Secondary Solidification Behavior of A356 Aluminum Alloy Prepared by the Self-Inoculation Method
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of Self-Inoculants
2.2. Slurry Preparation and Determination of Optimum Parameters
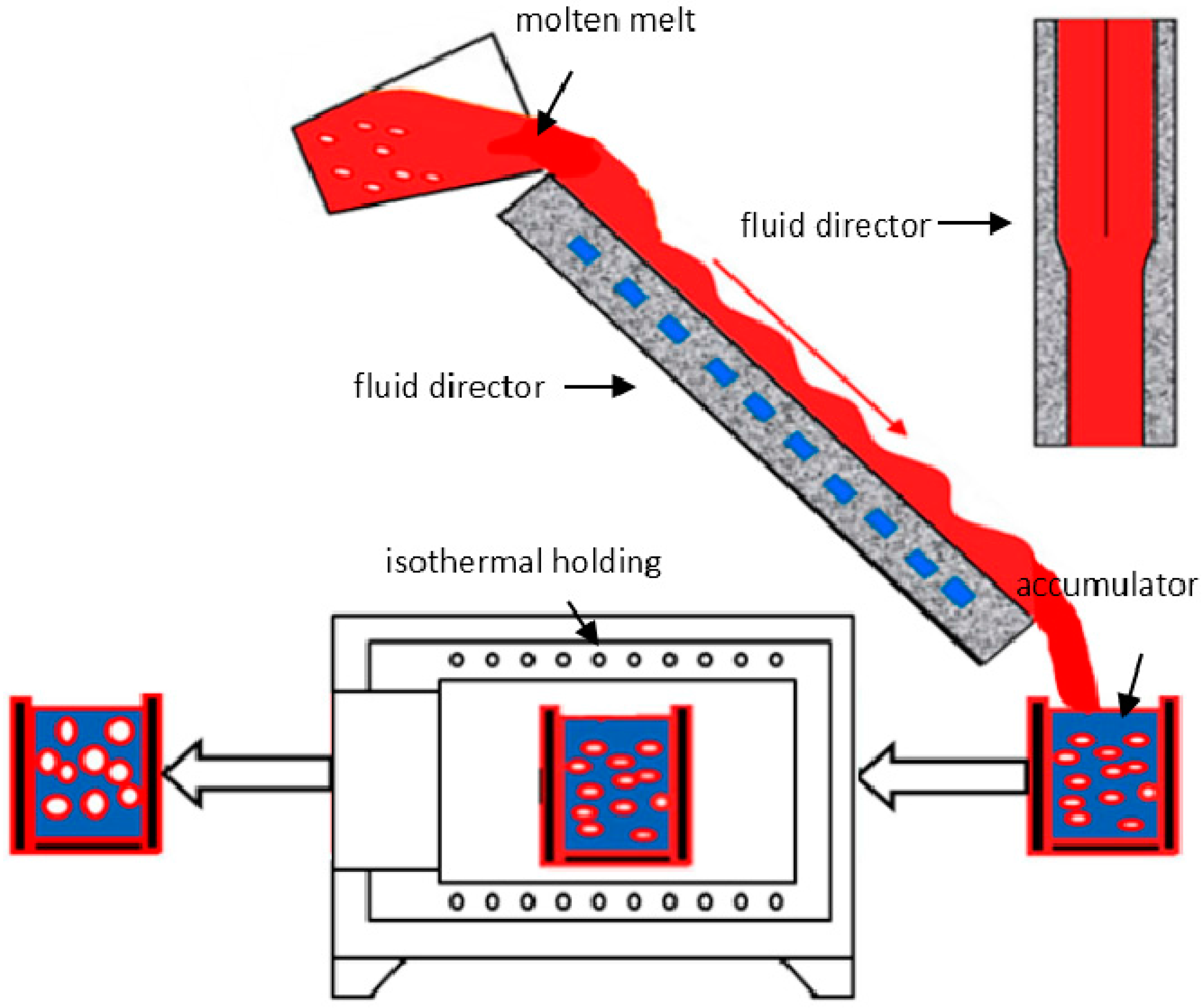
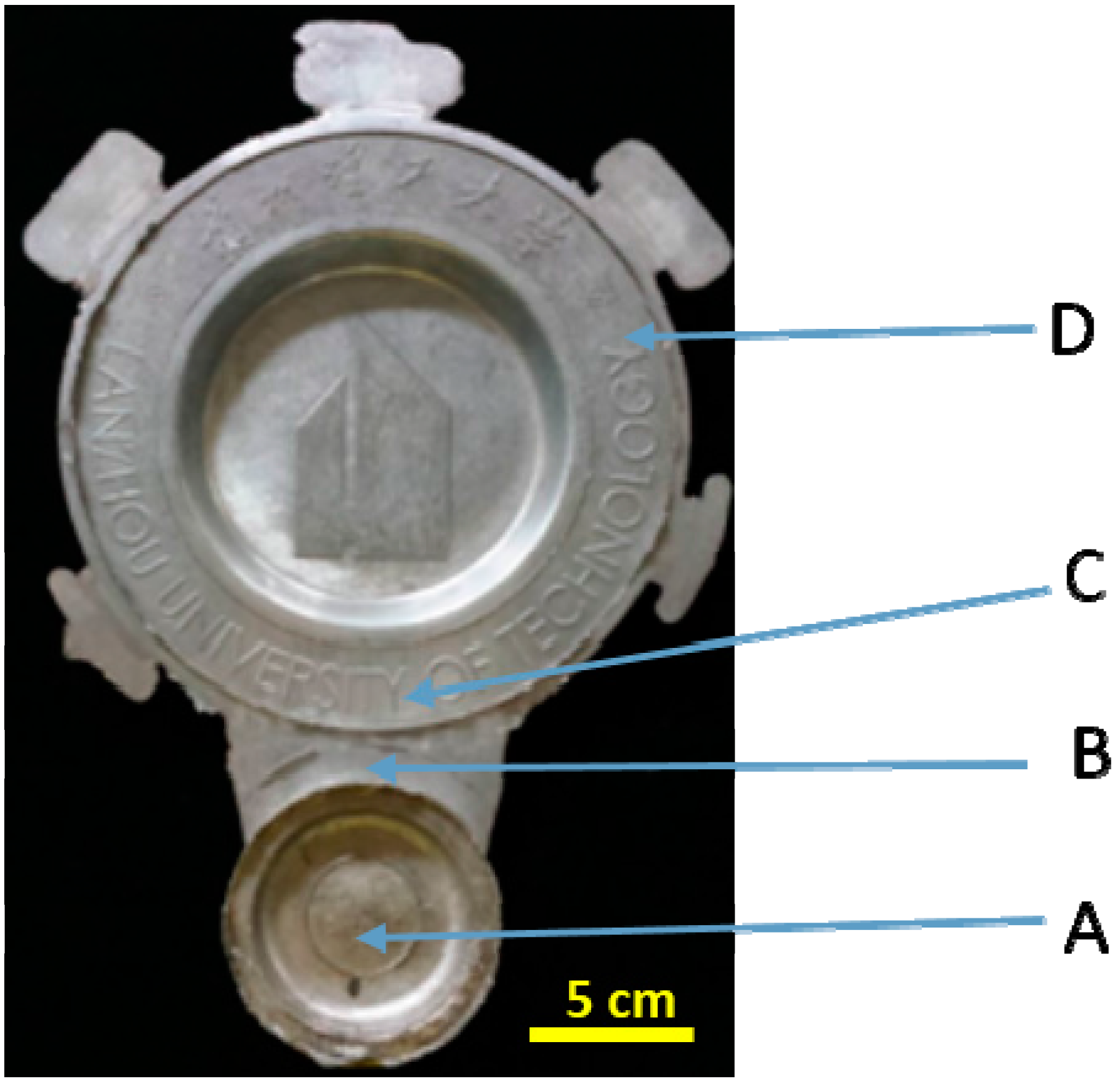
2.3. Die Casting Forming, Microstructure Observation and Quantitative Analysis
3. Results
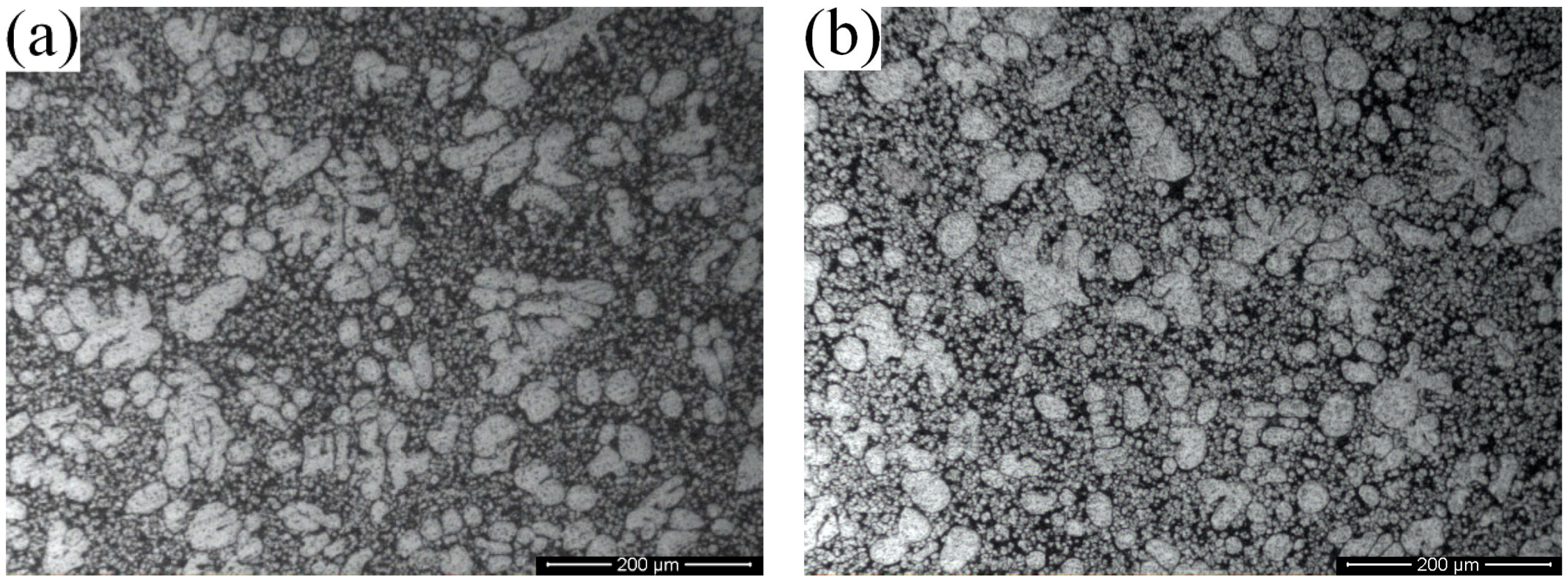
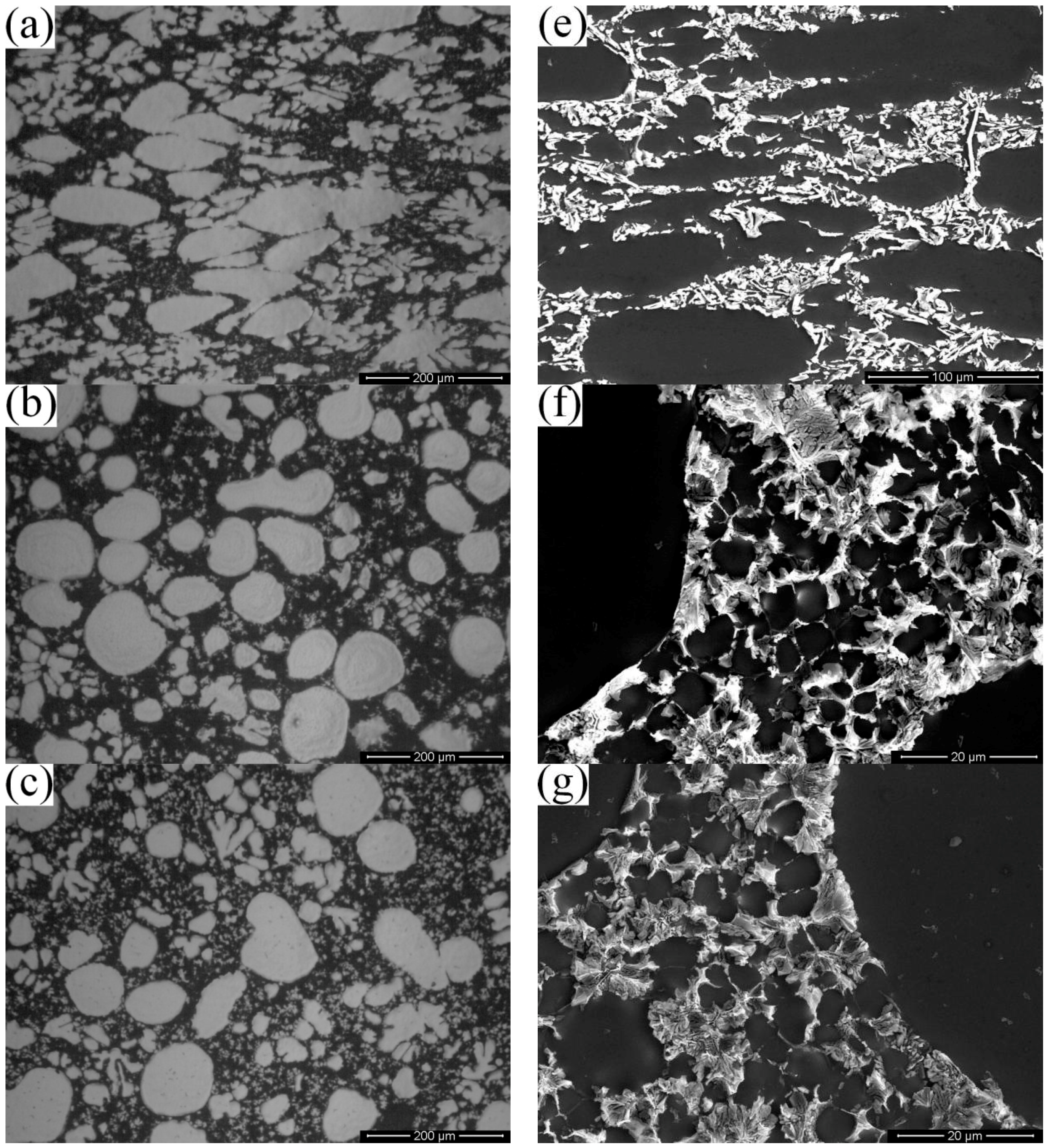
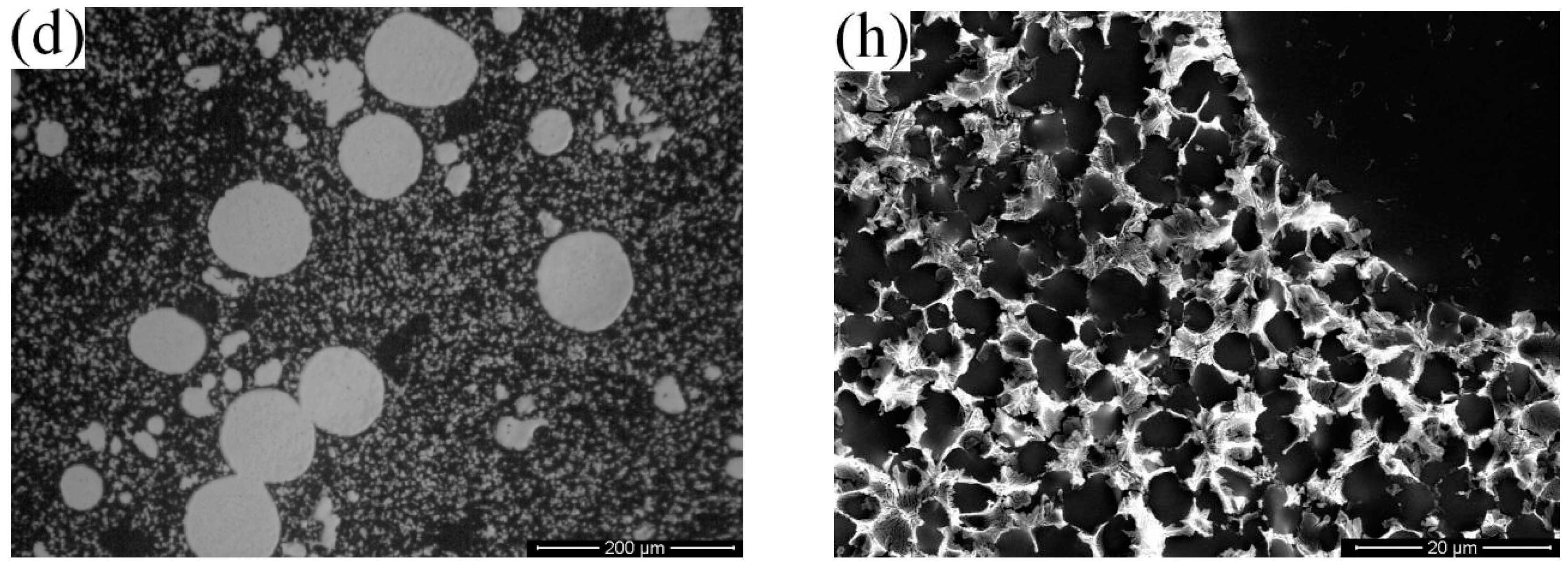
3.1. Microstructural Differences between High Pressure Die Casting (HPDC) and Rheo-Diecasting (RDC)
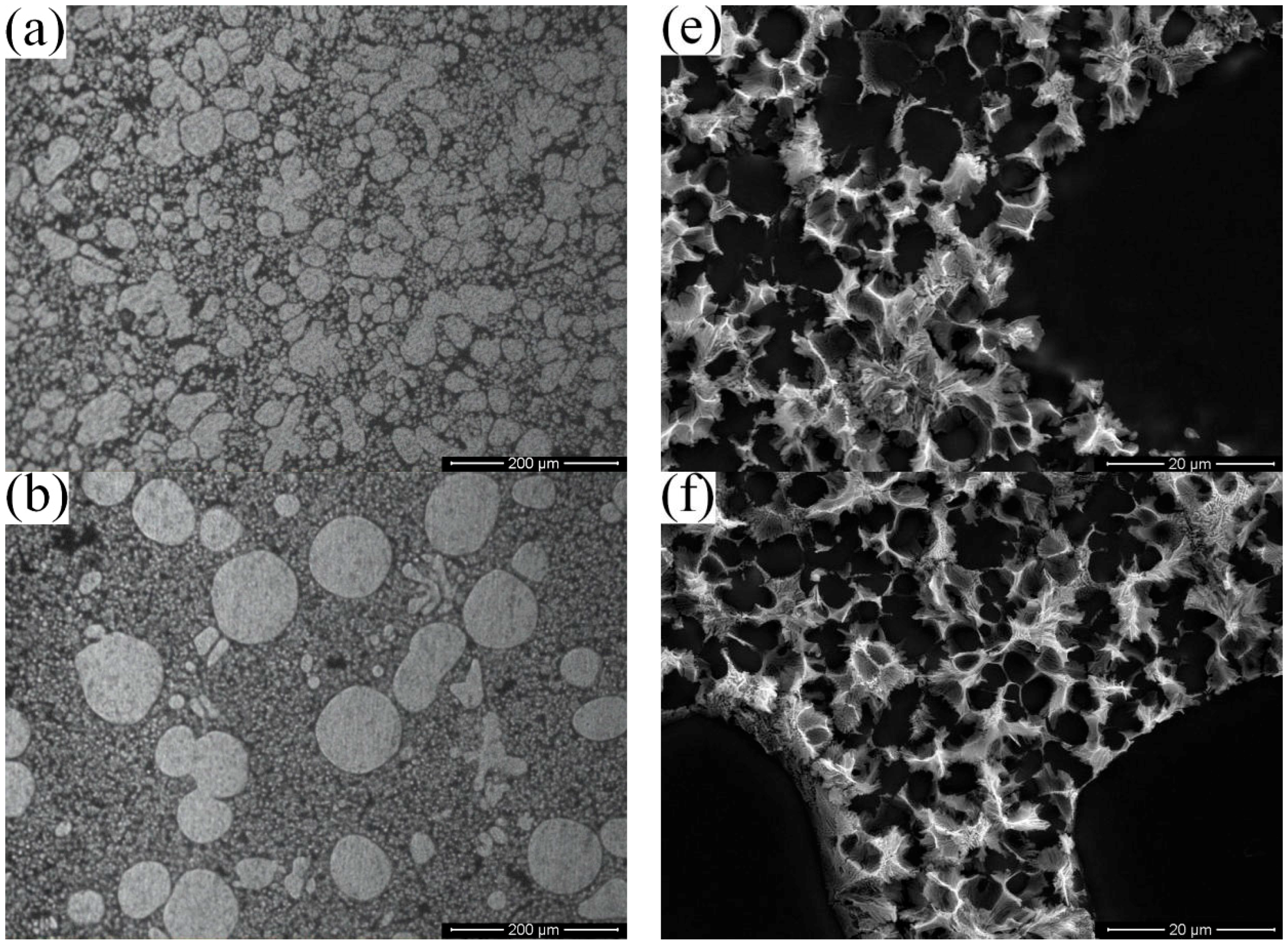
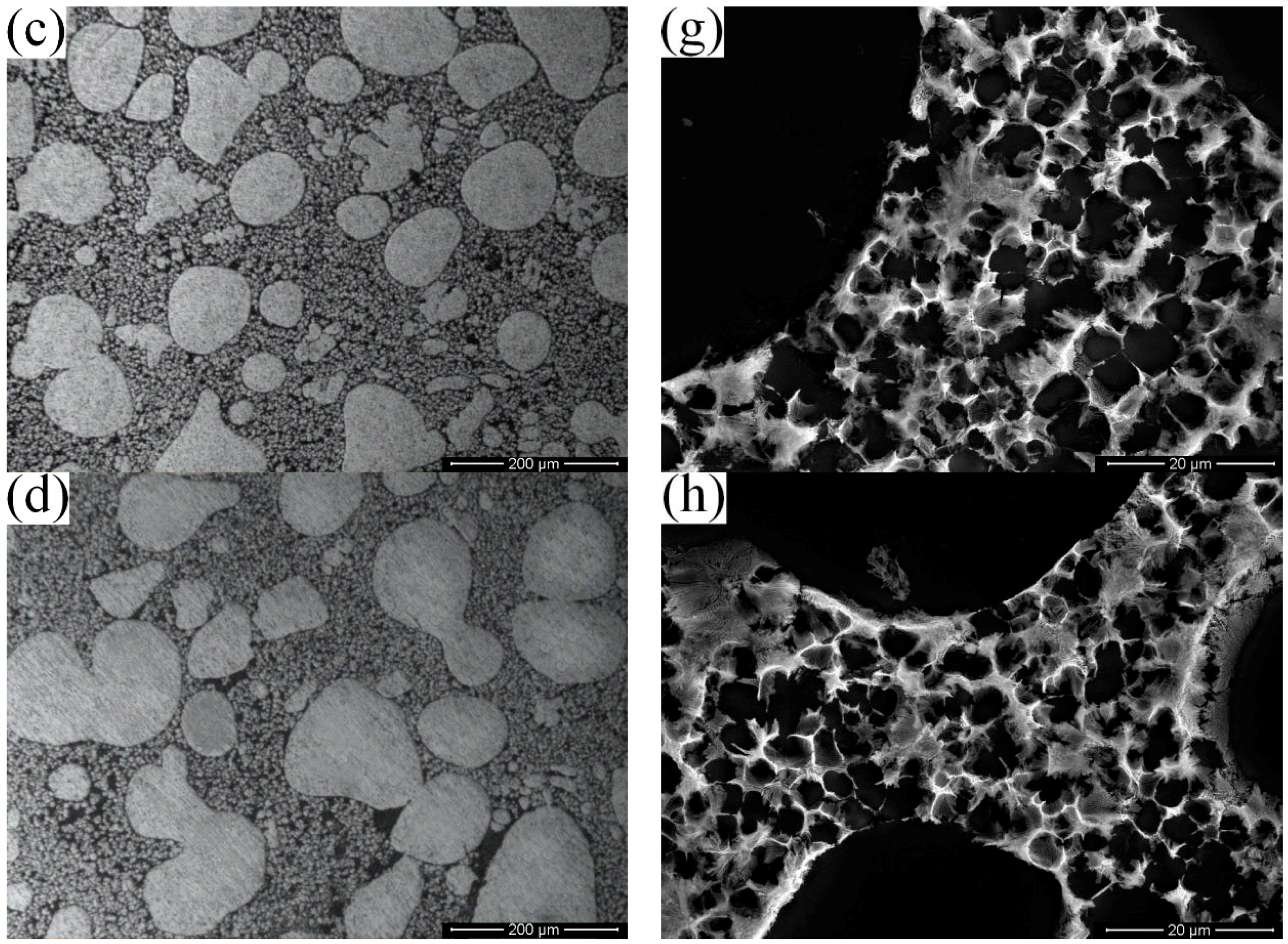
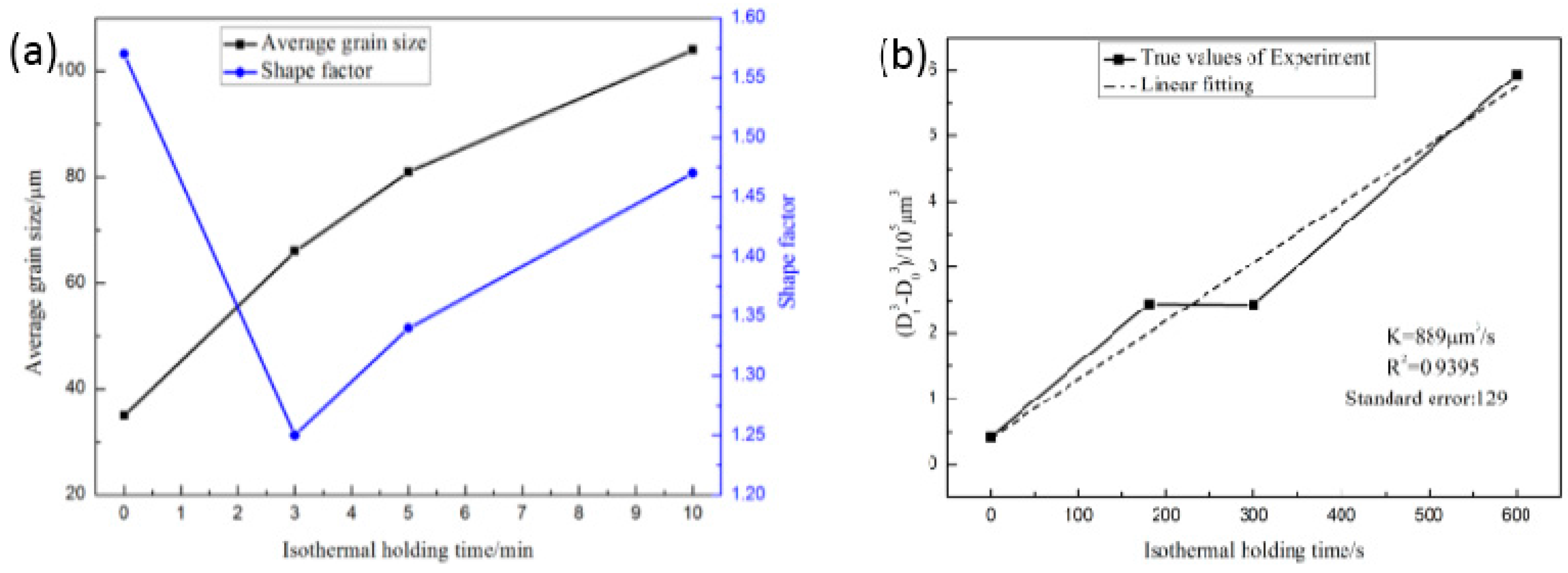
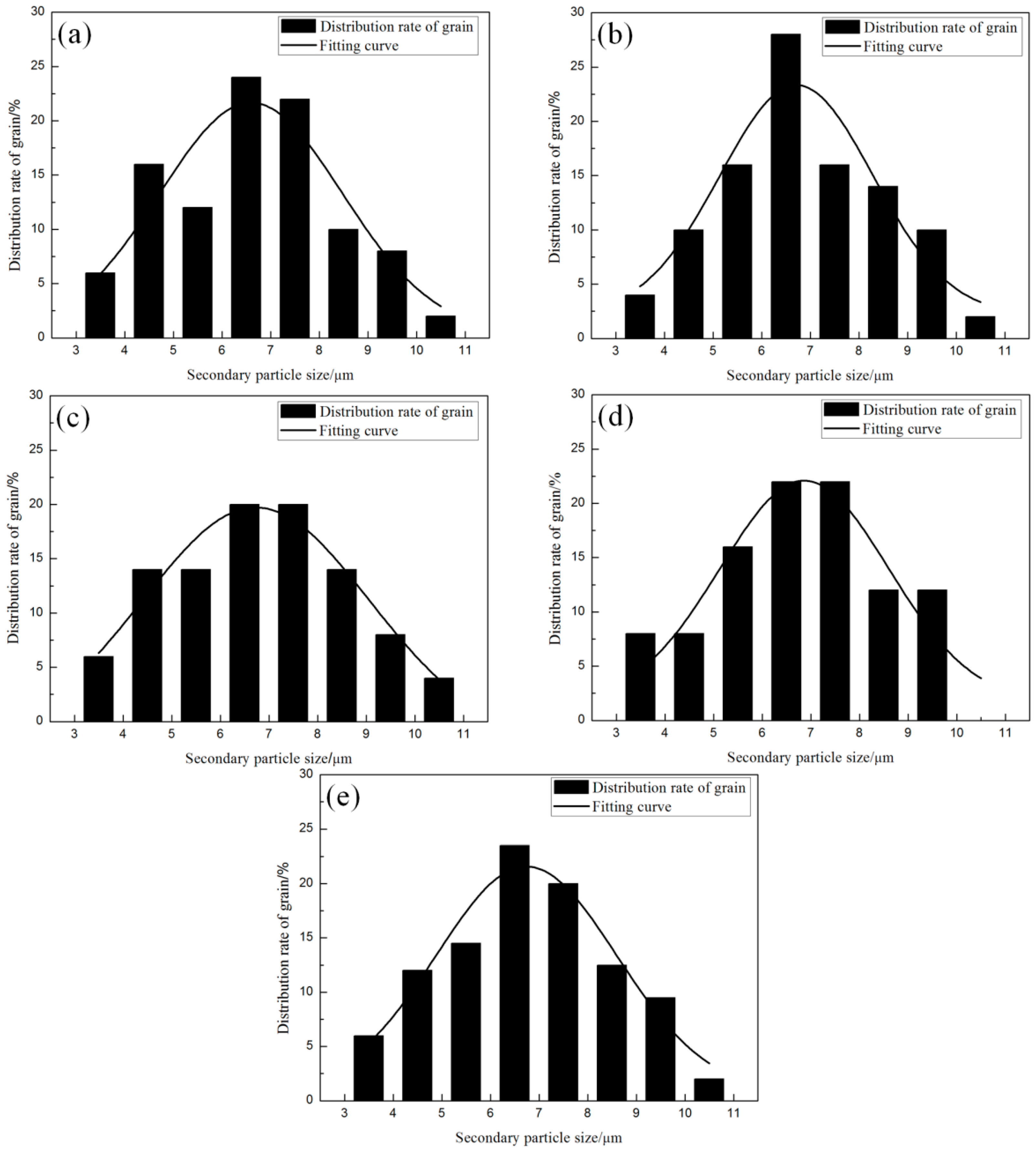
3.2. Effect of the Isothermal Holding Time on Primary α-Al Particles and Secondary Particles
3.3. Microstructures of α1 and α2 in Different Positions
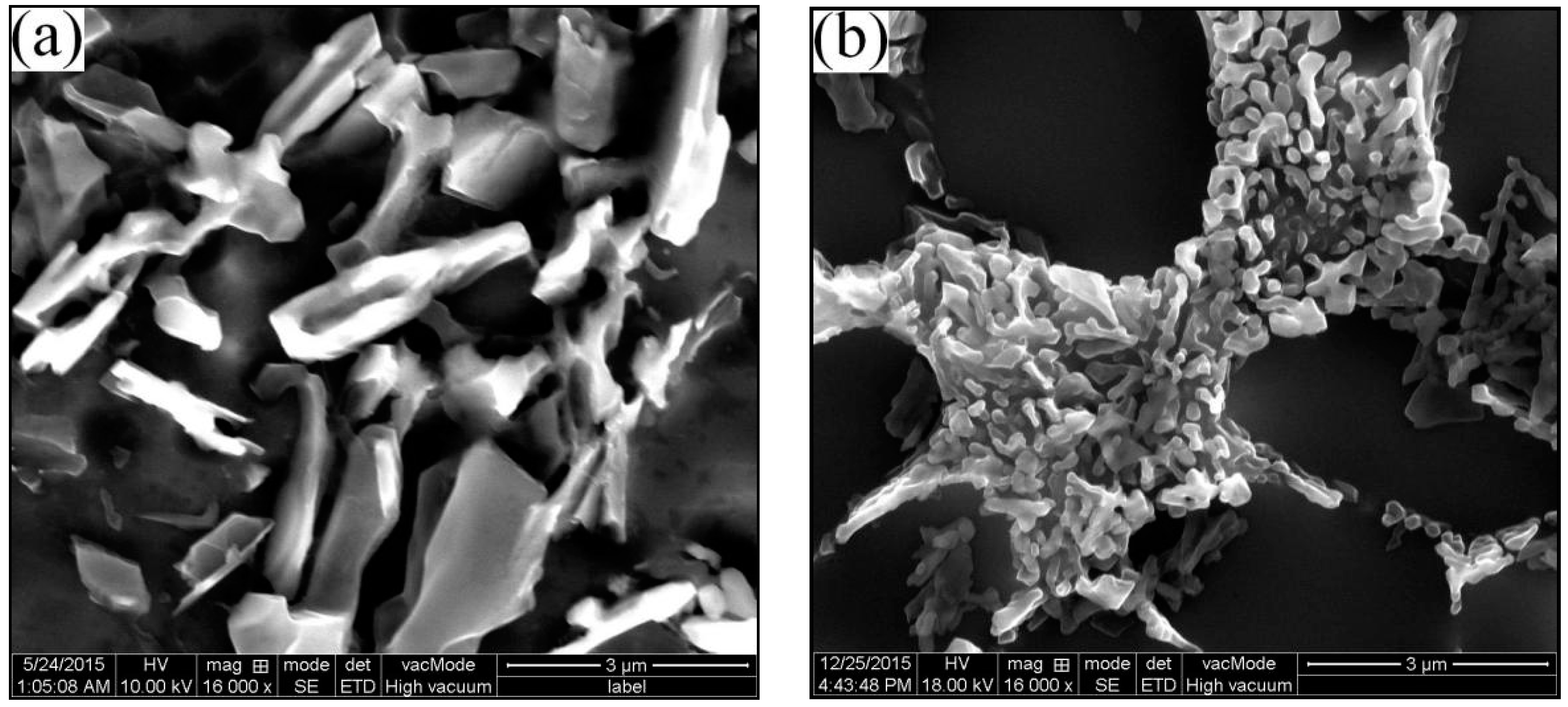
3.4. Eutectic Structures
3.4.1. Eutectic Morphologies of HPDC and RDC
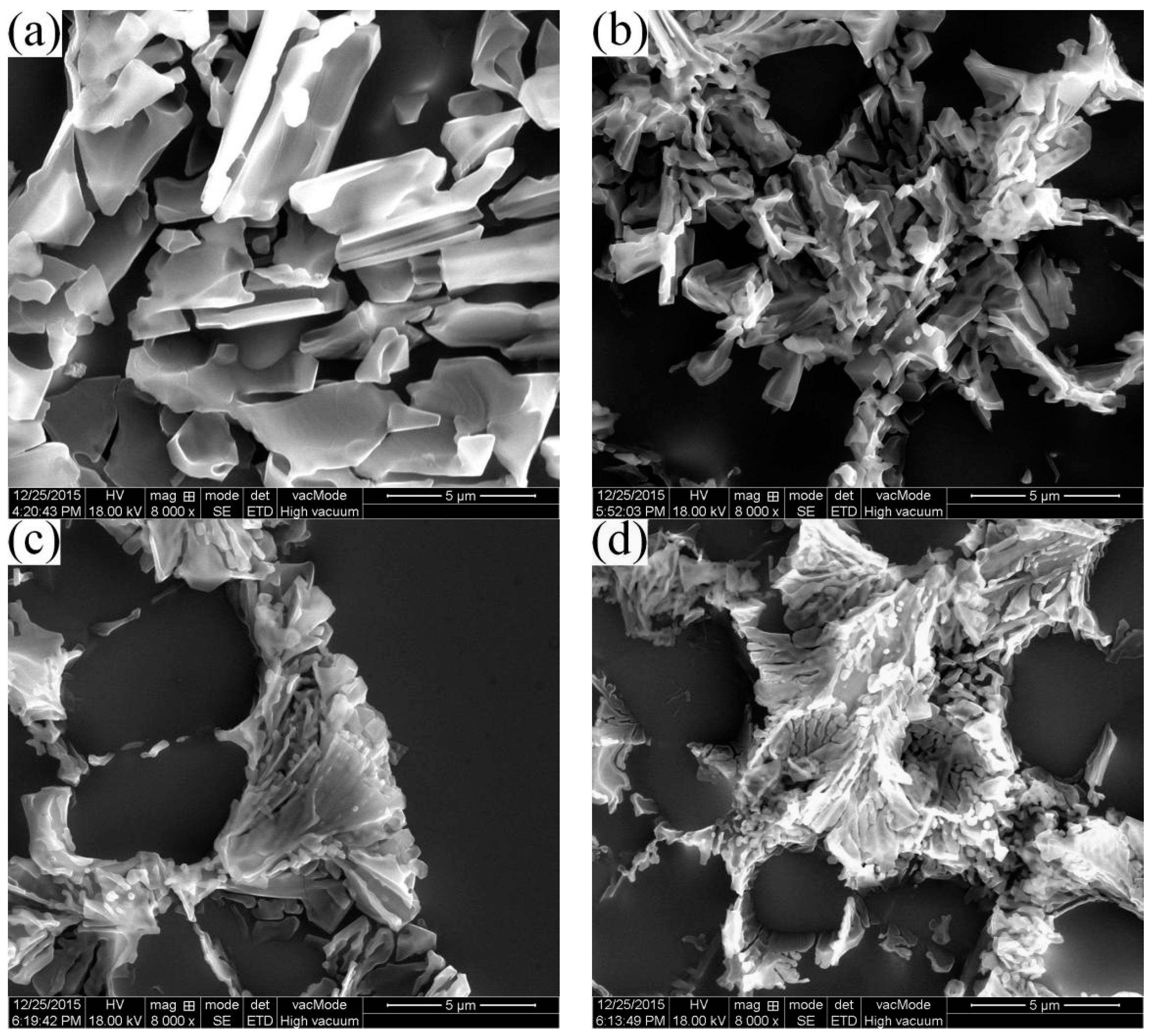
3.4.2. Morphology of Eutectic Structures in Different Positions
4. Discussion
4.1. Microstructural Formation of Semisolid RDC by Self-Inoculation Method (SIM)
4.2. Effect of the Isothermal Holding Time on α1 Particles
4.3. Formation of α2 Particles
4.4. Secondary Solidification Behavior
4.5. Effect of the Filling Distance on the α1 and α2 Particles
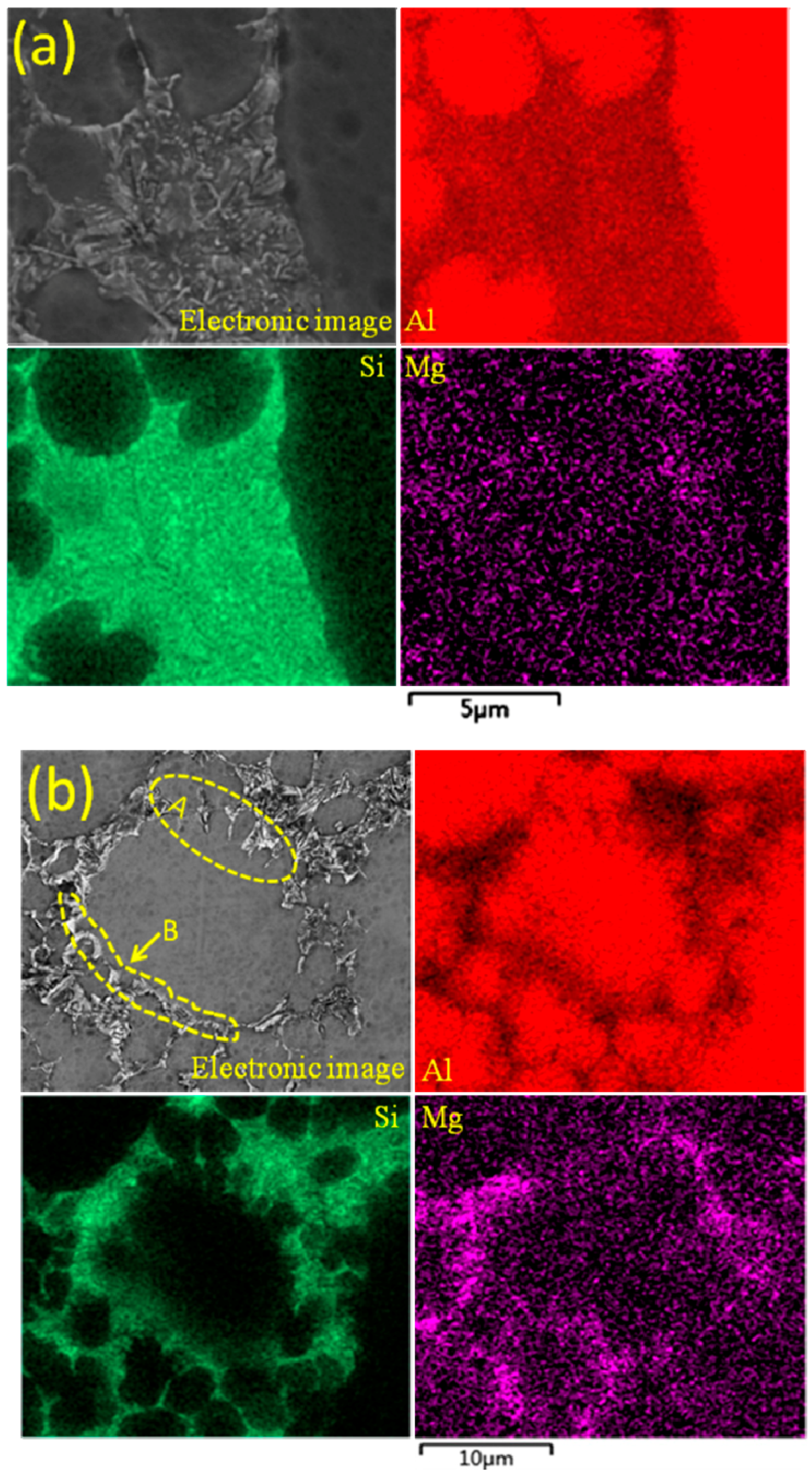
4.6. Formation of Eutectic Si
5. Conclusions
- Compared with dendritic microstructures produced by the HPDC process, the component with non-dendritic and uniformly distributed microstructures can be produced by the RDC process (combining SIM with HPDC).
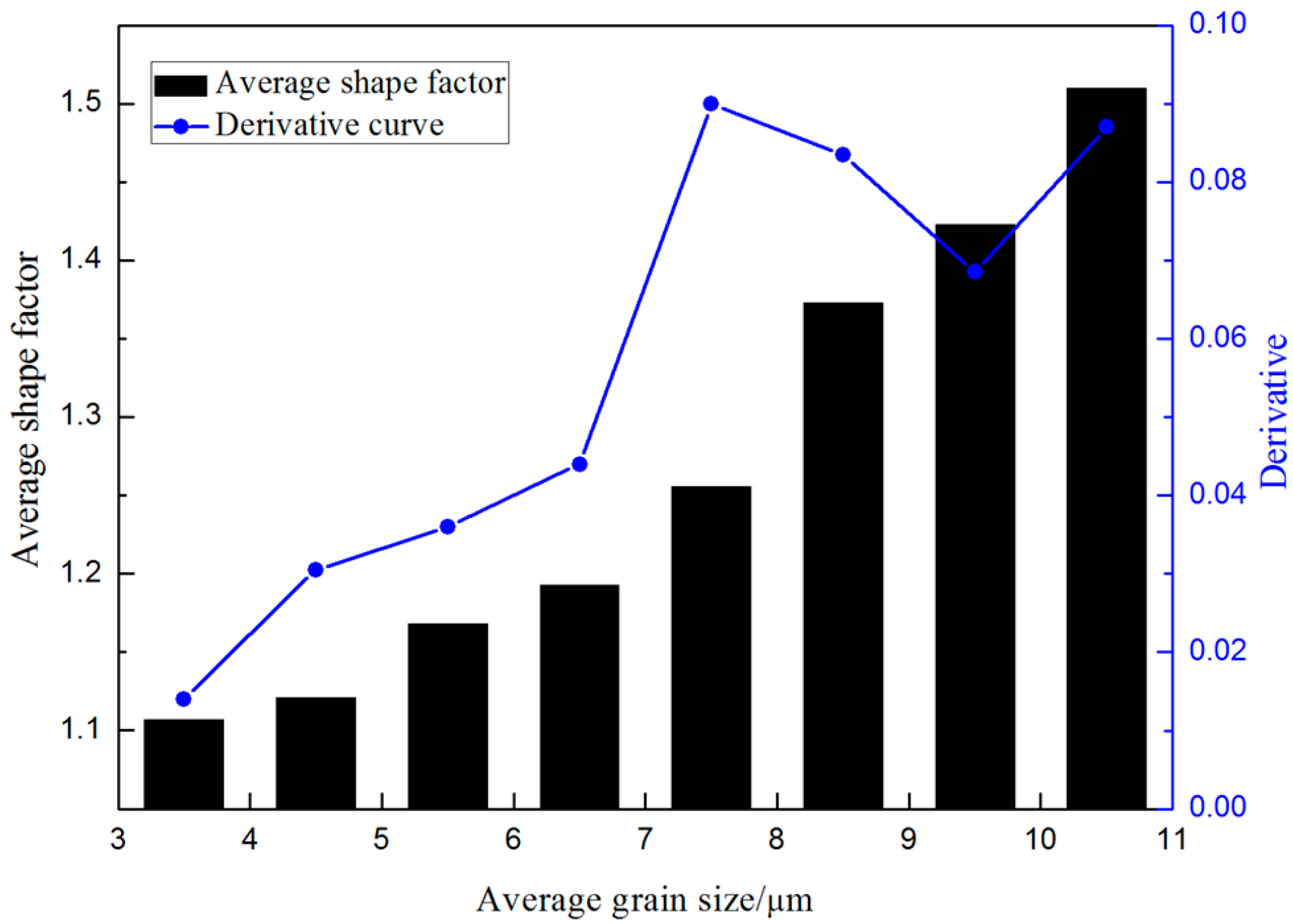
- The isothermal holding time of the slurry has a large effect on the primary particles, but has little effect on the secondary particles. The growth rate of the primary particles in the isothermal holding process conforms to the dynamic equation of Dt3 − D03 = Kt. The suitable holding time for rheo-diecasting of the A356 aluminum alloy is 3 min.
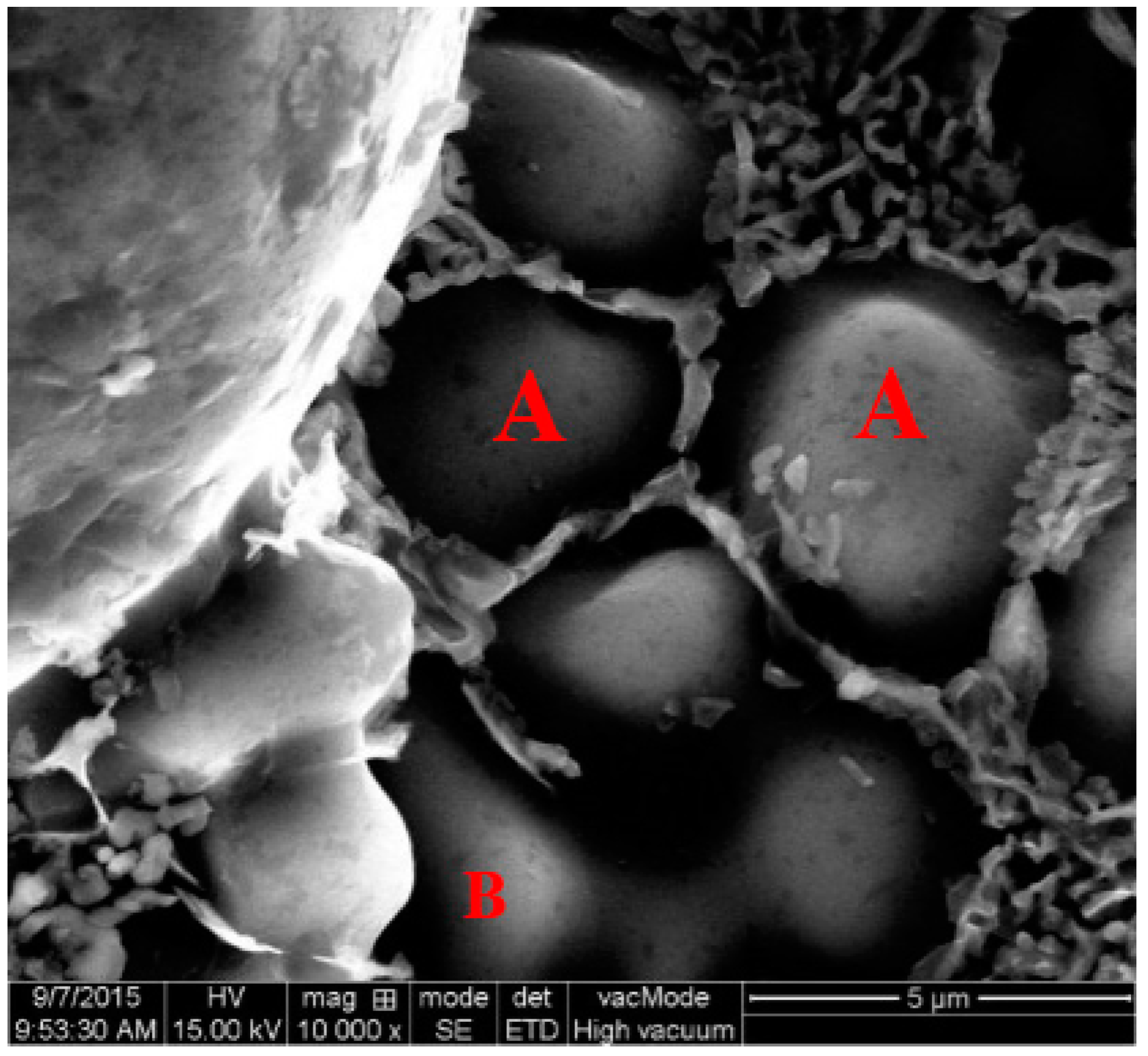
- The nucleation occurs throughout the entire remaining liquid due to the large cooling rate provided by the metallic die block. Meanwhile, the amount of Al atoms precipitated in the remaining liquid will grow and attach to the primary α-Al particles. Nuclei grow stably into globular particles with the limited grain size of 6.5 μm firstly, then both α1 and α2 particles show an unstable growth phenomenon due to the existence of constitutional undercooling.
- The average particle sizes and shape factors of both α1 and α2 decrease with the increase of the filling distance due to different cooling rates in different positions. The Si contents are gradually increasing in the α2 particles while there are no obvious differences in the α1 particles with the increase of the filling distance.
- The growth rate of the eutectic in RDC is 4 times faster than HPDC, which is mainly due to the limitation of the α2 particles in the RDC process. The average eutectic spacing decreases with the increase of the filling distance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flemings, M.C. Behavior of metal alloys in the semi-solid state. Metall. Mater. Trans. A 1991, 22A, 957–981. [Google Scholar] [CrossRef]
- Luo, S.J.; Jiang, Y.Z.; Li, Y.F.; Shan, W. Recognition of Semi-Solid Metal forming technologies. Spec. Cast. Nonferr. Alloys 2012, 7, 603–607. [Google Scholar]
- Eskin, D.G.; Katgerman, S.L. Mechanical properties in the semi-solid state and hot tearing of aluminum alloys. Prog. Mater. Sci. 2004, 5, 629–711. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.F. Research Progress of Semisolid Processing Technology. J. Harbin Univ. Sci. Technol. 2013, 2, 1–6. [Google Scholar]
- Zhao, J.W.; Wu, S.S. Microstructure and mechanical properties of rheo-diecasted A390 alloy. Trans. Nonferr. Met. Soc. China 2010, S3, s754–s757. [Google Scholar] [CrossRef]
- Martinez, R.A.; Flemings, M.C. Evolution of particle morphology in semisolid processing. Metall. Mater. Trans. A 2005, 8, 2205–2210. [Google Scholar] [CrossRef]
- Pan, Q.Y.; Findon, M.; Apelian, D. The Continuous Rheoconversion Process (CRP). In Proceedings of the 8th International Conference on Semi-Solid Process of Alloys and Composites, Limassol, Cyprus, 21–23 September 2004. [Google Scholar]
- Kaudmann, H.; Mundi, A.; Potzinger, R.; Uggowirzer, P.J.; Ishibashi, N. An update on the new rheo-casting-development work for Al and Mg alloys. Die Cast. Eng. 2002, 4, 16–19. [Google Scholar]
- Midson, S.P. Rheocasting processes for semi-solid casting of aluminum alloy. Die Cast. Eng. 2006, 1, 48–51. [Google Scholar]
- Li, Y.D.; Yang, J.; Ma, Y. Effect of pouring temperature on AM60 Mg alloy semi-solid slurry prepared by self-inoculation method (I). Trans. Nonferr. Met. Soc. China 2010, 6, 1046–1052. [Google Scholar]
- Hitchcock, M.; Wang, Y.; Fan, Z. Secondary solidification behaviour of the Al–Si–Mg alloy prepared by the rheo-diecasting process. Acta Mater. 2007, 5, 1589–1598. [Google Scholar] [CrossRef]
- Ji, S.; Das, A.; Fan, Z. Solidification behavior of the remnant liquid in the sheared semisolid slurry of Sn–15 wt % Pb alloy. Scr. Mater. 2002, 3, 205–210. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z. Extruded microstructure of Zn–5 wt % Al eutectic alloy processed by twin screw extrusion. J. Mater. Sci. Technol. 2012, 11, 1287–1294. [Google Scholar] [CrossRef]
- Reisi, M.; Niroumand, B. Growth of primary particles during secondary cooling of a rheocast alloy. J. Alloys Compd. 2009, 1–3, 643–647. [Google Scholar] [CrossRef]
- Zanler, S.; Ershov, A.; Rack, A.; Garciamareno, F.; Baumbach, T.; Banhart, J. Particle and liquid motion in semi-solid aluminium alloys: A quantitative in situ microradioscopy study. Acta Mater. 2013, 4, 1244–1253. [Google Scholar]
- Guan, R.G.; Chen, L.Q.; Li, J.P.; Wang, F.X. Dynamical solidification behaviors and metal flow during continuous semisolid extrusion process of AZ31 alloy. J. Mater. Sci. Technol. 2009, 3, 395–400. [Google Scholar]
- Zhao, Z.Y.; Guan, R.G.; Wang, X.; Liu, C.M. Microstructure formation mechanism during a novel semisolid rheo-rolling process of AZ91 magnesium alloy. Acta Metall. Sin. 2013, 4, 447–454. [Google Scholar] [CrossRef]
- Chen, Z.W.; Zhang, H.F.; Lei, Y.M. Secondary Solidification Behaviour of AA8006 Alloy Prepared by Suction Casting. J. Mater. Sci. Technol. 2011, 9, 769–775. [Google Scholar] [CrossRef]
- Xing, B.; Li, Y.D.; Feng, J.Y.; Hu, G.S.; Tang, C.L. Rheo-Cast Microstructure and Mechanical Properties of AM60 Alloy Produced by Self-Inoculation Rheo-Diecasting Process. Metals 2016, 3, 69. [Google Scholar] [CrossRef]
- Xing, B.; Li, Y.D.; Ma, Y.; Hao, Y. Evolution of rheocast microstructure of AZ31 alloy in semisolid state. China Foundry 2013, 4, 221–226. [Google Scholar]
- Li, Y.L.; Li, Y.D.; Li, C.; Wu, H.H. Microstructure characteristics and solidification behavior of wrought aluminum alloy 2024 rheo-diecast with self-inoculation method. China Foundry 2012, 4, 328–336. [Google Scholar]
- Li, Y.D.; Zhang, X.L.; Ma, Y.; Apelian, D.; Zhou, H.W.; Liu, X.H. Effect of mixing rate and temperature on primary Si phase of hypereutectic Al–20Si alloy during controlled diffusion solidification (CDS) process. China Foundry 2015, 3, 173–179. [Google Scholar]
- Deepak, K.S.; Mihira, A.; Mandai, A.; Chakraborty, M. Coarsening kinetics of semi-solid A356–5 wt % TiB2 in situ composite. Trans. Nonferr. Met. Soc. India 2015, 6, 1075–1080. [Google Scholar] [CrossRef]
- Voorhees, P.W.; Hardy, S.C. Ostwald ripening in a system with a high volume fraction of coarsening phase. Metall. Mater. Trans. A 1988, 11, 2713–2721. [Google Scholar]
- Hu, G.X.; Cai, X.; Rong, Y.H. Fundamentals of Materials Science; Shanghai Jiao Tong University Press: Shanghai, China, 2015; Volume 3, p. 232. [Google Scholar]
- Waterloo, G.; Jones, H. Microstructure and thermal stability of melt-spun Al–Nd and Al–Ce alloy ribbons. J. Mater. Sci. 1996, 31, 2301–2310. [Google Scholar] [CrossRef]
- Guo, Z.P.; Xiong, S.M. Effects of alloy materials and process parameters on the heat transfer coefficient at metal/die interface in high pressure die casting. Acta Metall. Sin. 2008, 4, 433–439. [Google Scholar]
- Li, Y.D.; Chen, T.J.; Ma, Y.; Yan, F.Y.; Hao, Y. Microstructural characteristic and secondary solidification behavior of AZ91D alloy prepared by thixoforming. Trans. Nonferr. Met. Soc. China 2008, 1, 18–23. [Google Scholar]
- Mullins, W.W.; Sekerka, R.F. Morphological stability of a partical growing by diffusion or heat flow. J. Appl. Phys. 1963, 2, 323–329. [Google Scholar] [CrossRef]
- Burden, M.H.; Hunt, J.D. Cellular and dendritic growth. J. Cryst. Growth 1974, 2, 109–116. [Google Scholar] [CrossRef]
- Hunt, J.D.; Lu, S.Z. Numerical modeling of cellular/dendritic array growth: Spacing and structure predictions. Metall. Mater. Trans. A 1996, 27, 611–623. [Google Scholar] [CrossRef]
- Chen, C.P.; Tsao, C.Y. Semi-solid deformation of non dendritic struction—I. Phenomenological behavior. Acta Mater. 1997, 5, 1955–1968. [Google Scholar] [CrossRef]
- Yang, W.; Liu, F.; Wang, H.F.; Lu, B.P.; Yang, G.C. Non-equilibrium transformation kinetics and primary grain size distribution in the rapid solidification of Fe–B hypereutectic alloy. J. Alloys Compd. 2011, 509, 2903–2908. [Google Scholar] [CrossRef]
- Jackson, K.A.; Hunt, J.D. Lamellar and rod eutectic growth. Trans. Metall. Soc. AIME 1966, 236, 1129–1142. [Google Scholar]
- Grugel, R.; Kurz, W. Growth of interdendritic eutectic in directionally solidified Al–Si alloys. Metall. Mater. Trans. A 1987, 18, 1137–1142. [Google Scholar] [CrossRef]
- Bayraktar, Y.; Ling, D.; Jones, H. Formation and segregation of primary silicon in Bridgman solidified Al–18.3 wt % Si alloy. J. Mater. Sci. 1995, 30, 5939–5943. [Google Scholar] [CrossRef]

| Si | Mg | Fe | Ti | Cu | Zn | Al |
|---|---|---|---|---|---|---|
| 7.06 | 0.27 | 0.115 | 0.097 | 0.001 | 0.01 | Balance |
| Positions | Primary Particles (α1) | Secondary Particles (α2) | ||
|---|---|---|---|---|
| Average Particle Size/μm | Shape Factor | Average Particle Size/μm | Shape Factor | |
| A | 73.32 | 1.63 | 35.34 | 2.02 |
| B | 63.37 | 1.36 | 8.21 | 1.65 |
| C | 63.17 | 1.32 | 7.42 | 1.57 |
| D | 55.48 | 1.25 | 6.74 | 1.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, Y.; Huang, X.; Ma, Y.; Guan, R. Secondary Solidification Behavior of A356 Aluminum Alloy Prepared by the Self-Inoculation Method. Metals 2017, 7, 233. https://doi.org/10.3390/met7070233
Li M, Li Y, Huang X, Ma Y, Guan R. Secondary Solidification Behavior of A356 Aluminum Alloy Prepared by the Self-Inoculation Method. Metals. 2017; 7(7):233. https://doi.org/10.3390/met7070233
Chicago/Turabian StyleLi, Ming, Yuandong Li, Xiaofeng Huang, Ying Ma, and Renguo Guan. 2017. "Secondary Solidification Behavior of A356 Aluminum Alloy Prepared by the Self-Inoculation Method" Metals 7, no. 7: 233. https://doi.org/10.3390/met7070233





