Effects of Q&P Processing Conditions on Austenite Carbon Enrichment Studied by In Situ High-Energy X-ray Diffraction Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Alloy
2.2. Diffraction Set-Up and Data Processing
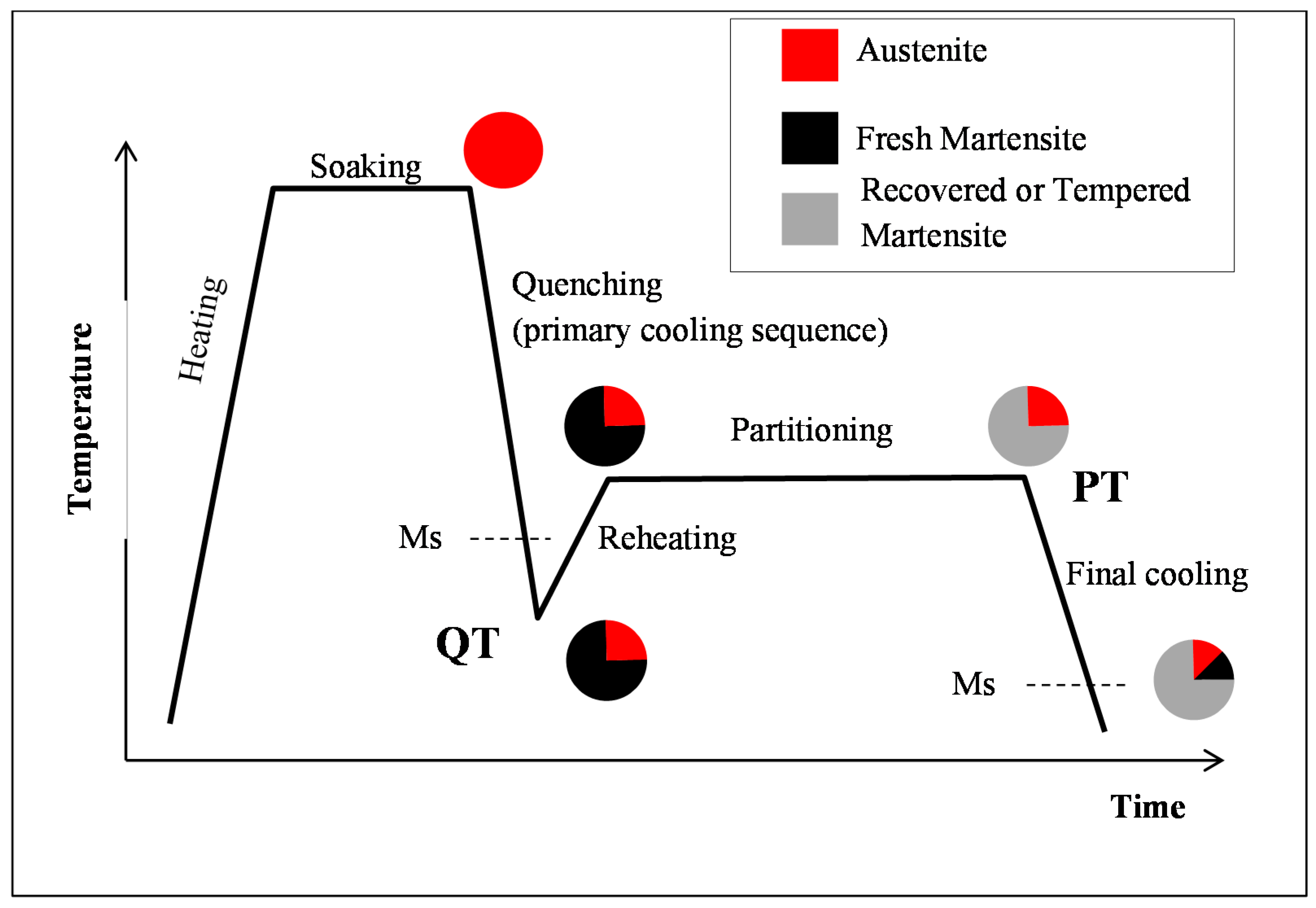
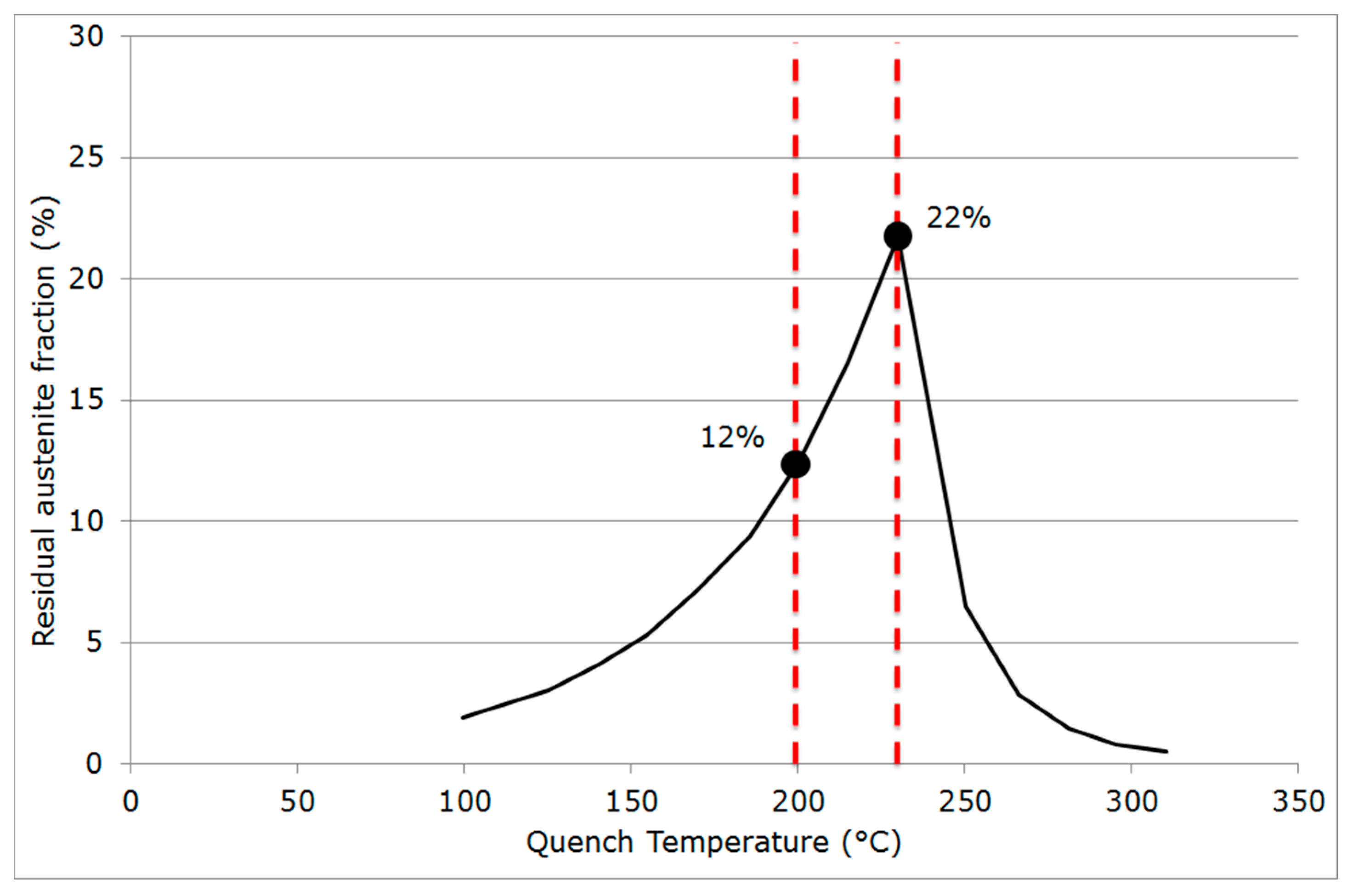
2.3. Quenching & Partitioning (Q&P) Processing Conditions
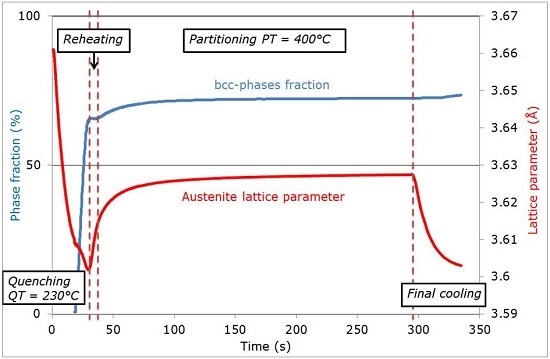
3. Results
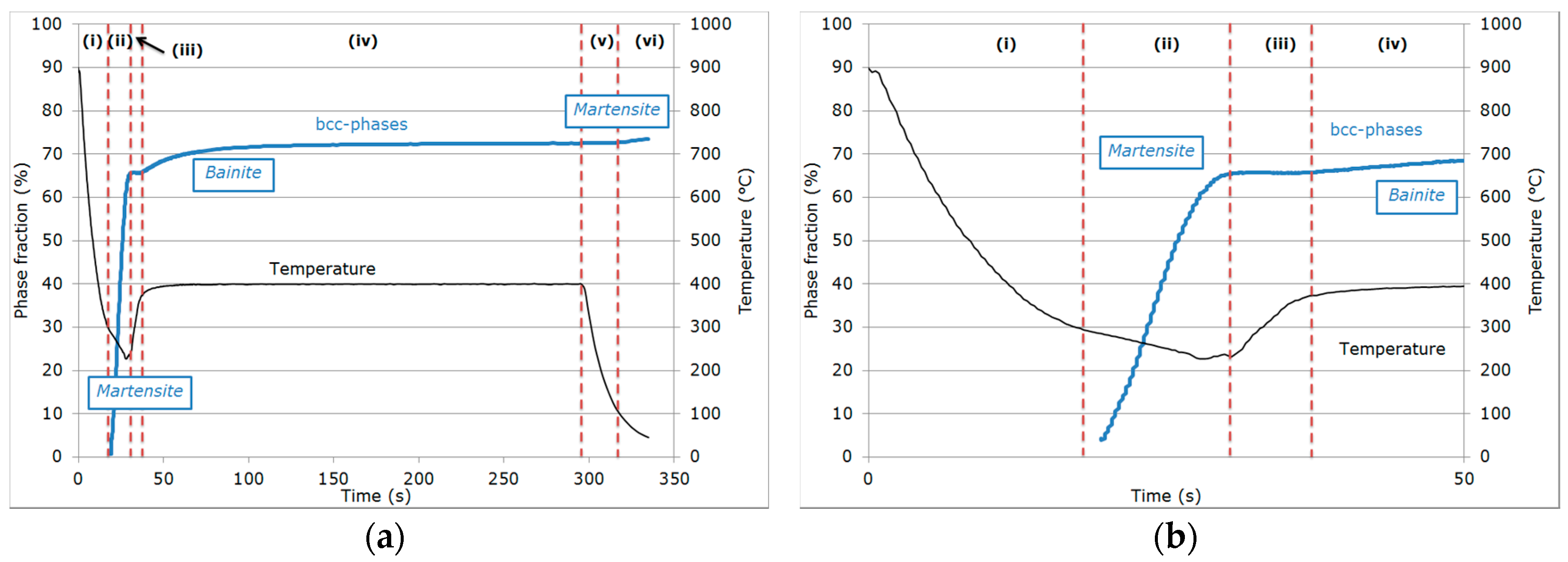
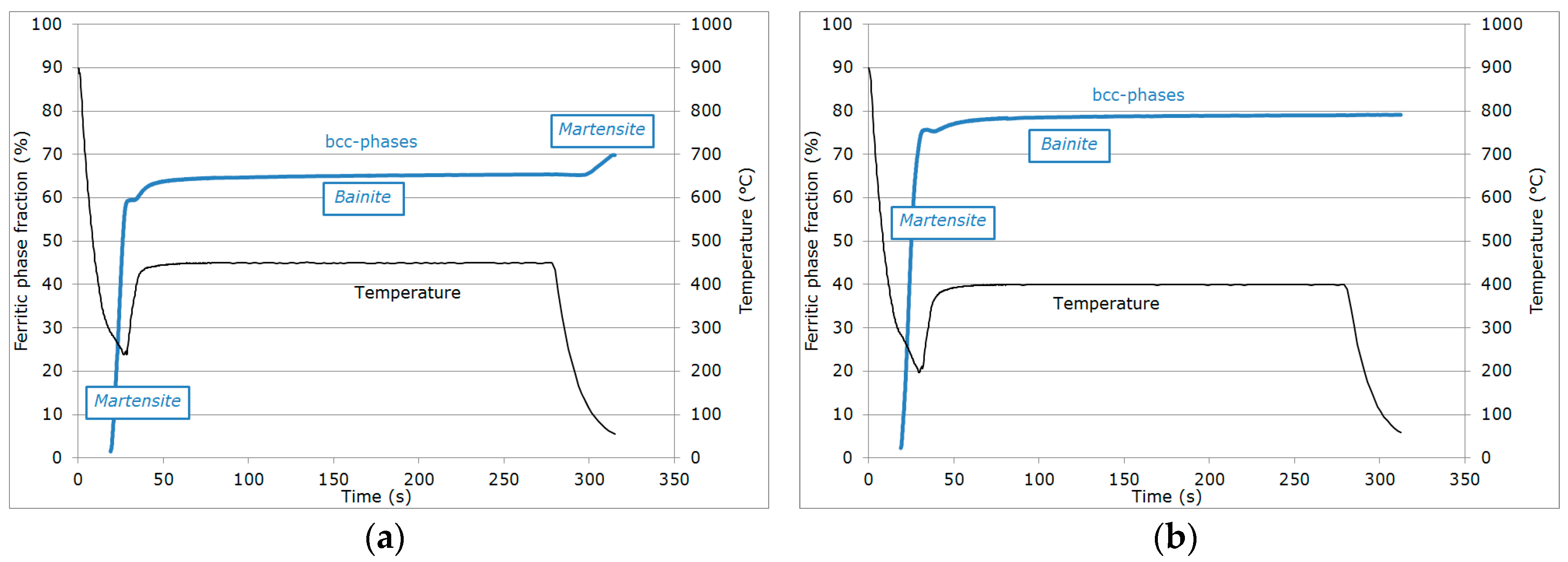
3.1. Evolution of Phase Fractions
- (i)
- During the first cooling step, the alloy remained fully austenitic above Ms.
- (ii)
- Primary martensitic transformation below initial Ms: The initial Ms temperature was estimated to be 295 °C from an extrapolation of the transformation kinetics. The final fraction of martensite before reheating was 65% (note that at this stage, the bcc phase was implicitly but obviously identified as martensite).
- (iii)
- Reheating step, with a duration of a few seconds, because of the regulation procedures of the heating device: The duration of this transient regime was lower than 5 s, before temperature stabilization for the partitioning step. Figure 3b reveals that during the reheating step, the phase fractions remained constant. For all studied conditions, the phase fractions remained constant during reheating up to about 370 °C.
- (iv)
- Partitioning step: Above 370 °C, a significant increase in the bcc phase fraction was observed during the partitioning step. The kinetics were initially fast during the first 50 s, progressively slowing down. Due to the experimental limitations in revealing tetragonality, the exact nature of this bcc phase was debatable as it could have corresponded to either bainite and/or athermal martensite. Indeed, there was strong evidence of both bainite and athermal martensite formation below Ms [7,31,32]. It was even stated that this isothermal product was neither purely martensitic nor purely bainitic [33]. One could even consider the simultaneous formation of both athermal martensite and bainite as an alternative. Indeed, the formation of athermal martensite could have a strong accelerating effect on the subsequent bainite formation by providing a higher density of potential nucleation sites. As a consequence, below Ms, the nature of the transformation products during isothermal holding was unclear. In addition, lower bainite and athermal martensite exhibited morphological similarities. The morphological criteria used by Somani et al. to distinguish bainite from athermal martensite (laths with wavy boundaries and ledge-like protrusions) [7] was discussed very recently by [34] in a convincing way. According to their analysis, below Ms, the driving force for bainite nucleation is so high that small units of bainite may grow from the initial martensite laths in the form of ledge-liked protrusions. Furthermore, bainitic ferrite can grow from the prior athermal martensite, maintaining a similar orientation relationship that could have contributed to the formation of the ledge-like protrusions that could have, in turn, given rise to a wavy appearance of the boundaries.
- (v)
- During the first part of the final cooling step, the fraction of the bcc phase identified as bainite remained constant down to 120 °C.
- (vi)
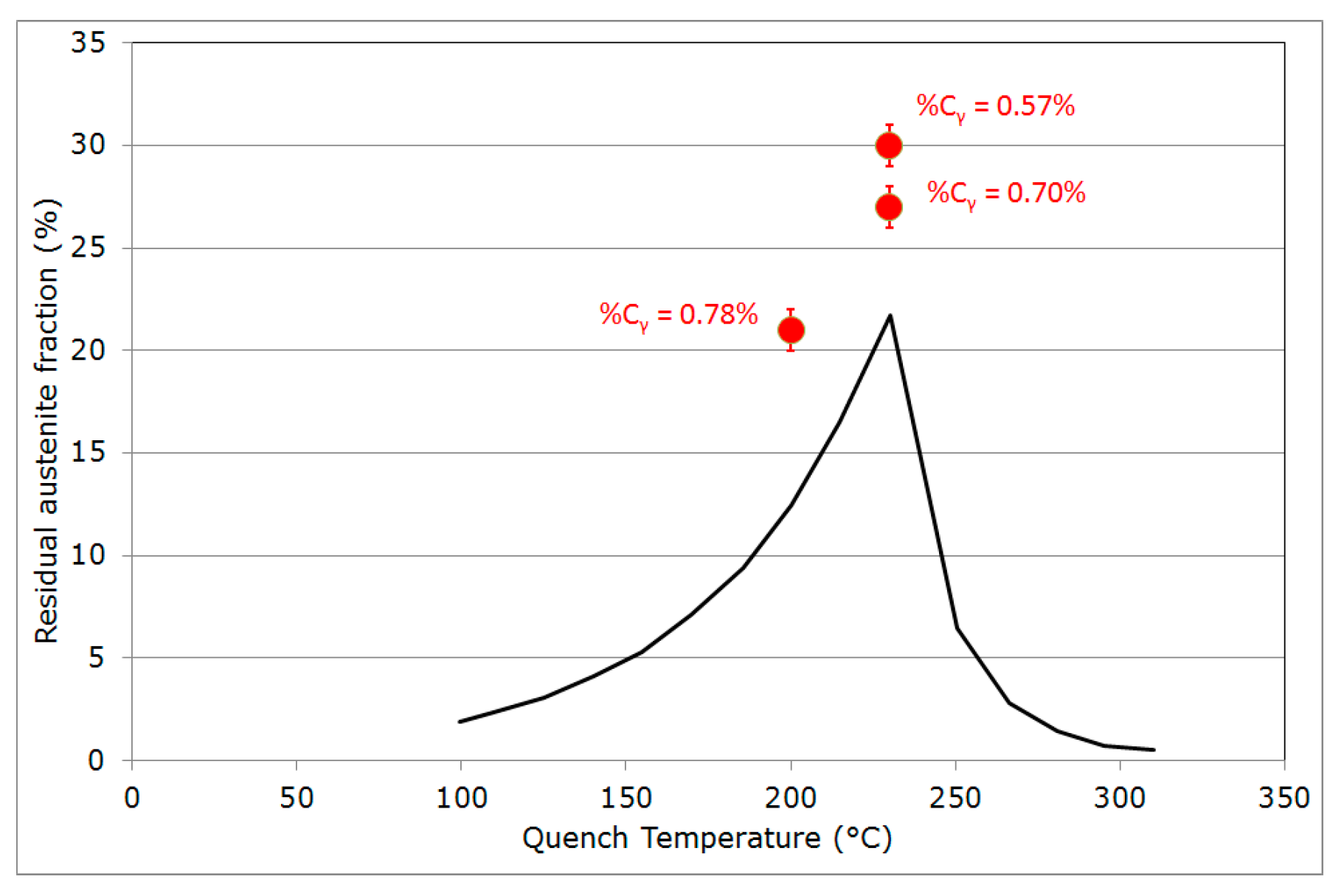
- Final martensitic transformation, evidenced by a 2% increase in the bcc phase fraction below 120 °C: Note that again, similarly to step (ii), the increase in the bcc phase was obviously attributed to martensite formation. The final fraction of retained austenite at RT was about 27%.
3.2. Evolution of Austenite Lattice Parameter
4. Discussion
4.1. Carbon Mass Balances
4.2. Critical Assessment of the Methods for Estimating Austenite Carbon Enrichment
5. Conclusions
- -
- After the first martensitic transformation during cooling down to the QT, no significant evolution is observed during the re-heating step-up to the PT.
- -
- During holding at the PT, an increase in the austenite lattice parameter is observed, resulting from the carbon redistribution from martensite and formation of ferritic bainite.
- -
- A final increase of the austenite lattice parameter is observed during the final quench, attributed to internal stresses resulting from differences in the thermal expansion between the different phases present (and potentially improving the TRIP ability of the steel).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnard, S.J.; Smith, G.D.W.; Sarikaya, M.; Thomas, G. Carbon atom distribution in a dual phase steel: An atom probe study. Scr. Metall. 1981, 15, 387–392. [Google Scholar] [CrossRef]
- Gouné, M.; Danoix, F.; Allain, S.; Bouaziz, O. Unambiguous carbon partitioning from martensite to austenite in Fe–C–Ni alloys during quenching and partitioning. Scr. Mater. 2013, 68, 1004–1007. [Google Scholar] [CrossRef]
- Matas, S.; Hehemann, M.F. Retained Austenite and the Tempering of Martensite. Nature 1960, 187, 685–686. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. Sci. 2004, 8, 219–237. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Edmonds, D.V.; He, K.; Rizzo, F.C.; De Cooman, B.C.; Matlock, D.K.; Speer, J.G. Quenching and partitioning martensite—A novel steel heat treatment. Mater. Sci. Eng. A 2006, 438–440, 25–34. [Google Scholar] [CrossRef]
- Somani, M.C.; Porter, D.A.; Karjalainen, L.P.; Misra, R.D.K. On Various Aspects of Decomposition of Austenite in a High-Silicon Steel During Quenching and Partitioning. Metall. Mater. Trans. A 2014, 45, 1247–1257. [Google Scholar] [CrossRef]
- De Moor, E.; Speer, J.G.; Matlock, D.K.; Kwak, J.H.; Lee, S.B. Quenching and Partitioning of CMnSi Steels Containing Elevated Manganese Levels. Steel Res. Int. 2012, 83, 322–327. [Google Scholar] [CrossRef]
- Xiong, X.C.; Chen, B.; Huang, M.X.; Wang, J.F.; Wang, L. The effect of morphology on the stability of retained austenite in a quenched and partitioned steel. Scr. Mater. 2013, 68, 321–324. [Google Scholar] [CrossRef]
- Ariza, E.A.; Nishikawa, A.S.; Goldenstein, H.; Tschiptschin, A.P. Characterization and methodology for calculating the mechanical properties of a TRIP-steel submitted to hot stamping and quenching and partitioning (Q&P). Mater. Sci. Eng. A 2016, 671, 54–69. [Google Scholar] [CrossRef]
- Zhao, H.S.; Li, W.; Zhu, X.; Lu, X.H.; Wang, L.; Zhou, S.; Jin, X.J. Analysis of the relationship between retained austenite locations and the deformation behavior of quenching and partitioning treated steels. Mater. Sci. Eng. A 2016, 649, 18–26. [Google Scholar] [CrossRef]
- HajyAkbary, F.; Sietsma, J.; Miyamoto, G.; Furuhara, T.; Santofimia, M.J. Interaction of carbon partitioning, carbide precipitation and bainite formation during the Q&P process in a low C steel. Acta Mater. 2016, 104, 72–83. [Google Scholar] [CrossRef]
- Pierce, D.T.; Coughlin, D.R.; Williamson, D.L.; Clarke, K.D.; Clarke, A.J.; Speer, J.G.; De Moor, E. Characterization of transition carbides in quench and partitioned steel microstructures by Mössbauer spectroscopy and complementary techniques. Acta Mater. 2015, 90, 417–430. [Google Scholar] [CrossRef]
- Toji, Y.; Miyamoto, G.; Raabe, D. Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation. Acta Mater. 2015, 86, 137–147. [Google Scholar] [CrossRef]
- Thomas, G.A.; Danoix, F.; Speer, J.G.; Thompson, S.W.; Cuvilly, F. Carbon Atom Re-Distribution during Quenching and Partitioning. ISIJ Int. 2014, 54, 2900–2906. [Google Scholar] [CrossRef]
- Clarke, A.J.; Speer, J.G.; Miller, M.K.; Hackenberg, R.E.; Edmonds, D.V.; Matlock, D.H.; Rizzo, F.C.; Clarke, K.D.; De Moor, E. Carbon partitioning to austenite from martensite or bainite during the quench and partition (Q&P) process: A critical assessment. Acta Mater. 2008, 56, 16. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Nguyen-Minh, T.; Zhao, L.; Petrov, R.; Sabirov, I.; Sietsma, J. New low carbon Q&P steels containing film-like intercritical ferrite. Mater. Sci. Eng. A 2010, 527, 6429–6439. [Google Scholar] [CrossRef]
- Toji, Y.; Matsuda, H.; Herbig, M.; Choi, P.P.; Raabe, D. Atomic-scale analysis of carbon partitioning between martensite and austenite by atom probe tomography and correlative transmission electron microscopy. Acta Mater. 2014, 65, 215–228. [Google Scholar] [CrossRef]
- Hell, J.C.; Dehmas, M.; Allain, S.; Prado, J.M.; Hazotte, A.; Chateau, J.P. Microstructure–Properties Relationships in Carbide-free Bainitic Steels. ISIJ Int. 2011, 51, 1724–1732. [Google Scholar] [CrossRef]
- Hell, J.C. Aciers Bainitiques Sans Carbure: Caractérisations Microstructurale Multi-Echelle et In Situ de la Transformation Austénite-Bainite et Relations Entre Microstructure et Comportement Mécanique. Ph.D. Thesis, Université Paul Verlaine, Metz, France, 10 November 2011. [Google Scholar]
- Caballero, F.G.; Allain, S.; Cornide, J.; Puerta Velásquez, J.D.; Garcia-Mateo, C.; Miller, M.K. Design of cold rolled and continuous annealed carbide-free bainitic steels for automotive application. Mater. Des. 2013, 49, 667–680. [Google Scholar] [CrossRef]
- Nishikawa, L.; Ogata, P.; Nishikawa, A.; Ramirez, M.; Goldenstein, H. Tempering Behaviour of a Quenched Microalloyed Pipeline Steel. In Proceedings of the TMS 2016 Conference, Nashville, TN, USA, 14–18 February 2016. [Google Scholar]
- Allain, S.Y.P.; Geandier, G.; Hell, J.C.; Soler, M.; Danoix, F.; Gouné, M. In-situ investigation of quenching and partitioning by High Energy X-Ray Diffraction experiments. Scr. Mater. 2017, 131, 15–18. [Google Scholar] [CrossRef]
- Epp, J.; Hirsch, T.; Curfs, C. In situ X-Ray Diffraction Analysis of Carbon Partitioning during Quenching of Low Carbon Steel. Metall. Mater. Trans. A 2012, 43, 2210–2217. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C. Modeling Start Curves of Bainite Formation. Metall. Mater. Trans. A 2010, 41, 285–296. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C. Bainite and martensite start temperature calculated with exponential carbon dependence. Mater. Sci. Technol. 2012, 28, 487–495. [Google Scholar] [CrossRef]
- The FIT2D Home Page. Available online: http://www.esrf.eu/computing/scientific/FIT2D/ (accessed on 11 April 2017).
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55. [Google Scholar] [CrossRef]
- Rementeria, R.; Jimenez, J.A.; Allain, S.Y.P.; Geandier, G.; Poplawsky, J.D.; Guo, W.; Urones-Garrote, E.; Garcia-Mateo, C.; Caballero, F.G. Quantitative assessment of carbon allocation anomalies in low temperature bainite. Acta Mater. 2017, 133, 333–345. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Nagakura, S. Crystal structure and morphology of the carbide precipitated from martensitic high carbon steel during the first stage of tempering. Acta Metall. 1972, 20, 846. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C.; Santofimia, M.J.; Sietsma, J. Experimental evidence for bainite formation below Ms in Fe–0.66C. Scr. Mater. 2008, 58, 488–491. [Google Scholar] [CrossRef]
- Kolmskog, P.; Borgenstam, A.; Hillert, M.; Hedstrom, P.; Babu, S.S.; Terasaki, H.; Komizo, Y.I. Direct Observation that Bainite can Grow Below Ms. Metall. Mater. Trans. A 2012, 43A, 4984–4988. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.J.; De Cooman, B.C. Microstructure of Low C Steel Isothermally Transformed in the Ms to Mf Temperature Range. Metall. Mater. Trans. A 2012, 43A, 4967–4983. [Google Scholar] [CrossRef]
- Navarro-López, A.; Hidalgo, J.; Sietsma, J.; Santofimia, M.J. Characterization of bainitic/martensitic structures formed in isothermal treatments below the Ms temperature. Mater. Charact. 2017, 128, 248–256. [Google Scholar] [CrossRef]
- Bigg, T.D.; Edmonds, D.V.; Eardley, E.S. Real-time structural analysis of quenching and partitioning (Q&P) in an experimental martensitic steel. J. Alloys Compd. 2013, 577, 695–698. [Google Scholar] [CrossRef]
- Lu, X.G.; Selleby, M.; Sundman, B. Assessments of molar volume and thermal expansion for selected bcc, fcc and hcp metallic elements. Calphad 2005, 29, 68–89. [Google Scholar] [CrossRef]
- Onink, M.; Brakrnan, C.M.; Tichelaar, F.D.; Mittemeijer, E.J.; Van der Zwaag, S. The lattice parameters of austenite and ferrite in FeC alloys as functions of carbon concentration and temperature. Scr. Metall. Mater. 1993, 29, 1011–1016. [Google Scholar] [CrossRef]
- Van Bohemen, S.C.M. The nonlinear lattice expansion of iron alloys in the range 100–1600 K. Scr. Mater. 2013, 69, 315–318. [Google Scholar] [CrossRef]
- Bruneseaux, F. Apport de la Diffraction des Rayons X à Haute Energie sur les Transformations de Phases, Application aux Alliages de Titanes. Ph.D. Thesis, The National Polytechnic Institute of Lorraine (INPL), Nancy, France, 16 May 2008. [Google Scholar]
- Ghosh, G.; Olson, G.B. The isotropic shear modulus of multicomponent Fe-base solid solutions. Acta. Mater. 2002, 50, 2655–2675. [Google Scholar] [CrossRef]
- Manjoine, M.J.; Voorhees, H.R. Compilation of Stress-Relaxation Data for Engineering Alloy; ASTM Data Series Publication DS 60; ASTM International: West Conshohocken, PA, USA, 1982. [Google Scholar]
- Arsenault, R.J.; Taya, M. Thermal residual stress in metal matrix composite. Acta Metall. 1987, 35, 651–659. [Google Scholar] [CrossRef]
- Lu, P. Further studies on Mori–Tanaka models for thermal expansion coefficients of composites. Polymer 2013, 54, 1691–1699. [Google Scholar] [CrossRef]

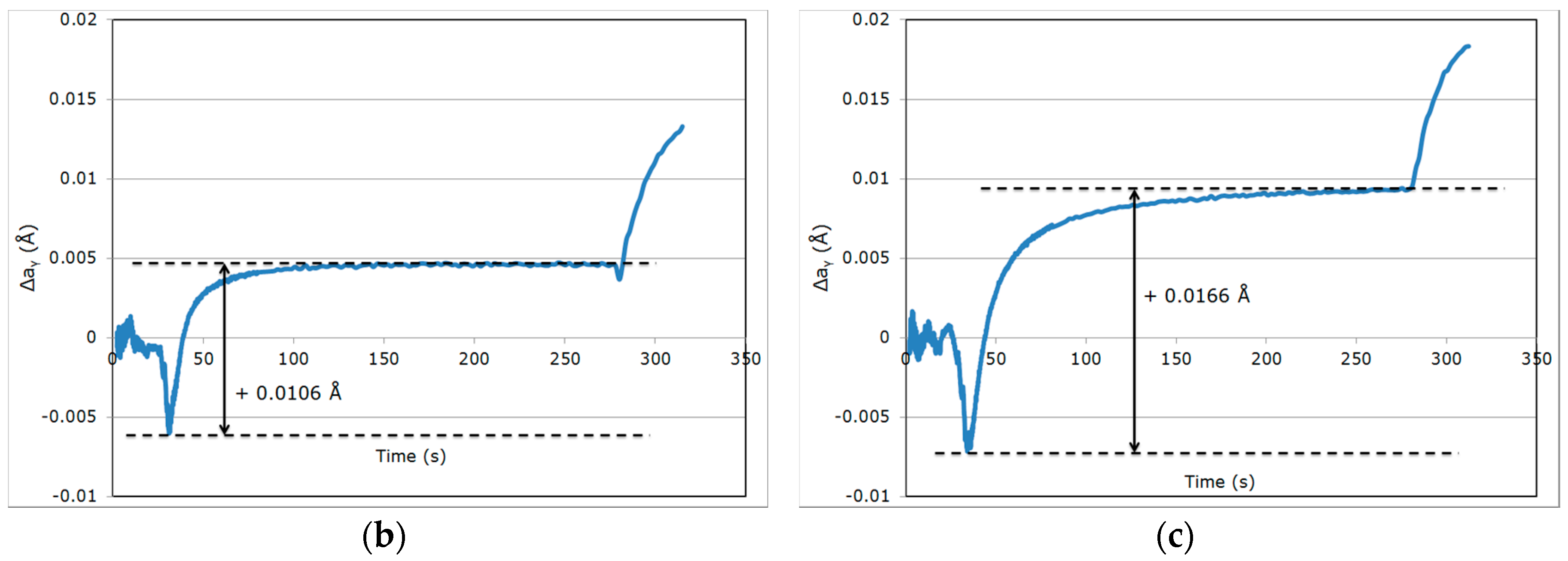
| Variation in Δaγ | Reference Cycle QT = 230 °C/PT = 400 °C | High Partitioning Temperature (PT) QT = 230 °C/PT = 450 °C | Low Quenching Temperature (QT) QT = 200 °C/PT = 400 °C |
|---|---|---|---|
| End of step (ii) | −0.0055 Å | −0.0060 Å | −0.0072 Å |
| During step (iv) | +0.0127 Å | +0.0106 Å | +0.0166 Å |
| During step (vi) | +0.0078 Å | +0.0087 Å | +0.0089 Å |
| Studied Cycles | Reference Cycle QT = 230 °C/PT = 400 °C | High PT QT = 230 °C/PT = 450 °C | Low QT QT = 200 °C/PT = 400 °C |
|---|---|---|---|
| Fγ (%) | 27 | 30 | 21 |
| Cγ (wt %) | 0.70 | 0.67 | 0.80 |
| Fγ × Cγ (wt %) | 0.19 | 0.20 | 0.17 |
| C0 − Fγ × Cγ (wt %) | 0.11 | 0.10 | 0.13 |
| Studied Cycles | Reference Cycle QT = 230 °C/PT = 400 °C | High PT QT = 230 °C/PT = 450 °C | Low QT QT = 200 °C/PT = 400 °C |
|---|---|---|---|
| aγ (Å) at RT | 3.6030 | 3.6013 | 3.6054 |
| Cγ (wt % using the Toji et al. formula) | 0.92 | 0.87 | 0.99 |
| C0 − Fγ × Cγ (wt %) | 0.05 | 0.04 | 0.09 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allain, S.Y.P.; Geandier, G.; Hell, J.-C.; Soler, M.; Danoix, F.; Gouné, M. Effects of Q&P Processing Conditions on Austenite Carbon Enrichment Studied by In Situ High-Energy X-ray Diffraction Experiments. Metals 2017, 7, 232. https://doi.org/10.3390/met7070232
Allain SYP, Geandier G, Hell J-C, Soler M, Danoix F, Gouné M. Effects of Q&P Processing Conditions on Austenite Carbon Enrichment Studied by In Situ High-Energy X-ray Diffraction Experiments. Metals. 2017; 7(7):232. https://doi.org/10.3390/met7070232
Chicago/Turabian StyleAllain, Sébastien Yves Pierre, Guillaume Geandier, Jean-Christophe Hell, Michel Soler, Frédéric Danoix, and Mohamed Gouné. 2017. "Effects of Q&P Processing Conditions on Austenite Carbon Enrichment Studied by In Situ High-Energy X-ray Diffraction Experiments" Metals 7, no. 7: 232. https://doi.org/10.3390/met7070232