Aluminium Wires Have the Free Air Balls (FABs): Electronic Flame-Off, Fracture Strength, Electrical Properties, and Bonding Characteristics of Nano Zn Film Al–Si Bonding Wires
Abstract
:1. Introduction
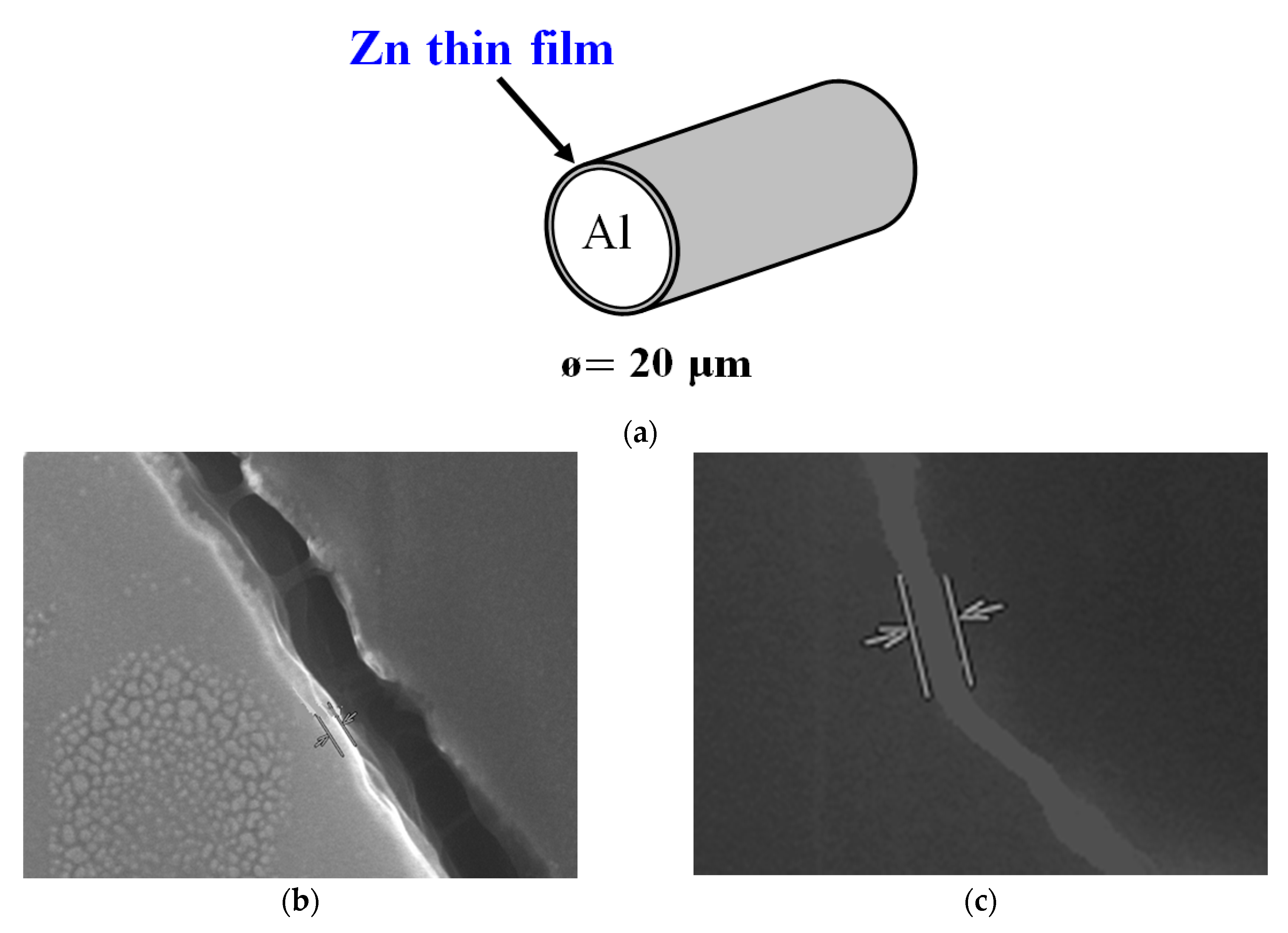
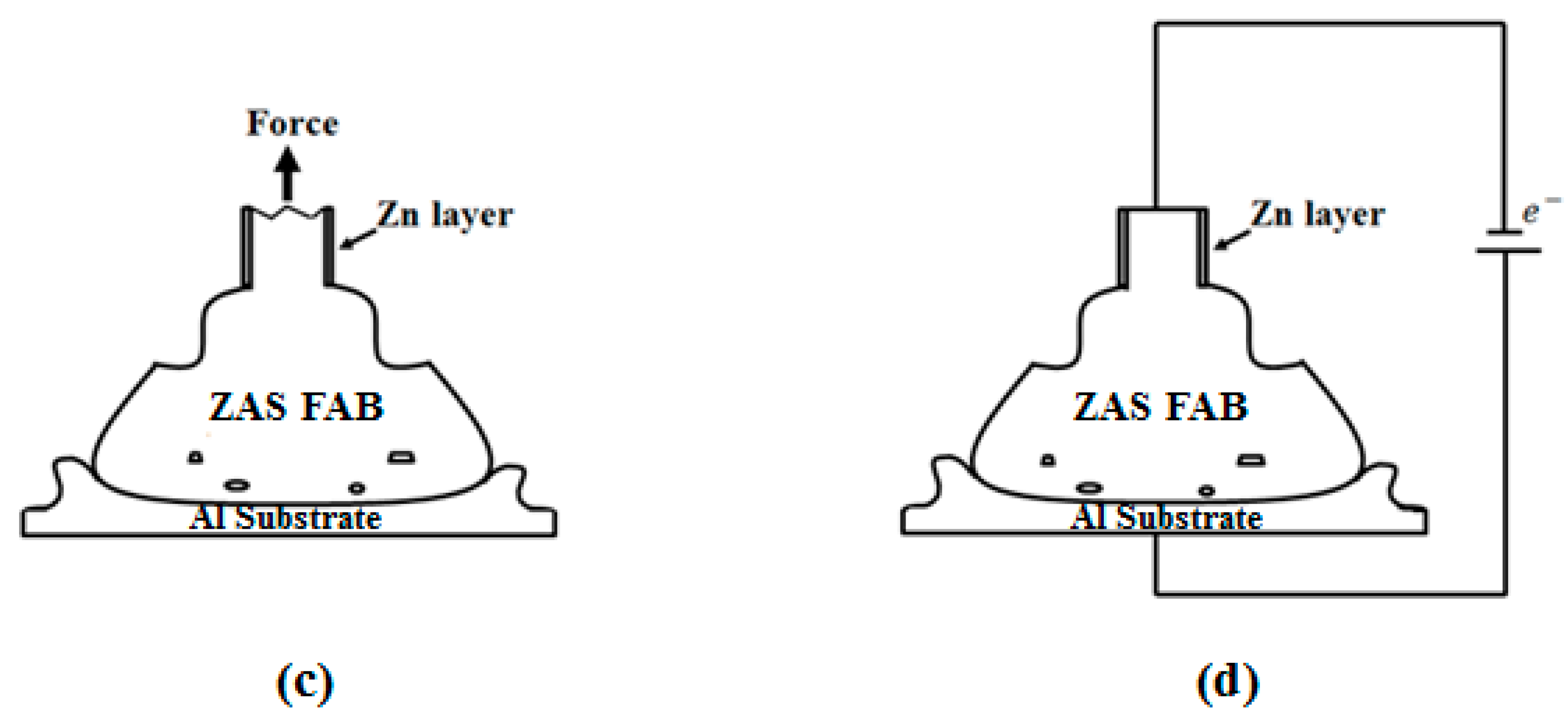
2. Experiment
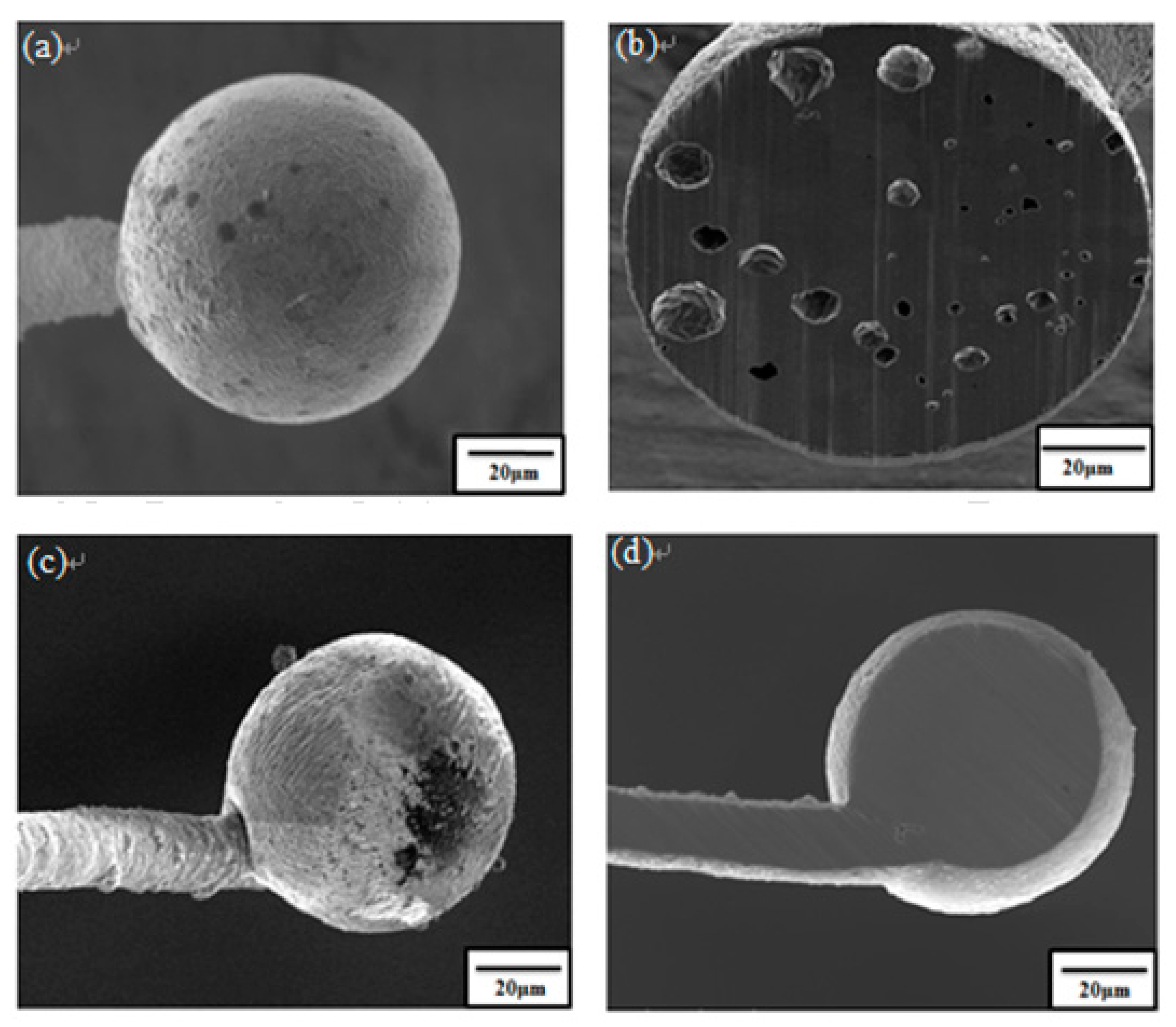
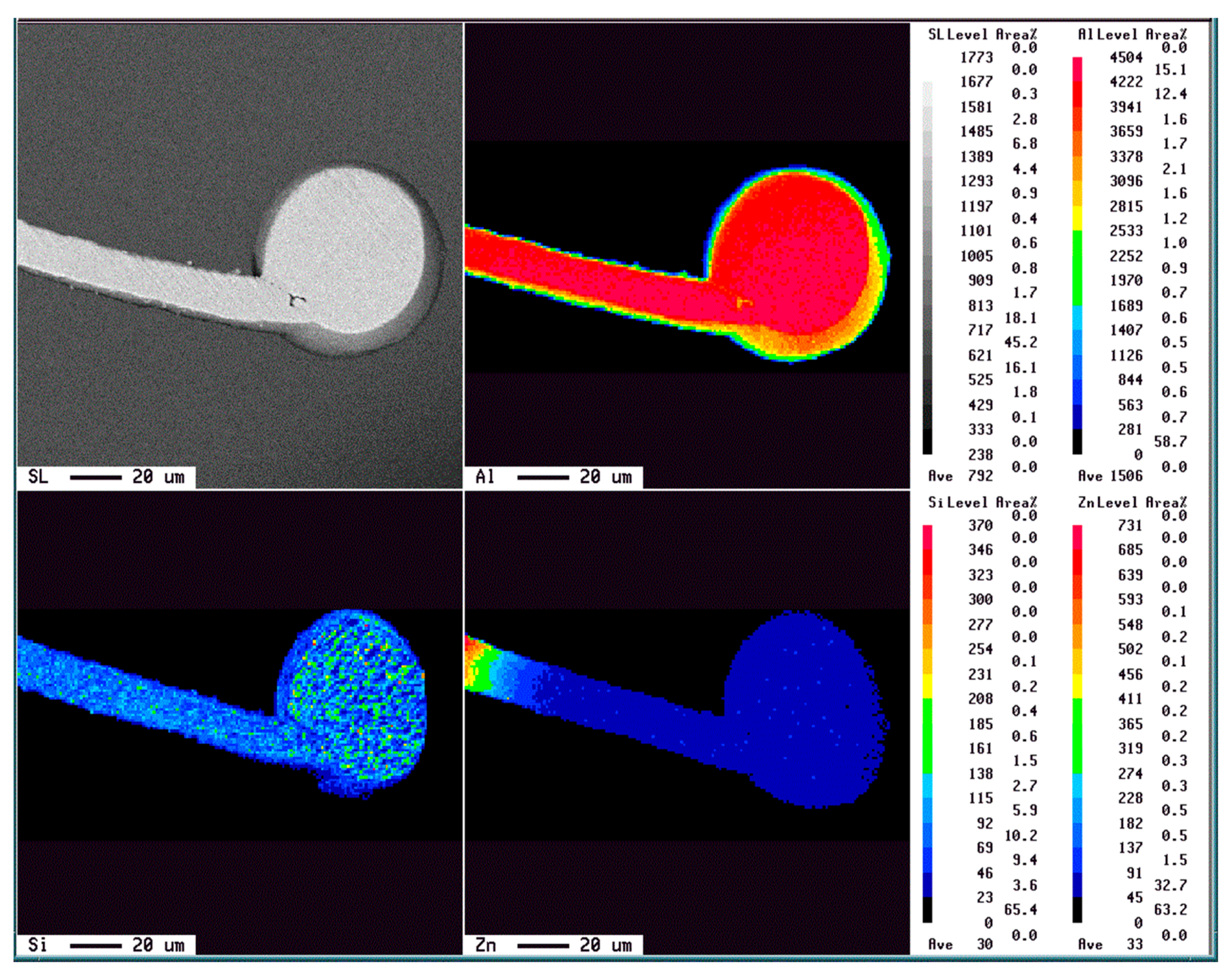
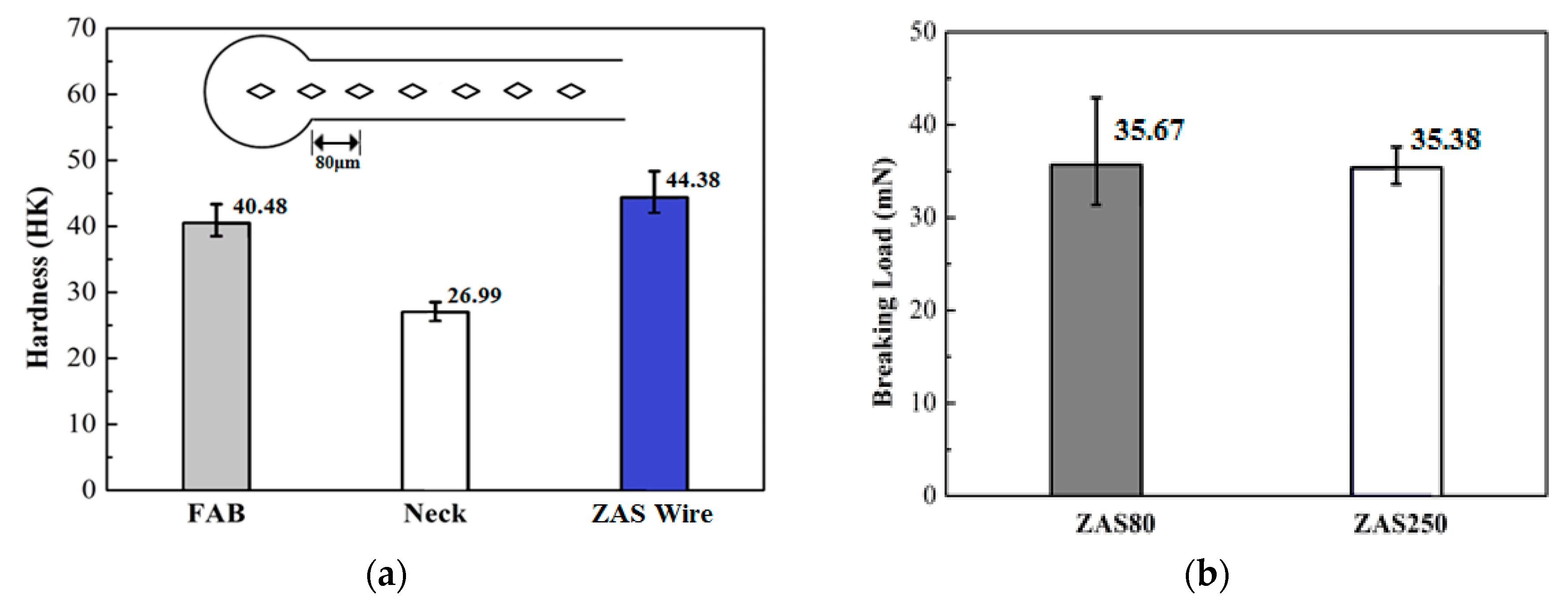
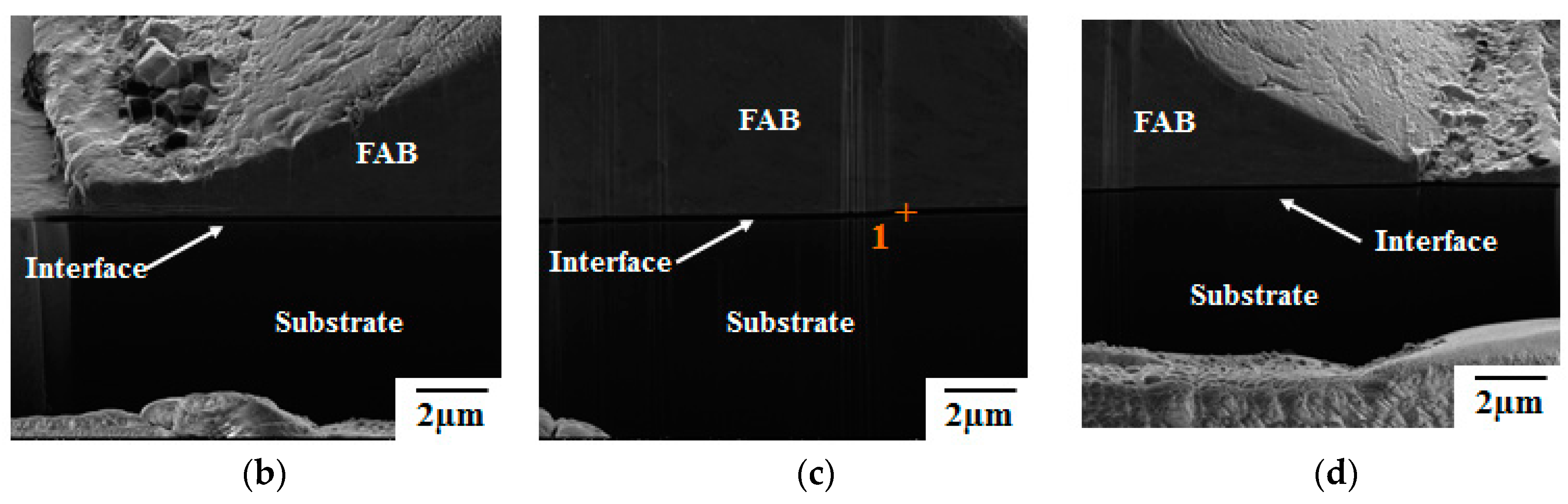
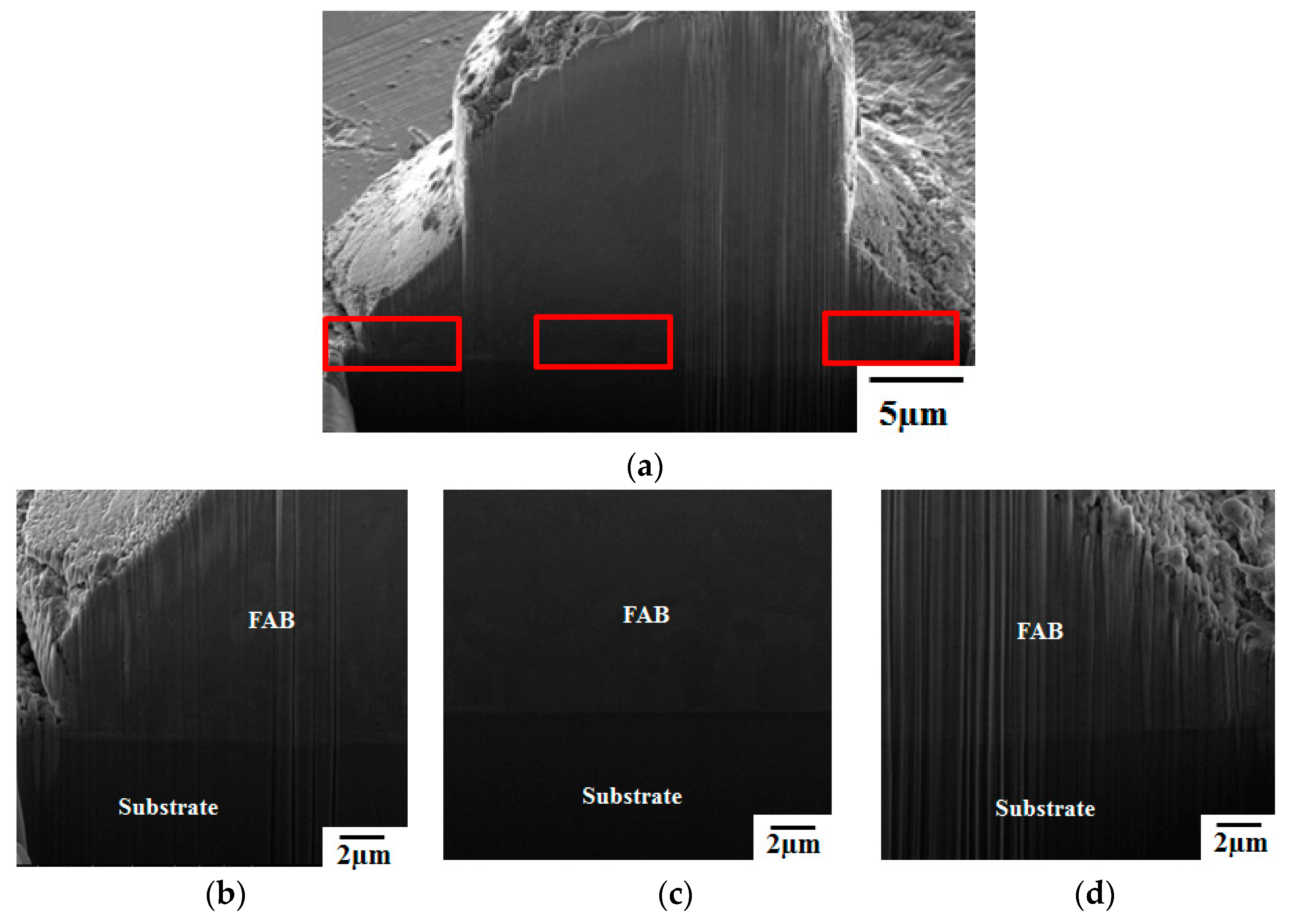
3. Results and Discussion
4. Discussion
- (1)
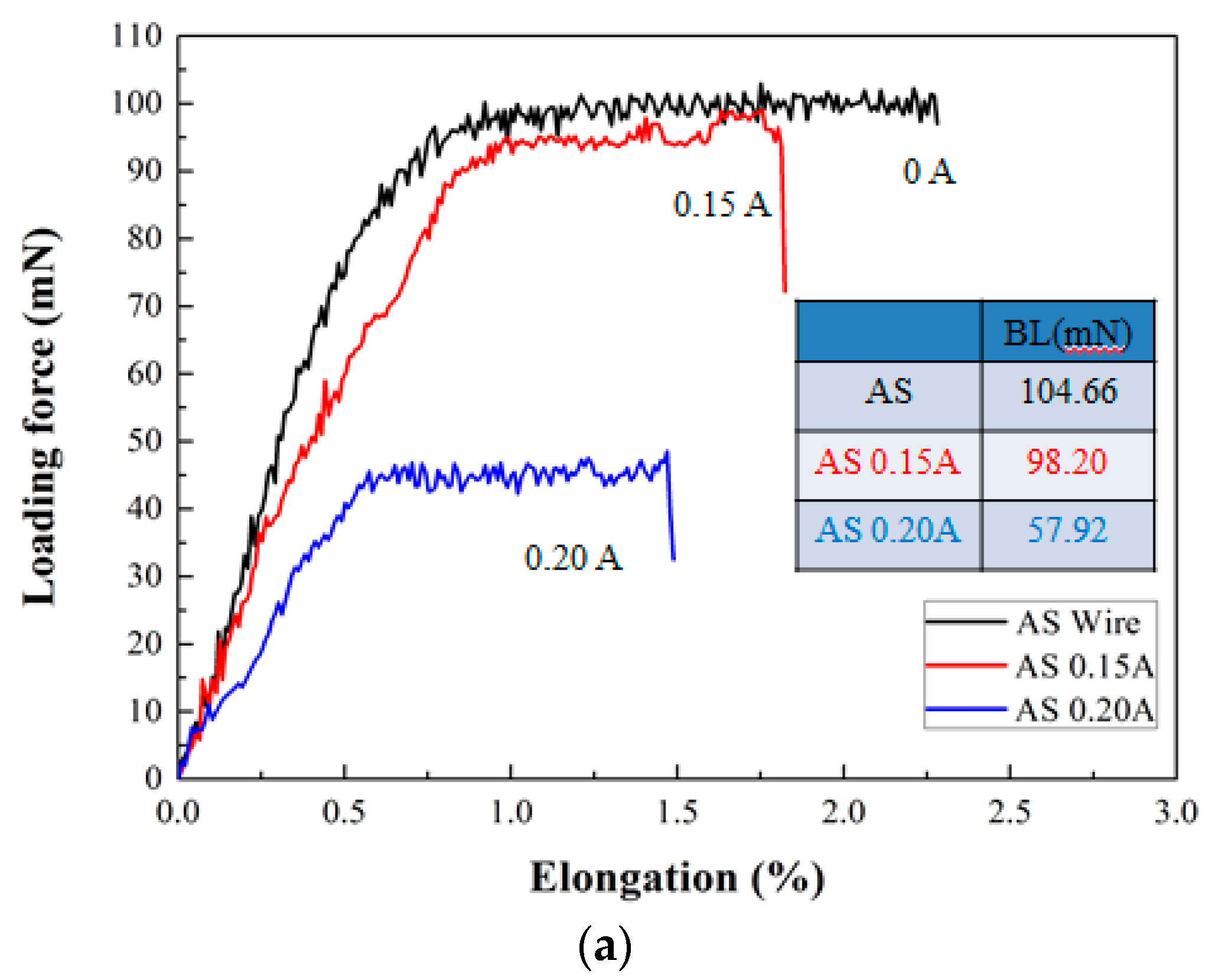
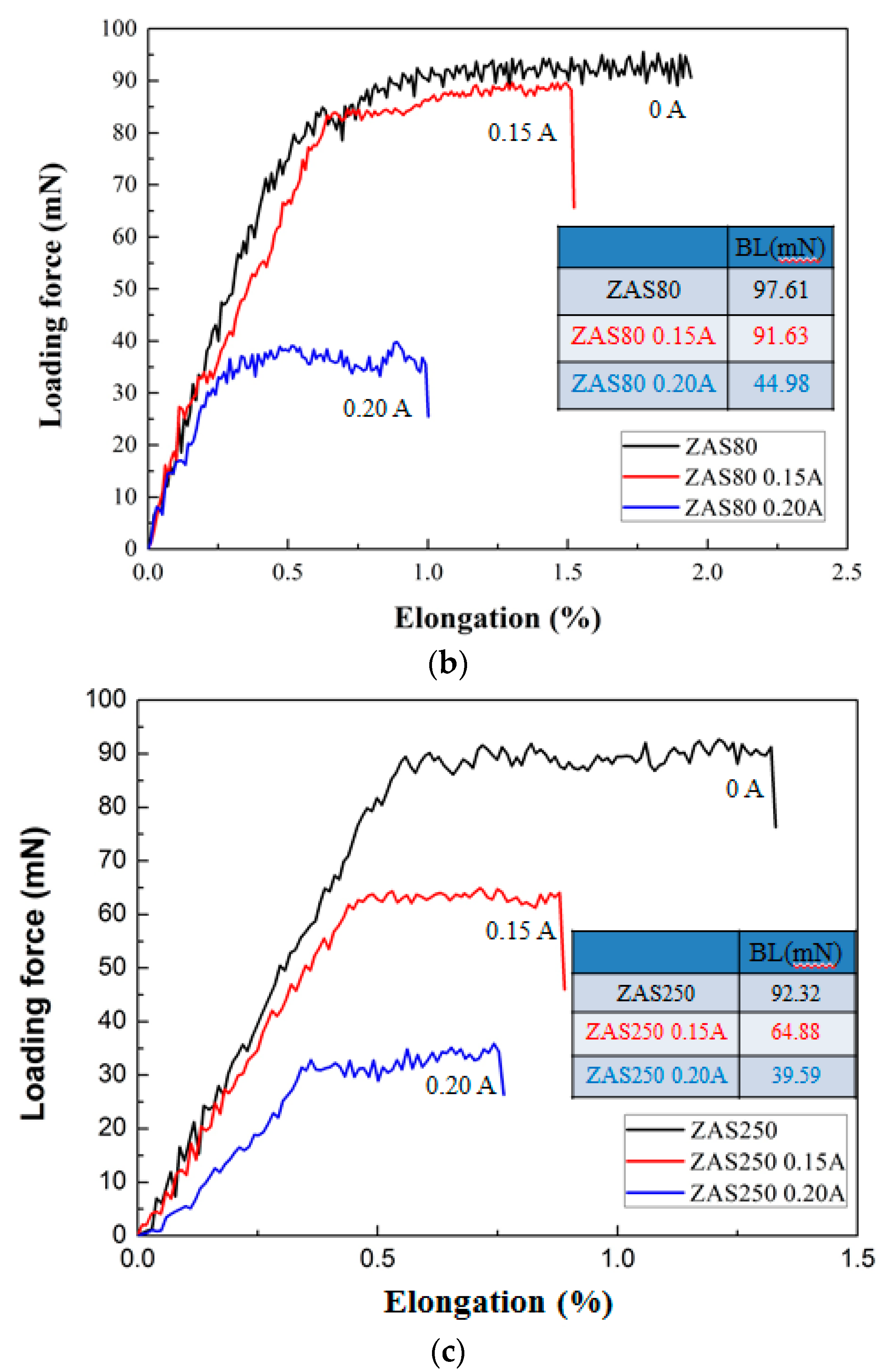
- Currently, Al wires cannot be used in wire bonding with ball bonding; however, coating Al–0.5 wt % Si wire with a Zn film with 80–250 nm can improve this problem. Further, the mechanical properties, bonding strengths and bias tensile strength of the 20 μm Zn-coated Al–0.5 wt % Si (ZAS) wires are stable.
- (2)
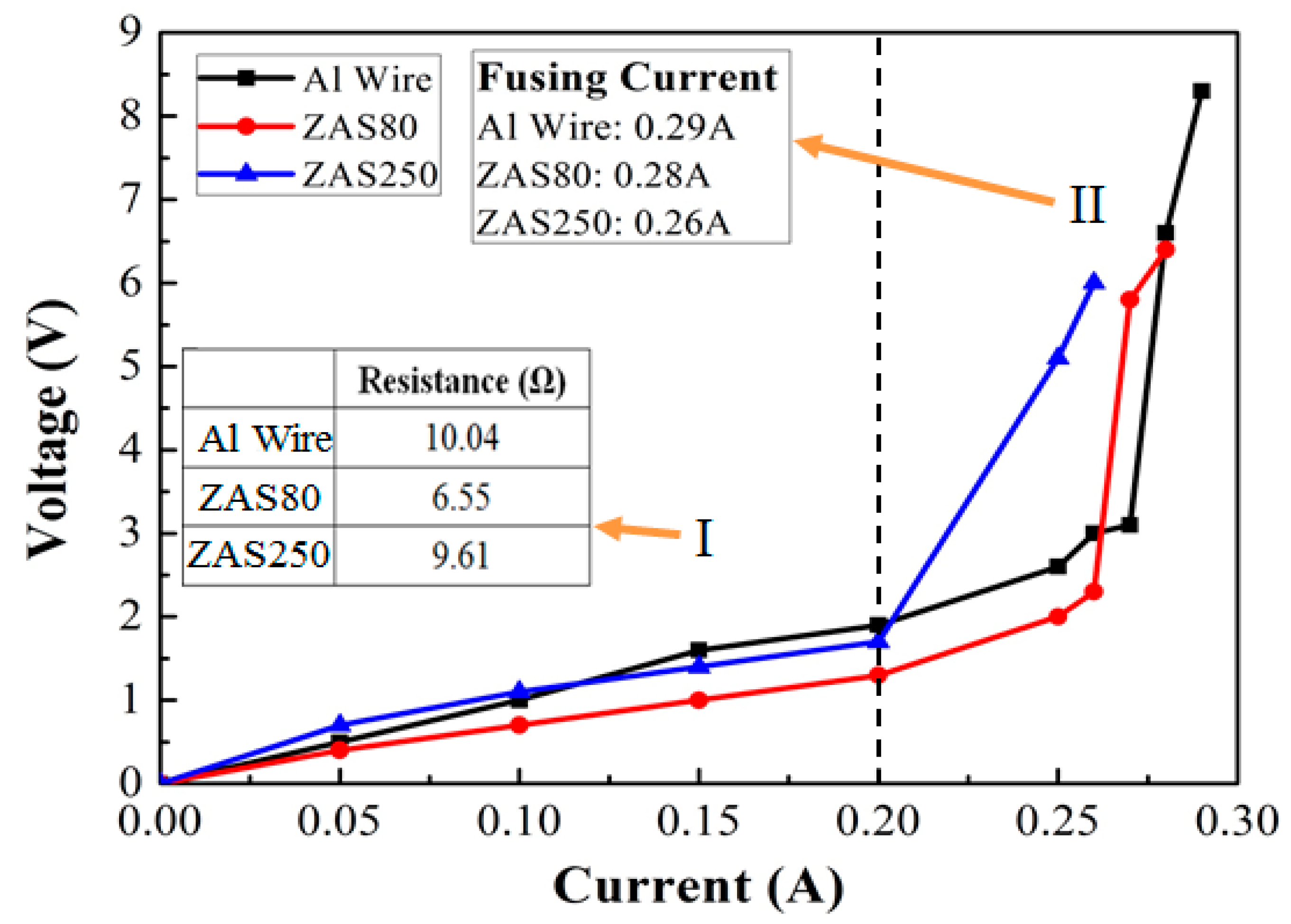
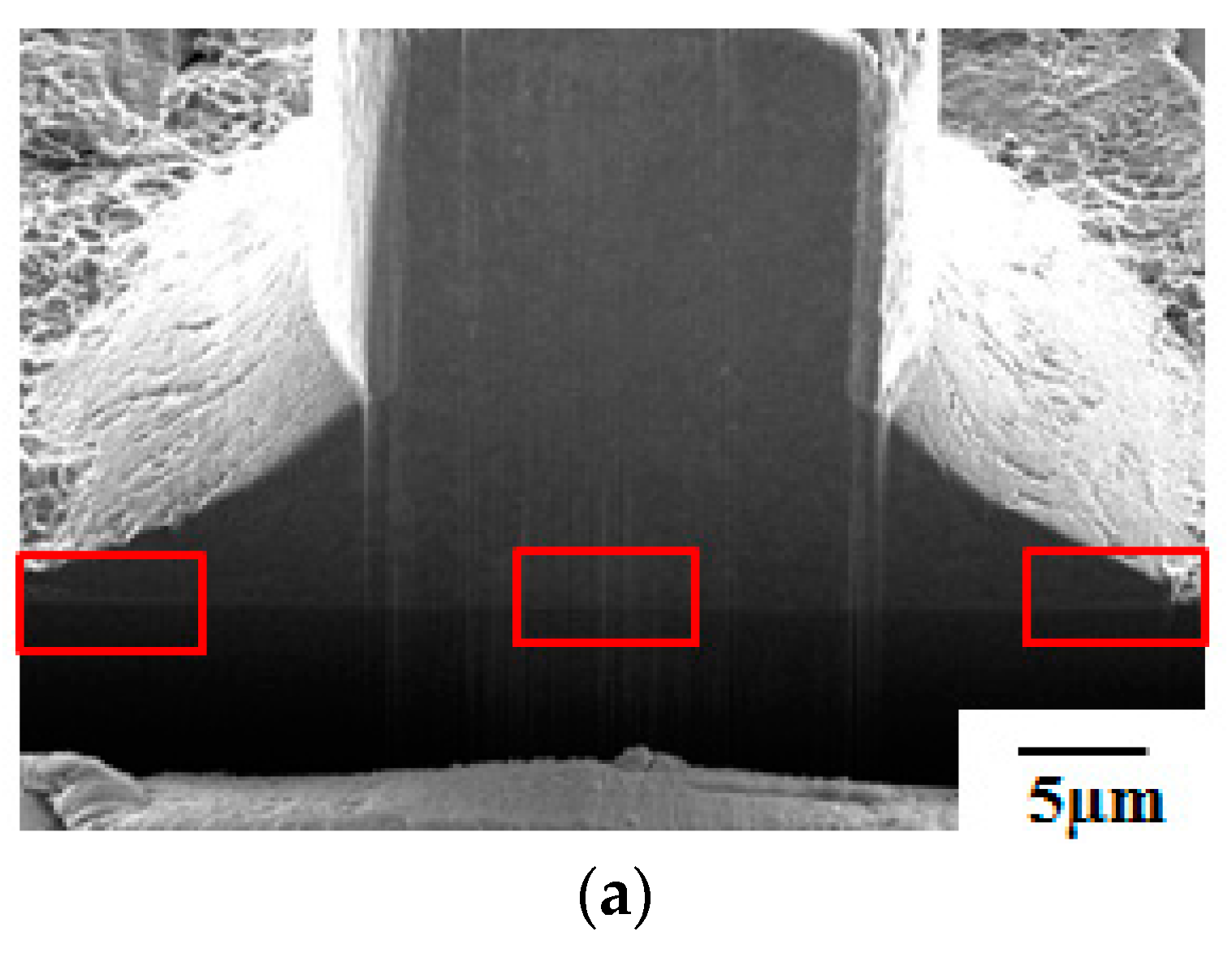
- Pores in the 80 nm ZAS wire FAB have no influence on bonding characteristics. Moreover, the low interface resistance, high fusing current, and excellent aging surface of the 80 nm ZAS wire hold potential for application in IC packaging.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Broll, M.S.; Geissler, U.; Höfer, J.; Schmitz, S.; Wittler, O.; Lang, K.D. Microstructural evolution of ultrasonic-bonded aluminum wires. Microelectron. Reliab. 2015, 55, 961–968. [Google Scholar] [CrossRef]
- Park, S.; Nagao, S.; Sugahara, T.; Suganuma, K. Mechanical stabilities of ultrasonic Al ribbon bonding on electroless nickel immersion gold finished Cu substrates. Jpn. J. Appl. Phys. 2014, 55, 069201. [Google Scholar] [CrossRef]
- So, H.; Senesky, D.G. Rapid fabrication and packaging of AlGaN/GaN high-Temperature ultraviolet photodetectors using direct wire bond in Rapid fabrication and packaging of AlGaN/GaN high-Temperature ultraviolet photodetectors using direct wire bonding. J. Phys. D Appl. Phys. 2016, 49, 285109. [Google Scholar] [CrossRef]
- Lum, I.; Marey, M.; Zhou, Y. Footprint Study of Ultrasonic Wedge-Bonding with Aluminum Wire on Copper Substrate. J. Electron. Mater. 2006, 35, 433–442. [Google Scholar] [CrossRef]
- Table of Electrical Resistivity and Conductivity. Available online: http://chemistry.about.com/od/moleculescompounds/a/Table-Of-Electrical-Resistivity-And-Conductivity.htm (accessed on 23 April 2017).
- Salleh, M.A.A.M.; McDonald, S.D.; Yasuda, H.; Sugiyama, A.; Nogita, K. Rapid Cu6Sn5 growth at liquid Sn/solid Cu interfaces. Scr. Mater. 2015, 100, 17–20. [Google Scholar] [CrossRef]
- Chen, K.J.; Hung, F.Y.; Lui, T.S.; Chen, L.H.; Qiu, D.W.; Chou, T.L. Microstructure and electrical mechanism of Sn–xAg–Cu PV-Ribbon for solar cells. Microelectron. Eng. 2014, 116, 33–39. [Google Scholar] [CrossRef]
- Alemdag, Y.; Beder, M. Microstructural, mechanical and tribological properties of Al–7Si–(0–5)Zn alloys. Mater. Des. 2014, 63, 159–167. [Google Scholar] [CrossRef]
- Gencer, Y.; EmreGulec, A. The effect of Zn on the microarc oxidation coating behavior of synthetic Al–Zn binary alloys. J. Alloys Compd. 2012, 525, 159–165. [Google Scholar] [CrossRef]
- Hsueh, H.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H.; Chen, K.J. Intermetallic phase on the interface of Ag–Au–Pd/Al Structure. Adv. Mater. Sci. Eng. 2014, 2014, 925768. [Google Scholar] [CrossRef]
- Tseng, Y.W.; Hung, F.Y.; Lui, T.S. Microstructure, tensile and electrical properties of gold-coated silver bonding wire. Microelectron. Reliab. 2015, 55, 608–612. [Google Scholar] [CrossRef]
- Tseng, Y.W.; Hung, F.Y.; Lui, T.S. Effect of annealing on the microstructure and bonding interface properties of Ag–2Pd alloy wire. Microelectron. Reliab. 2015, 55, 1256–1261. [Google Scholar] [CrossRef]
- Chen, K.J.; Hung, F.Y.; Lui, T.S.; Chang, S.J.; Hu, Z.S. The low-temperature crystallization and interface characteristics of ZnInSnO/In films using a bias-crystallization mechanism. J. Nanomater. 2012, 2012, 272387. [Google Scholar] [CrossRef]
- Hsueh, H.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. Effect of the direct current on microstructure, tensile property and bonding strength of pure silver wires. Microelectron. Reliab. 2013, 51, 1159–1163. [Google Scholar] [CrossRef]
- Hsueh, H.W.; Hung, F.Y.; Lui, T.S. A Study on electromigration inducing intergranular fracture of fine silver alloy wires. Appl. Phys. Lett. 2017, 110. [Google Scholar] [CrossRef]
- Tseng, Y.W.; Hung, F.Y.; Lui, T.S. Thermoelectric mechanism and interface characteristics of cyanide-free nano gold-coated silver wire. J. Electron. Mater. (JEM) 2016, 45, 624–630. [Google Scholar]
- Olivia, W.S.; Liang, X.; George, P.; Orkid, C.; BiolInorg, J. Structures and Free Energy Landscapes of Aqueous Zinc(II)-Bound Amyloid-β(1-40) and Zinc(II)-Bound Amyloid-β(1-42) With Dynamics. Chemistry 2012, 17, 927–938. [Google Scholar]
- Lumley, R.N.; Schaffer, G.B. Anomalous pore morphologies in liquid-phase-sintered Al–Zn alloys. Metall. Mater. Trans. 1999, 30, 1682–1685. [Google Scholar] [CrossRef]
- Hung, F.Y.; Wang, Y.T.; Chen, L.H.; Lui, T.S. Recrystallization Effect and Electric Flame-Off Characteristic of Thin Copper Wire. Mater. Trans. 2006, 47, 1776–1781. [Google Scholar] [CrossRef]
- Huang, I.T.; Hung, F.Y.; Lui, T.S.; Chen, L.H.; Hsueh, H.W. A Study on the Tensile Fracture Mechanism of 15 μm Copper Wire after EFO Process. Microelectron. Reliab. 2011, 51, 25–29. [Google Scholar] [CrossRef]
- Zhang, K.S.; Bai, J.B.; Francžois, D. Ductile fracture of materials with high void volumefraction. Int. J. Solids Struct. 1999, 36, 3407–3425. [Google Scholar] [CrossRef]
- Hung, F.Y.; Chen, L.H.; Lui, T.S. A study on the particle erosion of upper bainitic ADI. Wear 2002, 252, 985–991. [Google Scholar] [CrossRef]



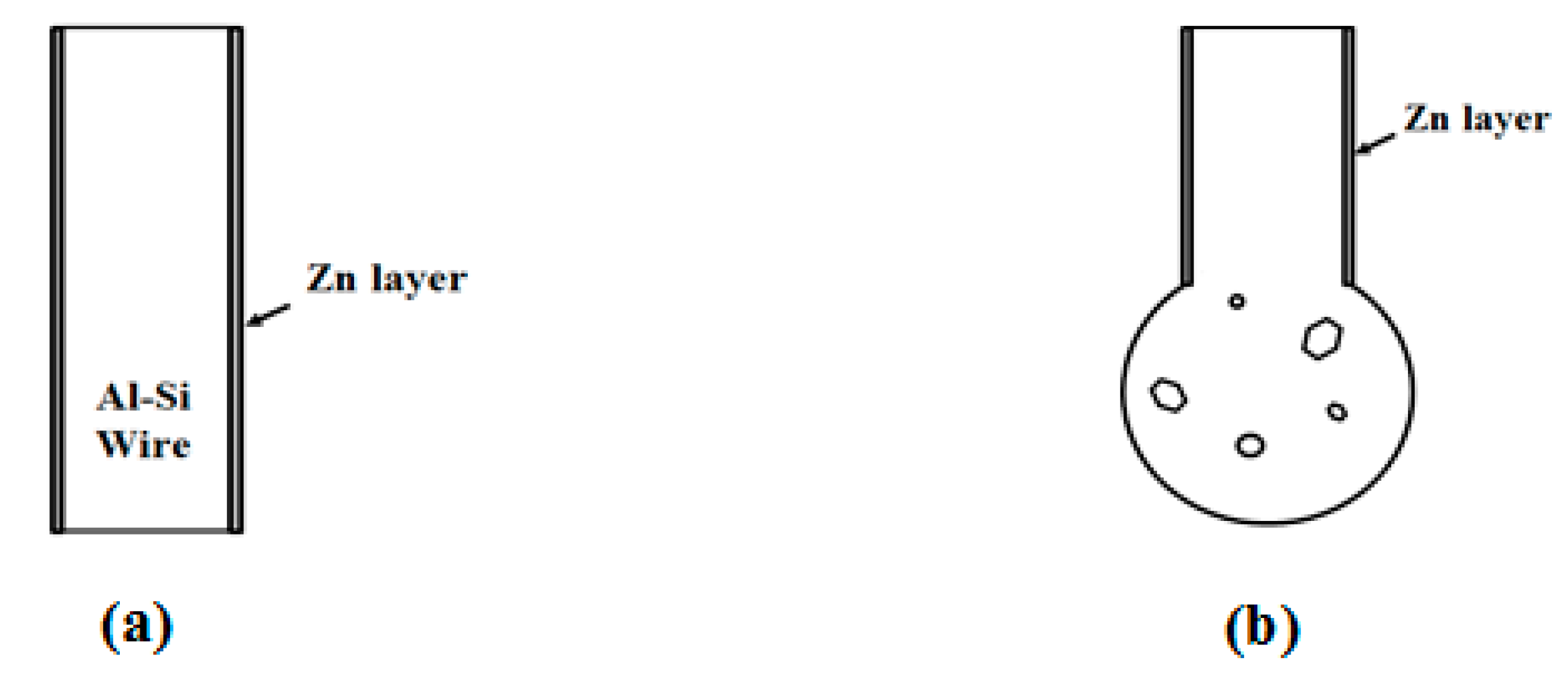
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, F.-Y.; Lui, T.-S.; Chu, K.-M.; Tseng, Y.-W. Aluminium Wires Have the Free Air Balls (FABs): Electronic Flame-Off, Fracture Strength, Electrical Properties, and Bonding Characteristics of Nano Zn Film Al–Si Bonding Wires. Metals 2017, 7, 152. https://doi.org/10.3390/met7050152
Hung F-Y, Lui T-S, Chu K-M, Tseng Y-W. Aluminium Wires Have the Free Air Balls (FABs): Electronic Flame-Off, Fracture Strength, Electrical Properties, and Bonding Characteristics of Nano Zn Film Al–Si Bonding Wires. Metals. 2017; 7(5):152. https://doi.org/10.3390/met7050152
Chicago/Turabian StyleHung, Fei-Yi, Truan-Sheng Lui, Kuan-Ming Chu, and Yi-Wei Tseng. 2017. "Aluminium Wires Have the Free Air Balls (FABs): Electronic Flame-Off, Fracture Strength, Electrical Properties, and Bonding Characteristics of Nano Zn Film Al–Si Bonding Wires" Metals 7, no. 5: 152. https://doi.org/10.3390/met7050152





