.org/10.3390/met7040129
Liu C, Yang S, Li J, Ni H, Zhang X. The Influence of FeO on the Reaction between Fe–Al–Ca Alloy and Al2O3–CaO–FeO Oxide during Heat Treatment at 1473 K. Metals. 2017; 7(4):129. https://doi.org/10.3390/met7040129
Chicago/Turabian StyleLiu, Chengsong, Shufeng Yang, Jingshe Li, Hongwei Ni, and Xueliang Zhang. 2017. "The Influence of FeO on the Reaction between Fe–Al–Ca Alloy and Al2O3–CaO–FeO Oxide during Heat Treatment at 1473 K" Metals 7, no. 4: 129. https://doi.org/10.3390/met7040129






