Investigation of Dendrite Coarsening in Complex Shaped Lamellar Graphite Iron Castings
Abstract
:1. Introduction
2. Materials and Methods
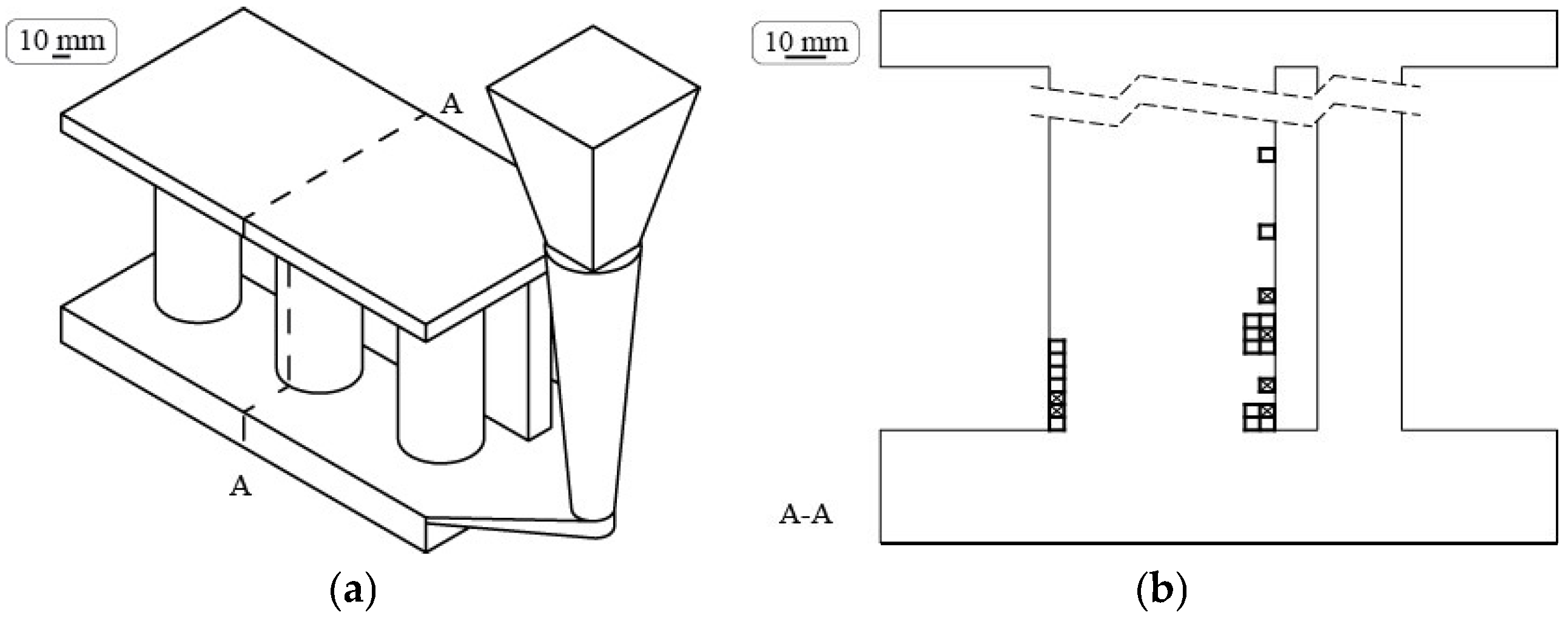
2.1. Shrinkage Porosity (SP)
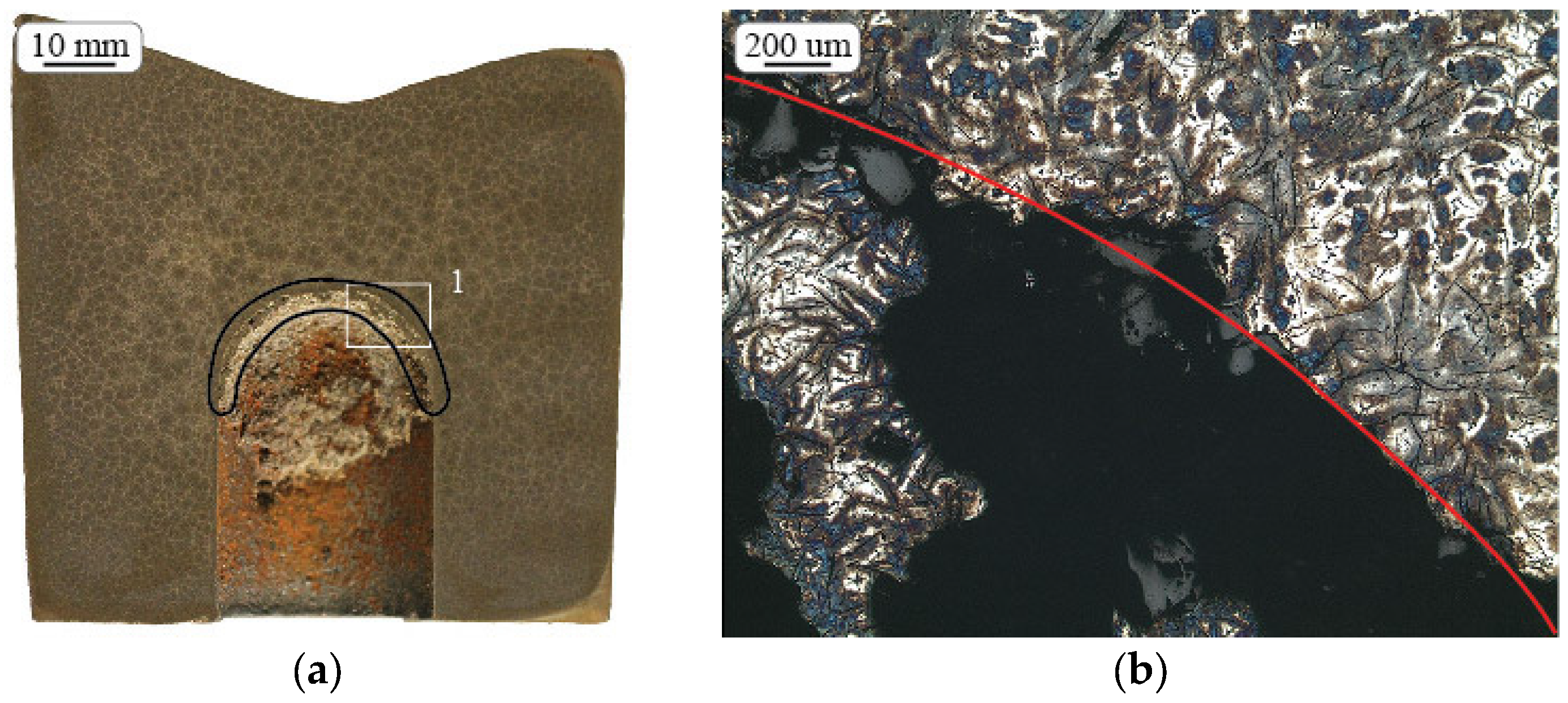
2.2. Metal Expansion Penetration (MEP)
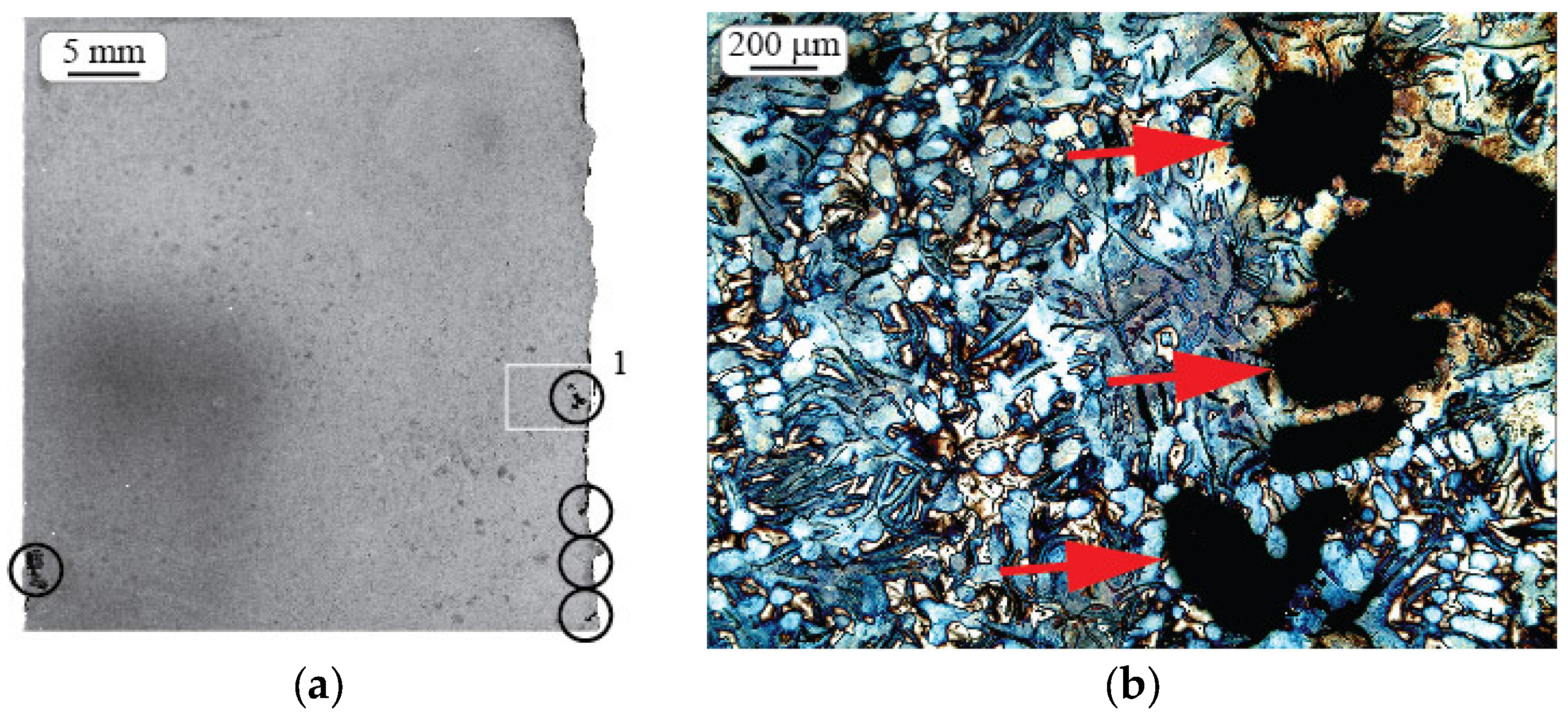
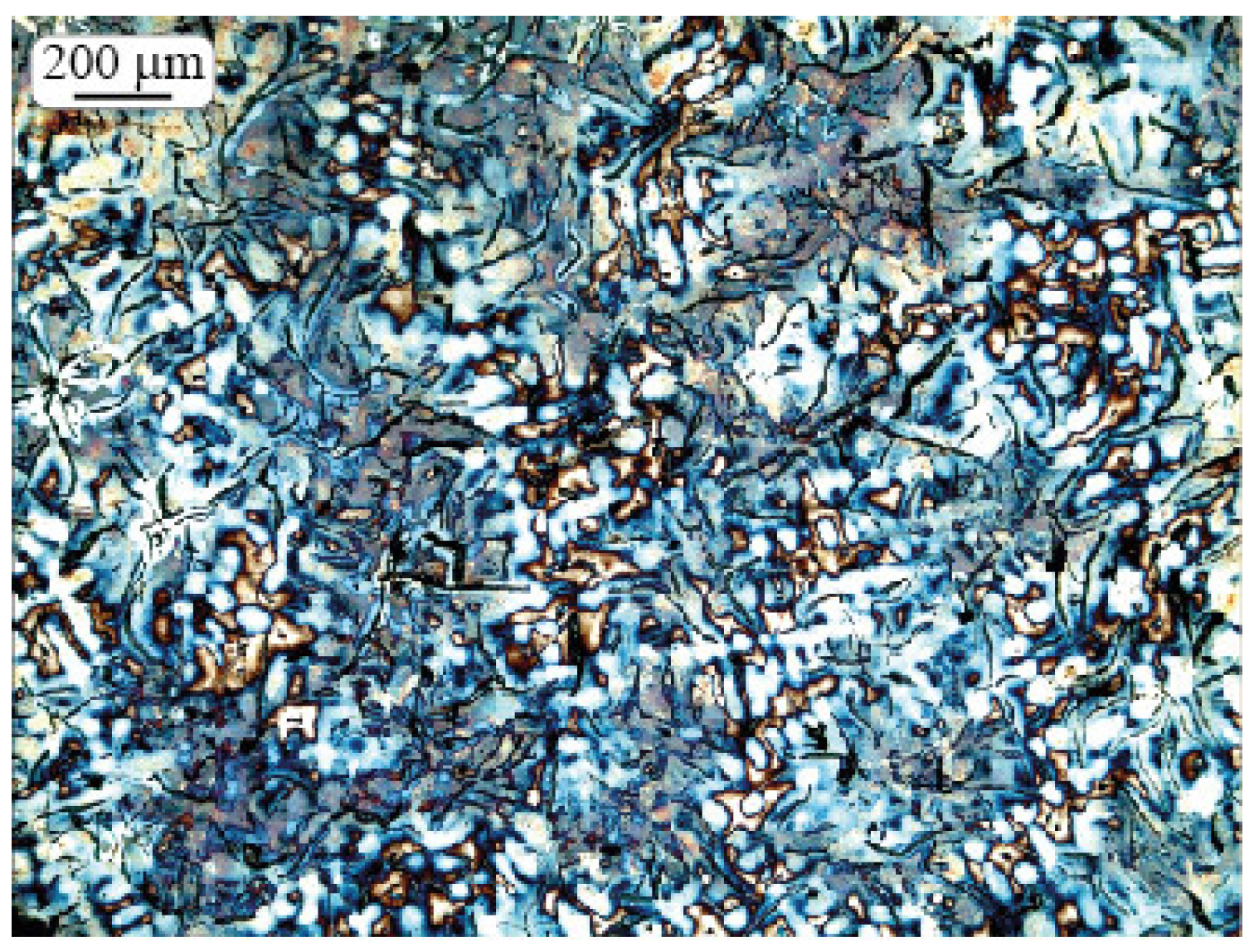
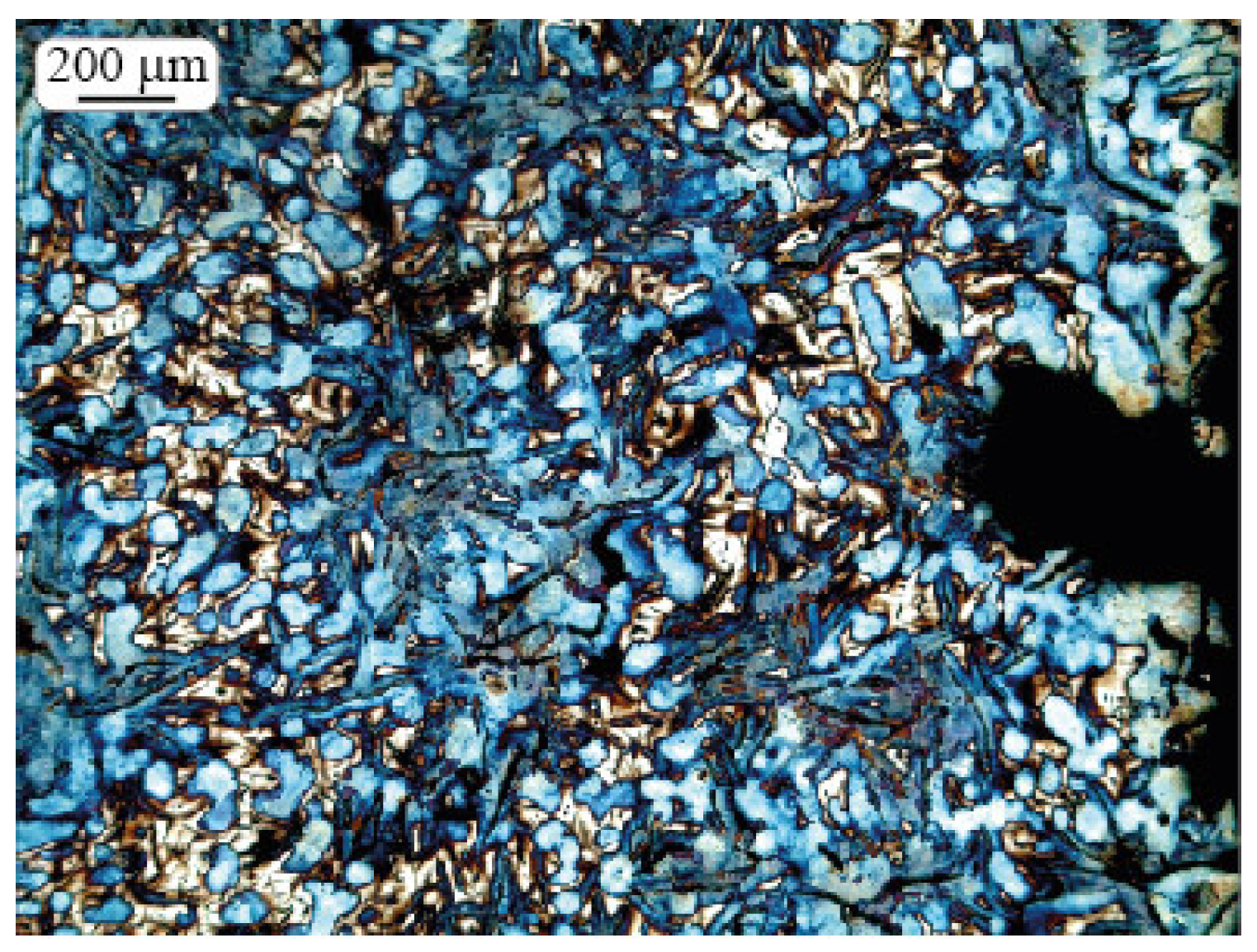
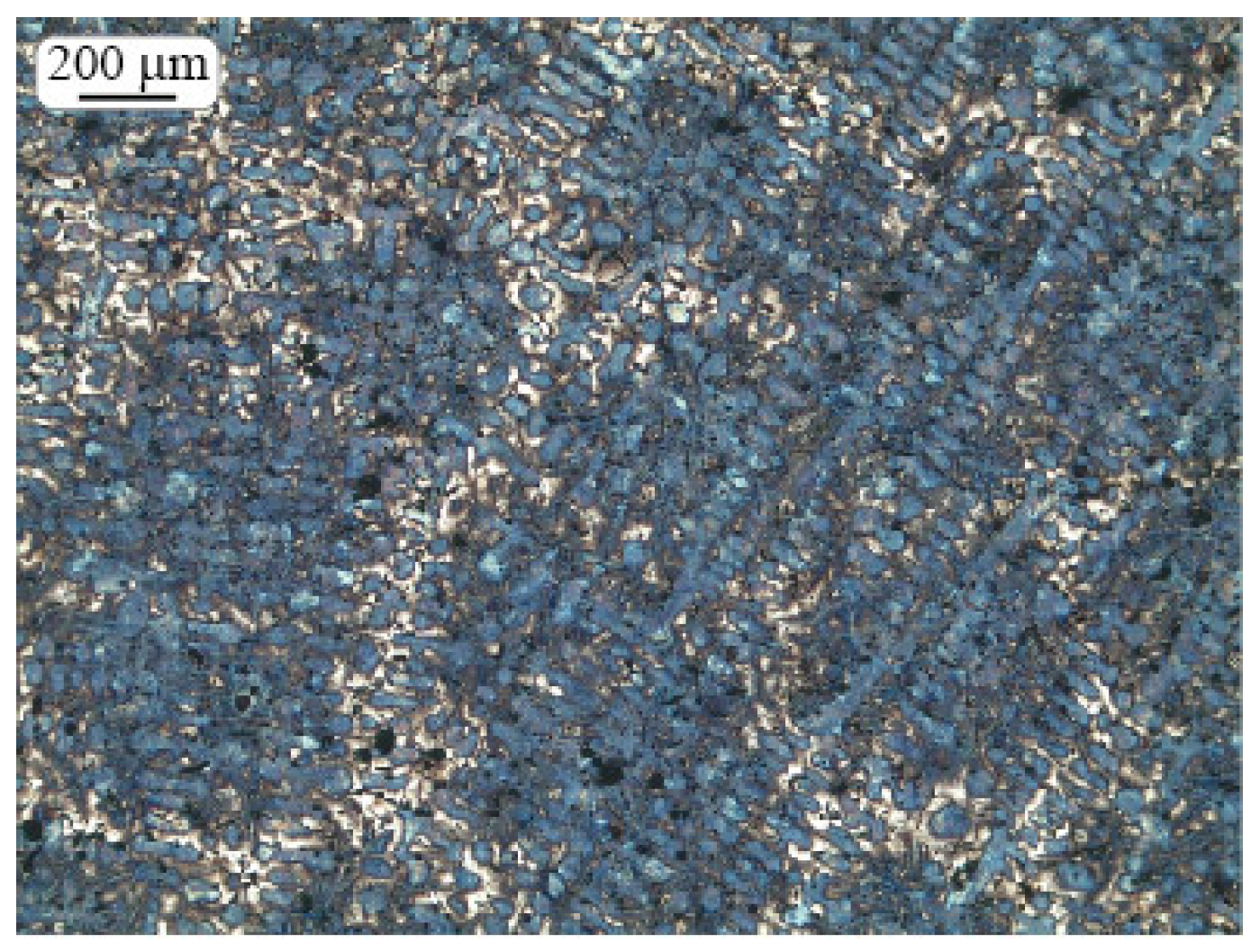
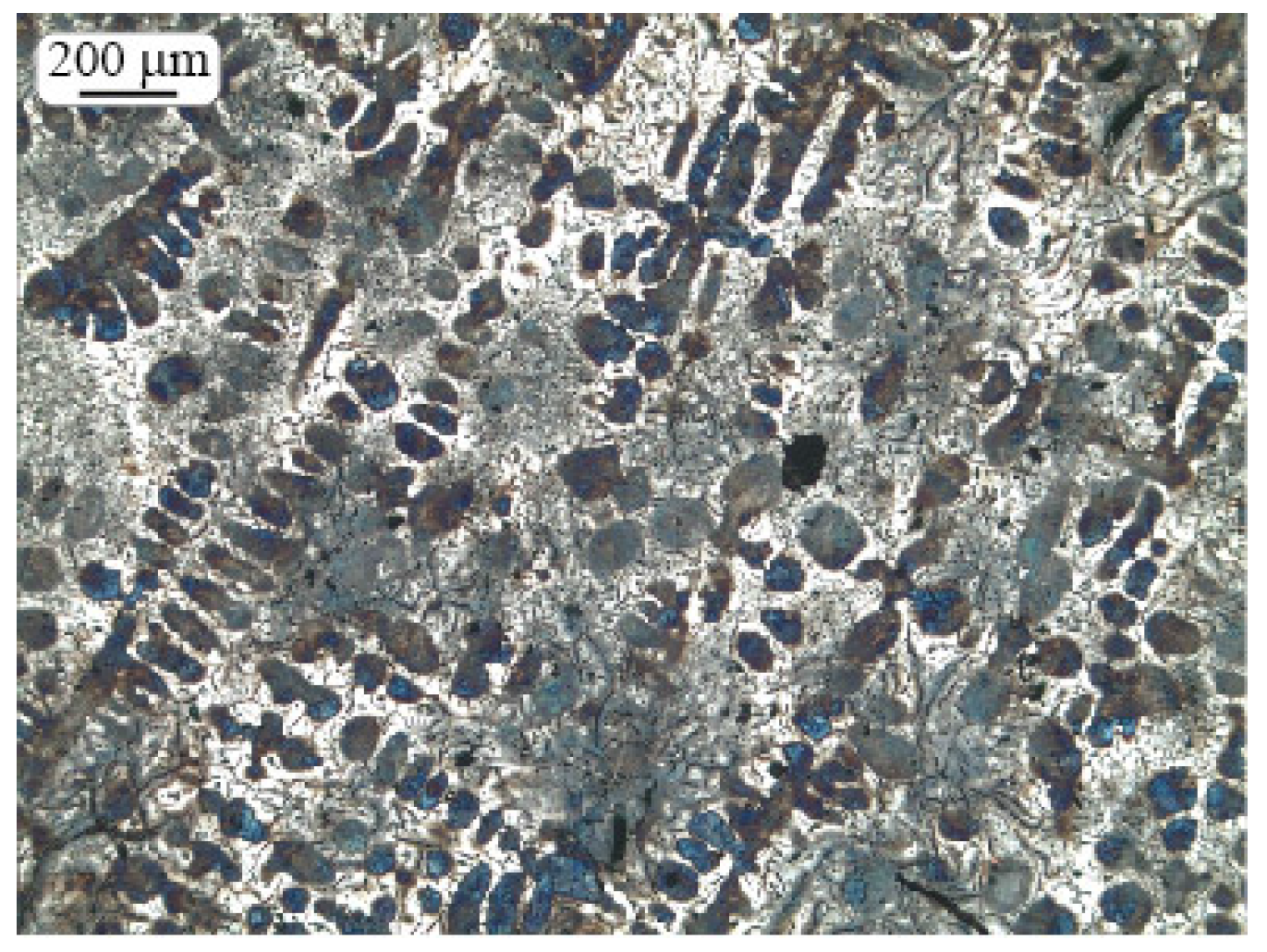
2.3. Microstructure Investigation
2.4. Morphologic Parameters
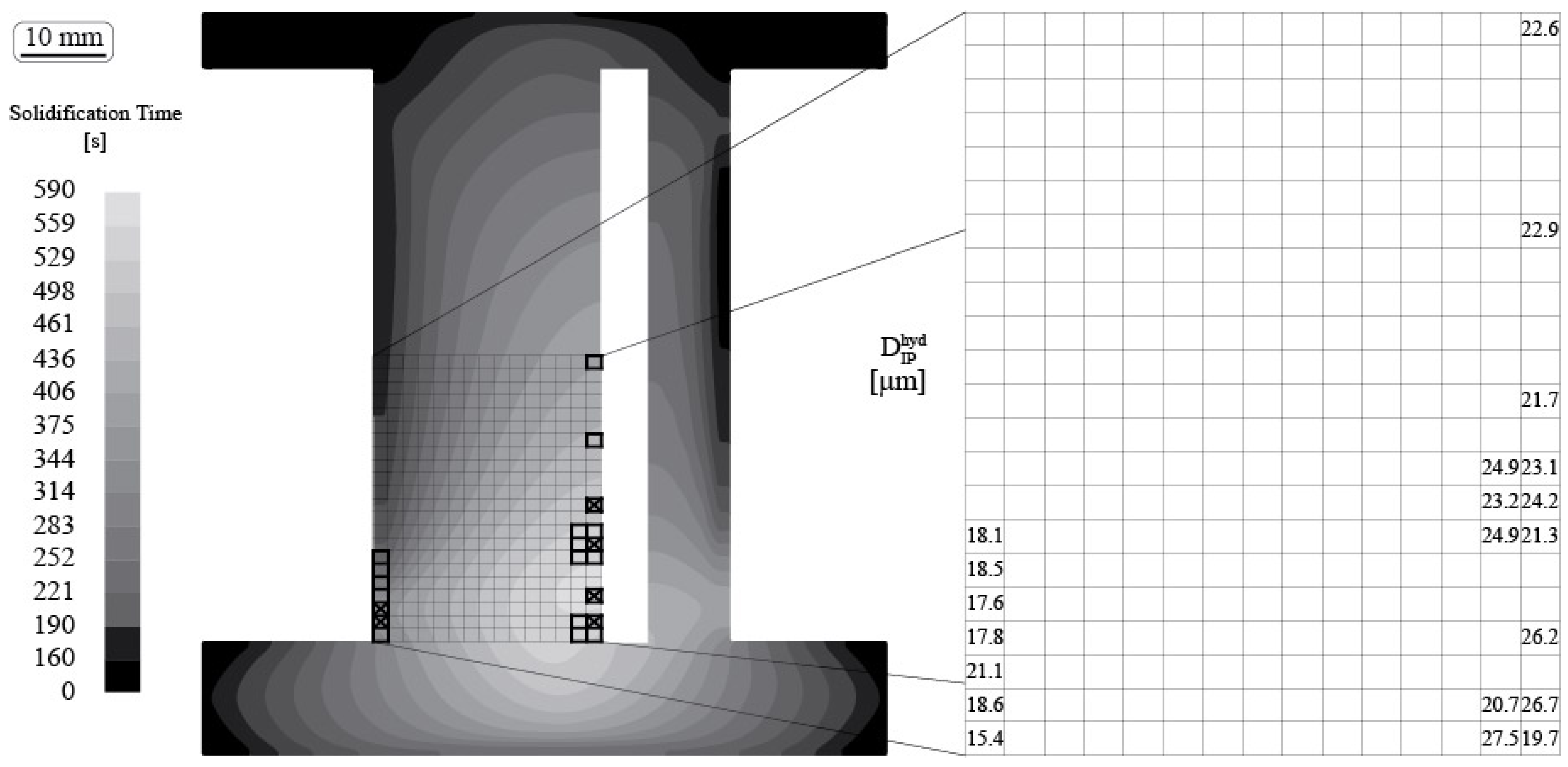
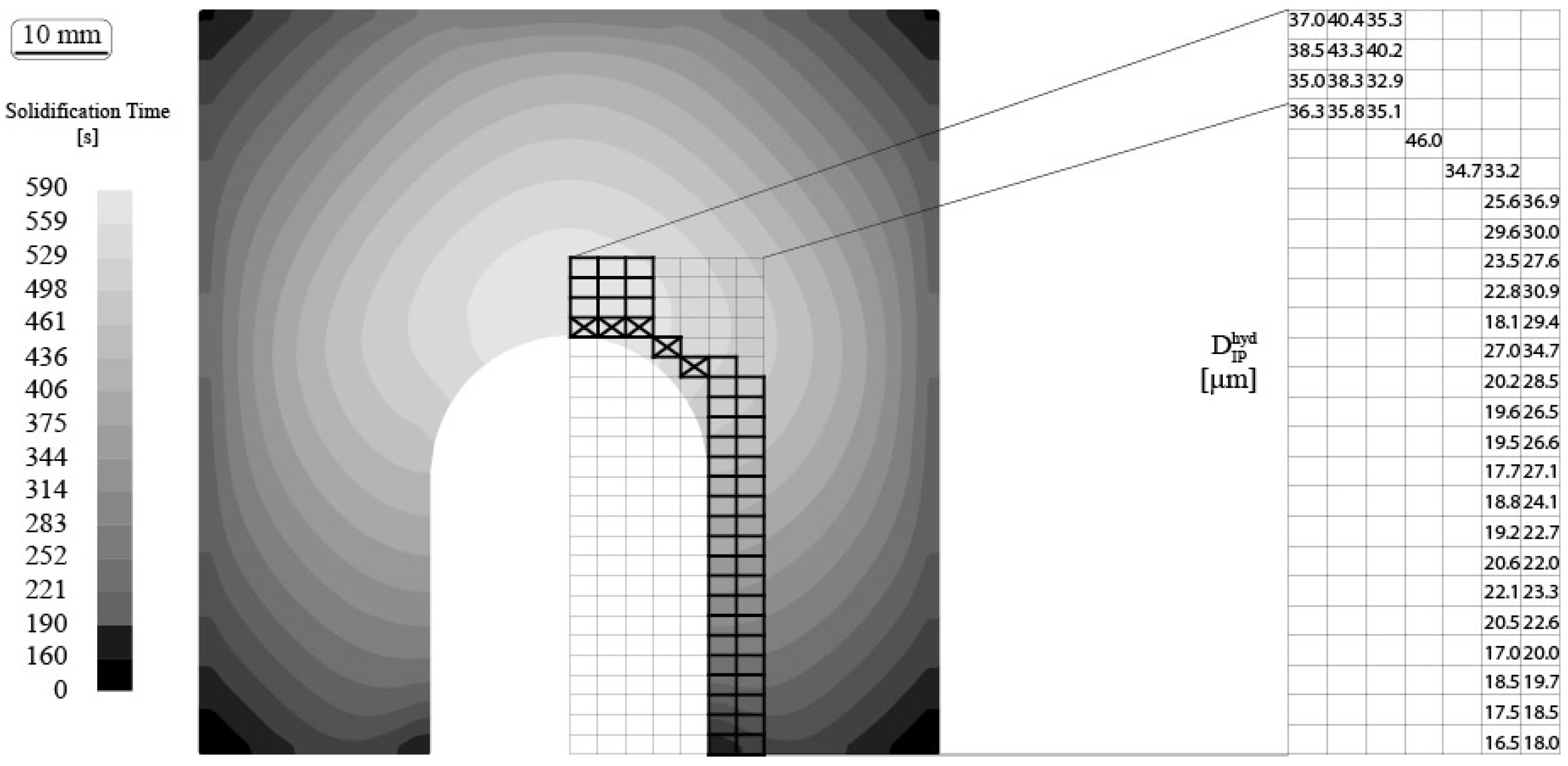
2.5. Numerical Simulation of the Local Solidification Time
3. Results and Discussion
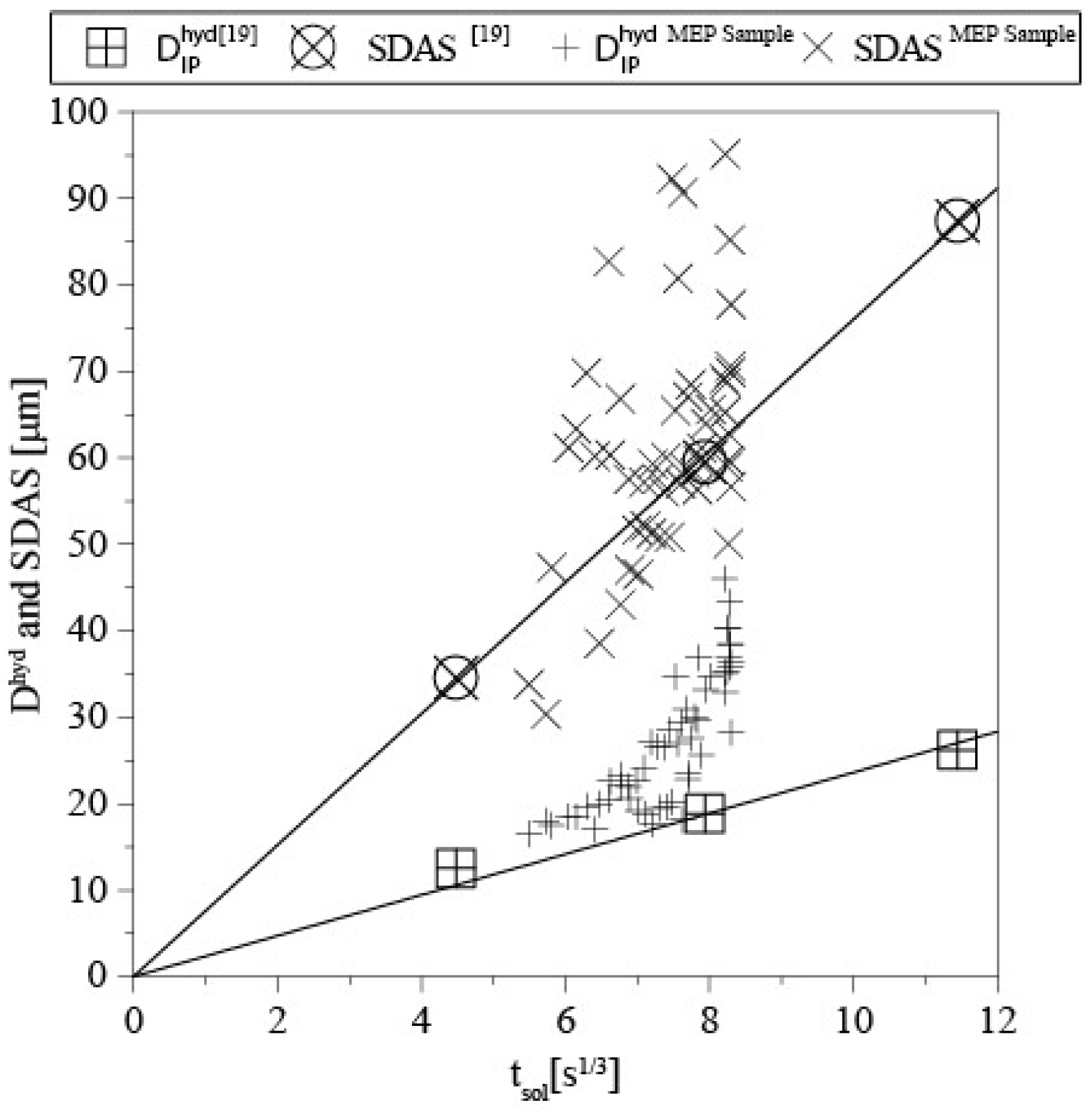
3.1. Shrinkage Porosity (SP)
3.2. Metal Expansion Penetration (MEP)
4. Conclusions
- (1)
- The coarsening phenomena was observed in two different complex shaped castings.
- (2)
- The local solidification time influences the austenite morphology in complex shaped castings, which is in agreement with previous observations in compact shaped cylindrical castings.
- (3)
- A variation in local solidification time within the same complex shaped casting leads to the formation of a morphological gradient, which can be considered as the transport path for the intradendritic liquid in forming a shrinkage porosity defect or a metal expansion penetration defect.
- (4)
- The morphological parameter observations in complex shaped casting are disturbed due to morphological changes like a dendrite fragmentation and coalescence.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
- Alloy
- Material: GJL-150
| Temperature (°C) | CP (J·kg−1 K−1) | fS | k (W·m−1·K−1) | ρ (kg·m−3) |
|---|---|---|---|---|
| 1 | 450 | - | 54.0 | 7100.0 |
| 25 | 467 | - | - | - |
| 100 | 506 | - | 52.5 | 7074.5 |
| 200 | 563 | - | 51.0 | 7049.1 |
| 300 | 621 | - | 50.0 | 7023.8 |
| 400 | 663 | - | 49.0 | 6998.6 |
| 500 | 741 | - | 48.5 | - |
| 600 | 851 | - | - | - |
| 700 | 1036 | - | - | - |
| 725 | 1100 | - | - | - |
| 810 | 744 | - | - | - |
| 900 | 774 | - | - | - |
| 1000 | 804 | - | - | 6850.6 |
| 1100 | 830 | - | - | - |
| 1160 | 844 | 1.000 | 40.0 | 6814.0 |
| 1161 | - | 0.830 | - | - |
| 1162 | - | 0.530 | - | - |
| 1163 | - | 0.180 | - | - |
| 1164 | - | 0.040 | - | - |
| 1166 | - | 0.030 | - | - |
| 1168 | - | 0.020 | - | - |
| 1170 | - | 0.010 | - | - |
| 1173 | 740 | 0.000 | 38.0 | 6882.0 |
| 1200 | 747 | - | - | - |
| 1255 | - | - | - | 6813.9 |
| 1300 | 778 | - | - | - |
| 1355 | - | - | - | 6745.3 |
| 1400 | 813 | - | - | - |
| 1500 | 854 | - | - | - |
| 1600 | 871 | - | - | - |
| 1700 | 872 | - | - | - |
| 2000 | 872 | - | 38.0 | 6310.2 |
| Temperature (°C) | CP (J·kg−1·K−1) | ρ (kg·m−3) | k (W·m−1·K−1) |
|---|---|---|---|
| 1 | 700 | 1500.0 | 0.91 |
| 20 | - | - | 0.90 |
| 25 | 741 | - | - |
| 50 | - | - | 0.87 |
| 99 | 850 | - | - |
| 100 | 850 | 1495.0 | 0.82 |
| 101 | 850 | - | - |
| 127 | 886 | - | - |
| 150 | - | - | 0.79 |
| 200 | - | 1493.0 | 0.77 |
| 227 | 991 | - | - |
| 250 | - | - | 0.76 |
| 300 | - | 1490.0 | 0.74 |
| 326 | 1082 | - | - |
| 327 | 1082 | - | - |
| 400 | - | 1487.0 | 0.70 |
| 427 | 1167 | - | - |
| 500 | - | 1478.0 | 0.70 |
| 526 | 1248 | - | - |
| 527 | 1248 | - | - |
| 573 | 1285 | - | - |
| 574 | 1160 | - | - |
| 600 | - | 1476.0 | 0.70 |
| 627 | 1162 | - | - |
| 700 | - | - | 0.71 |
| 727 | 1167 | - | - |
| 750 | - | - | 0.73 |
| 800 | - | - | 0.76 |
| 827 | 1172 | - | - |
| 850 | - | - | 0.79 |
| 900 | - | - | 0.83 |
| 927 | 1177 | - | - |
| 950 | - | - | 0.88 |
| 1000 | - | - | 0.93 |
| 1027 | 1181 | - | - |
| 1050 | - | - | 0.99 |
| 1100 | - | - | 1.05 |
| 1127 | 1186 | - | - |
| 1150 | - | - | 1.12 |
| 1200 | 1.20 | ||
| 1227 | 1190 | - | - |
| 1327 | 1194 | - | - |
| 1600 | - | - | 1.40 |
| 2000 | 1250 | 1470.0 | 1.50 |
| Temperature (°C) | h (W·m−2·K−1) |
|---|---|
| 1 | 300 |
| 600 | 500 |
| 1100 | 600 |
| 1200 | 800 |
| 2000 | 800 |
- SP sample: 183520 metal cells out of 2473692 cells
- MEP sample: 34120 metal cells out of 206424 cells
| Sample Name | Component Name | Volume (L) | Weight (kg) |
|---|---|---|---|
| SP sample | casting | 1.93 | 13.68 |
| SP sample | mold | 16.59 | 24.87 |
| MEP sample | casting | 0.37 | 2.61 |
| MEP sample | mold | 1.2 | 1.8 |
| Sample Name | Pouring Time (s) | Simulation Time (s) | Δt (s) | Solidification and Cooling |
|---|---|---|---|---|
| SP sample | 10 | 600 | 1 | time dependent |
| MEP sample | 6 | 600 | 1 | time dependent |
Appendix B
| Capital letters | |
| Ai | Area of the intradendritic phase (m2) |
| Heat capacity (J·kg−1·K−1) | |
| Hydraulic diameter of the intradendritic phase (m) | |
| Hydraulic diameter of the intradendritic space (m) | |
| L | Latent heat of solidification (J·kg−1) |
| Pi | Perimeter of the intradendritic phase (m) |
| Released latent heat (J·m−3) | |
| Released latent heat during solidification (W·m−3) | |
| T | Temperature (°C) |
| Small letter | |
| fS | Fraction solidified metal (-) |
| k | Thermal conductivity (W·m−1·K−1) |
| t | Time (s) |
| tsol | Solidification time (s) |
| Greek letters | |
| ρ | Density (kg·m−3) |
| λ2 | Secondary dendrite arm spacing (μm) |
References
- Modern Casting Staff. 50th Census of World Casting Production. In Modern Casting; American Foundry Society: Schaumburg, IL, USA, 2016; pp. 25–29. [Google Scholar]
- Levelink, H.G.; Julien, F.P. Penetration and shrinkage by interaction of solidifying cast iron and casting mold—Part 1. AFS Cast Met. Res. J. 1973, 9, 56–63. [Google Scholar]
- Levelink, H.G.; Julien, F.P. Penetration and shrinkage by interaction of solidifying cast iron and casting mold—Part 2. AFS Cast Met. Res. J. 1973, 9, 105–109. [Google Scholar]
- Dugic, I.; Svensson, I.L. An investigation of the effect of inoculants on the metal expansion penetration in grey iron. Int. J. Cast Met. Res. 1999, 11, 333–338. [Google Scholar] [CrossRef]
- Diószegi, A.; Dugic, I. The mechanisms of metal expansion penetration in grey cast iron. In Proceedings of the Eight International Symposium on Science and Processing of Cast Iron, Beijing, China, 16–19 October 2006; pp. 92–97. [Google Scholar]
- Diószegi, A.; Dugic, I.; Svensson, I.L. Metal expansion penetration on concave casting surfaces of grey cast iron cylinder heads. AFS Trans. 2007, 115, 609–615. [Google Scholar]
- Elmquist, L.; Adolfsson, S.; Diószegi, A. Characterizing shrinkage porosity in gray cast iron using microstructure investigation. In AFS Transactions; American Foundry Society: Schaumburg, IL, USA, 2008; Volume 116, pp. 691–703. [Google Scholar]
- Elmquist, L.; Diószegi, A. Shrinkage porosity and its relation to solidification structure of grey cast iron parts. Int. J. Cast Met. Res. 2010, 23, 44–50. [Google Scholar] [CrossRef]
- Elmquist, L.; Soivio, K.; Diószegi, A. Cast iron solidification structure and how it is related to defect formation. Mater. Sci. Forum 2014, 790, 441–446. [Google Scholar] [CrossRef]
- Rivera, G.L.; Boeri, R.E.; Sikora, J.A. Solidification of gray cast iron. Scr. Mater. 2004, 50, 331–335. [Google Scholar] [CrossRef]
- Rivera, G.; Calvillo, P.; Boeri, R.; Houbaert, Y.; Sikora, J. Examination of the solidification macrostructure of spheroidal and flake graphite cast irons using DAAS and ESBD. Mater. Charact. 2008, 59, 1342–1348. [Google Scholar] [CrossRef]
- Glicksman, M.E.; Voorhees, P.W. Ostwald Ripening and Relaxation in Dendritic Structures. Metall. Trans. A 1984, 15, 995–1001. [Google Scholar] [CrossRef]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Lacaze, J.; Lesoult, G. Birth, Growth and Ripening of Solidification Structures. Acta Stereol. 1986, 5, 331–336. [Google Scholar]
- Kammer, D.; Voorhees, P.W. The morphological evolution of dendritic microstructures during coarsening. Acta Mater. 2006, 54, 1549–1558. [Google Scholar] [CrossRef]
- Hernando, J.C.; Diószegi, A. An Overview of Isothermal Coarsening in Hypoeutectic Lamellar Cast Iron. In Advances in the Science and Engineering of Casting Solidification: An MPMD Symposium Honoring Doru Michael Stefanescu; Nastac, L., Liu, B., Fredriksson, H., Lacaze, J., Hong, C., Catalina, A.V., Buhrig-Polaczek, A., Monroe, C., Sabau, A.S., Ruxanda, R.E.L., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 295–302. [Google Scholar]
- Hernando, J.C.; Domeij, B.; Diószegi, A. Influence of Ti and Mo additions on the isothermal coarsening process of primary austenite in Lamellar Graphite Iron. In Proceedings of the 5th Decennial International Conference on Solidification Processing, Old Windsor, UK, 25–28 July 2017. [Google Scholar]
- Hernando, J.C.; Ghassemali, E.; Diószegi, A. The Morphological Evolution of Primary Austenite during Isothermal Coarsening. 2017, unpublished work. [Google Scholar]
- Lora, R.; Diószegi, A. Dynamic Coarsening of 3.3C-1.9Si Gray Cast Iron. Metall. Mater. Trans. A 2012, 43, 5165–5172. [Google Scholar] [CrossRef]
- Voort, G.F.V. Color Metallography. In ASM Handbook, Vol. 9: Metallography and Microstructures; ASM International: Materials Park, OH, USA, 2004; pp. 493–512. [Google Scholar]
- Vazehrad, S.; Elfsberg, J.; Diószegi, A. Study of microstructure and silicon segregation in cast iron using color etching and electron microprobe analysis. Mater. Charact. 2015, 104, 132–138. [Google Scholar] [CrossRef]
- Flemings, M.C. Coarsening in Solidification Processing. Mater. Trans. 2005, 46, 895–900. [Google Scholar] [CrossRef]
- Diószegi, A.; Fourlakidis, V.; Lora, R. Austenite Dendrite Morphology in Lamellar Cast Iron. In Proceedings of the SPCI 10, Mar Del Plata, Argentina, 10–13 November 2014. [Google Scholar]
- MAGMASoft Standard Manual; MAGMA Giessereitechnologie GmbH: Aachen, Germany, 2012.
- Hattel, J. Fundamentals of Numerical Modelling of Casting Processes, 1st ed.; Polyteknisk Forlag: Kongens Lyngby, Denmark, 2005. [Google Scholar]


| Element | C | Si | Mn | P | S | Cr | Mo |
|---|---|---|---|---|---|---|---|
| Content in SP sample (wt %) | 3.28 | 1.96 | 0.64 | 0.03 | 0.06 | 0.26 | 0.05 |
| Content in MEP sample (wt %) | 3.18 | 1.77 | 0.56 | 0.05 | 0.09 | 0.15 | 0.24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svidró, P.; Diószegi, A.; Pour, M.S.; Jönsson, P. Investigation of Dendrite Coarsening in Complex Shaped Lamellar Graphite Iron Castings. Metals 2017, 7, 244. https://doi.org/10.3390/met7070244
Svidró P, Diószegi A, Pour MS, Jönsson P. Investigation of Dendrite Coarsening in Complex Shaped Lamellar Graphite Iron Castings. Metals. 2017; 7(7):244. https://doi.org/10.3390/met7070244
Chicago/Turabian StyleSvidró, Péter, Attila Diószegi, Mohsen Saffari Pour, and Pär Jönsson. 2017. "Investigation of Dendrite Coarsening in Complex Shaped Lamellar Graphite Iron Castings" Metals 7, no. 7: 244. https://doi.org/10.3390/met7070244






