The Nitrocarburising Response of Low Temperature Bainite Steel
Abstract
:1. Introduction
2. Materials and Methods
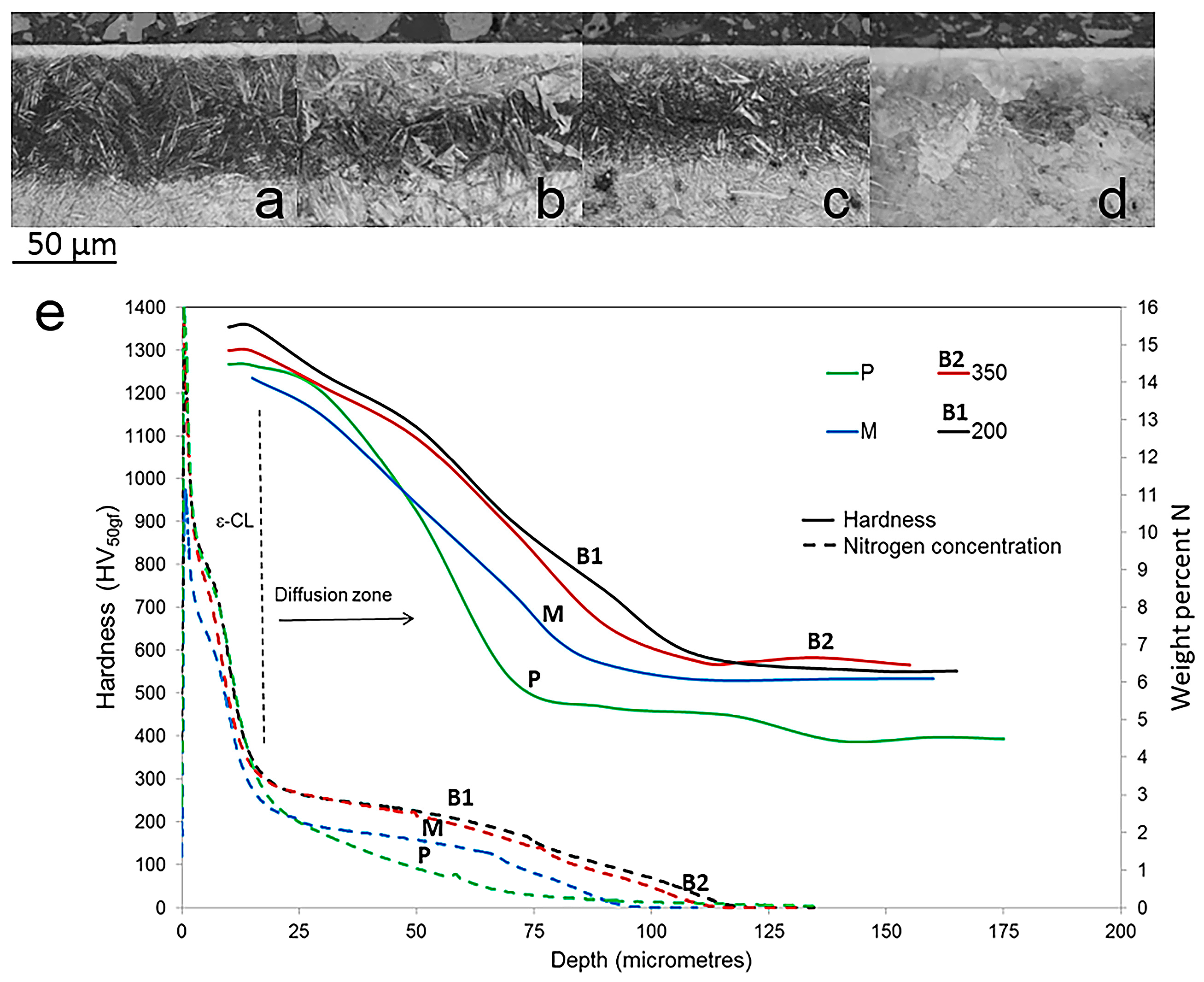
3. Results and Discussion
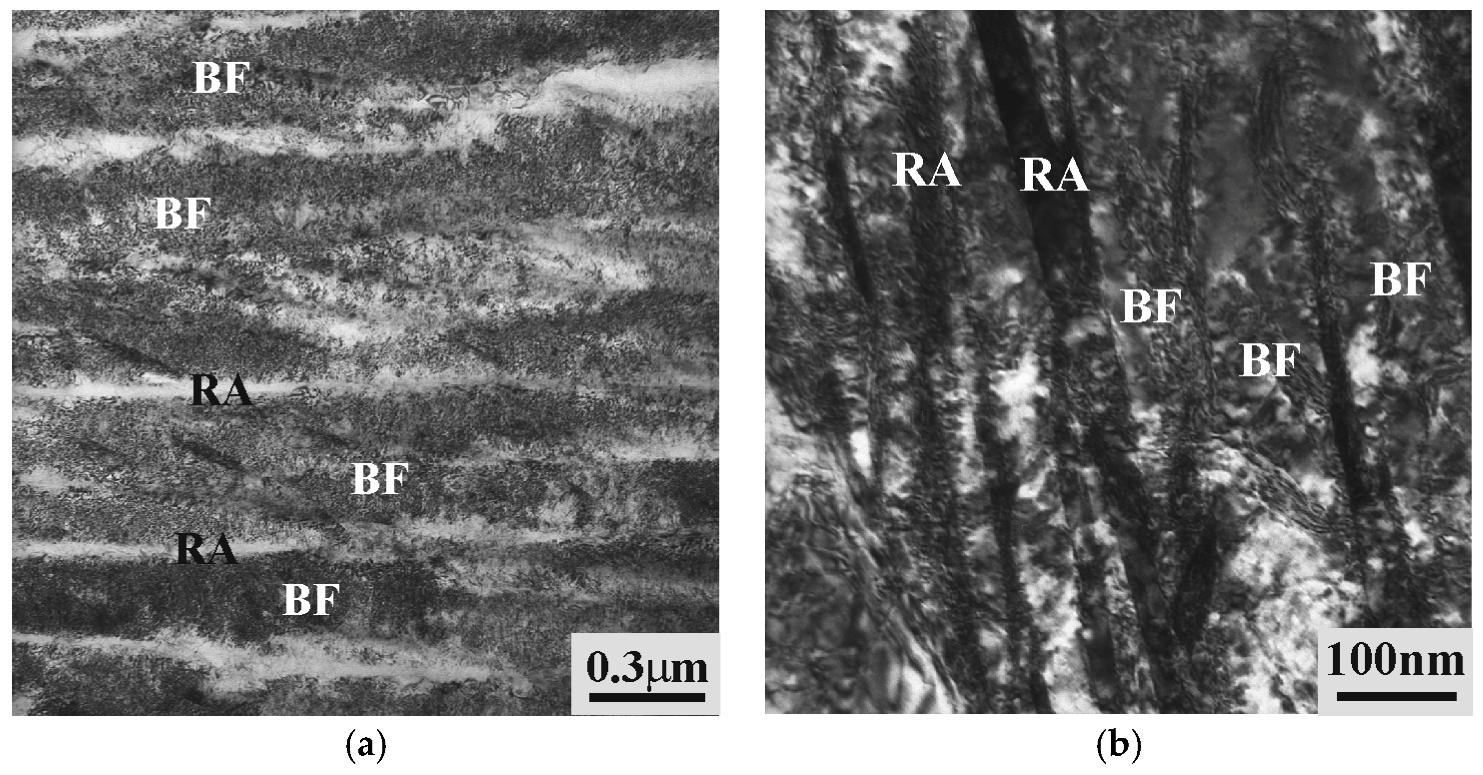
3.1. Characterization of Initial Micostructures
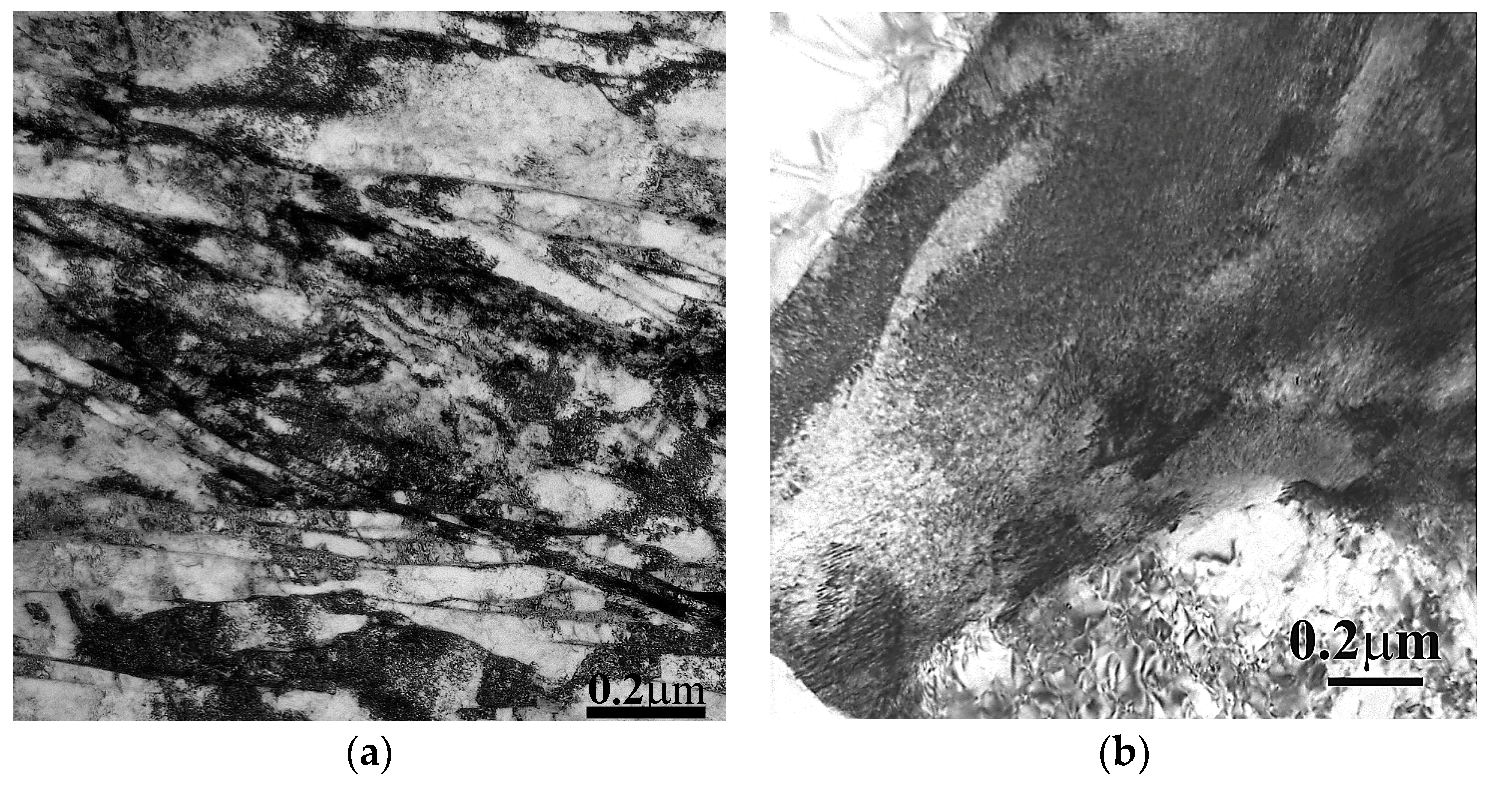
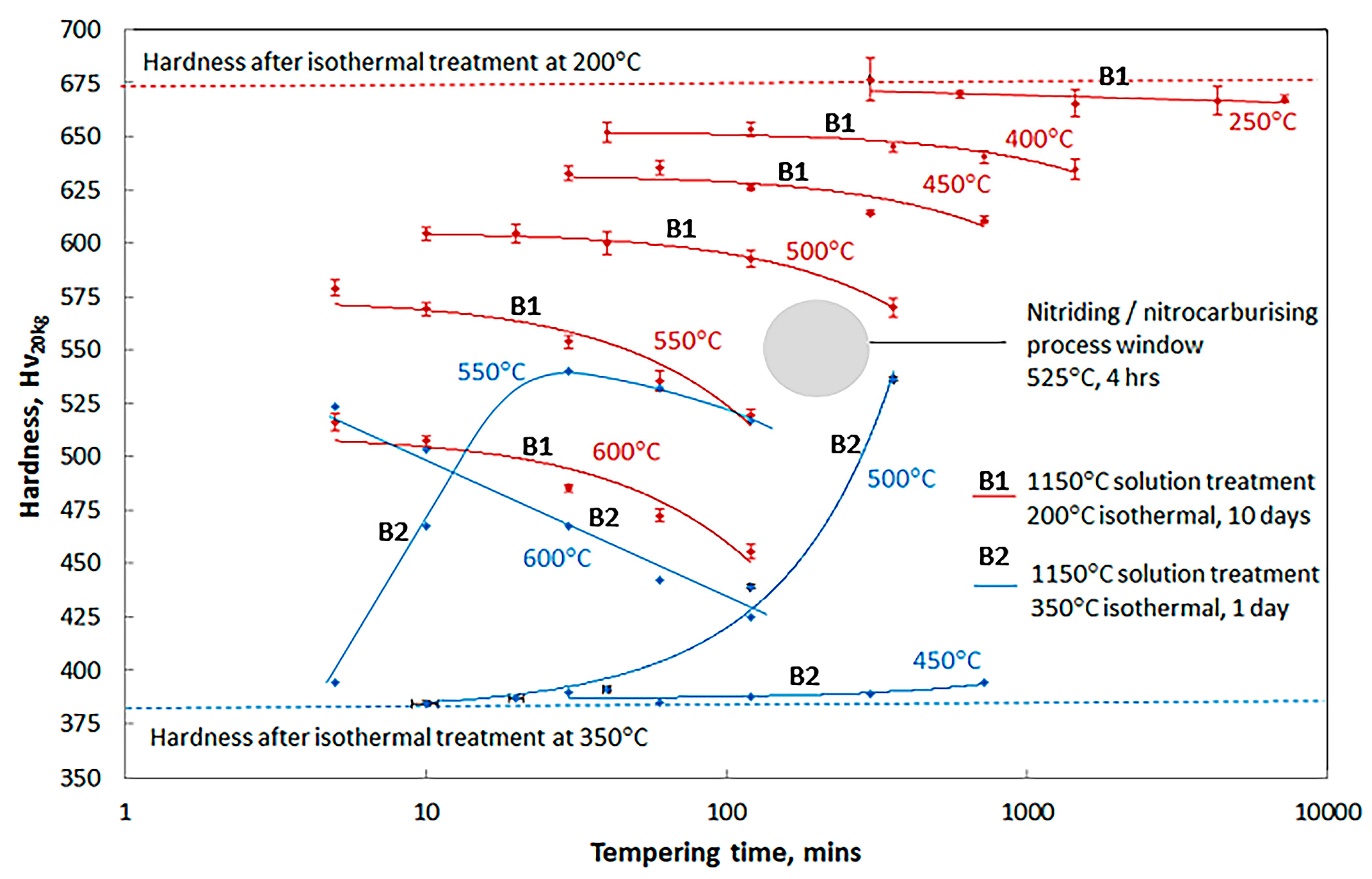
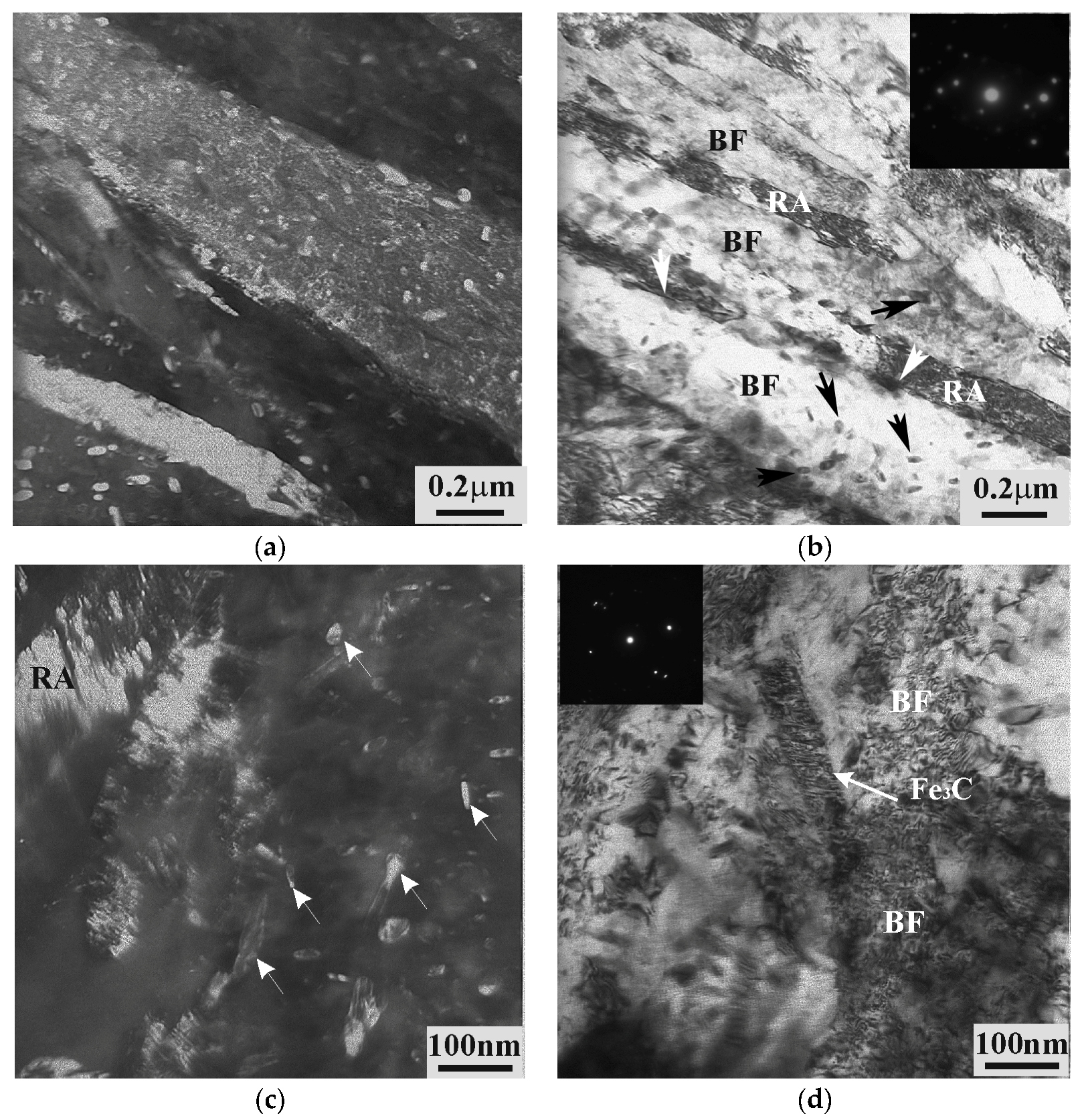
3.2. Effect of Tempering on Microstructure and Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strength bainitic steels. Mater. Sci. Technol. 2001, 17, 517–522. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels, 2nd ed.; Institute of Materials: London, UK, 2001. [Google Scholar]
- Garcia-Mateo, C.; Caballero, F.G. Ultra-high-strength bainitic steels. ISIJ Int. 2005, 45, 1736–1740. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Beladi, H.; Xiong, X.Y.; Adachi, Y.; Hodgson, P.D. Nano-scale microstructural characterization of a nanobainitc steel. Acta Mater. 2011, 59, 5511–5522. [Google Scholar] [CrossRef]
- Beladi, H.; Adachi, Y.; Timokhina, I.; Hodgson, P.D. Crystallographic analysis of nanobainitic steels. Scr. Mater. 2009, 60, 455–460. [Google Scholar] [CrossRef]
- Beladi, H.; Tari, V.; Timokhina, I.B.; Cizek, P.; Rohrer, G.S.; Rollett, A.; Hodgson, P.D. On the crystallographic characteristics of nanobainitic steel. Acta Mater. 2017, 127, 426–437. [Google Scholar] [CrossRef]
- Wang, T.S.; Yang, J.; Shang, C.J.; Li, X.Y.; Lv, B.; Zhang, M.; Zhang, F.C. Sliding friction surface microstructure and wear resistance of 9SiCr steel with low-temperature austempering treatment. Surf. Coat. Technol. 2008, 202, 4036–4040. [Google Scholar] [CrossRef]
- Narayanaswamy, B.; Hodgson, P.; Timokhina, I.; Beladi, H. The impact of retained austenite characteristics on the two-body abrasive wear behavior of ultra-high strength bainitic steels. Metall. Mater. Trans. 2016, 47, 4883–4895. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Clarke, A.J.; Garcia-Mateo, C. Examination of carbon partitioning into austenite during tempering of bainite. Scr. Mater. 2010, 63, 442–445. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C.; Capdevila, C.; Babu, S.S. Redistribution of alloying elements during tempering of a nanocrystalline steel. Acta Mater. 2008, 56, 188–189. [Google Scholar] [CrossRef]
- Budinski, K.G. Surface Engineering for Wear Resistance; Prentice-Hall: Upper Saddle River, NJ, USA, 1988. [Google Scholar]
- Tong, W.P.; Tao, N.R.; Wang, Z.B.; Lu, J.; Lu, K. Nitriding iron at lower temperatures. Science 2003, 299, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lu, J.; Wang, L.; Xu, T.; Xue, Q. Surface nanocrystallization by surface mechanical attrition treatment and its effect on structure and properties of plasma nitrided AISI 321 stainless steel. Acta Mater. 2006, 54, 5599–5605. [Google Scholar] [CrossRef]
- Tong, W.P.; Tao, N.R.; Wang, Z.B.; Zhang, H.W.; Lu, J.; Lu, K. The formation of ε-Fe3–2N phase in a nanocrystalline Fe. Scr. Mater. 2004, 50, 647–650. [Google Scholar] [CrossRef]
- Tong, W.P.; Han, Z.; Wang, L.M.; Lu, J.; Lu, K. Low-temperature nitriding of 38CrMoAl steel with a nanostructured surface layer induced by surface mechanical attrition treatment. Surf. Coat. Technol. 2008, 202, 4957–4963. [Google Scholar] [CrossRef]
- Peet, M.J.; Babu, S.S.; Miller, M.K.; Bhadeshia, H.K.D.H. Tempering of low-temperature bainite. Metall. Mater. Trans. A 2017, 48, 1–9. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of low temperature bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Cullity, B.D.; Weymouth, J.W. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Publishing: Boston, MA, USA, 1978; p. 555. [Google Scholar]
- Fonda, R.W.; Spanos, G.; Vandermeer, R.A. Observations of plate martensite in a low carbon steel. Scr. Metall. Mater. 1994, 31, 683–686. [Google Scholar] [CrossRef]
- Morito, S.; Huang, X.; Furuhara, T.; Maki, T.; Hansen, N. The morphology and crystallography of lath martensite in alloy steels. Acta Mater. 2006, 54, 5323–5331. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabijanic, D.; Timokhina, I.; Beladi, H.; Hodgson, P. The Nitrocarburising Response of Low Temperature Bainite Steel. Metals 2017, 7, 234. https://doi.org/10.3390/met7070234
Fabijanic D, Timokhina I, Beladi H, Hodgson P. The Nitrocarburising Response of Low Temperature Bainite Steel. Metals. 2017; 7(7):234. https://doi.org/10.3390/met7070234
Chicago/Turabian StyleFabijanic, Daniel, Ilana Timokhina, Hossein Beladi, and Peter Hodgson. 2017. "The Nitrocarburising Response of Low Temperature Bainite Steel" Metals 7, no. 7: 234. https://doi.org/10.3390/met7070234



