Enhanced Surface Precipitates on Ultrafine-Grained Titanium in Physiological Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
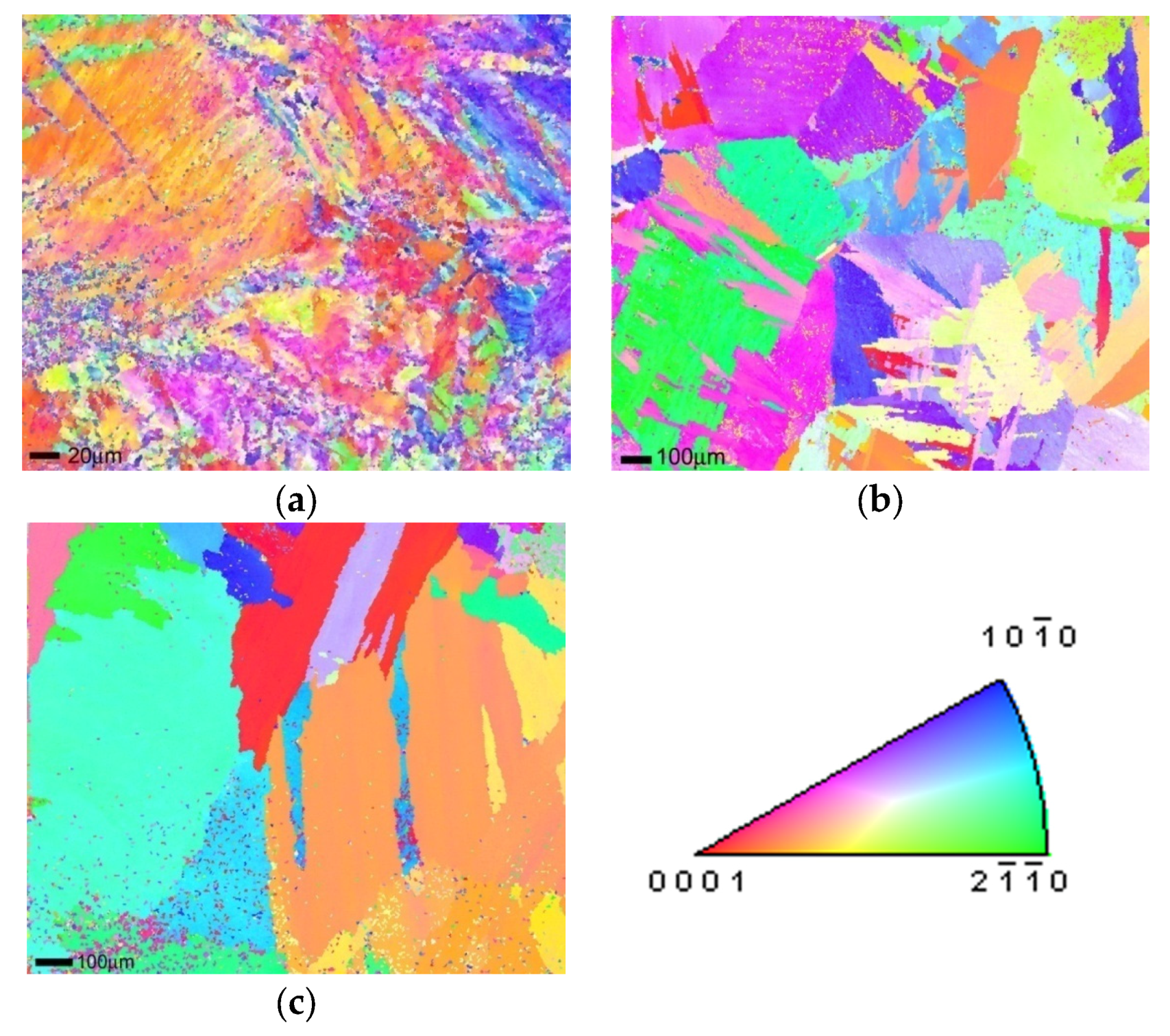
3.1. Microstructure
3.2. Electrochemical Impedance Spectroscopy (EIS) Results
3.3. Response to Soaking
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, T.N.; Balakrishnan, A.; Lee, B.C.; Kim, W.S.; Dvorankova, B.; Smetana, K.; Park, J.K.; Panigrahi, B.B. In vitro fibroblast response to ultra fine grained titanium produced by a severe plastic deformation process. J. Mater. Sci. Mater. Med. 2008, 19, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kim, Y.-J.; Park, C.H.; Lee, D.-H.; Kod, Y.G.; Jang, J.-H.; Lee, C.S. Enhanced osteoblast response to an equal channel angular pressing-processed pure titanium substrate with microrough surface topography. Acta Biomater. 2009, 5, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Estrin, Y.S.; Ivanova, E.P.; Michalska, A.; Truong, V.K.; Lapovok, R.; Boyd, R. Accelerated stem cell attachment to ultrafine grained titanium. Acta Biomater. 2011, 7, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, S.; Azari, F.; Li, H.; Bateni, M.R.; Szpunar, J.A.; Vali, H.; Tabrizian, M. The significance of crystallographic texture of titanium alloy substrates on pre-osteoblast responses. Biomaterials 2006, 27, 3532–3539. [Google Scholar] [CrossRef] [PubMed]
- Maleki-Ghaleh, H.; Hajizadeh, K.; Hadjizadeh, A.; Shakeri, M.S.; Alamdari, S.; Ghobadi Masoudfar, S.; Aghaie, E.; Javidi, M.; Zdunek, J.; Kurzydlowski, K.J. Electrochemical and cellular behavior of ultrafine-grained titanium in vitro. Mater. Sci. Eng. C 2014, 39, 299–304. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Li, Z.; Diao, X.; Xin, H.; Zhang, Q.; Jia, X.; Wu, Y.; Li, K.; Guo, Y. In vitro and in vivo studies of ultrafine-grain Ti as dental implant material processed by ECAP. Mater. Sci. Eng. C 2016, 67, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in vitro biocompatibility of ultrafine-grained titanium with hierarchical porous surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Lu, G.; Bernasek, S.L.; Schwartz, J. Oxidation of a polycrystalline titanium surface by oxygen and water. Surf. Sci. 2000, 458, 80–90. [Google Scholar] [CrossRef]
- Alkhateeb, E.; Virtanen, S. Influence of surface self-modification in Ringer’s solution on the passive behavior of titanium. J. Biomed. Mater. Res. Part A 2005, 75, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Asperilla, L.; Garcia-Alonso, M.C.; Escudero, M.L. Study of the interaction of inorganic and organic compounds of cell culture medium with a Ti surface. Acta Biomater. 2010, 2, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Matykina, E.; Arrabal, R; Valiev, R.Z.; Molina-Aldareguia, J.M.; Belov, P.; Sabirov, I. Electrochemical anisotropy of nanostructured titanium for biomedical implants. Electrochim. Acta 2015, 176, 1221–1232. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Mueller, Y.; Forster, D.; Virtanen, S. Electrochemical characterization of passive film on Ti alloys under simulated biological conditions. Electrochim. Acta 2002, 47, 1913–1923. [Google Scholar] [CrossRef]
- Hanawa, T.; Ota, M. Calcium phosphate naturally formed on titanium in electrolyte solution. Biomaterials 1991, 12, 767–774. [Google Scholar] [CrossRef]
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Biomed. Mater. Res. 1998, 40, 530–538. [Google Scholar] [CrossRef]
- Wu, W.; Nancollas, G.H. Kinetics of heterogeneous nucleation of calcium phosphates on anatase and rutile. J. Colloid Interface Sci. 1998, 199, 206–211. [Google Scholar] [CrossRef]
- Lima, J.; Sousa, S.R.; Ferreira, A.; Barbosa, M.A. Interactions between calcium, phosphate, and albumin on the surface of titanium. J. Biomed. Mater. Res. 2001, 55, 45–53. [Google Scholar] [CrossRef]
- Healy, K.E.; Ducheyne, P. Hydration and preferential molecular adsorption on titanium in vitro. Biomaterials 1992, 13, 553–561. [Google Scholar] [CrossRef]
- Skale, S.; Dolecek, V.; Slemnik, M. Substitution of the constant phase element by Warburg impedance for protective coating. Corros. Sci. 2007, 49, 1045–1055. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Raman, V.; Rajendran, N. Corrosion behaviour of Ti–6Al–7Nb and Ti–6Al–4V ELI alloys in the simulated body fluid solution by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 52, 839–846. [Google Scholar] [CrossRef]
- Yuan, X.X.; Xu, N.X. Determination of hydrogen diffusion coefficient in metal hydride electrode by modified Warburg impedance. J. Alloys Comp. 2001, 329, 115–120. [Google Scholar] [CrossRef]
- Cvijović-Alagić, I.; Cvijović, Z.; Bajat, J.; Rakin, M. Composition and processing effects on the electrochemical characteristics of biomedical titanium alloys. Corros. Sci. 2014, 83, 245–254. [Google Scholar] [CrossRef]
- Assis, S.L.; Wolynec, S.; Costa, I. The electrochemical behaviour of Ti–13Nb–13Zr alloy in various solutions. Mater. Corros. 2008, 59, 739–743. [Google Scholar] [CrossRef]
- Alves, V.A.; Reis, R.Q.; Santos, I.C.B.; Souza, D.G.; Goncalves, T.F.; Pereirada-Silva, M.A.; Rossi, A.; Silva, L.A. In situ impedance spectroscopy study of the electrochemical corrosion of Ti and Ti–6Al–4V in simulated body fluid at 25 °C and 37 °C. Corros. Sci. 2009, 51, 2473–2482. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST) XPS Database Selected Element Search Menu. Available online: http://srdata.nist.gov/xps/selEnergyType.aspx (accessed on 5 October 2015).
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomenon, 2nd ed.; Elsevier: Oxford, UK, 2004; pp. 12–24. [Google Scholar]
- Oliveira, N.C.M.; Moura, C.C.G.; Zanetta-Barbosa, D.; Mendonça, D.B.S.; Cooper, L.; Mendonça, G.; Dechichi, P. Effects of titanium surface nodization with CaP incorporation on human osteoblastic response. Mater. Sci. Eng. C 2013, 33, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Carlier, A.; Chai, Y.C.; Moesen, M.; Theys, T.; Schrooten, J.; Van Oosterwyck, H.; Geris, L. Designing optimal calcium phosphate scaffold-cell combinations using an integrative model-based approach. Acta Biomater. 2011, 7, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Chun, J.-S.; Lee, W.-K. Association of collagen with calcium phosphate promoted osteogenic responses of osteoblast-like MG63 cells. Colloids Surf. B Biointerfaces 2011, 83, 245–253. [Google Scholar] [CrossRef] [PubMed]
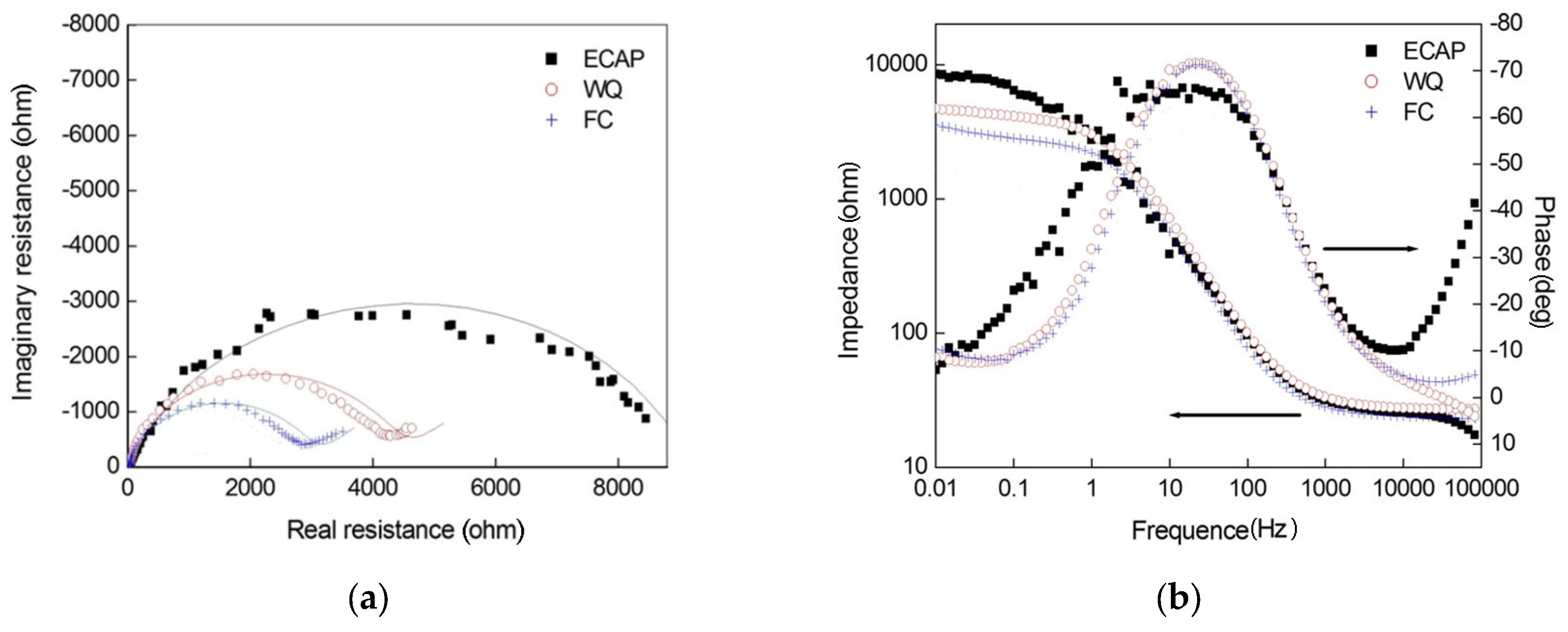
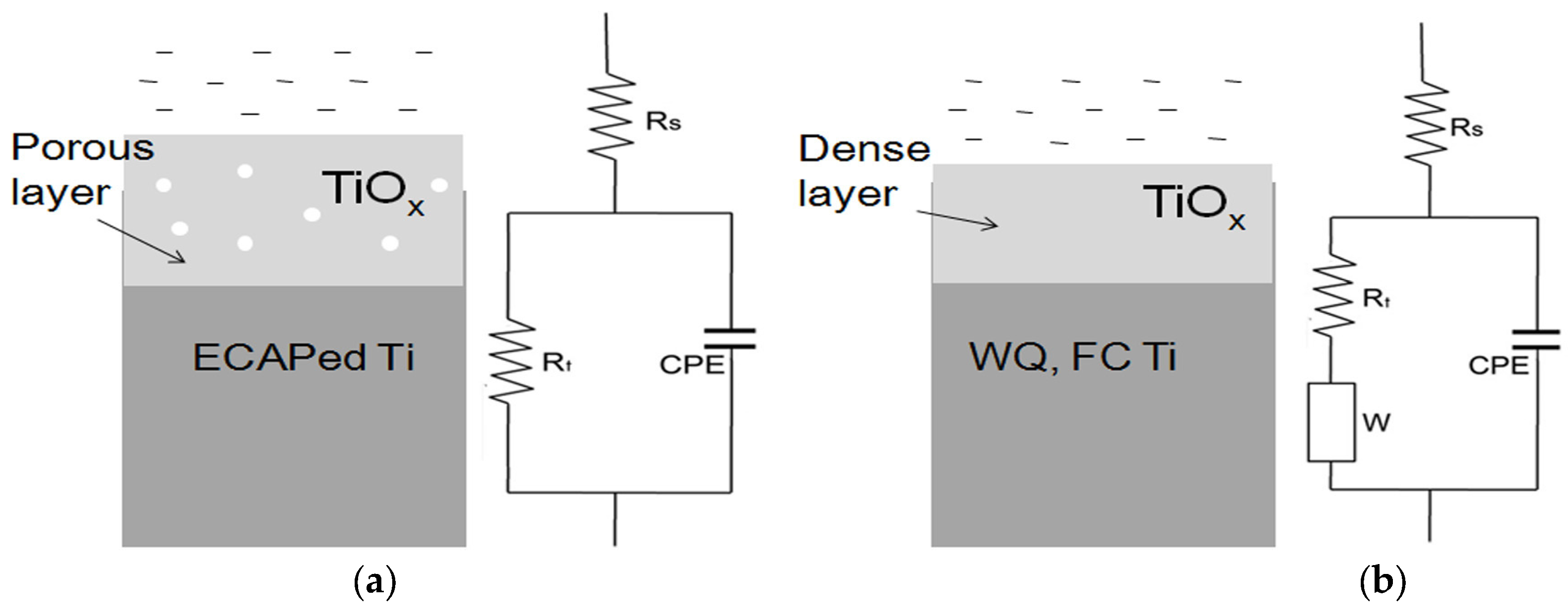
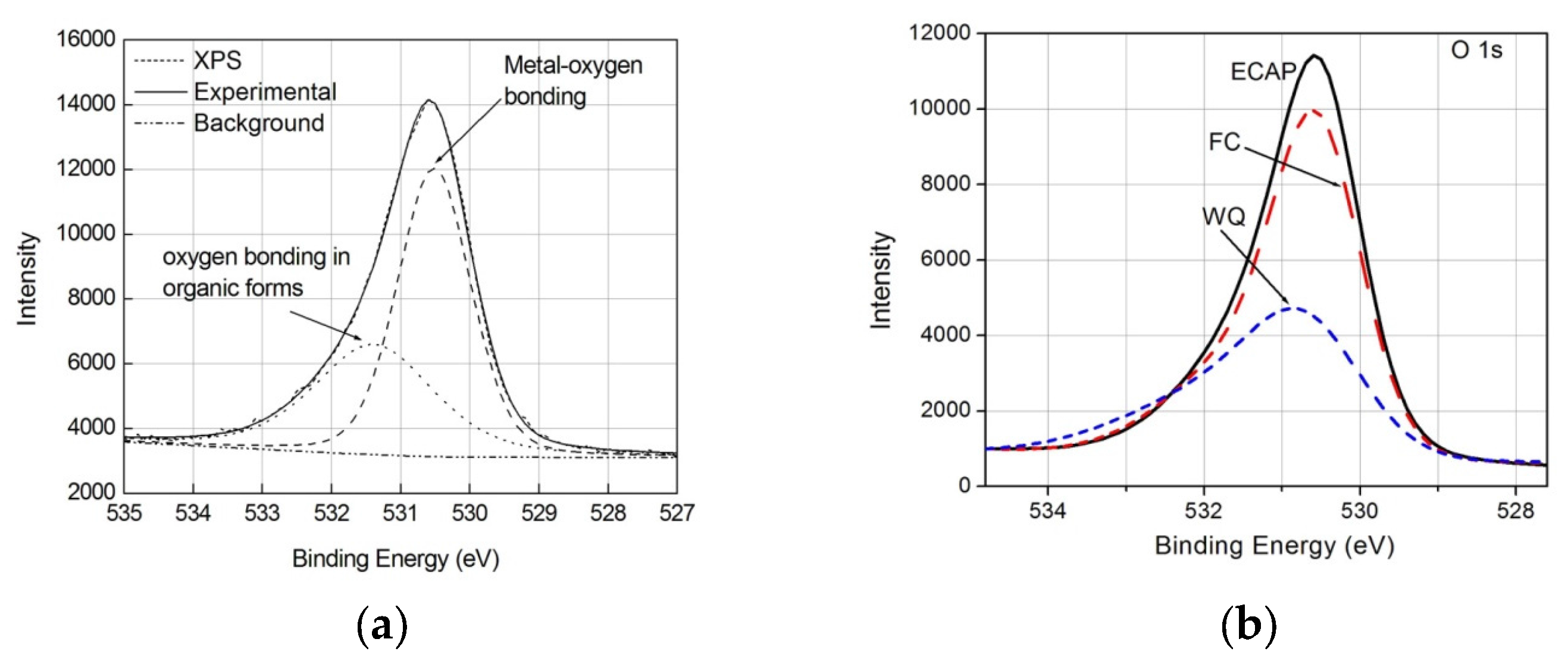

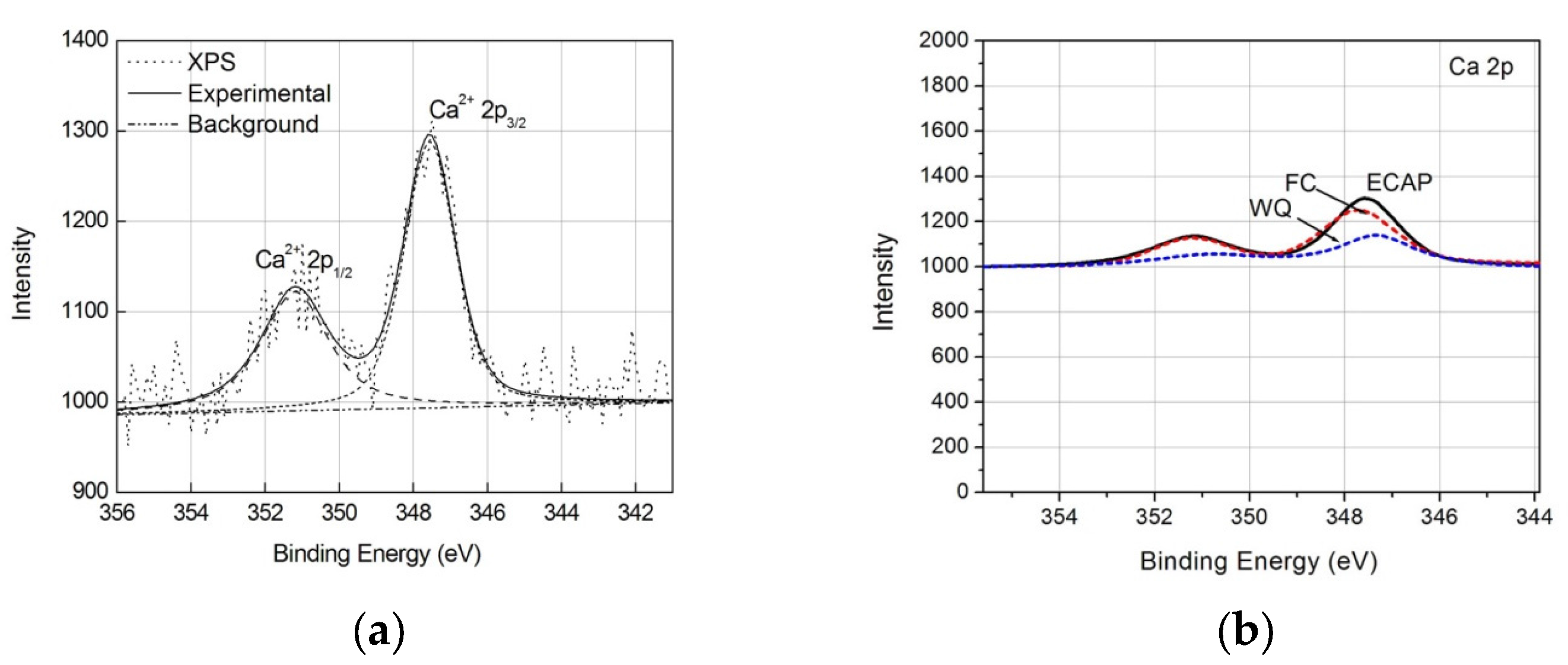
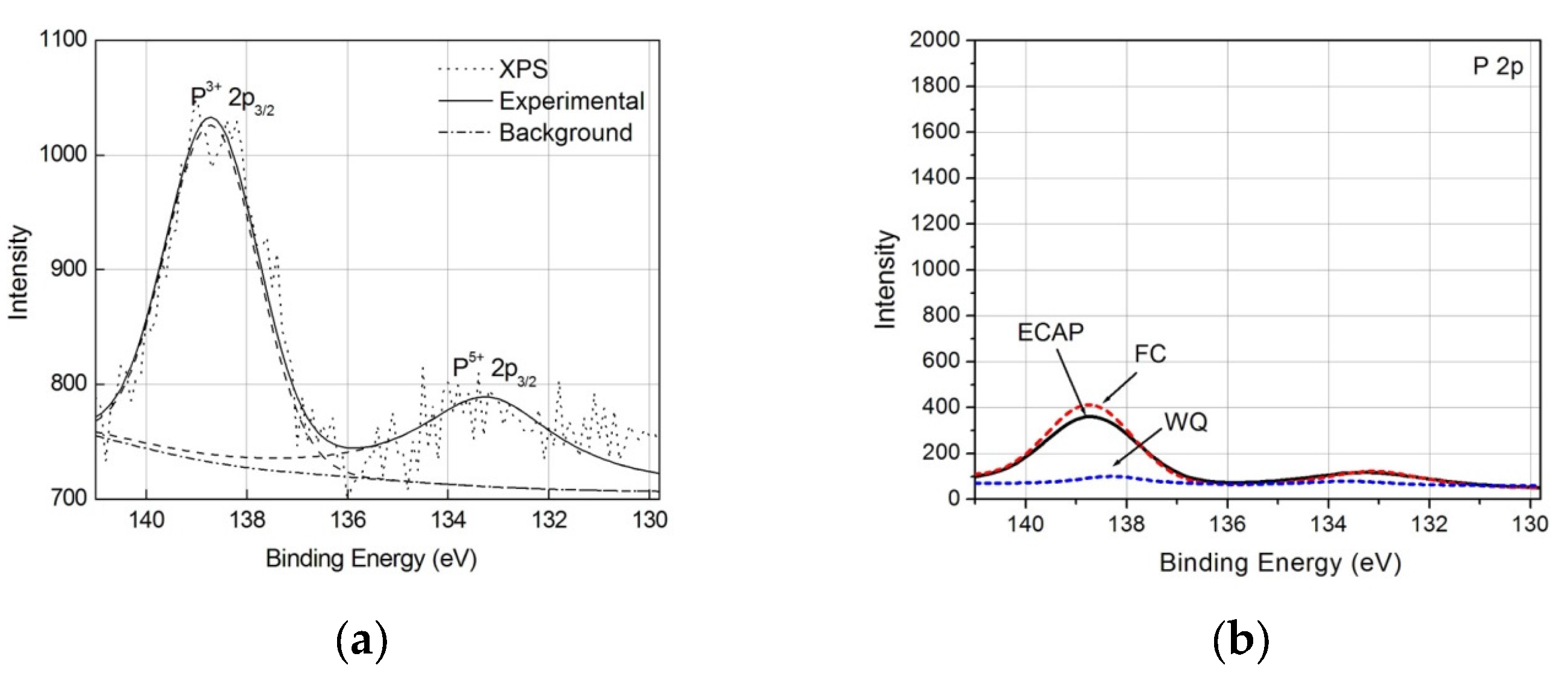
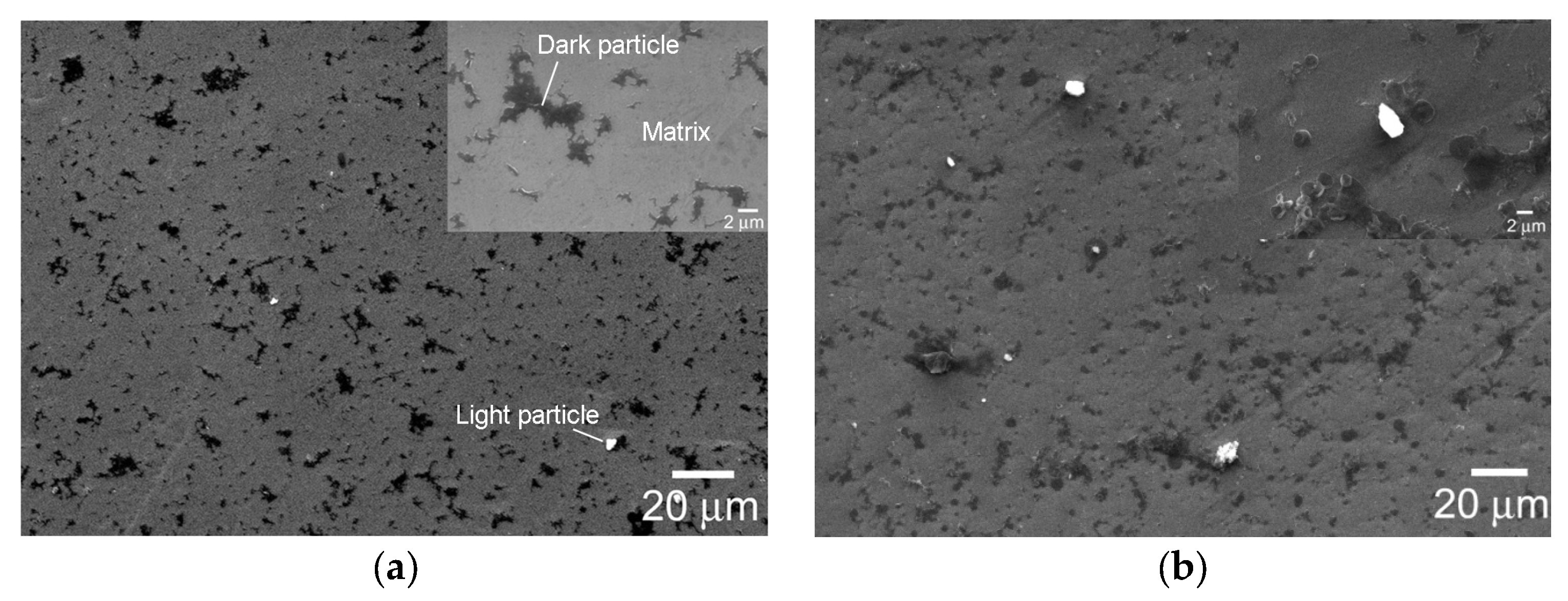

| Specimen Types | Rs (Ω·cm2) | Q (Ω−1·cm−2·sn) | n | Rt (Ω·cm2) | σ (Ω−1·cm−2·s0.5) |
|---|---|---|---|---|---|
| FC | 21.82 + 2.27/−4.17 | (5.54 + 2.34/−1.28) × 10−5 | 0.863 + 0.034/−0.035 | 1201.13 + 467.9/−45.5 | 0.0063 + 0.0019/−0.0021 |
| WQ | 25.19 + 2.66/−3.39 | (4.42 + 3.04/−2.12) × 10−5 | 0.882 + 0.048/−0.054 | 4452.0 + 664/−157 | 0.0049 + 0.0012/−0.0020 |
| ECAP | 21.5 ± 5.93 | (7.85 ± 1.07) × 10−5 | 0.773 ± 0.019 | 6043.5 ± 733.5 | - |
| Sample | O 1s | Ti 2p | Ca 2p | P 2p | N 1s |
|---|---|---|---|---|---|
| ECAP | 36.2 | 20.3 | 0.5 | 1.0 | 1.6 |
| FC | 31.2 | 17.5 | 0.5 | 0.4 | 1.7 |
| WQ | 22.2 | 17.2 | <0.1 | <0.1 | 4.3 |
| Specimens | Measured Places | O | Ti | P | Ca | Ca:P |
|---|---|---|---|---|---|---|
| ECAP-1 * | Matrix | 13.72 | 74.18 | 0.152 | 0 | - |
| Dark particle | 16.08 | 55.66 | 0.127 | 0.016 | 1:7.7 | |
| ECAP-2 | Matrix | 12.34 | 72.18 | 0 | 0.019 | - |
| Dark particle 1 | 17.55 | 46.86 | 0.076 | 0.015 | 1:5.2 | |
| Dark particle 2 | 13.73 | 54.24 | 0.114 | 0.024 | 1:4.7 | |
| FC-1 | Matrix | 3.49 | 92.54 | 0.015 | 0.023 | 1:0.6 |
| Light particle | 12.84 | 58.83 | 0.295 | 0.441 | 1:0.7 | |
| Dark particle | 8.01 | 61.89 | 0.033 | 0.043 | 1:0.8 | |
| FC-2 | Matrix | 4.27 | 91.00 | 0.116 | 0 | - |
| Light particle | 53.40 | 9.29 | 0 | 0 | - | |
| Dark particle | 6.28 | 78.04 | 0.026 | 0 | - | |
| WQ-1 | Matrix | 4.36 | 82.86 | 0.068 | 0 | - |
| Light particle | 37.88 | 16.36 | 0.081 | 0.070 | 1:1.2 | |
| WQ-2 | Matrix | 4.00 | 91.44 | 0.145 | 0 | - |
| Dark particle 1 | 6.18 | 81.33 | 0.147 | 0 | - | |
| Dark particle 2 | 3.61 | 90.37 | 0.187 | 0 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wang, L.; Zou, C.-H. Enhanced Surface Precipitates on Ultrafine-Grained Titanium in Physiological Solution. Metals 2017, 7, 245. https://doi.org/10.3390/met7070245
Zhou Q, Wang L, Zou C-H. Enhanced Surface Precipitates on Ultrafine-Grained Titanium in Physiological Solution. Metals. 2017; 7(7):245. https://doi.org/10.3390/met7070245
Chicago/Turabian StyleZhou, Qing, Lei Wang, and Cheng-Hong Zou. 2017. "Enhanced Surface Precipitates on Ultrafine-Grained Titanium in Physiological Solution" Metals 7, no. 7: 245. https://doi.org/10.3390/met7070245




