Effect of Anode Pulse-Width on the Microstructure and Wear Resistance of Microarc Oxidation Coatings
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Testing and Characterization
3. Results and Discussion
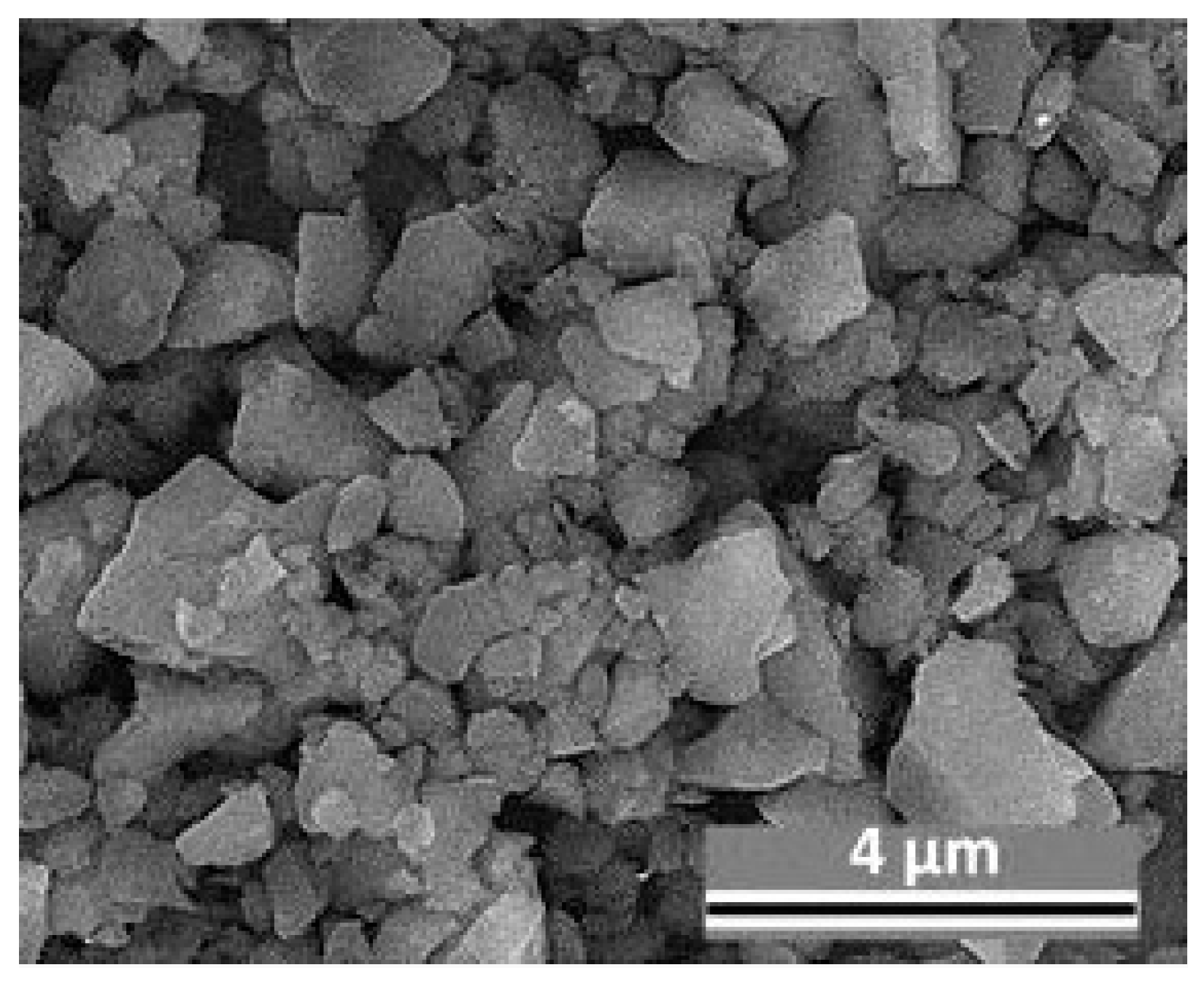
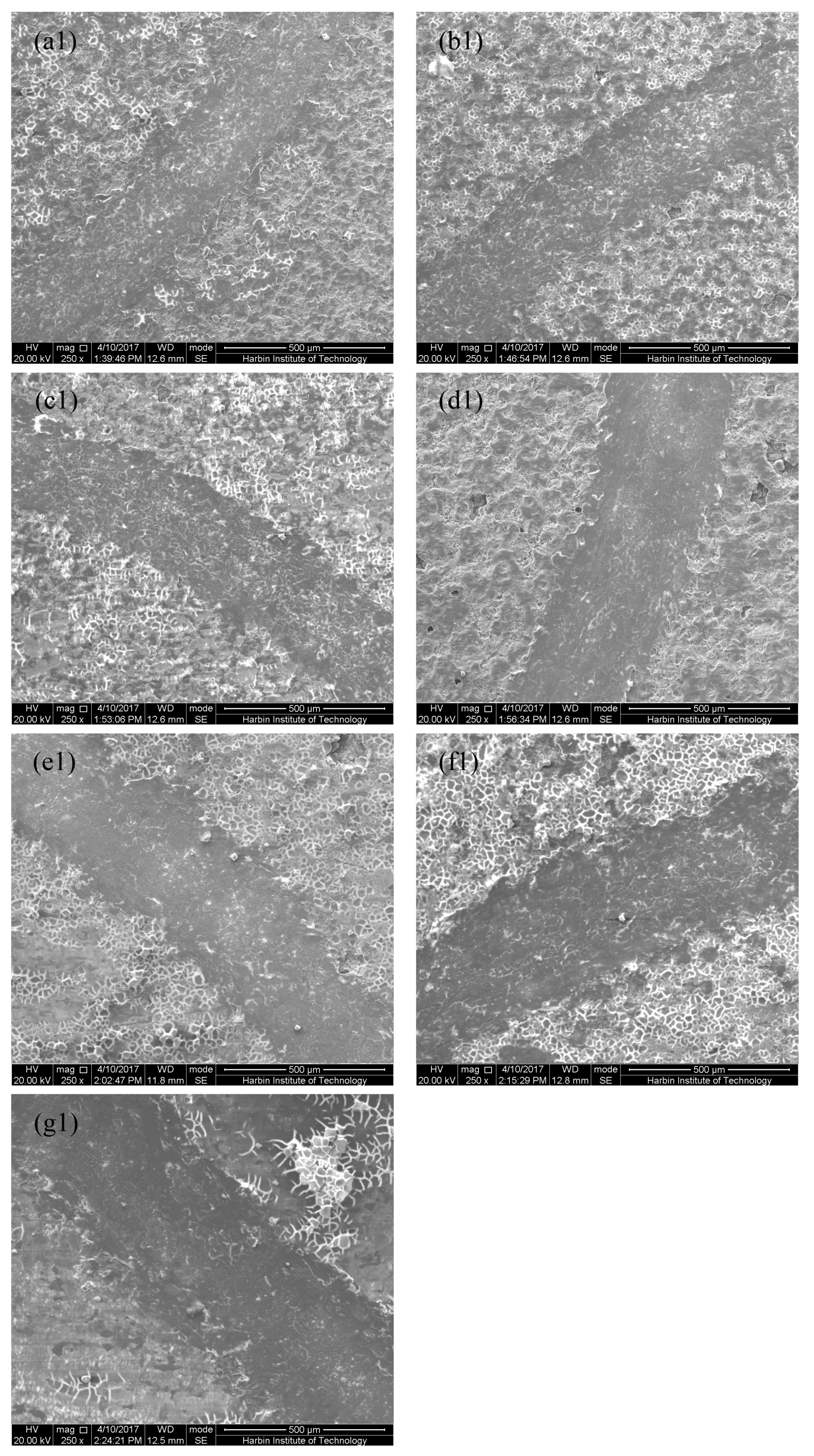
3.1. Microstructure of MAO Coatings and MAO Self-Lubricating Composite Coatings
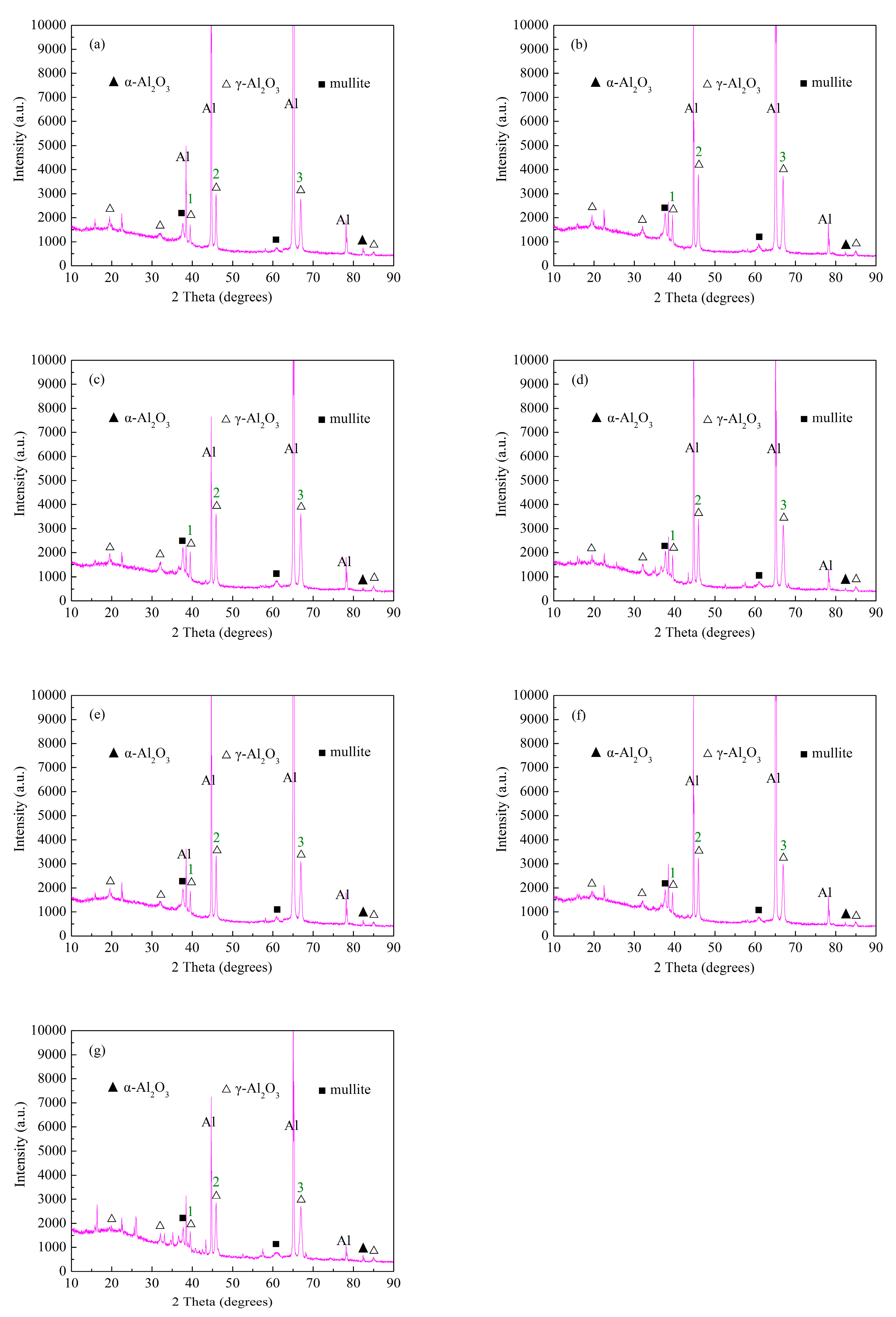
3.2. Effect of Anode Pulse-Width on the Phase Structure of MAO Ceramic Coatings
3.3. Effect of Anode Pulse-Width on the Adhesion Strength of MAO Ceramic Coatings
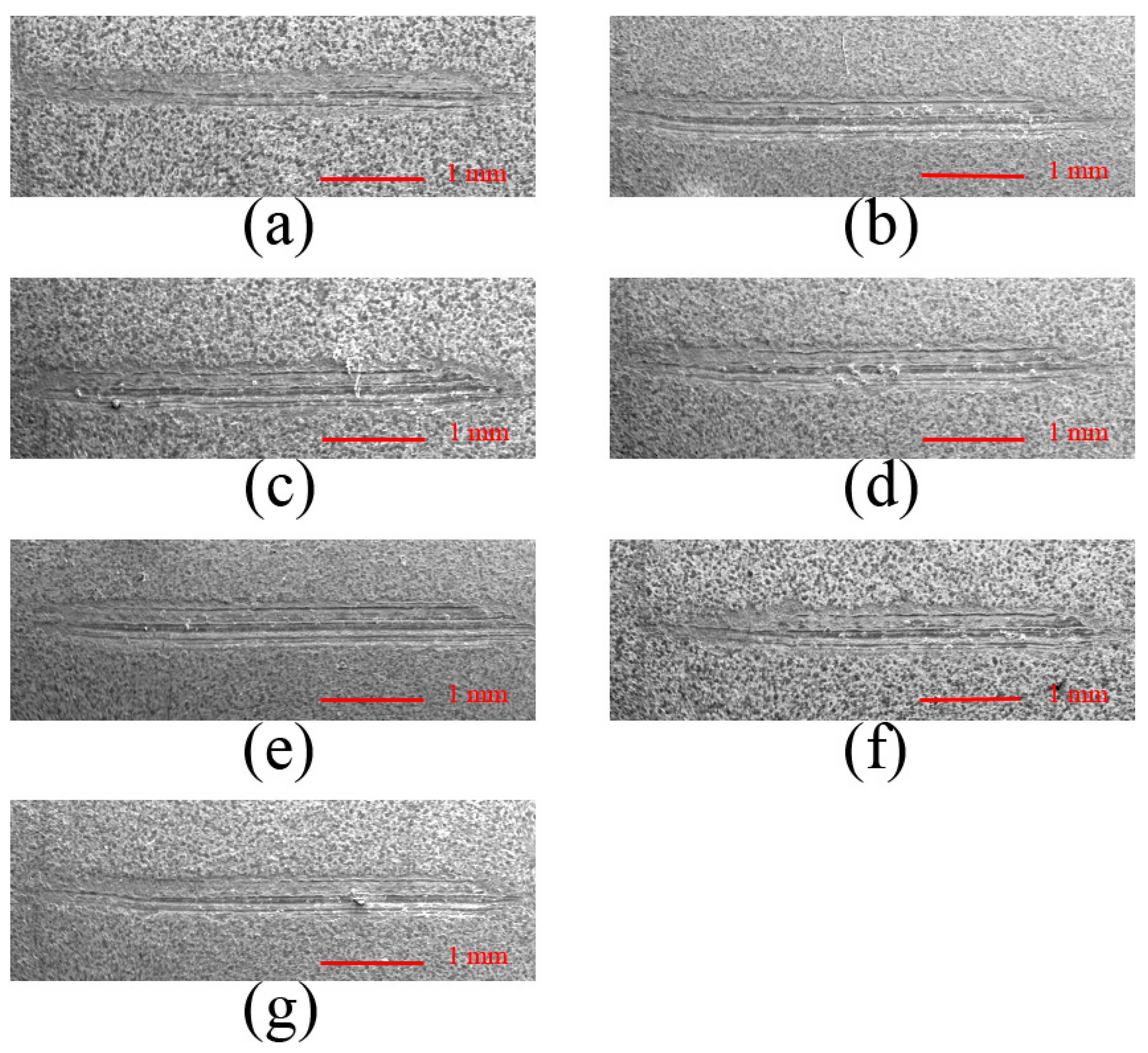
3.4. Effect of Anode Pulse-Width on the Wear Resistance of MAO Ceramic Coatings
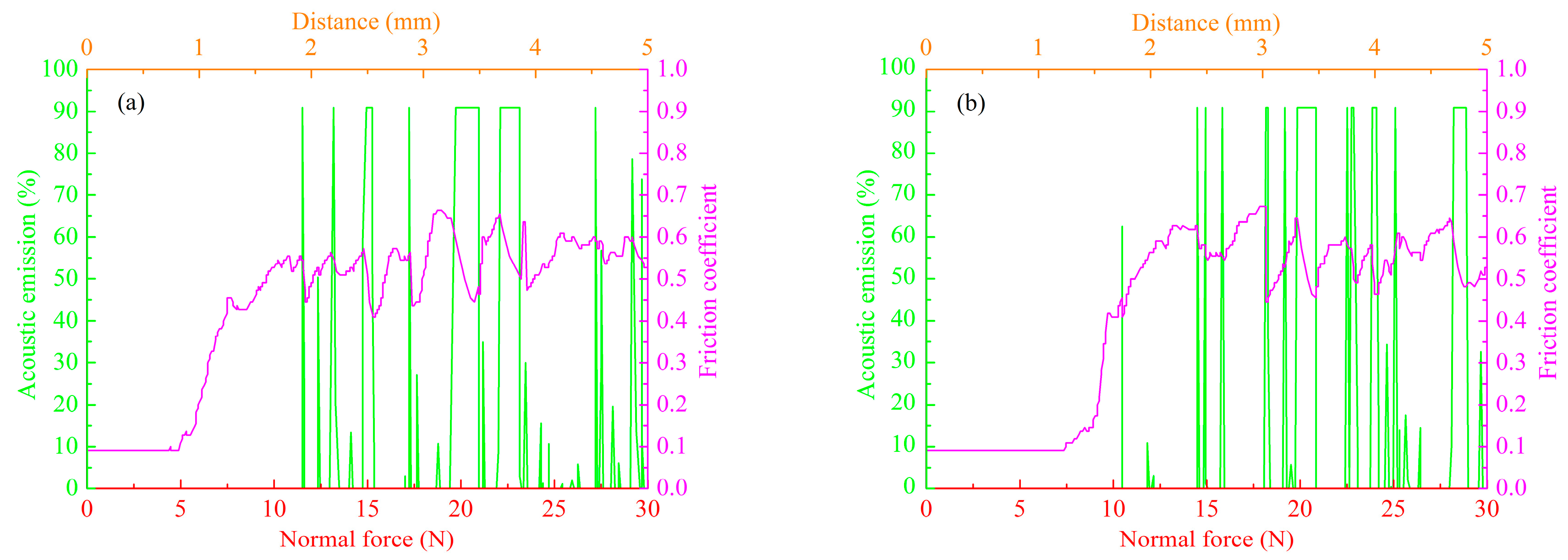
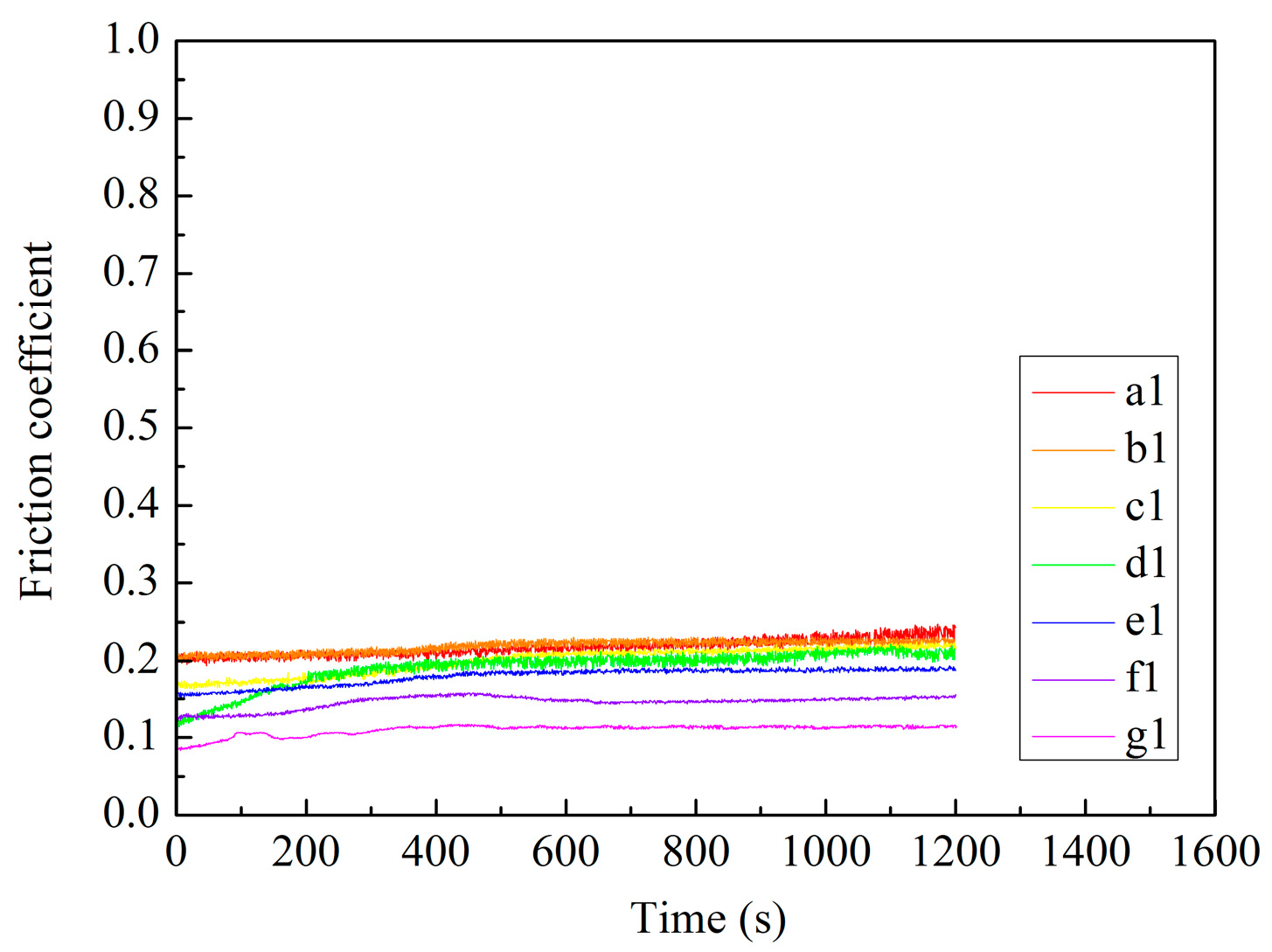
3.5. Tribological Properties of MAO Self-Lubricating Composite Coatings
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.S.; Guo, X.W.; Li, X.D.; Ge, D.Y. Improvement on the fatigue performance of 2024-T4 alloy by synergistic coating technology. Materials 2014, 7, 3533–3546. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.P.; Guo, Y.C.; Yang, Z.; Wang, J.L. Ceramic coating formation on high Si containing Al alloy by PEO process. Surf. Eng. 2016, 32, 428–434. [Google Scholar] [CrossRef]
- Rao, R.N.; Das, S.; Mondal, D.P.; Dixit, G. Effect of heat treatment on the sliding wear behavior of aluminium alloy (Al–Zn–Mg) hard particle composite. Tribol. Int. 2010, 43, 330–339. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lee, J.L.; Kuo, T.h.; Kuo, S.N.; Tseng, K.H. The influence of sodium tungstate concentration and anodizing conditions on microarc oxidation (MAO) coatings for aluminum alloy. Surf. Coat. Technol. 2012, 206, 3437–3443. [Google Scholar] [CrossRef]
- Polat, A.; Makaraci, M.; Usta, M. Influence of sodium silicate concentration on structural and tribological properties of microarc oxidation coatings on 2017A aluminum alloy substrate. J. Alloys Compd. 2010, 504, 519–526. [Google Scholar] [CrossRef]
- Nimura, K.; Sugawara, T.; Jibiki, T.; Ito, S.; Shima, M. Surface modification of aluminum alloy to improve fretting wear properties. Tribol. Int. 2016, 93, 702–708. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, L.; Qi, Y.L.; Cai, W.; Jiang, Z.H. Self-lubricating Al2O3/PTFE composite coating formation on surface of aluminium alloy. Surf. Coat. Technol. 2010, 204, 3315–3318. [Google Scholar] [CrossRef]
- Tsutumi, Y.; Niinomi, M.; Nakai, M.; Shimabukuro, M.; Ashida, M.; Chen, P.; Doi, H.; Hanawa, T. Electrochemical surface treatment of a β-titanium alloy to realize an antibacterial property and bioactivity. Metals 2016, 6, 76. [Google Scholar] [CrossRef]
- Yavari, S.A.; Necula, B.S.; Fratila-Apachitei, L.E.; Duszczyk, J.; Apachitei, I. Biofunctional surfaces by plasma electrolytic oxidation on titanium biomedical alloys. Surf. Eng. 2016, 32, 411–417. [Google Scholar] [CrossRef]
- Han, O.S.; Hwang, M.J.; Song, Y.H.; Song, H.J.; Park, Y.J. Effects of surface structure and chemical composition of binary Ti alloys on cell differentiation. Metals 2016, 6, 150. [Google Scholar] [CrossRef]
- Mioč, U.B.; Stojadinović, S.; Nedić, Z. Characterization of bronze surface layer formed by microarc oxidation process in 12-tungstophosphoric acid. Materials 2010, 3, 110–126. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xue, Z.G.; Wang, Q.; Wu, X.Q.; Matykina, E.; Skeldon, P.; Thompson, G.E. New findings on properties of plasma electrolytic oxidation coatings from study of an Al-Cu-Li alloy. Electrochim. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Chen, M.A.; Ou, Y.C.; Yu, C.Y.; Xiao, C.; Liu, S.Y. Corrosion performance of epoxy/BTESPT/MAO coating on AZ31 alloy. Surf. Eng. 2016, 32, 38–46. [Google Scholar] [CrossRef]
- Guan, Y.J.; Xia, Y.; Xu, F.T. Interface fracture property of PEO ceramic coatings on aluminum alloy. Surf. Coat. Technol. 2008, 202, 4204–4209. [Google Scholar] [CrossRef]
- Martin, J.; Leone, P.; Nomine, A.; Renaux, D.V.; Henrion, G.; Belmonte, T. Influence of electrolyte ageing on the plasma electrolytic oxidation of aluminium. Surf. Coat. Technol. 2015, 269, 36–46. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Dehghanian, C.; Baradaran, A. Preparation of ceramic coating on Ti substrate by Plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance. Appl. Surf. Sci. 2011, 257, 2617–2624. [Google Scholar] [CrossRef]
- Jin, F.Y.; Chu, P.K.; Xu, G.D.; Zhao, J.; Tang, D.; Tong, H.H. Structure and mechanical properties of magnesium alloy treated by micro-arc discharge oxidation using direct current and high-frequency bipolar pulsing modes. Mater. Sci. Eng. A 2006, 435–436, 123–126. [Google Scholar] [CrossRef]
- Bayati, M.R.; Moshfegh, A.Z.; Golestani-Fard, F. Effect of electrical parameters on morphology, chemical composition, and photoactivity of the nano-porous titania layers synthesized by pulse-microarc oxidation. Electrochim. Acta 2010, 55, 2760–2766. [Google Scholar] [CrossRef]
- Montazeri, M.; Dehghanian, C.; Shokouhfar, M.; Baradaran, A. Investigation of the voltage and time effects on the formation of hydroxyapatite-containing titania prepared by plasma electrolytic oxidation on Ti–6Al–4V alloy and its corrosion behavior. Appl. Surf. Sci. 2011, 257, 7268–7275. [Google Scholar] [CrossRef]
- Mu, M.; Zhou, X.J.; Xiao, Q.; Liang, J.; Huo, X.D. Preparation and tribological properties of self-lubricating TiO2/graphite composite coating on TI6Al4V alloy. Appl. Surf. Sci. 2012, 258, 8570–8576. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Su, J.F.; Xie, X. A study of the interactive effects of hybrid current modes on the tribological properties of a PEO (plasma electrolytic oxidation) coated AM60B Mg-alloy. Surf. Coat. Technol. 2013, 215, 421–430. [Google Scholar] [CrossRef]
- Wu, H.H.; Lu, X.Y.; Long, B.H.; Wang, X.Q.; Wang, J.B.; Jin, Z.S. The effects of cathodic and anodic voltages on the characteristics of porous nanocrystalline titania coatings fabricated by microarc oxidation. Mater. Lett. 2005, 59, 370–375. [Google Scholar] [CrossRef]
- Li, Q.B.; Liang, J.; Liu, B.X.; Peng, Z.J.; Wang, Q. Effects of cathodic voltages on structure and wear resistance of plasma electrolytic oxidation coatings formed on aluminium alloy. Appl. Surf. Sci. 2014, 297, 176–181. [Google Scholar] [CrossRef]
- Yin, B.; Peng, Z.J.; Liang, J.; Jin, K.J.; Zhu, S.Y.; Yang, J.; Qiao, Z.H. Tribological behavior and mechanism of self-lubricating wear-resistant composite coatings fabricated by one-step plasma electrolytic oxidation. Tribol. Int. 2016, 97, 97–107. [Google Scholar] [CrossRef]
- Qin, Y.K.; Xiong, D.S.; Li, J.L. Characterization and friction behavior of LST/PEO duplex-treated Ti6Al4V alloy with burnished MoS2 film. Appl. Surf. Sci. 2015, 347, 475–484. [Google Scholar] [CrossRef]
- Wang, S.Y.; Si, N.C.; Xia, Y.P.; Liu, L. Influence of nano-SiC on microstructure and property of MAO coating formed on AZ91D magnesium alloy. Trans. Nonferr. Met. Soc. China 2015, 25, 1926–1934. [Google Scholar] [CrossRef]
- Hussein, R.O.; Xie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behavior during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Tekin, K.C.; Malayoglu, U.; Shrestha, S. Tribological behaviour of plasma electrolytic oxide coatings on Ti6Al4V and cp-Ti alloys. Surf. Eng. 2016, 32, 435–442. [Google Scholar] [CrossRef]
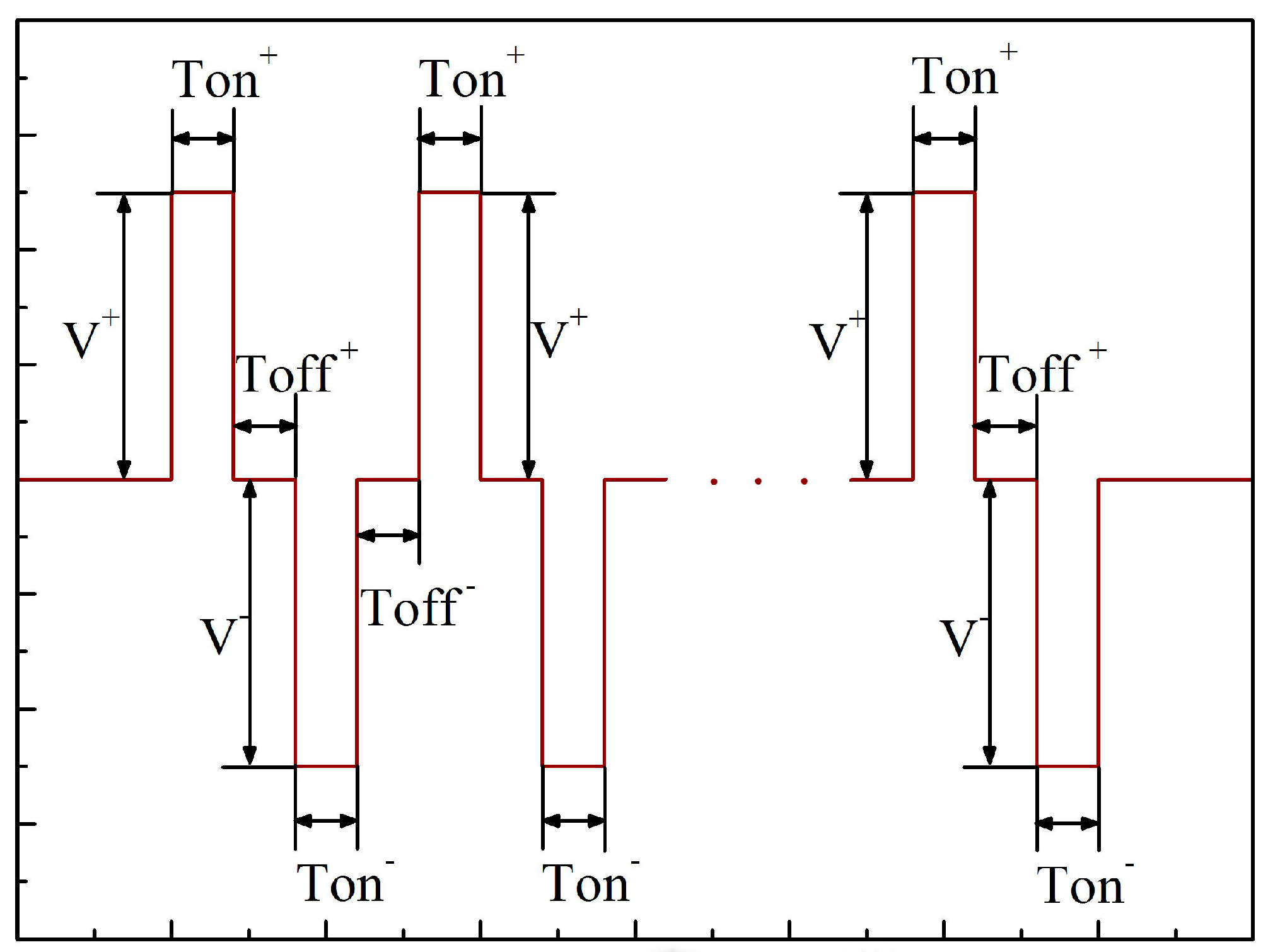
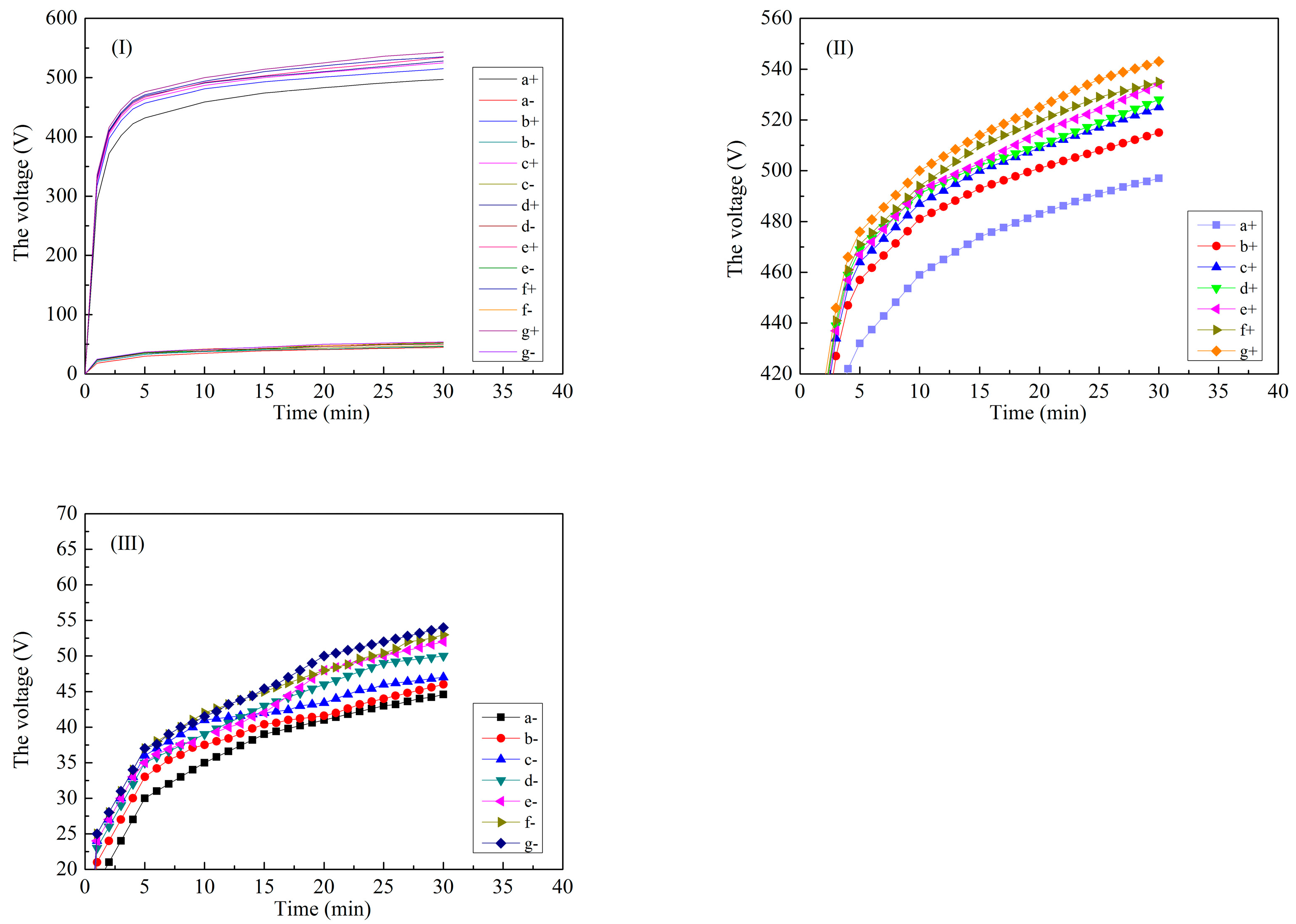
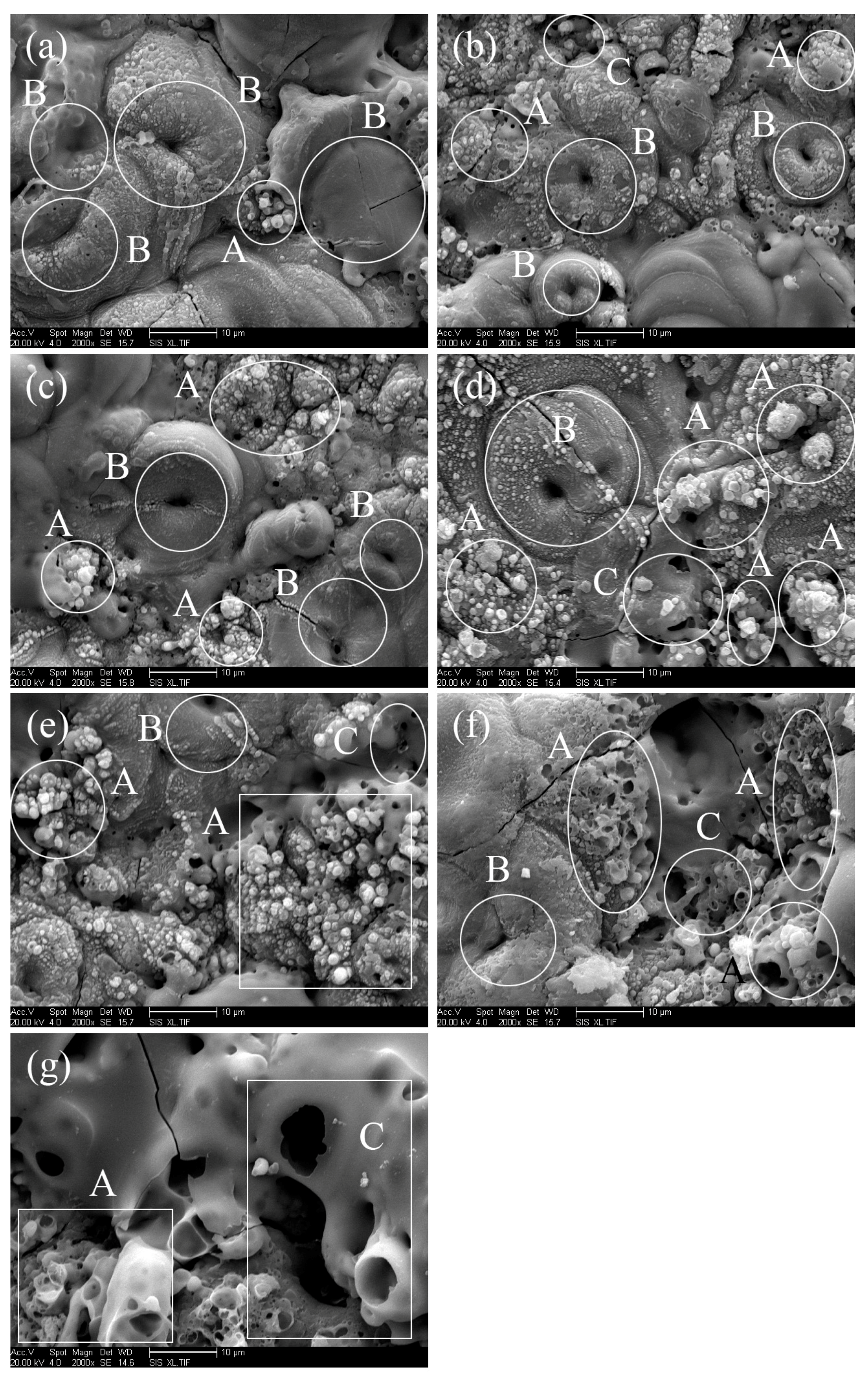
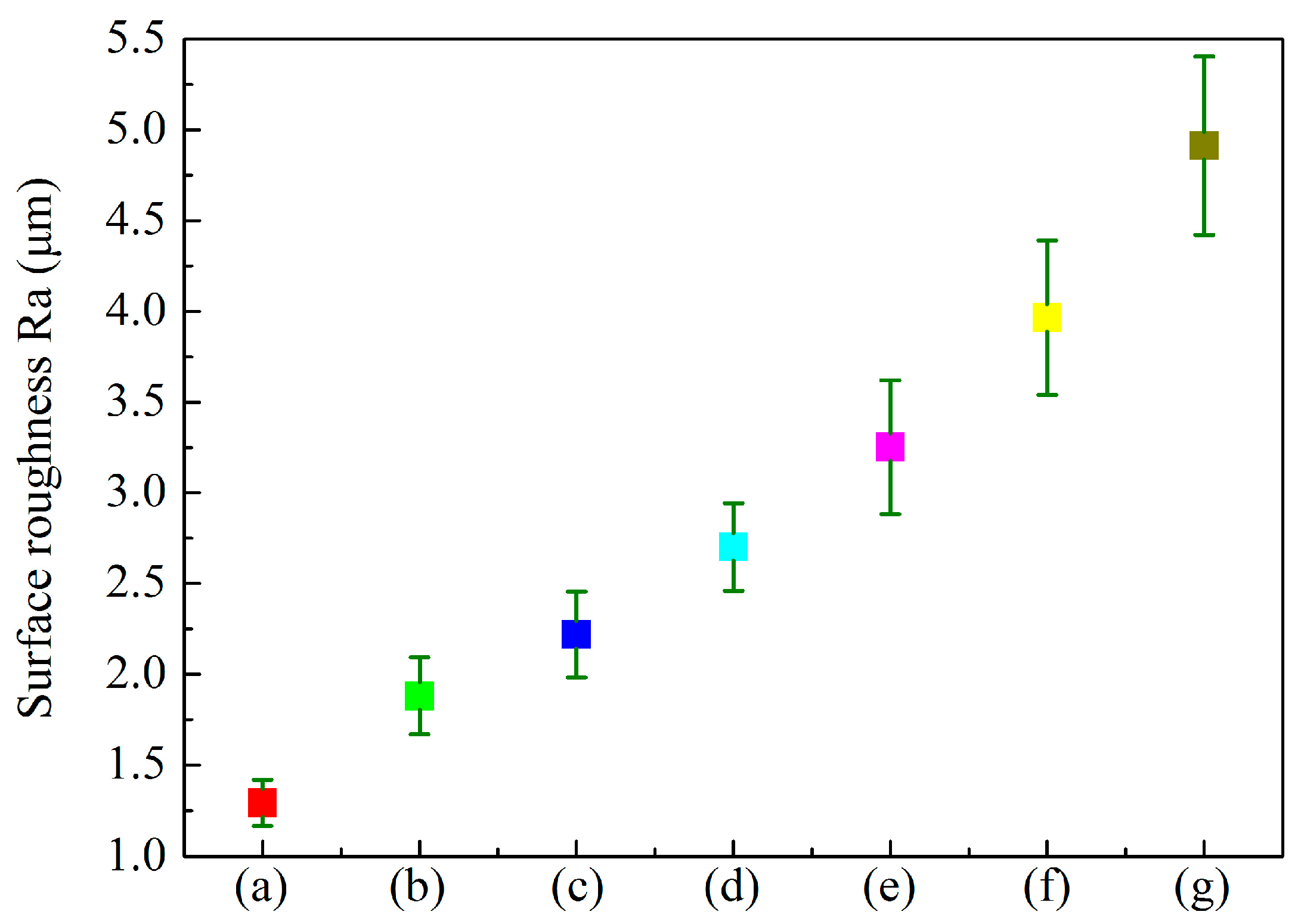
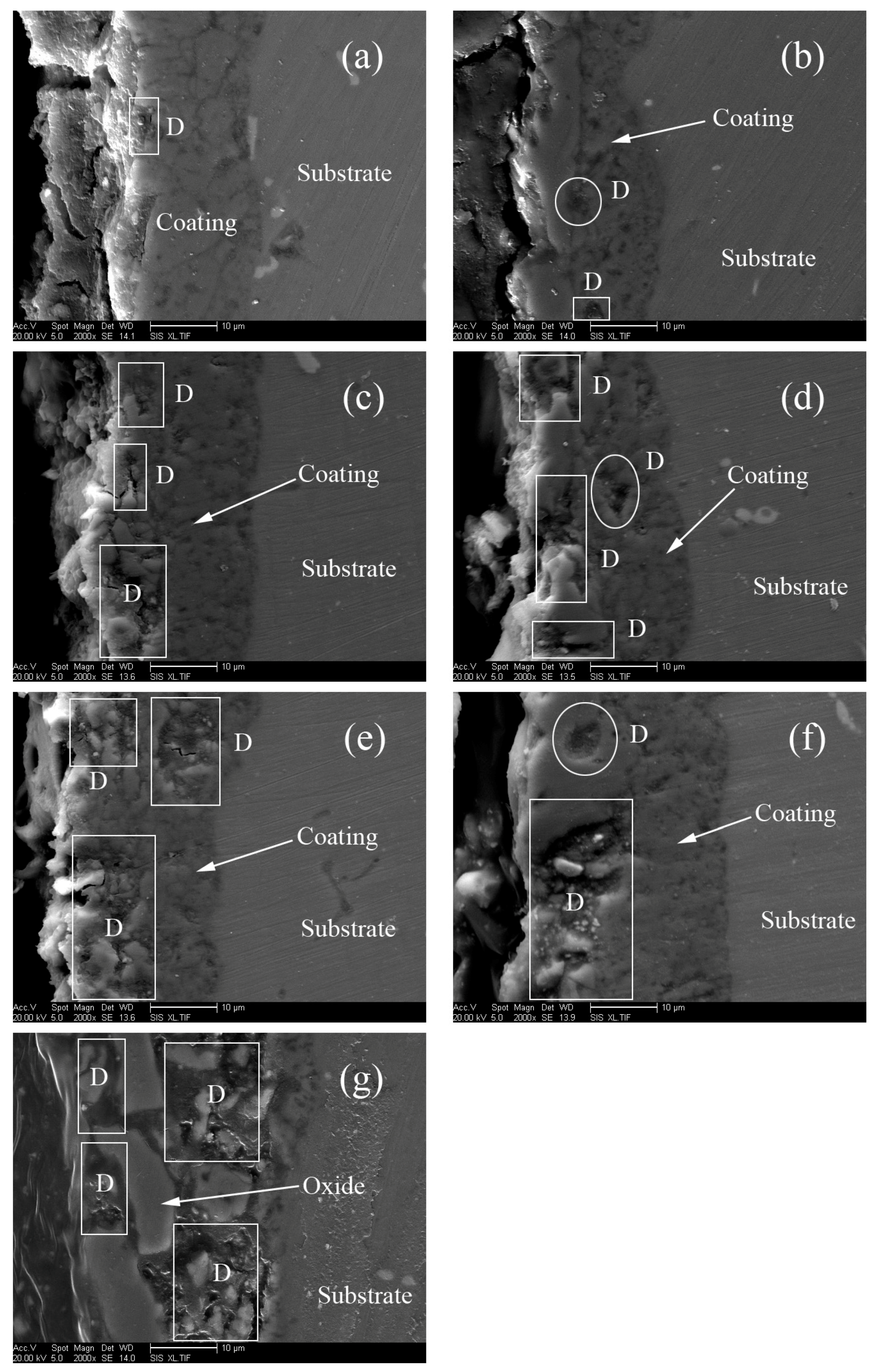
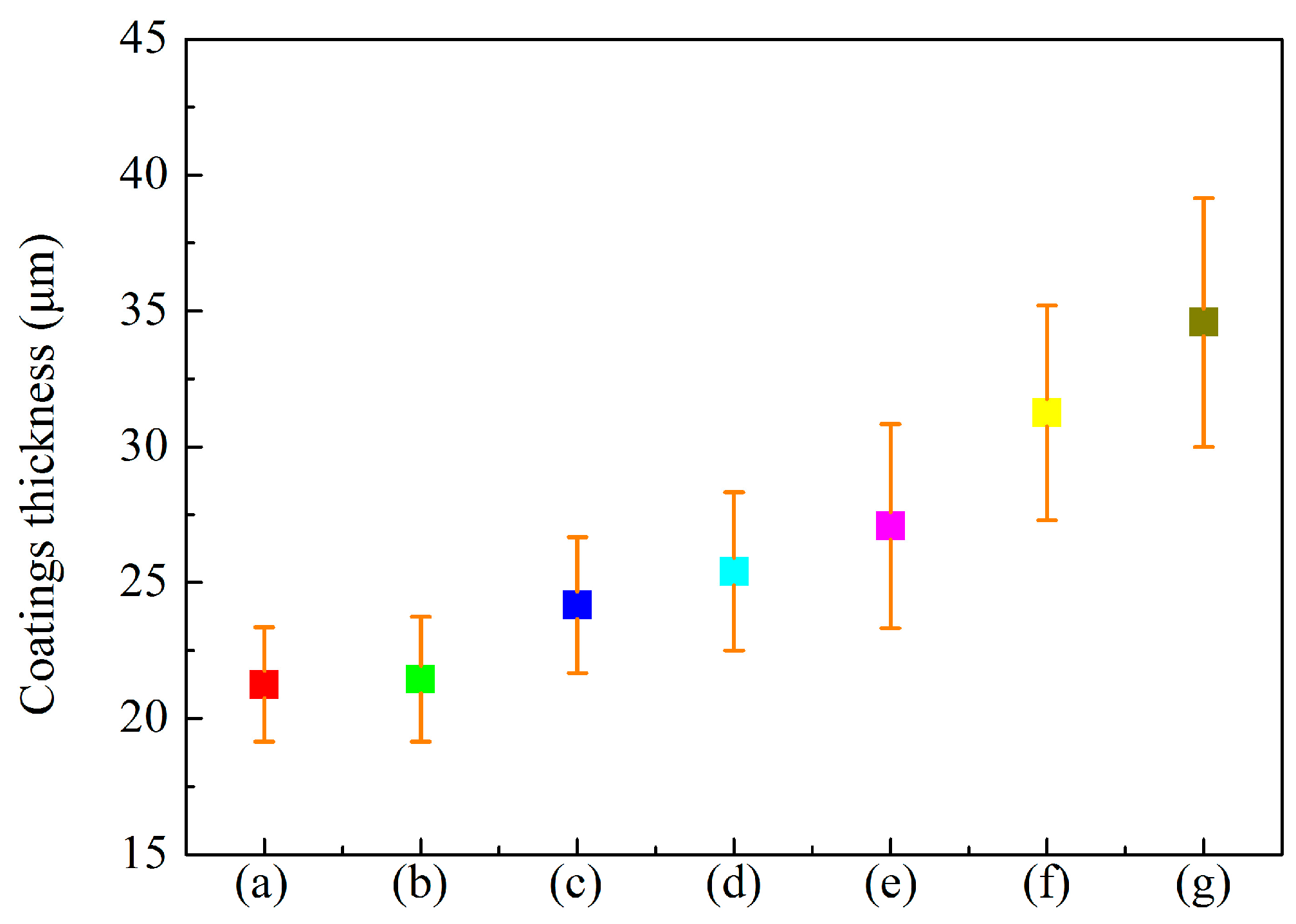
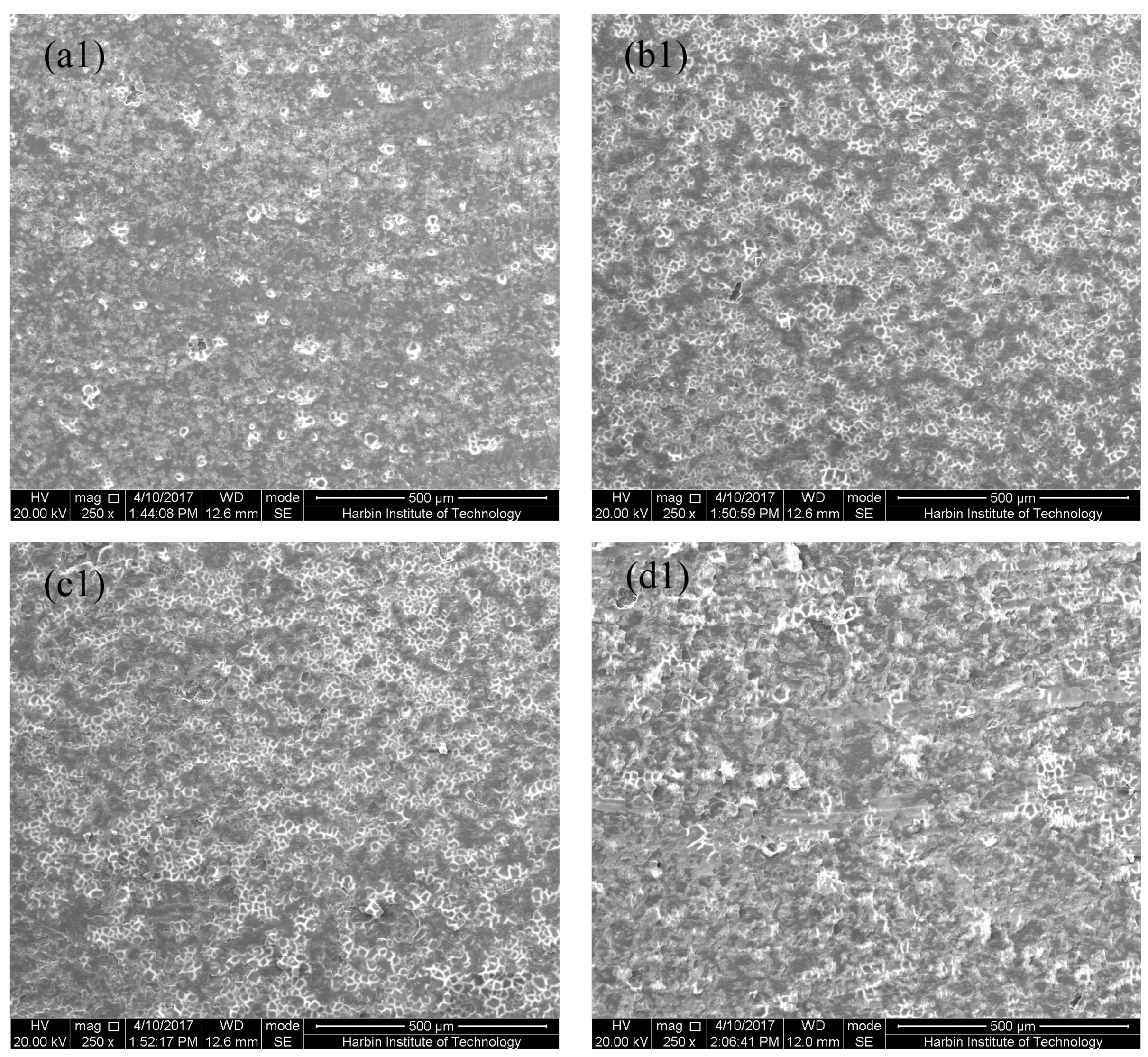
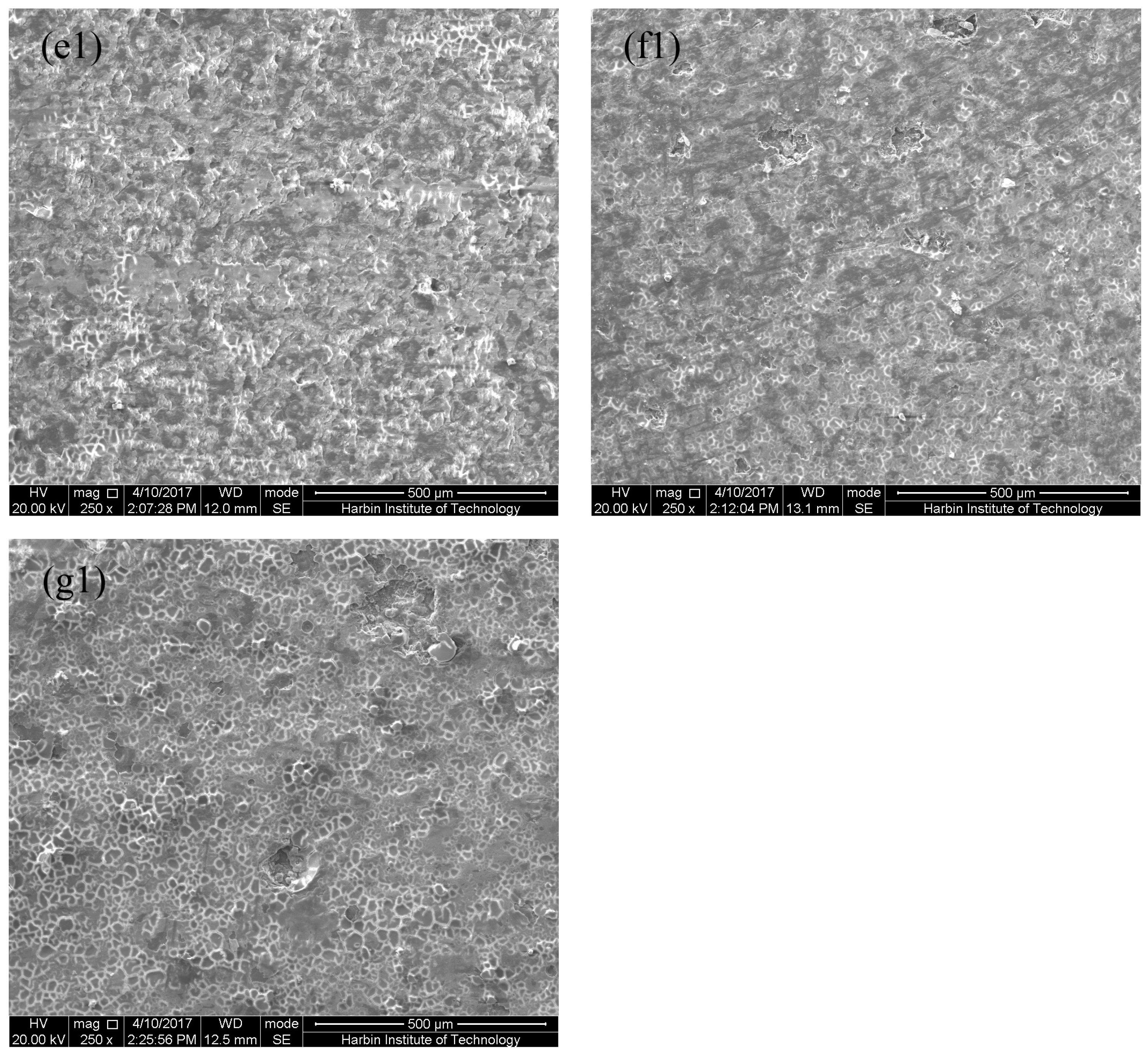
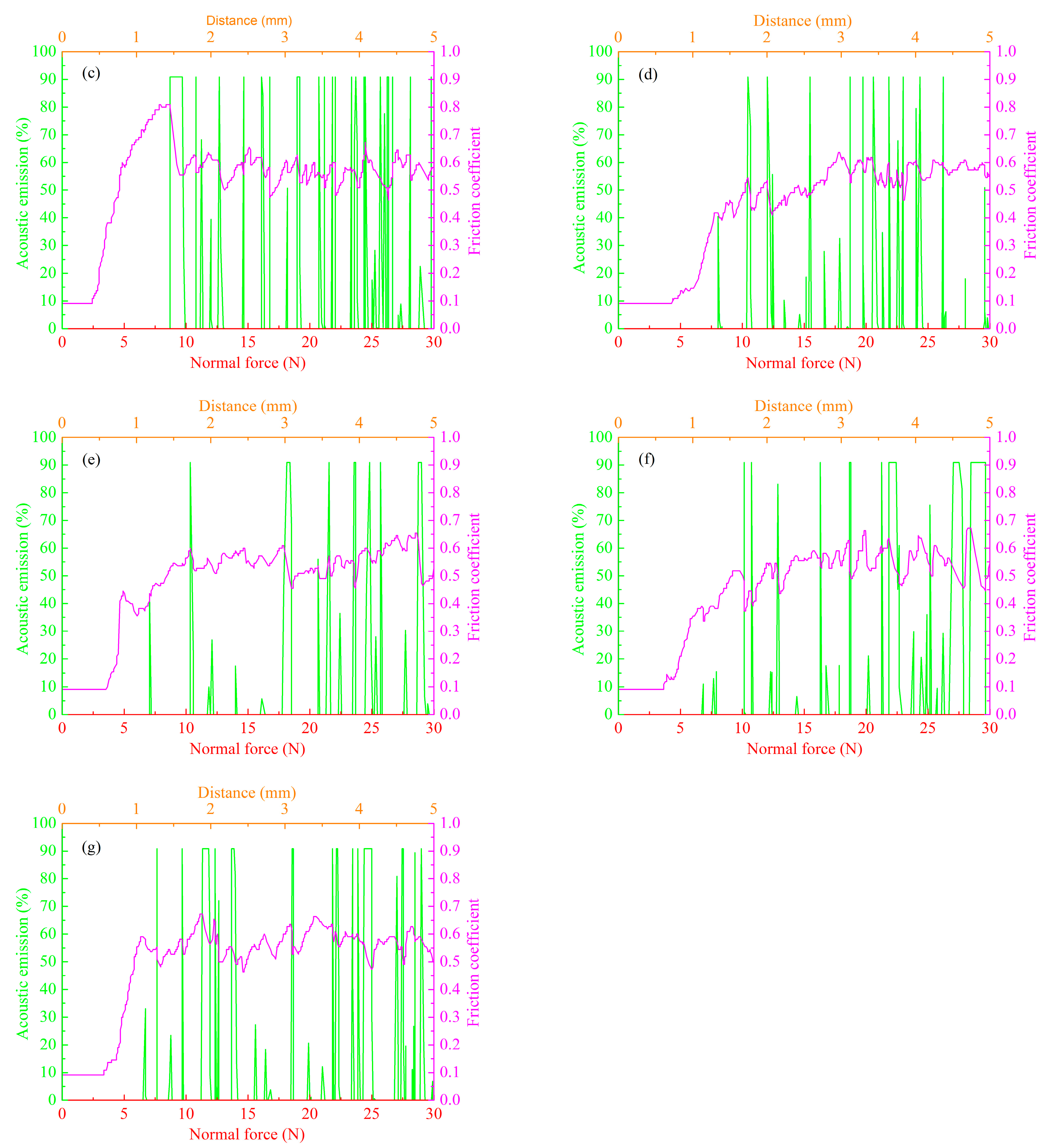
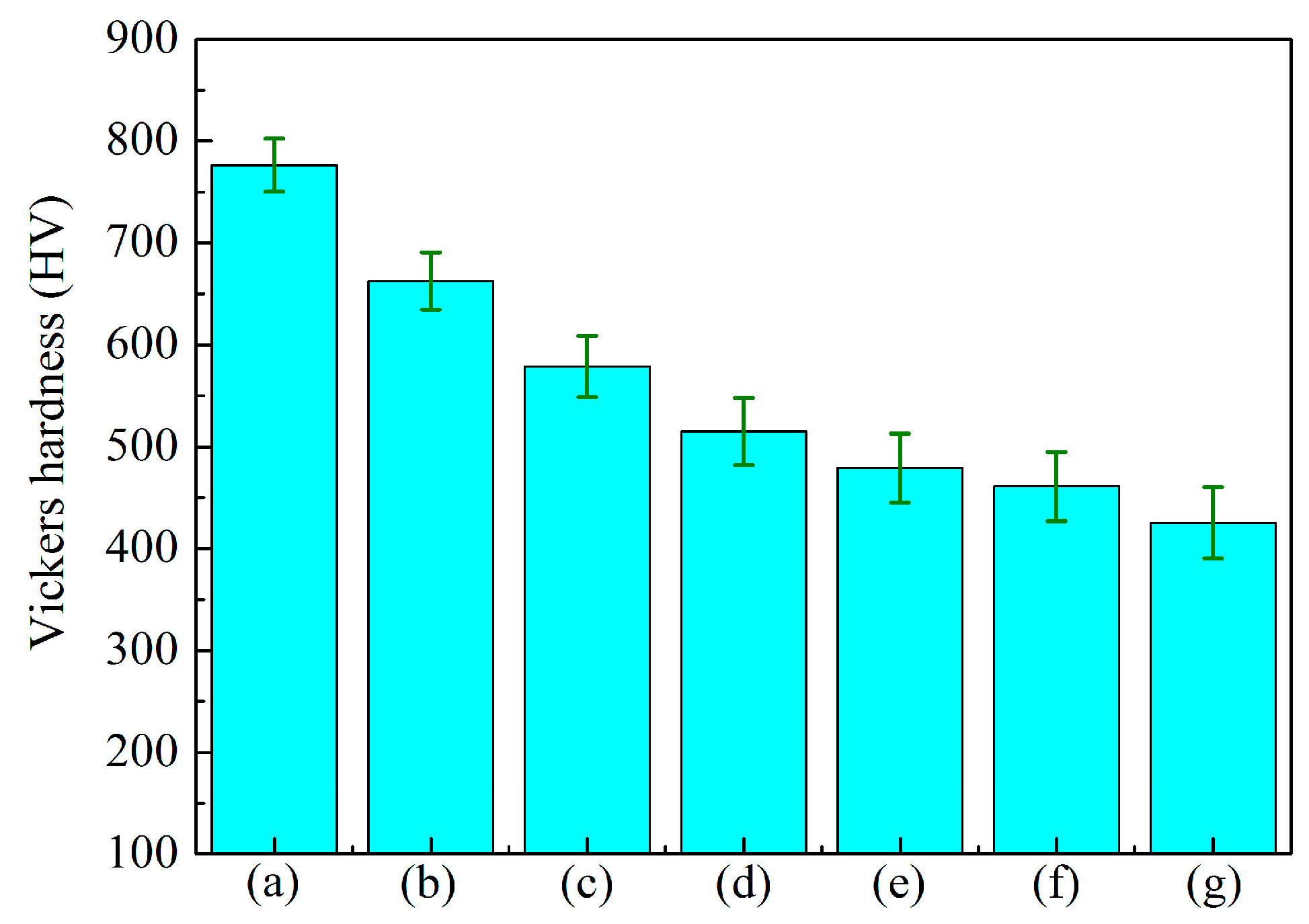

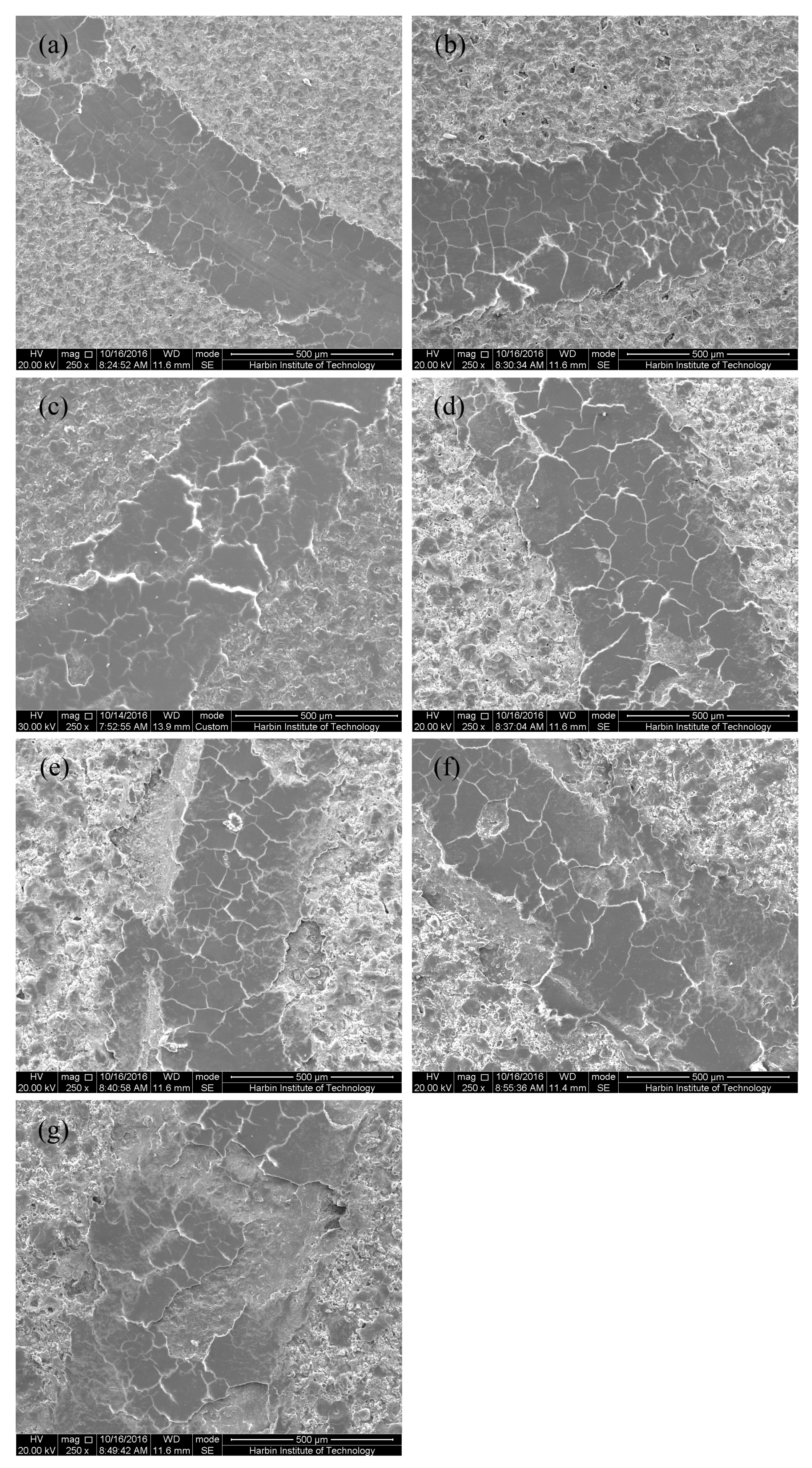
| Labels | Ton+ (μs) | Toff+ (μs) | Ton− (μs) | Toff− (μs) | V+ (V) | V− (V) | I+ (A/dm2) | I− (A/dm2) | Time (min) |
|---|---|---|---|---|---|---|---|---|---|
| a (a1) | 1000 | 300 | 2000 | 300 | Figure 2 | Figure 2 | 37 | 9 | 30 |
| b (b1) | 1500 | 300 | 2000 | 300 | Figure 2 | Figure 2 | 37 | 9 | 30 |
| c (c1) | 2000 | 300 | 2000 | 300 | Figure 2 | Figure 2 | 37 | 9 | 30 |
| d (d1) | 2500 | 300 | 2000 | 300 | Figure 2 | Figure 2 | 37 | 9 | 30 |
| e (e1) | 3000 | 300 | 2000 | 300 | Figure 2 | Figure 2 | 37 | 9 | 30 |
| f (f1) | 3500 | 300 | 2000 | 300 | Figure 2 | Figure 2 | 37 | 9 | 30 |
| g (g1) | 4000 | 300 | 2000 | 300 | Figure 2 | Figure 2 | 37 | 9 | 30 |
| Labels | Intensity (a.u., arbitrary unit) | ||||||
|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | |
| 1 | 1720 | 2110 | 2032 | 1895 | 1879 | 1818 | 1671 |
| 2 | 2988 | 3789 | 3607 | 3404 | 3335 | 3253 | 2853 |
| 3 | 2789 | 3731 | 3606 | 3163 | 3099 | 2991 | 2701 |
| Coatings | Lc (N) |
|---|---|
| A (Figure 10a) | 11.58 |
| B (Figure 10b) | 10.50 |
| C (Figure 10c) | 8.83 |
| D (Figure 10d) | 8.17 |
| E (Figure 10e) | 7.17 |
| F (Figure 10f) | 6.83 |
| G (Figure 10g) | 6.67 |
| Coatings | Wear Time (s) | Wear Track Width (μm) | Wear Track Depth (μm) | Wear Rate (mm3·N−1·m−1) |
|---|---|---|---|---|
| a | 1200 | 420.56 | 3.19 | 0.13 × 10−5 |
| b | 1200 | 490.65 | 3.73 | 0.15 × 10−5 |
| c | 1200 | 560.75 | 4.26 | 0.18 × 10−5 |
| d | 1200 | 654.21 | 4.97 | 0.20 × 10−5 |
| e | 1200 | 700.94 | 5.32 | 0.21 × 10−5 |
| f | 1200 | 724.30 | 5.50 | 0.22 × 10−5 |
| g | 1200 | 794.39 | 6.03 | 0.24 × 10−5 |
| Coatings | Wear Time (s) | Wear Track Width (μm) | Wear Track Depth (μm) | Wear Rate (mm3·N−1·m−1) |
|---|---|---|---|---|
| a1 | 1200 | 380.47 | 2.92 | 0.12 × 10−5 |
| b1 | 1200 | 357.10 | 2.81 | 0.11 × 10−5 |
| c1 | 1200 | 333.74 | 2.73 | 0.10 × 10−5 |
| d1 | 1200 | 403.83 | 3.16 | 0.13 × 10−5 |
| e1 | 1200 | 427.20 | 3.24 | 0.13 × 10−5 |
| f1 | 1200 | 473.92 | 3.69 | 0.15 × 10−5 |
| g1 | 1200 | 520.65 | 4.15 | 0.17 × 10−5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-W.; Di, S.-C. Effect of Anode Pulse-Width on the Microstructure and Wear Resistance of Microarc Oxidation Coatings. Metals 2017, 7, 243. https://doi.org/10.3390/met7070243
Li Z-W, Di S-C. Effect of Anode Pulse-Width on the Microstructure and Wear Resistance of Microarc Oxidation Coatings. Metals. 2017; 7(7):243. https://doi.org/10.3390/met7070243
Chicago/Turabian StyleLi, Zhen-Wei, and Shi-Chun Di. 2017. "Effect of Anode Pulse-Width on the Microstructure and Wear Resistance of Microarc Oxidation Coatings" Metals 7, no. 7: 243. https://doi.org/10.3390/met7070243




