Precipitation Behavior of ωo Phase in Ti-37.5Al-12.5Nb Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Single ωo Variant Nucleated at the α2 Boundary
4.2. Thin γ Plates Precipitated within the α2 Phase
5. Conclusions
- Only one ωo variant preferentially nucleates at the α2 boundaries. This is because the minimum misfit exists at the α2/ωo interface if the OR between these two phases is: <> α2//<0001> ωo; {} α2//{} ωo.
- Precipitate-free regions are observed at the α2 boundaries. EDS results indicate that the ωo precipitates are more concentrated in Nb than βo-matrix. The preferred nucleation of the ωo variant causes solute depletion surrounding the α2 plates, which inhibits the nucleation and growth of new ωo precipitates in the un-precipitated regions.
- Thin γ plates precipitate within the α2 phase. These fine γ plates can relieve the distortion caused by the mismatch at the α2/ωo interface.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Transformation Matrices B from βo to ωo
| Variants | Orientation Relationship | Orientation Matrix A of the βo Phase |
|---|---|---|
| A1 | (111)βo//(0001)ωo; [] βo//[] ωo | |
| A2 | ()βo//(0001)ωo; [] βo//[] ωo | |
| A3 | ()βo//(0001)ωo; [] βo//[] ωo | |
| A4 | ()βo//(0001)ωo; [] βo//[] ωo |
Appendix A.2. Transformation Matrices C from α2 to βo
| B1 | B2 | B3 | B4 | |
|---|---|---|---|---|
| [uvtw] | [uvtw] | [uvtw] | [uvtw] | |
| C1 | 0.54 | 0 | 0 | 0.54 |
| −0.54 | −0.54 | 0 | −0.54 | |
| 0 | 0.54 | 0 | 0 | |
| 0.277 | −0.28 | 0.83 | −0.28 | |
| C2 | 0.54 | 0 | 0 | 0.54 |
| 0 | 0 | −0.54 | 0 | |
| −0.54 | 0 | 0.54 | −0.54 | |
| −0.28 | −0.83 | 0.28 | 0.28 | |
| C3 | −0.61 | 0 | 0 | −0.61 |
| 0.11 | 0.11 | 0.50 | 0.11 | |
| 0.50 | −0.11 | −0.50 | 0.50 | |
| 0.20 | 0.82 | −0.42 | −0.20 | |
| C4 | 0.06 | 0 | 0 | 0.06 |
| −0.50 | −0.50 | 0.44 | −0.50 | |
| 0.44 | 0.50 | −0.44 | 0.44 | |
| 0.48 | 0.42 | 0.54 | −0.48 | |
| C5 | 0.06 | 0 | 0 | 0.06 |
| 0.44 | 0.44 | −0.50 | 0.44 | |
| −0.50 | −0.44 | 0.50 | −0.50 | |
| −0.48 | −0.54 | −0.42 | 0.48 | |
| C6 | −0.61 | 0 | 0 | −0.61 |
| 0.50 | 0.50 | 0.11 | 0.50 | |
| 0.11 | −0.50 | −0.11 | 0.11 | |
| −0.20 | 0.42 | −0.82 | 0.20 |
| B1 | B2 | B3 | B4 | |
|---|---|---|---|---|
| [uvtw] | [uvtw] | [uvtw] | [uvtw] | |
| C1 | 0 | 0.67 | 0.67 | 0 |
| 0.33 | −0.33 | −0.33 | −0.33 | |
| −0.33 | −0.33 | −0.33 | 0.33 | |
| 0.68 | 0 | 0 | 0.68 | |
| C2 | 0 | 0.67 | 0.67 | 0 |
| 0.33 | −0.33 | −0.33 | −0.33 | |
| −0.33 | −0.33 | −0.33 | 0.33 | |
| 0.68 | 0 | 0 | 0.68 | |
| C3 | 0 | 0.67 | 0.67 | 0 |
| 0.33 | −0.33 | −0.33 | −0.33 | |
| −0.33 | −0.33 | −0.33 | 0.33 | |
| 0.68 | 0 | 0 | 0.68 | |
| C4 | 0 | 0.67 | 0.67 | 0 |
| 0.33 | −0.33 | −0.33 | −0.33 | |
| −0.33 | −0.33 | −0.33 | 0.33 | |
| 0.68 | 0 | 0 | 0.68 | |
| C5 | 0 | 0.67 | 0.67 | 0 |
| 0.33 | −0.33 | −0.33 | −0.33 | |
| −0.33 | −0.33 | −0.33 | 0.33 | |
| 0.68 | 0 | 0 | 0.68 | |
| C6 | 0 | 0.67 | 0.67 | 0 |
| 0.33 | −0.33 | −0.33 | −0.33 | |
| −0.33 | −0.33 | −0.33 | 0.33 | |
| 0.68 | 0 | 0 | 0.68 |
References
- Kim, Y.W. Gamma titanium aluminides: Their status and future. JOM 1995, 47, 39–42. [Google Scholar] [CrossRef]
- Paul, J.D.H.; Appel, F.; Wagner, R. The compression behaviour of niobium alloyed γ-titanium alumindies. Acta Mater. 1998, 46, 1075–1085. [Google Scholar] [CrossRef]
- Liu, Z.C.; Lin, J.P.; Li, S.J.; Chen, G.L. Effect of Nb and Al on the microstructures and mechanical properties of high Nb containing TiAl base alloys. Intermetallics 2002, 10, 653–659. [Google Scholar] [CrossRef]
- Stark, A.; Bartels, A.; Clemens, H.; Schimansky, F.P. On the Formation of Ordered ω-phase in High Nb Containing γ-TiAl Based Alloys. Adv. Eng. Mater. 2008, 10, 929–934. [Google Scholar] [CrossRef]
- Stark, A.; Oehring, M.; Pyczak, F.; Schreyer, A. In situ observation of various phase transformation paths in Nb-rich TiAl alloys during quenching with different rates. Adv. Eng. Mater. 2011, 13, 700–704. [Google Scholar] [CrossRef]
- Kainuma, R.; Fujita, Y.; Mitsui, H.; Ohnuma, I.; Ishida, K. Phase equilibria among α (hcp), β (bcc) and γ (L10) phases in Ti–Al base ternary alloys. Intermetallics 2000, 8, 855–867. [Google Scholar] [CrossRef]
- Chen, G.L.; Xu, X.J.; Teng, Z.K.; Wang, Y.L.; Lin, J.P. Microsegregation in high Nb containing TiAl alloy ingots beyond laboratory scale. Intermetallics 2007, 15, 625–631. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Boettinger, W.J.; Burton, B.P.; Biancaniello, F.S.; Shoemaker, C.B. The formation of ordered ω-related phases in alloys of composition Ti4Al3Nb. Acta Metall. Mater. 1990, 38, 931–943. [Google Scholar] [CrossRef]
- Song, L.; Zhang, L.Q.; Xu, X.J.; Sun, J.; Lin, J.P. Omega phase in as-cast high-Nb-containing TiAl alloy. Scr. Mater. 2013, 68, 929–932. [Google Scholar] [CrossRef]
- Song, L.; Xu, X.J.; You, L.; Liang, Y.F.; Wang, Y.L.; Lin, J.P. Ordered α2 to ωo phase transformations in high Nb-containing TiAl alloys. Acta Mater. 2015, 91, 330–339. [Google Scholar] [CrossRef]
- Schloffer, M.; Rashkova, B.; Schöberl, T.; Schwaighofer, E.; Zhang, Z.; Clemens, H.; Mayer, S. Evolution of the ωo phase in a β-stabilized multi-phase TiAl alloy and its effect on hardness. Acta Mater. 2014, 64, 241–252. [Google Scholar] [CrossRef]
- Huang, Z.W. Ordered ω phases in a 4Zr–4Nb-containing TiAl-based alloy. Acta Mater. 2008, 56, 1689–1700. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, D.; Wu, X. Thermal stability of the omega phase in Zr-containing TiAl alloys. J. Alloys Compd. 2009, 475, 134–138. [Google Scholar] [CrossRef]
- Gil, I.; Morris, M.A.; Morris, D.G. The effect of heat treatments on the microstructural stability of the intermetallic Ti–46.5Al–2W–0.5Si. Intermetallics 2001, 9, 373–385. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Lupinc, V.; Guo, J.T. Microstructural stability of the intermetallic Ti–45Al–2W–0.5Si–0.5B in the 800–980 °C temperature range. Mater. Sci. Eng. A 2003, 354, 97–105. [Google Scholar] [CrossRef]
- Yin, W.M.; Lupinc, V.; Battezzati, L. Microstructural study of a γ-TiAl based alloy containing W and Si. Mater. Sci. Eng. A 1997, 239–240, 713–721. [Google Scholar] [CrossRef]
- Huang, Z.W. Thermal stability of Ti–44Al–4Nb–4Hf–0.2Si–1B alloy. Intermetallics 2013, 37, 11–21. [Google Scholar] [CrossRef]
- Vohra, Y.K.; Sikka, S.K.; Menon, E.S.K.; Krishnan, R. Direct evidence of intermediate state during alpha-omega transformation in Ti–V alloy. Acta Metall. 1980, 28, 683–685. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sikka, S.K.; Chidambaram, R. On orientation relations between α and ω phases in Zr by texture studies using neutron diffraction method. Scr. Metall. 1985, 19, 1167–1169. [Google Scholar] [CrossRef]
- Bystrzanowski, S.; Bartels, A.; Stark, A.; Gerling, R.; Schimansky, F.P.; Clemens, H. Evolution of microstructure and texture in Ti–46Al–9Nb sheet material during tensile flow at elevated temperatures. Intermetallics 2010, 18, 1046–1055. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, H. Martensite in a TiAl alloy quenched from beta phase field. Intermetallics 2015, 56, 87–95. [Google Scholar] [CrossRef]
- Song, L.; Xu, X.J.; You, L.; Liang, Y.F.; Lin, J.P. Phase transformation and decomposition mechanisms of the βo(ω) phase in cast high Nb containing TiAl alloy. J. Alloys Compd. 2014, 616, 483–491. [Google Scholar] [CrossRef]
- Klein, T.; Rashkova, B.; Holec, D.; Clemens, H.; Mayer, S. Silicon distribution and silicide precipitation during annealing in an advanced multi-phase γ-TiAl based alloy. Acta Mater. 2016, 110, 236–245. [Google Scholar] [CrossRef]
- Ye, T.; Song, L.; Gao, S.B.; Liang, Y.F.; Wang, Y.L.; Lin, J.P. Precipitation behavior of the ωo phase in an annealed high Nb–TiAl alloy. J. Alloys Compd. 2017, 701, 882–891. [Google Scholar] [CrossRef]
- Zhang, L.C.; Cheng, T.T.; Aindow, M. Nucleation of the lamellar decomposition in a Ti–44Al–4Nb–4Zr alloy. Acta Mater. 2004, 52, 191–197. [Google Scholar] [CrossRef]
- Denquin, A.; Naka, S. Phase transformation mechanisms involved in two-phase TiAl-based alloys—I. Lambellar structure formation. Acta Mater. 1996, 44, 343–352. [Google Scholar] [CrossRef]
- Mayer, S.; Petersmann, M.; Fischer, F.D.; Clemens, H.; Waitz, T.; Antretter, T. Experimental and theoretical evidence of displacive martensite in an intermetallic Mo-containing γ-TiAl based alloy. Acta Mater. 2016, 115, 242–249. [Google Scholar] [CrossRef]
- Wang, S.C.; Aindow, M.; Starink, M.J. Effect of self-accommodation on α/α boundary populations in pure titanium. Acta Mater. 2003, 51, 2485–2503. [Google Scholar] [CrossRef]
- Liu, H.W.; Luo, C.P.; Yuan, Y.; Liu, J.M.; Liu, Z.G. Interpretation of the morphology and crystallography of precipitate γ1-Ti4Nb3Al9 in L10-TiAl(Nb)-based intermetallics by invariant line theory. Intermetallics 2007, 15, 1497–1503. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.Y.; Cao, S.; Pan, Y.; Huang, M.D.; Hu, Q.M.; Sun, Q.Y.; Xiao, L.; Sun, J. Strong deformation anisotropies of ω-precipitates and strengthening mechanisms in Ti–10V–2Fe–3Al alloy micropillars: Precipitates shearing vs precipitates disordering. Acta Mater. 2016, 117, 68–80. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Tichelaar, F.D.; Schapink, F.W.; Xu, Q.; Chen, C.Q. An evidence of stress-induced α2→γ transformation in a γ-TiAl based alloy. Scr. Metall. 1995, 32, 981–985. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhang, L.C.; Chen, G.L.; Ye, H.Q.; Nieh, T.G. Deformation-induced γ ↔ α2 phase transformation in a hot-forged Ti–45Al–10Nb alloy. Mater. Sci. Eng. A 1997, 239–240, 287–292. [Google Scholar] [CrossRef]

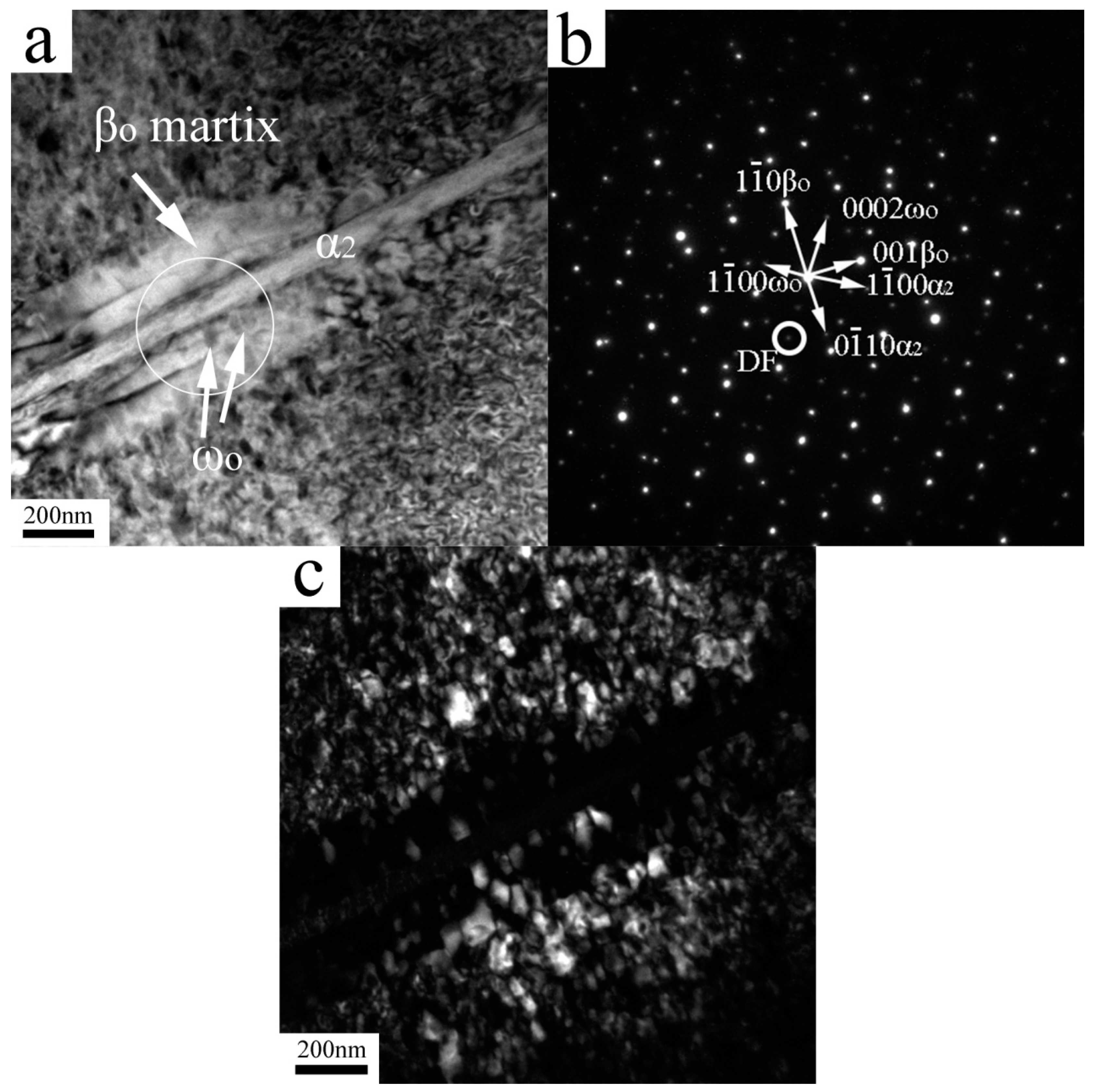
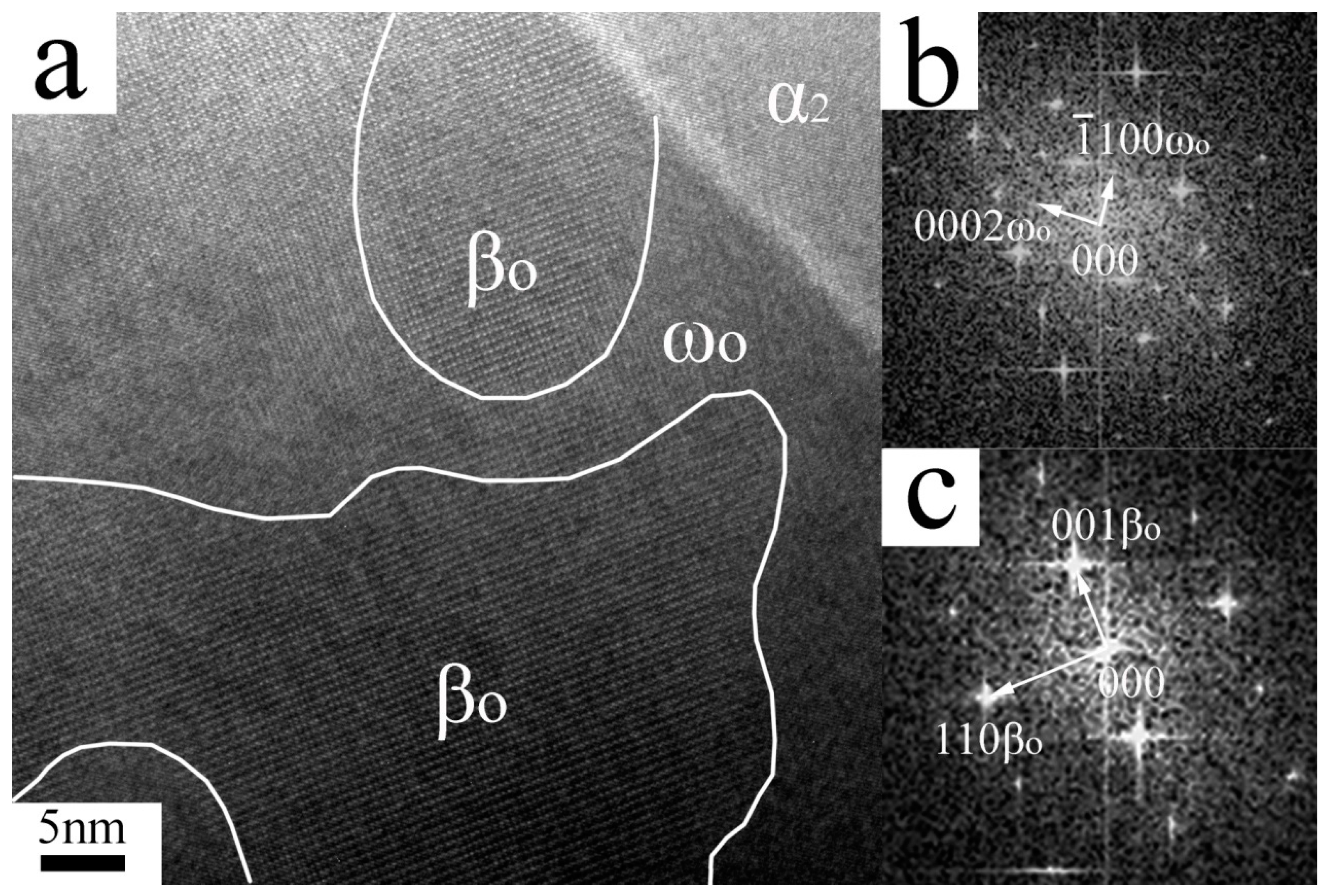
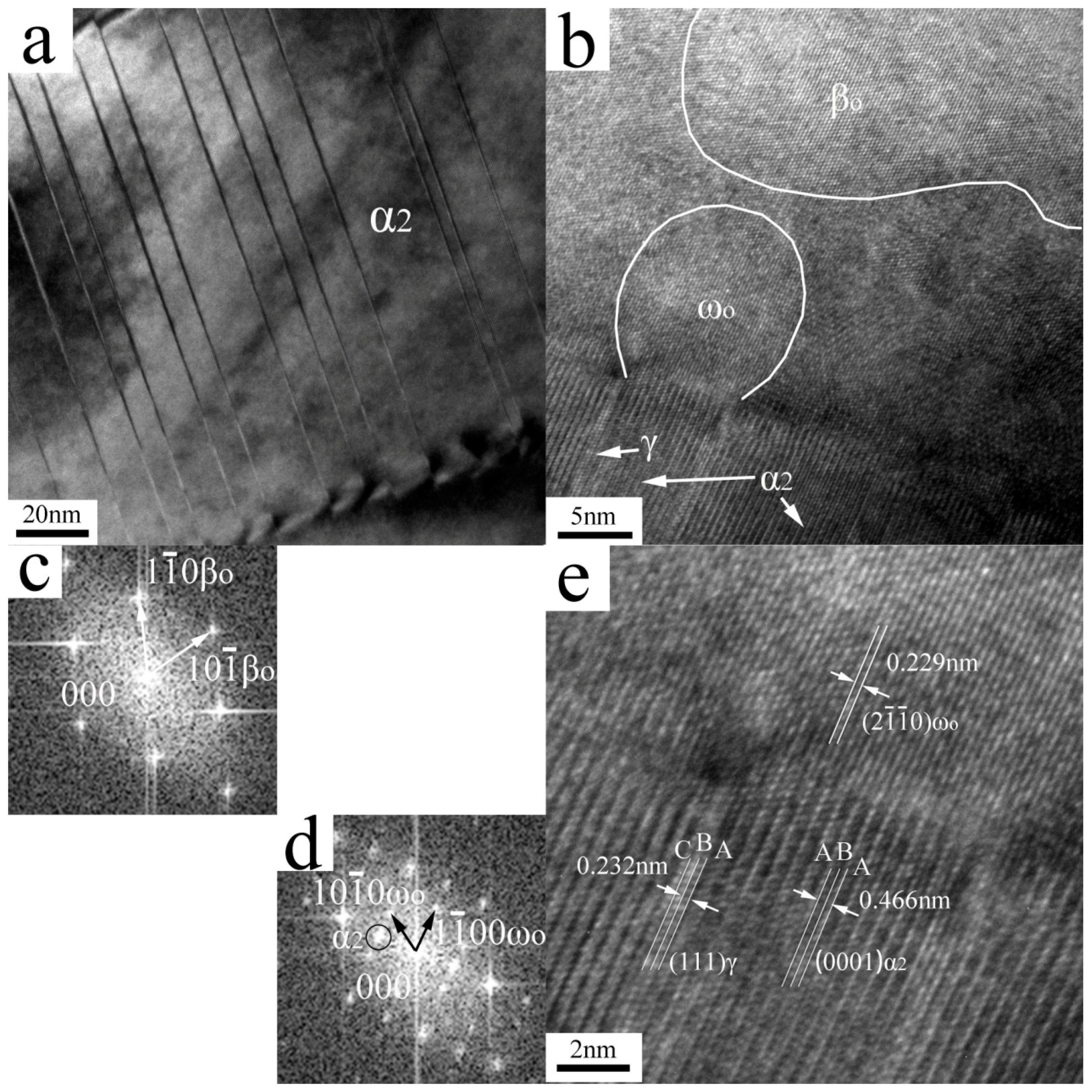
| Elements | Ti | Al (at%) | Nb (at%) | O (wt%) | N (wt%) |
|---|---|---|---|---|---|
| Composition | Bal. | 37.0 | 13.0 | 0.018 | 0.0086 |
| Regions | Composition (at %) | ||
|---|---|---|---|
| Ti | Al | Nb | |
| βo martix | 49.9 ± 0.8 | 38.5 ± 0.5 | 11.6 ± 0.8 |
| ωo | 49.5 ± 0.7 | 36.7 ± 0.5 | 13.8 ± 0.8 |
| α2 plate | 51.1 ± 0.8 | 38.1 ± 0.5 | 10.8 ± 0.8 |
| Variants | Orientation Relationship | Transformation Matrices B for βo to ωo |
|---|---|---|
| B1 | (111) βo//(0001) ωo; [] βo//[] ωo | |
| B2 | () βo//(0001) ωo; [] βo//[] ωo | |
| B3 | () βo//(0001) ωo; [] βo//[] ωo | |
| B4 | () βo//(0001) ωo; [] βo//[] ωo |
| Variants | Orientation Relationship | Transformation Matrices C for α2 to βo |
|---|---|---|
| C1 | (110) βo//(0001) α2; [] βo//[] α2 | |
| C2 | (110) βo//(0001) α2; [] βo//[] α2 | |
| C3 | (110) βo//(0001) α2; [] βo//[] α2 | |
| C4 | (110) βo//(0001) α2; [] βo//[] α2 | |
| C5 | (110) βo//(0001) α2; [] βo//[] α2 | |
| C6 | (110) βo//(0001) α2; [] βo//[] α2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Song, L.; Quan, M.; He, J.; Lin, J. Precipitation Behavior of ωo Phase in Ti-37.5Al-12.5Nb Alloy. Metals 2017, 7, 192. https://doi.org/10.3390/met7060192
Ye T, Song L, Quan M, He J, Lin J. Precipitation Behavior of ωo Phase in Ti-37.5Al-12.5Nb Alloy. Metals. 2017; 7(6):192. https://doi.org/10.3390/met7060192
Chicago/Turabian StyleYe, Teng, Lin Song, Maohua Quan, Jianping He, and Junpin Lin. 2017. "Precipitation Behavior of ωo Phase in Ti-37.5Al-12.5Nb Alloy" Metals 7, no. 6: 192. https://doi.org/10.3390/met7060192






