A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution
Abstract
:1. Introduction
2. Background Knowledge about Thiosulfate Consumption
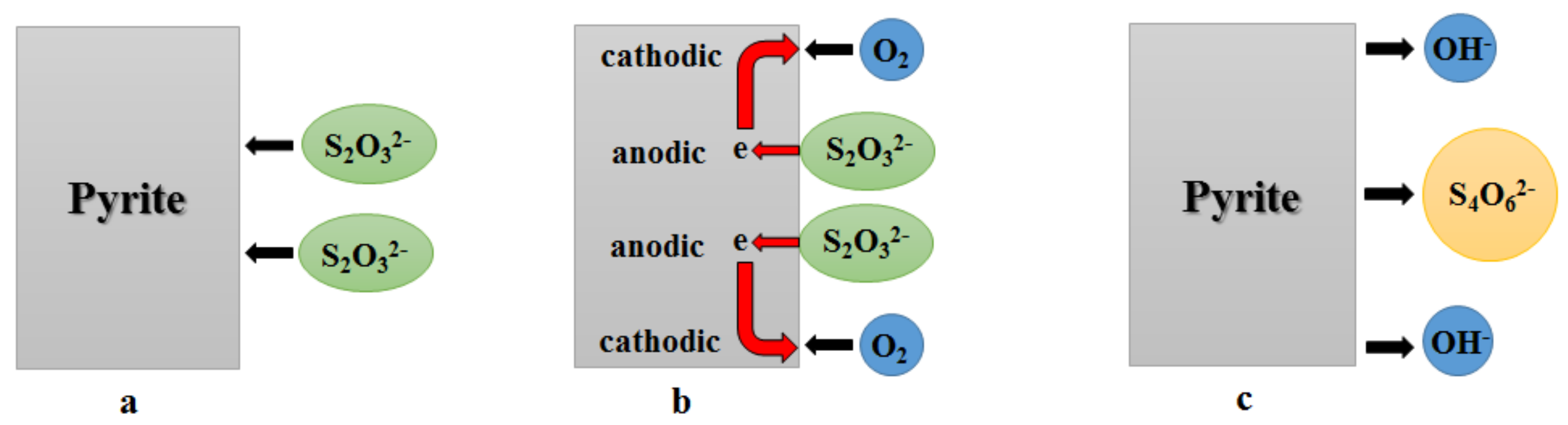
2.1. Electrochemical-Catalytic Mechanism of Thiosulfate Leaching
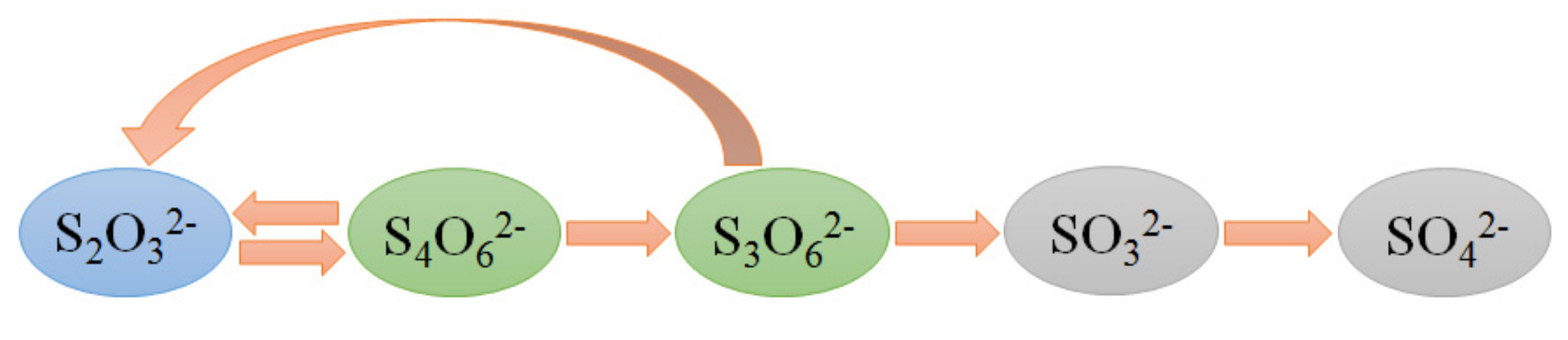
2.2. Route of Thiosulfate Decomposition
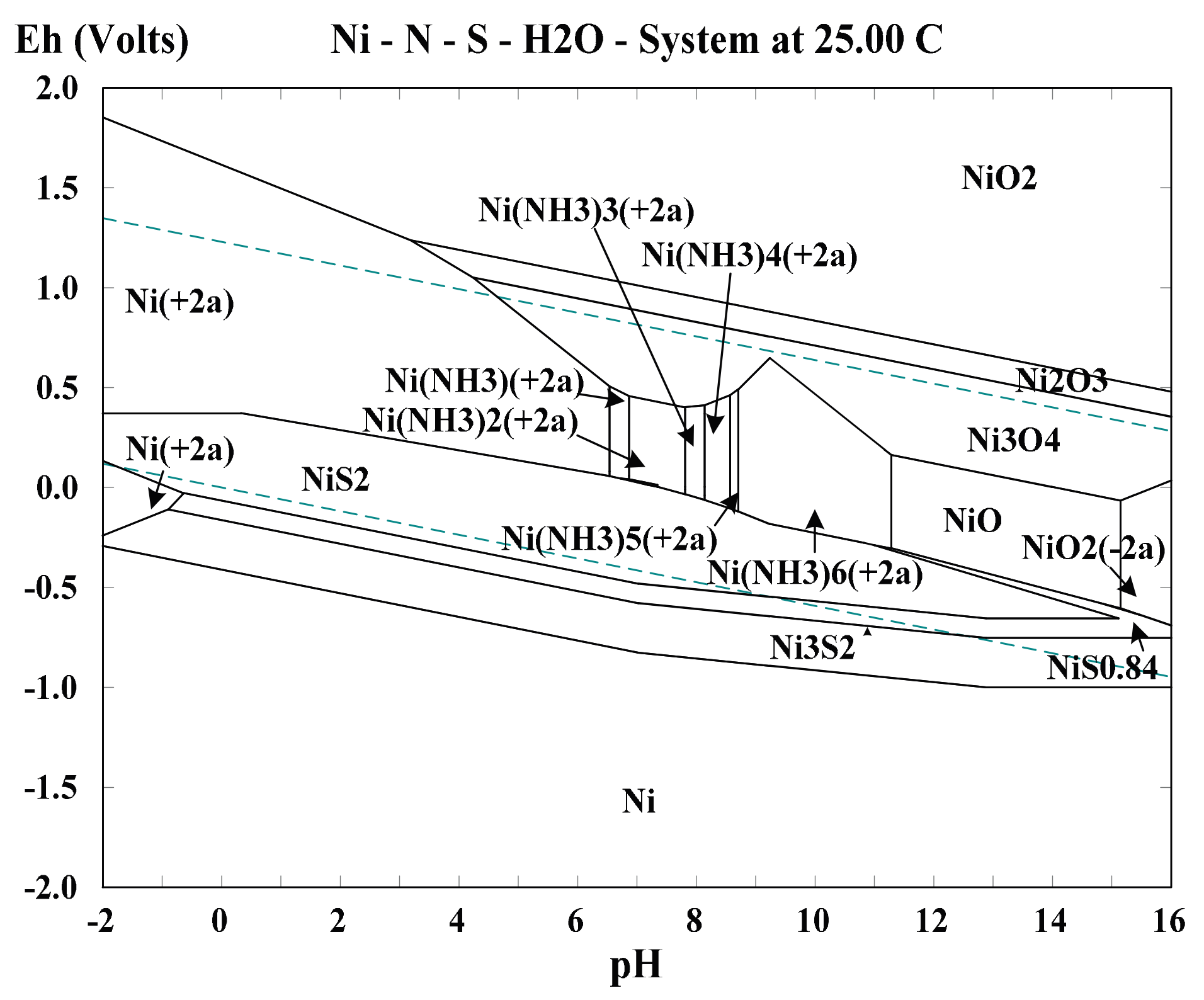
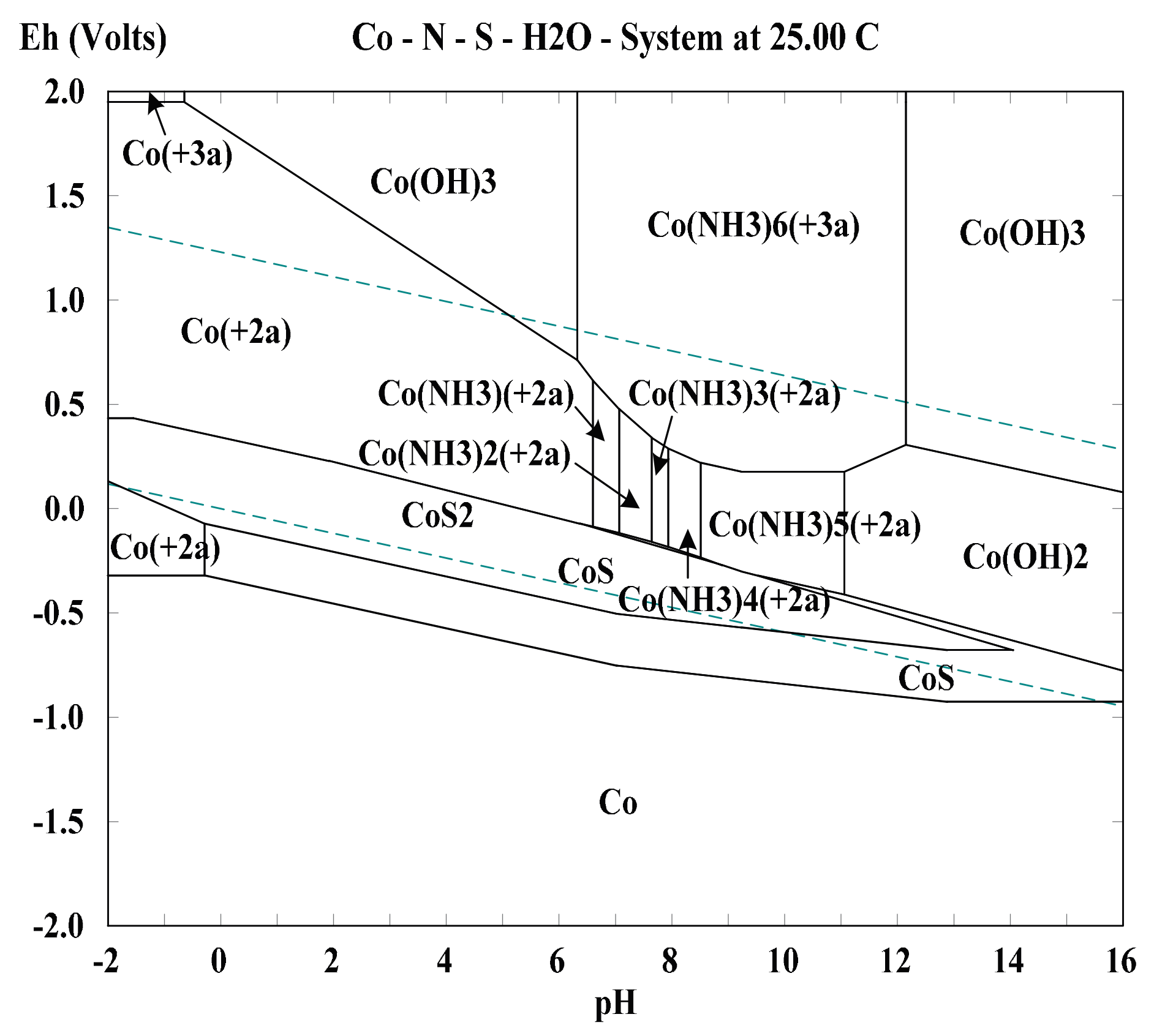
2.3. Effect of Associated Minerals
3. Measures for the Reduction of Thiosulfate Consumption
3.1. The Control of Reaction Conditions
3.2. The Use of Additives
3.2.1. Inorganic Additives
3.2.2. Organic Macromolecular Additives
3.3. The Generation of Thiosulfate In Situ
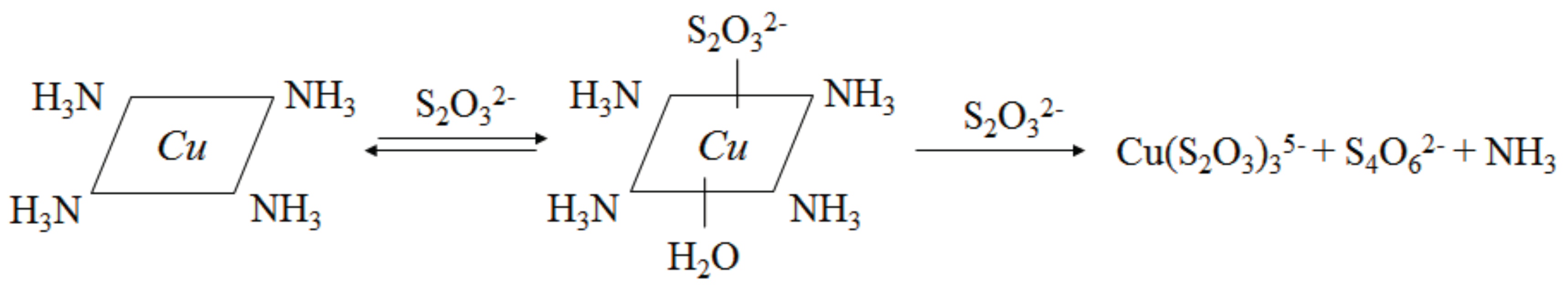
3.4. The Replacement of Traditional Cupric-Ammonia Catalysis
4. Gold Recovery from Pregnant Thiosulfate Leach Solution
4.1. Activated Carbon Adsorption, Cementation, Electrowinning, and Solvent Extraction
4.2. Resin Adsorption
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Muir, D.M. A review of the selective leaching of gold from oxidised copper-gold ores with ammonia-cyanide and new insights for plant control and operation. Miner. Eng. 2011, 24, 576–582. [Google Scholar] [CrossRef]
- Jiang, T. Chemistry of Extractive Metallurgy of Gold; Hunan Science and Technology Press: Changsha, China, 1998. [Google Scholar]
- Hasab, M.G.; Rashchi, F.; Raygan, S. Chloride-hypochlorite leaching and hydrochloric acid washing in multi-stages for extraction of gold from a refractory concentrate. Hydrometallurgy 2014, 142, 56–59. [Google Scholar] [CrossRef]
- Hasab, M.G.; Raygan, S.; Rashchi, F. Chloride-hypochlorite leaching of gold from a mechanically activated refractory sulfide concentrate. Hydrometallurgy 2013, 138, 59–64. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Li, G.; Jiang, T. Fluidized roasting-stage leaching of a silver and gold bearing polymetallic sulfide concentrate. Hydrometallurgy 2014, 147, 79–82. [Google Scholar] [CrossRef]
- Chen, X. Associated Sulfide Minerals in Thiosulfate Leaching of Gold: Problems and Solutions. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, September 2008. [Google Scholar]
- Öncel, M.S.; İnce, M.; Bayramoǧlu, M. Leaching of silver from solid waste using ultrasound assisted thiourea method. Ultrason. Sonochem. 2005, 12, 237–242. [Google Scholar] [CrossRef]
- Örgül, S.; Atalay, Ü. Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore. Hydrometallurgy 2002, 67, 71–77. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Chai, L. Research status and prospect of gold leaching in alkaline thiourea solution. Miner. Eng. 2006, 19, 1301–1306. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of carboxymethyl cellulose (CMC). Miner. Eng. 2011, 24, 115–121. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Breuer, P.L.; Chu, C.K. The importance of controlling oxygen addition during the thiosulfate leaching of gold ores. Int. J. Miner. Process. 2003, 72, 323–330. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Li, G. Stage leaching of a complex polymetallic sulfide concentrate: A focus on the extractions of Ag and Au. Hydrometallurgy 2016, 159, 87–94. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Yin, W.; Jiang, T.; Li, G. Thiosulfate leaching of Au, Ag and Pd from a high Sn, Pb and Sb bearing decopperized anode slime. Hydrometallurgy 2016, 164, 278–287. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Genik-Sas-Berezowsky, R.M.; Sefton, V.B.; Gormely, L.S. Recovery of Precious Metals from Metal Sulphides. U.S. Patent 4,070,182, 24 January 1978. [Google Scholar]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Muir, D.M.; Aylmore, M.G. Thiosulphate as an alternative to cyanide for gold processing–issues and impediments. Trans. Inst. Min. Metall. Sect. C 2004, 113, 2–12. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A review of ammoniacal thiosulfate leaching of gold: An update useful for further research in Non-cyanide gold lixiviants. Miner. Process Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Fleming, C.A.; McMullen, J.; Thomas, K.G.; Wells, J.A. Recent advances in the development of an alternative to the cyanidation process: Thiosulfate leaching and resin in pulp. Miner. Metall. Proc. 2001, 20, 1–9. [Google Scholar]
- Marchbank, A.R.; Thomas, K.G.; Dreisinger, D.; Fleming, C. Gold Recovery from Refractory Carbonaceous Ores by Pressure Oxidation and Thiosulfate Leaching. U.S. Patent 5,536,297, 28 July 1996. [Google Scholar]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Liu, S.; Li, G. The development of an environmentally friendly leaching process of a high C, As and Sb bearing sulfide gold concentrate. Miner. Eng. 2016, 89, 138–147. [Google Scholar] [CrossRef]
- Wan, R.Y.; LeVier, K.M. Solution chemistry factors for gold thiosulfate heap leaching. Int. J. Miner. Process. 2003, 72, 311–322. [Google Scholar] [CrossRef]
- Yen, W.T.; Stogran, K.; Fujita, T. Gold extraction from a copper bearing ore by thiosulphate leaching. Resour. Process. 1996, 43, 83–87. [Google Scholar] [CrossRef]
- Black, S.B. The Thermodynamic Chemistry of the Aqueous Copper-Ammonia Thiosulfate System. Ph.D. Thesis, Murdoch University, Perth, Australia, 2006. [Google Scholar]
- Jiang, T.; Chen, J.; Xu, S. Electrochemistry and mechanism of leaching gold with ammoniacal thiosulfate. In Proceedings of the XVIII International Mineral Processing Congress, Australasian Institute of Mining and Metallurgy, Sydney, Australia, 23–28 May 1993. [Google Scholar]
- Jiang, T.; Chen, J.; Xu, S. A kinetic study of gold leaching with thiosulphate. Hydrometall. Fundam. Technol. Innov. 1993, 119–126. [Google Scholar]
- Ter-Arakelyan, K. On technological expediency of sodium thiosulphate usage for gold extraction from raw material. lzv. VUZ Tsvetn. Metall. 1984, 5, 72–76. [Google Scholar]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Equation for thiosulphate yield during pyrite oxidation. Miner. Eng. 2015, 74, 105–111. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper (II) in ammoniacal thiosulphate solutions in the presence of additives. Part I: A review of the effect of hard–soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115, 1–20. [Google Scholar] [CrossRef]
- Senanayake, G. The role of ligands and oxidants in thiosulfate leaching of gold. Gold Bull. 2005, 38, 170–179. [Google Scholar] [CrossRef]
- Chu, C.K.; Breuer, P.L.; Jeffrey, M.I. The impact of thiosulfate oxidation products on the oxidation of gold in ammonia thiosulfate solutions. Miner. Eng. 2003, 16, 265–271. [Google Scholar] [CrossRef]
- Ahern, N. Thiosulfate Degradation during Gold Leaching in Ammoniacal Thiosulfate Solutions: A Focus Trithionate. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, October 2005. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. Ammoniacal thiosulphate leaching of gold in the presence of pyrite. Hydrometallurgy 2006, 82, 126–132. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Effect of hematite on thiosulphate leaching of gold. Int. J. Miner. Process. 2007, 82, 138–147. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Leaching behaviour of sulphides in ammoniacal thiosulphate systems. Hydrometallurgy 2002, 63, 189–200. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The stability of thiosulfate in the presence of pyrite in low-temperature aqueous solutions. Geochim. Cosmochim. Acta 1995, 59, 4605–4622. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Li, G. Effect of common associated sulfide minerals on thiosulfate leaching of gold and the role of humic acid additive. Hydrometallurgy 2017, 171, 44–52. [Google Scholar] [CrossRef]
- Yen, W.T.; Aghamirian, M.; Deschenes, G.; Theben, S. Gold extraction from mild refractory ore using ammonium thiosulfate. In Proceedings of the International Symposium on Gold Recovery, Montreal, QC, Canada, 3–6 May 1998. [Google Scholar]
- Chen, X. Thiosulfate Stability in Gold Leaching Process. Master’s Thesis, Queen’s University, Kingston, ON, Canada, May 2001. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. The role of oxygen in thiosulphate leaching of gold. Hydrometallurgy 2007, 85, 193–202. [Google Scholar] [CrossRef]
- Molleman, E.; Dreisinger, D. The treatment of copper–gold ores by ammonium thiosulfate leaching. Hydrometallurgy 2002, 66, 1–21. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W.T. Using nickel as a catalyst in ammonium thiosulfate leaching for gold extraction. Mater. Trans. 2004, 45, 516–526. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.C.; Huynh, T.H.; Jeong, J.; Pandey, B.D. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Zhang, Y. Effect of galena on thiosulfate leaching of gold. Hydrometallurgy 2017, 171, 157–164. [Google Scholar] [CrossRef]
- Sun, W. Mechanism and Applications of Potential-Controlled Flotation in Lime Adjust High Alkali Pulp. Ph.D. Thesis, Central South University, Changsha, China, December 2001. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of orthophosphate and polyphosphate. Hydrometallurgy 2011, 106, 38–45. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Effect of thiosulphate salts on ammoniacal thiosulphate leaching of gold. Hydrometallurgy 2010, 105, 120–126. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of ethylenediaminetetraacetic acid (EDTA). Miner. Eng. 2010, 23, 143–150. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. The role of amino acids in the thiosulphate leaching of gold. Miner. Eng. 2011, 24, 1022–1024. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, B.; Li, Q.; Jiang, T.; Zhang, X.; Li, G.; Guo, Y.; Chen, X.; Fan, X.; Huang, Z.; et al. A Method of Thiosulfate Leaching of Gold with the Additive of Ammonium Alcohol Polyvinyl Phosphate. China Patent No. 201510223605.1, 12 April 2017. [Google Scholar]
- Allard, B. A comparative study on the chemical composition of humic acids from forest soil, agricultural soil and lignite deposit: Bound lipid, carbohydrate and amino acid distributions. Geoderma 2006, 130, 77–96. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Jiang, T.; Li, Q.; Zhang, X.; Wang, D. Improved thiosulfate leaching of a refractory gold concentrate calcine with additives. Hydrometallurgy 2015, 152, 214–222. [Google Scholar] [CrossRef]
- Fang, Z.H.; Li, Z.J.; Shi, W.; Han, B.L. Gold leaching of a residue containing elemental sulfur with lime added under pressurized oxygen. Chin. J. Process Eng. 2002, 2, 17–20. [Google Scholar]
- Zhou, S. Research on the Alkaline Pressure Leaching of Gold Based on Situ Generated Thiosulfate. Master’s Thesis, Central South University, Changsha, China, May 2016. [Google Scholar]
- Hojjatie, M.M.; Lockhart, C.L.F.; Dimitriadis, A.; Van Cauwenbergh, J.; Van Dael, R. Continuous Process for Preparation of Calcium Thiosulfate Liquid Solution. U.S. Patent 8,454,929, 4 June 2013. [Google Scholar]
- Choi, Y.; Kondos, P.; Aylmore, M.G.; McMullen, J.; Van Weert, G. Thiosulfate Generation in situ in Precious Metal Recovery. U.S. Patent 7,572,317, 11 August 2009. [Google Scholar]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Dissolution of gold during pyrite oxidation reaction. Miner. Eng. 2016, 87, 2–9. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Liu, S.; Zhou, S.; Li, G.; Guo, Y.; Chen, X.; Fan, X.; et al. A Technology of Gold Leaching from Sulfide Gold Ores/Concentrates. China Patent 201510223325.0, 5 April 2017. [Google Scholar]
- Chandra, I.; Jeffrey, M.I. A fundamental study of ferric oxalate for dissolving gold in thiosulfate solutions. Hydrometallurgy 2005, 77, 191–201. [Google Scholar] [CrossRef]
- Heath, J.A.; Jeffrey, M.I.; Zhang, H.G.; Rumball, J.A. Anaerobic thiosulfate leaching: Development of in situ gold leaching systems. Miner. Eng. 2008, 21, 424–433. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Zhong, J.; Nie, Y.; Xiang, P. The copper–ethanediamine–thiosulphate leaching of gold ore containing limonite with cetyltrimethyl ammonium bromide as the synergist. Hydrometallurgy 2014, 150, 178–183. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W.T. Gold recovery from nickel catalyzed ammonium thiosulfate solution by strongly basic anion exchange resin. Mater. Trans. 2003, 44, 2099–2107. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Motekaitis, R.J. Critically Selected Constants of Metal Complexes Database; Version 5.0 Software; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Zhou, Z. Fundament of Colloid Chemistry; Beijing University Press: Beijing, China, 1987. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. The role of heavy metal ions in gold dissolution in the ammoniacal thiosulphate system. Hydrometallurgy 2002, 64, 231–246. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhou, S.; Liu, X.; Li, G.; Guo, Y.; Chen, X.; Fan, X.; et al. A Technology of Thiosulfate Leaching of Gold with the Catalysis of Nickel-Citrate. China Patent 201510229799.6, 7 September 2016. [Google Scholar]
- Xu, B.; Li, Q.; Liu, X.; Jiang, T.; Yang, Y.; Min, X.; Li, G.; Guo, Y.; Fan, X.; Zhang, Y.; et al. A Method of Thiosulfate Leaching of Gold with the Catalysis of Cobalt-Ethylene Diamine Tetraacetic Acid. China Patent 201610018078.5, 15 May 2017. [Google Scholar]
- Gallagher, N.P.; Hendrix, J.L.; Milosavljevic, E.B.; Nelson, J.H.; Solujic, L. Affinity of activated carbon towards some gold(I) complexes. Hydrometallurgy 1990, 25, 305–316. [Google Scholar] [CrossRef]
- Lulham, J.; Lindsay, D. Separation Process. International Patent No. WO/1991/011539, 8 August 1991. [Google Scholar]
- Guerra, E.; Dreisinger, D.B. A study of the factors affecting copper cementation of gold from ammoniacal thiosulphate solution. Hydrometallurgy 1999, 51, 155–172. [Google Scholar] [CrossRef]
- Choo, W.L.; Jeffrey, M.I. An electrochemical study of copper cementation of gold(I) thiosulfate. Hydrometallurgy 2004, 71, 351–362. [Google Scholar] [CrossRef]
- Hiskey, J.B.; Lee, J. Kinetics of gold cementation on copper in ammoniacal thiosulfate solution. Hydrometallurgy 2003, 69, 45–56. [Google Scholar] [CrossRef]
- Lee, J. Gold Cementation on Copper in Thiosulfate Solution: Kinetic, Electrochemical, and Morphological Studies. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, December 2003. [Google Scholar]
- Zhao, J.; Wu, Z. Extraction of gold from thiosulfate solutions with alkyl phosphorus esters. Hydrometallurgy 1997, 46, 363–372. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Z. Extraction of gold from thiosulfate solutions using amine mixed with neutral donor reagents. Hydrometallurgy 1998, 48, 133–144. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Z.; Chen, J. Solvent extraction of gold in thiosulfate solutions with amines. Solvent Extr. Ion Exch. 1998, 16, 527–543. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Z.; Chen, J. Gold extraction from thiosulfate solutions using mixed amines. Solvent Extr. Ion Exch. 1998, 16, 1407–1420. [Google Scholar] [CrossRef]
- Mohansingh, R. Adsorption of Gold from Gold Copper Ammonium Thiosulfate Complex onto Activated Carbon and Ion Exchange Resins. Master’s Thesis, University of Nevada, Reno, NV, USA, May 2000. [Google Scholar]
- Zhang, H.; Dreisinger, D.B. The recovery of gold from ammoniacal thiosulfate solutions containing copper using ion exchange resin columns. Hydrometallurgy 2004, 72, 225–234. [Google Scholar] [CrossRef]
- Fleming, C.A. Hydrometallurgy of precious metals recovery. Hydrometallurgy 1992, 30, 127–162. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Hewitt, D.M.; Dai, X.; Brunt, S.D. Ion exchange adsorption and elution for recovering gold thiosulfate from leach solutions. Hydrometallurgy 2010, 100, 136–143. [Google Scholar] [CrossRef]
- Zhang, H.; Dreisinger, D.B. The adsorption of gold and copper onto ion-exchange resins from ammoniacal thiosulfate solutions. Hydrometallurgy 2002, 66, 67–76. [Google Scholar] [CrossRef]
- Kononova, O.N.; Kholmogorov, A.G.; Kononov, Y.S.; Pashkov, G.L.; Kachin, S.V.; Zotova, S.V. Sorption recovery of gold from thiosulphate solutions after leaching of products of chemical preparation of hard concentrates. Hydrometallurgy 2001, 59, 115–123. [Google Scholar] [CrossRef]

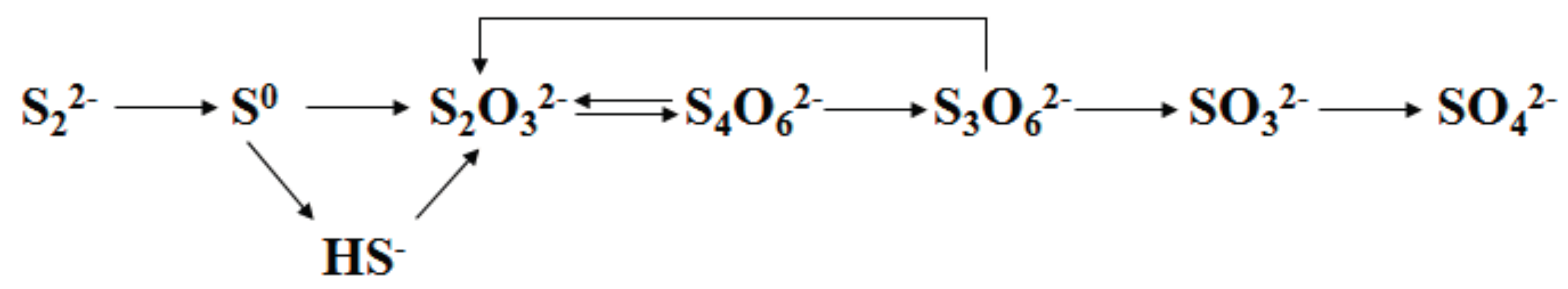
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals 2017, 7, 222. https://doi.org/10.3390/met7060222
Xu B, Kong W, Li Q, Yang Y, Jiang T, Liu X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals. 2017; 7(6):222. https://doi.org/10.3390/met7060222
Chicago/Turabian StyleXu, Bin, Wenhao Kong, Qian Li, Yongbin Yang, Tao Jiang, and Xiaoliang Liu. 2017. "A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution" Metals 7, no. 6: 222. https://doi.org/10.3390/met7060222



