Characteristics of Cold and Hot Pressed Iron Aluminum Powder Metallurgical Alloys
Abstract
:1. Introduction
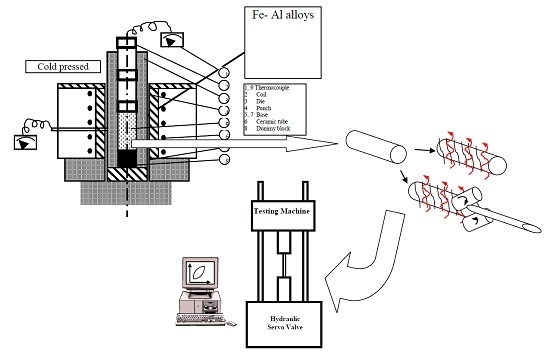
2. Experimental Procedures
3. Results and Discussion
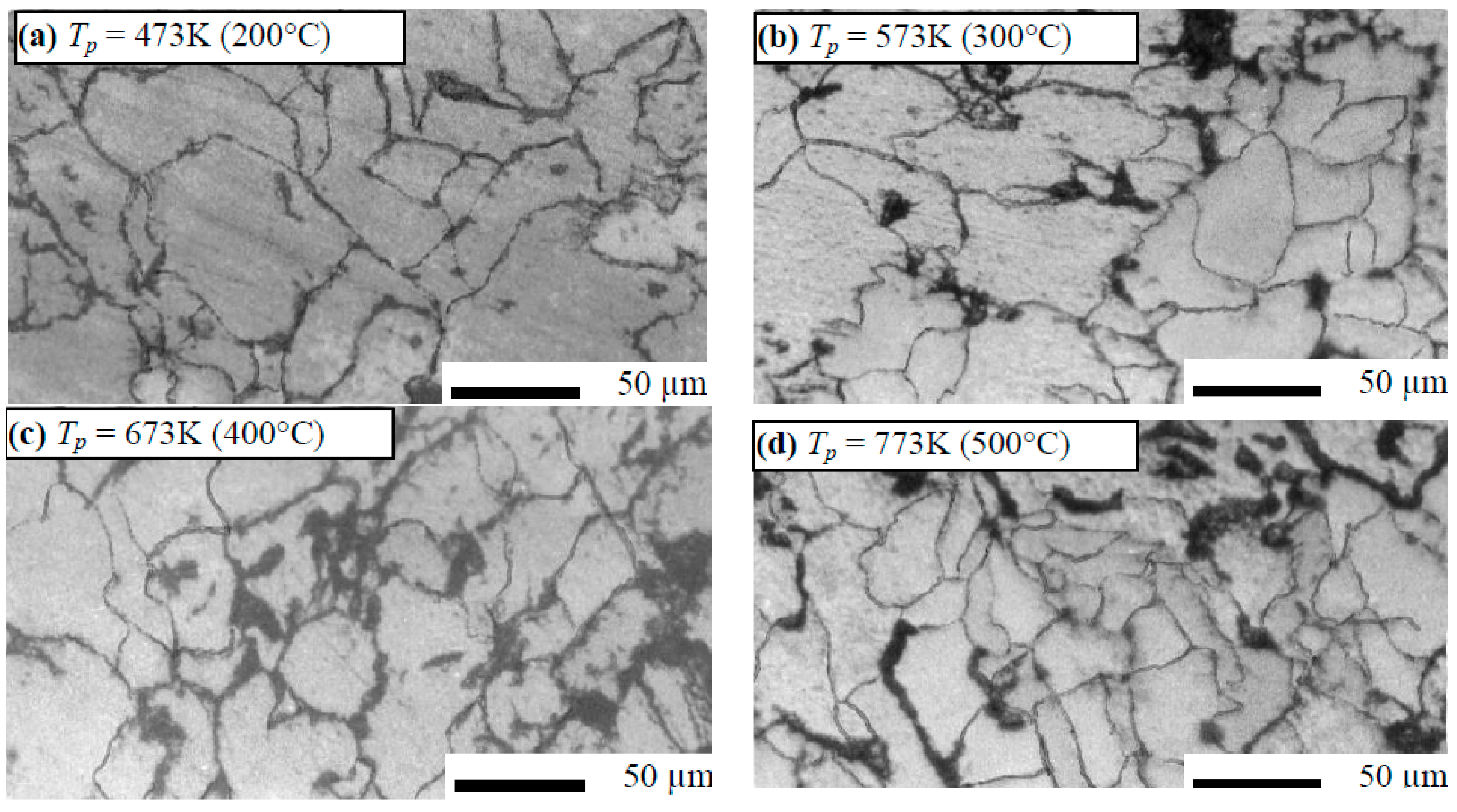
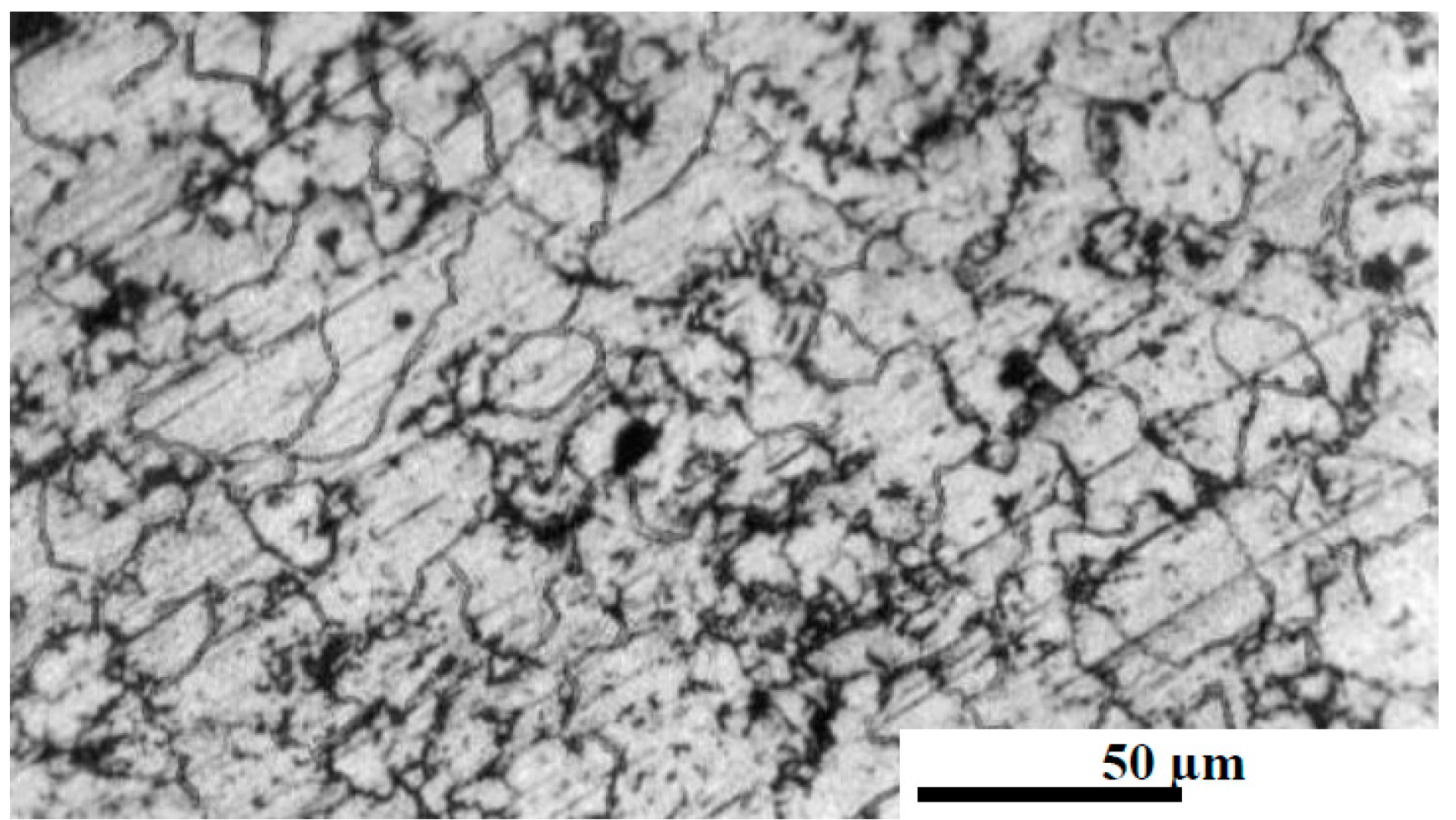
3.1. Microstrautral Optical Investigations
3.1.1. Cold Pressed
3.1.2. Hot Pressed
3.2. Hardness and Radial Crushing Strength
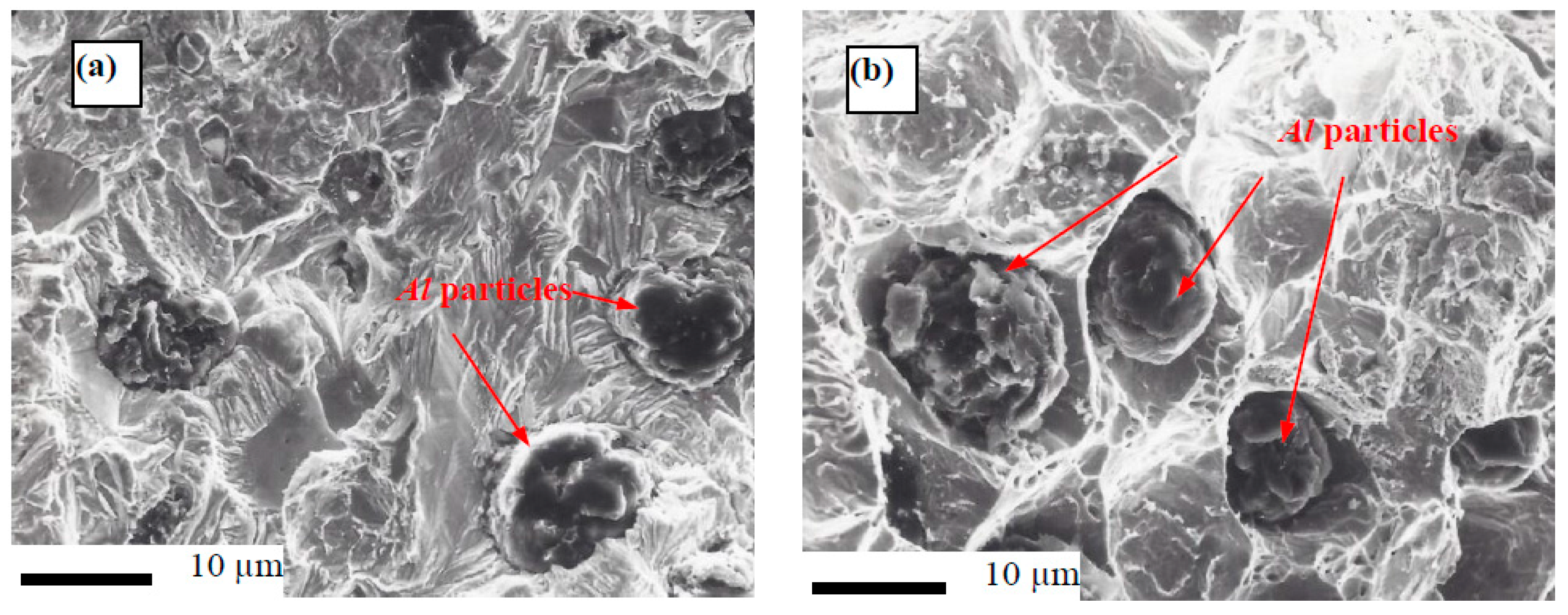
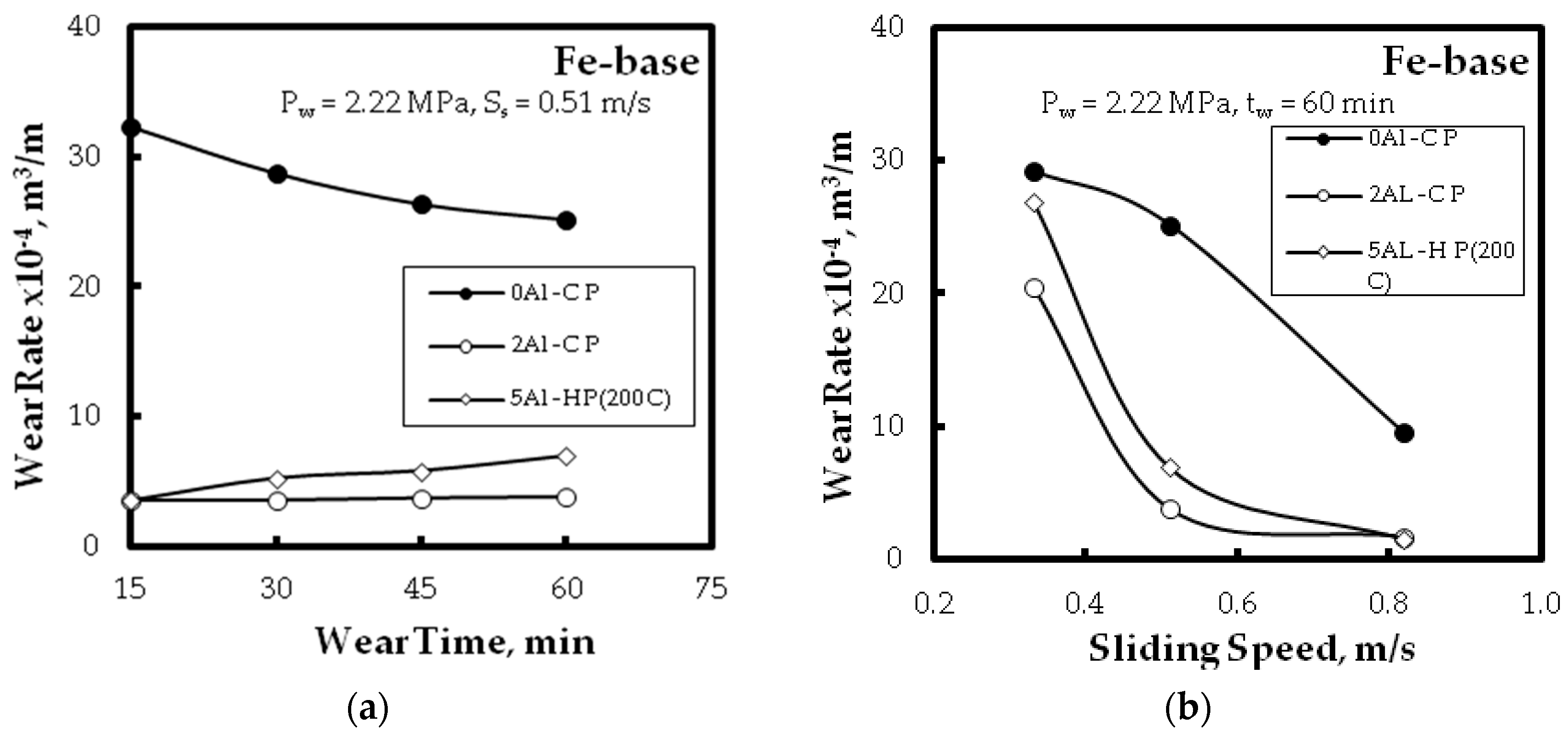
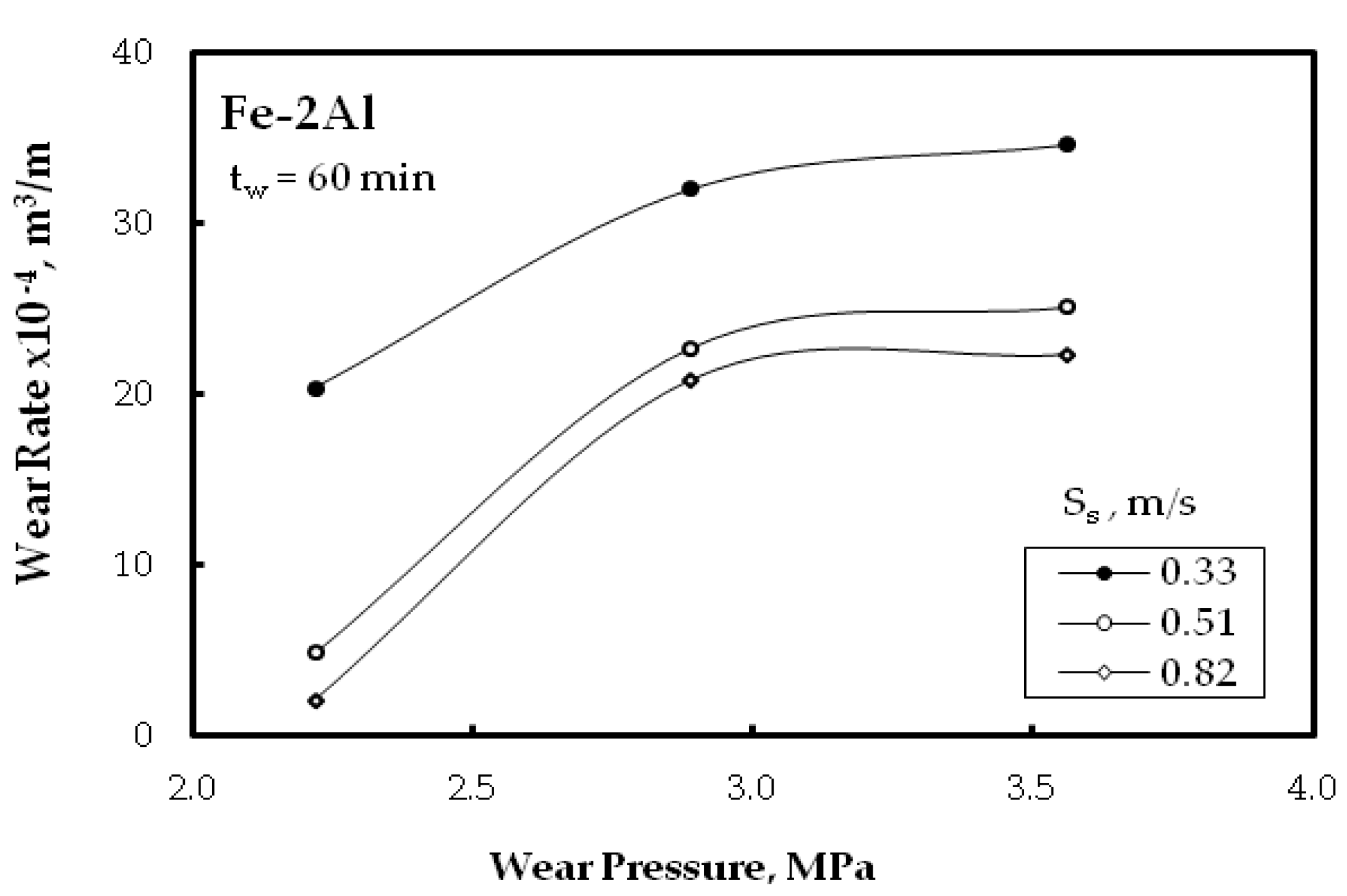
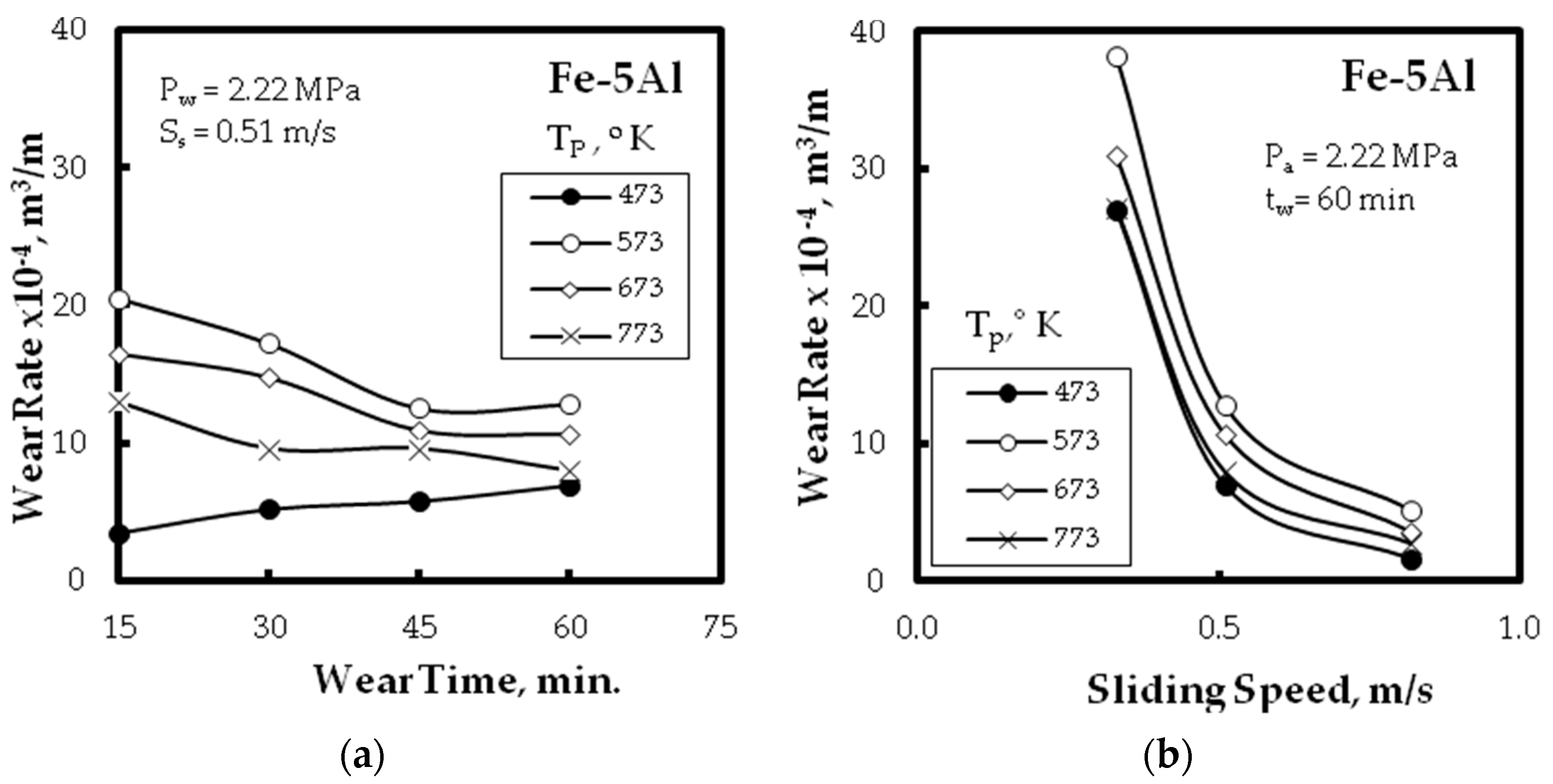
3.3. Wear Resitance
4. Conclusions
- Under laboratory conditions and without the use of lubricant or binder, it was possible to produce Fe-Al with Al additives ranging within 1, 2, 3, 5 and 10 in wt. % alloys, using two powder metallurgy techniques, namely, cold and hot pressed, having comparable theoretical density and properties of the solid metals. Uniform distribution of dispersed phase inside the alloy structure was obtained.
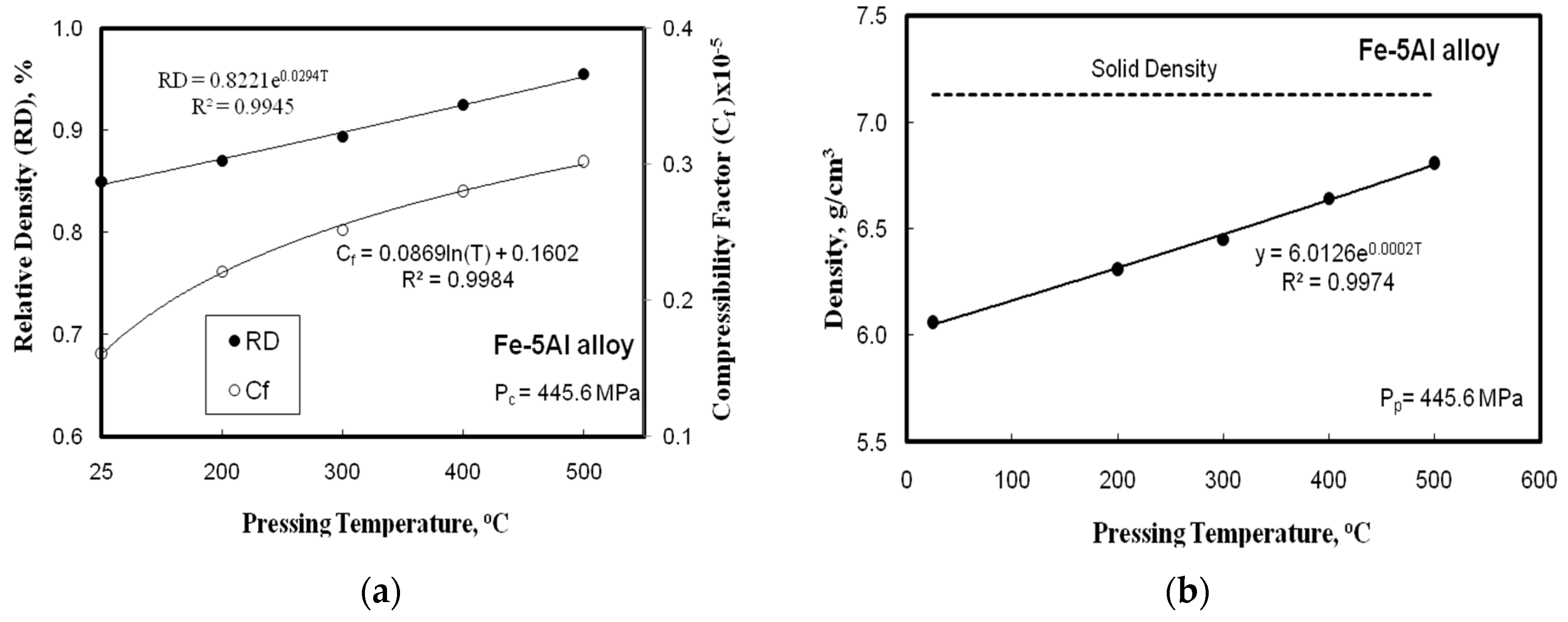
- By augmenting the Al content to 10 wt. % in the base Fe-based matrix, the Brinell hardness number was reduced from 780 to 690 MPa and the radial strength from 380 to 228 MPa, with a reduction of 11.5%, and 40%, respectively. The reduction of the compressibility factor of hot pressed powder alloys was detected with increasing the hot pressing temperature up to 500 °C.
- Improvement of the wear resistance was observed, with augmenting the Al powder to the Fe matrix up to five times compared with the alloy without Al additions for different wear parameters: wear time, and sliding speed. This was also detected for the different PM technique.
- The hot compact of Fe-Al alloys of about 95% theoretical density can be obtained from the metal powders, by employing a pressure of about 445.6 MPa and temperature of 500 °C. These alloys had higher density, better wear resistance, and homogenous structure than the parts produced by separate compaction and sintering obtained after elemental powders were added.
Author Contributions
Conflicts of Interest
References
- Caballero, E.S.; Cintas, J.; Cuevas, F.G.; Montes, J.M.; Ternero, F. Influence of Milling Atmosphere on the Controlled Formation of Ultrafine Dispersoids in Al-Based MMCs. Metals 2016, 6, 224. [Google Scholar] [CrossRef]
- Luka, F.; Vilemova, M.; Nevrla, B.; Klecka, J.; Chraska, T. Properties of Mechanically Alloyed W-Ti Materials with Dual Phase Particle Dispersion. Metals 2017, 7, 3. [Google Scholar] [CrossRef]
- Chen, C.L.; Lin, C.H. A Study on the Aging Behavior of Al6061 Composites Reinforced with Y2O3 and TiC. Metals 2017, 7, 11. [Google Scholar] [CrossRef]
- El-Hadek, M.A. Numerical simulation of the inertia friction welding process of dissimilar materials. Metall. Trans. B 2014, 45, 2346–2356. [Google Scholar] [CrossRef]
- Nassef, A.; El-Hadek, M. Mechanics of hot pressed aluminum composites. Int. J. Adv. Manuf. Technol. 2015, 76, 1905–1912. [Google Scholar] [CrossRef]
- Nassef, A.; El-Hadek, M. Microstructure and Mechanical Behavior of Hot Pressed Cu-Sn Powder Alloys. Adv. Mater. Sci. Eng. 2016, 2016, 9796169. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Köster, W.; Tonn, W. The Iron Corner of the Iron-Manganese-Aluminium System. Arch. Eisenhuettenwes 1933, 7, 365–366. [Google Scholar]
- James, P.J. Precipitation of the Carbide-FEMN-3 ALC IN AN Iron-Aluminium Alloy. J. Iron Steel Inst. 1969, 207, 54–57. [Google Scholar]
- Furushima, R.; Katou, K.; Shimojima, K.; Hosokawa, H.; Mikami, M.; Matsumoto, A. Effect of η-phase and FeAl composition on the mechanical properties of WC–FeAl composites. Intermetallics 2015, 66, 120–126. [Google Scholar] [CrossRef]
- Amaya, M.; Romero, J.M.; Martinez, L.; Pérez, R. Mechanical Properties of Spray-Atomized FeAl40 at. % Al Alloys. In Materials Characterization; Springer International Publishing: Cham, Switzerland, 2015; Volume 5, pp. 199–207. [Google Scholar]
- Trotter, G.; Baker, I. The effect of aging on the microstructure and mechanical behavior of the alumina-forming austenitic stainless steel Fe–20Cr–30Ni–2Nb–5Al. Mater. Sci. Eng. A 2015, 627, 270–276. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A.; Motz, C. A Review on the Properties of Iron Aluminide Intermetallics. Crystals 2016, 6, 10. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kainuma, R.; Omori, T.; Ishida, K. Alloy Design for Fe-Ni-Co-Al-based Superelastic Alloys. Mater. Today Proc. 2015, 2, S485–S492. [Google Scholar] [CrossRef]
- Ikeda, O.; Ohnuma, I.; Kainuma, R.; Ishida, K. Phase equilibria and stability of ordered BCC phases in the Fe-rich portion of the Fe–Al system. Intermetallics 2001, 9, 755–761. [Google Scholar] [CrossRef]
- Kim, H.; Suh, D.W.; Kim, N.J. Fe–Al–Mn–C lightweight structural alloys: A review on the microstructures and mechanical properties. Sci. Technol. Adv. Mater. 2013, 14, 014205. [Google Scholar] [CrossRef] [PubMed]
- Frommeyer, G.; Bruex, U. Microstructures and mechanical properties of high-strength Fe-Mn-Al-C light-weight TRIPLEX steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Sutou, Y.; Kamiya, N.; Umino, R.; Ohnuma, I.; Ishida, K. High-strength Fe-20Mn-Al-C-based alloys with low density. ISIJ Int. 2010, 50, 893–899. [Google Scholar] [CrossRef]
- Greer, A.L.; Rutherford, K.L.; Hutchings, I.M. Wear resistance of amorphous alloys and related materials. Int. Mater. Rev. 2002, 47, 87–112. [Google Scholar] [CrossRef]
- Maupin, H.E.; Wilson, R.D.; Hawk, J.A. Wear deformation of ordered Fe-Al intermetallic alloys. Wear 1993, 162, 432–440. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, Z.; Ma, S.; Zhang, W.; Liu, W. Sliding wear behavior of Fe–Al and Fe–Al/WC coatings prepared by high velocity arc spraying. Wear 2004, 257, 1089–1095. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.H. Sliding wear behavior of Fe 3 Al-based alloys. Mater. Sci. Eng. A 1998, 258, 319–324. [Google Scholar] [CrossRef]
- Chonglin, W. Discussion on radial crushing strength testing. Powder Metall. Technol. 1996, 8, 206–211. [Google Scholar]
- Candela, N.; Plaza, R.; Rosso, M.; Velasco, F.; Torralba, J.M. Radial crushing strength and microstructure of molybdenum alloyed sintered steels. J. Mater. Process. Technol. 2001, 119, 7–13. [Google Scholar] [CrossRef]
- Larker, H.T.; Larker, R. Hot isostatic pressing. Mater. Sci. Technol. 1991. [Google Scholar] [CrossRef]
- Leuenberger, H.; Rohera, B.D. Fundamentals of powder compression. I. The compactibility and compressibility of pharmaceutical powders. Pharm. Res. 1986, 3, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hryha, E.; Dudrova, E.; Bengtsson, S. Influence of powder properties on compressibility of prealloyed atomised powders. Powder Metall. 2008, 51, 340–342. [Google Scholar] [CrossRef]
- El-Hadek, M.A.; Kaytbay, S.H. Fracture properties of SPS tungsten copper powder composites. Metall. Trans. A 2013, 44, 544–551. [Google Scholar] [CrossRef]
- Kaytbay, S.; El-Hadek, M. Wear resistance and fracture mechanics of WC–Co composites. Int. J. Mater. Res. 2014, 105, 557–565. [Google Scholar] [CrossRef]
- El-Hadek, M.A.; Kassem, M. Failure behavior of Cu–Ti–Zr-based bulk metallic glass alloys. J. Mater. Sci. 2009, 44, 1127–1136. [Google Scholar] [CrossRef]
- El-Hadek, M.; Kaytbay, S. Characterization of copper carbon composites manufactured using the electroless precipitation process. Mater. Manuf. Process. 2013, 28, 1003–1008. [Google Scholar]
- El-Katatny, S.M.; Nassef, A.E.; El-Domiaty, A.; El-Garaihy, W.H. Fundamental Analysis of Cold Die Compaction of Reinforced Aluminum Powder. Int. J. Eng. Tech. Res. 2015, 3, 180–184. [Google Scholar]
- Ahari, F. Flexible High Radial Strength Stent. U.S. Patent 6,264,685, 24 July 2001. [Google Scholar]
- Chtourou, H.; Guillot, M.; Gakwaya, A. Modeling of the metal powder compaction process using the cap model. Part I. Experimental material characterization and validation. Int. J. Solids Struct. 2002, 39, 1059–1075. [Google Scholar] [CrossRef]
- Bocchini, G.F. Warm compaction of metal powders: Why it works, why it requires a sophisticated engineering approach. Powder Metall. 2013, 42, 171–180. [Google Scholar] [CrossRef]
- Wang, J.; Xing, J.; Cao, L.; Su, W.; Gao, Y. Dry sliding wear behavior of Fe3Al alloys prepared by mechanical alloying and plasma activated sintering. Wear 2010, 268, 473–480. [Google Scholar] [CrossRef]
- Sharma, G.; Limaye, P.K.; Ramanujan, R.V.; Sundararaman, M.; Prabhu, N. Dry-sliding wear studies of Fe3Al-ordered intermetallic alloy. Mater. Sci. Eng. A 2004, 386, 408–414. [Google Scholar] [CrossRef]
- Dhokey, N.B.; Rane, K.K. Wear behavior and its correlation with mechanical properties of TiB2 reinforced aluminium-based composites. Adv. Tribol. 2011, 2011, 837469. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical performance of the Al x CoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0. 5Fe alloy with boron addition. Metall. Trans. A 2004, 35, 1465–1469. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chuang, M.H.; Chen, S.K.; Huang, Y.S. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall. Trans. A 2006, 37, 1363–1369. [Google Scholar] [CrossRef]


| Radial Strength MPa | Brinell Hardness Number | Al Content in Fe-Based Alloys wt. % |
|---|---|---|
| 380.44 | 780 | 0 |
| 369.78 | 751 | 1 |
| 344.67 | 735 | 1.5 |
| 310.11 | 722 | 2 |
| 270.23 | 714 | 2.5 |
| 245.11 | 705 | 3 |
| 235.14 | 698 | 5 |
| 230.21 | 691 | 7.5 |
| 228.43 | 690 | 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassef, A.; El-Garaihy, W.H.; El-Hadek, M. Characteristics of Cold and Hot Pressed Iron Aluminum Powder Metallurgical Alloys. Metals 2017, 7, 170. https://doi.org/10.3390/met7050170
Nassef A, El-Garaihy WH, El-Hadek M. Characteristics of Cold and Hot Pressed Iron Aluminum Powder Metallurgical Alloys. Metals. 2017; 7(5):170. https://doi.org/10.3390/met7050170
Chicago/Turabian StyleNassef, Ahmed, Waleed H. El-Garaihy, and Medhat El-Hadek. 2017. "Characteristics of Cold and Hot Pressed Iron Aluminum Powder Metallurgical Alloys" Metals 7, no. 5: 170. https://doi.org/10.3390/met7050170