Simultaneous Removal of Hg(II) and Phenol Using Functionalized Activated Carbon Derived from Areca Nut Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Activated Carbon
2.3. Characterization of Materials
2.4. Batch Adsorption Experiment
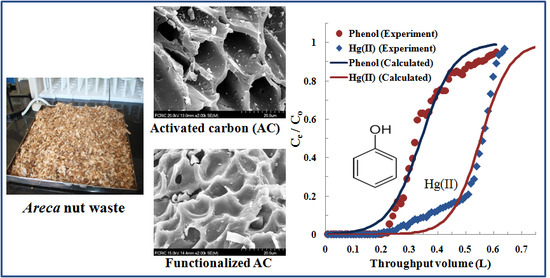
2.5. Fixed-Bed Column Adsorption
3. Results and Discussion
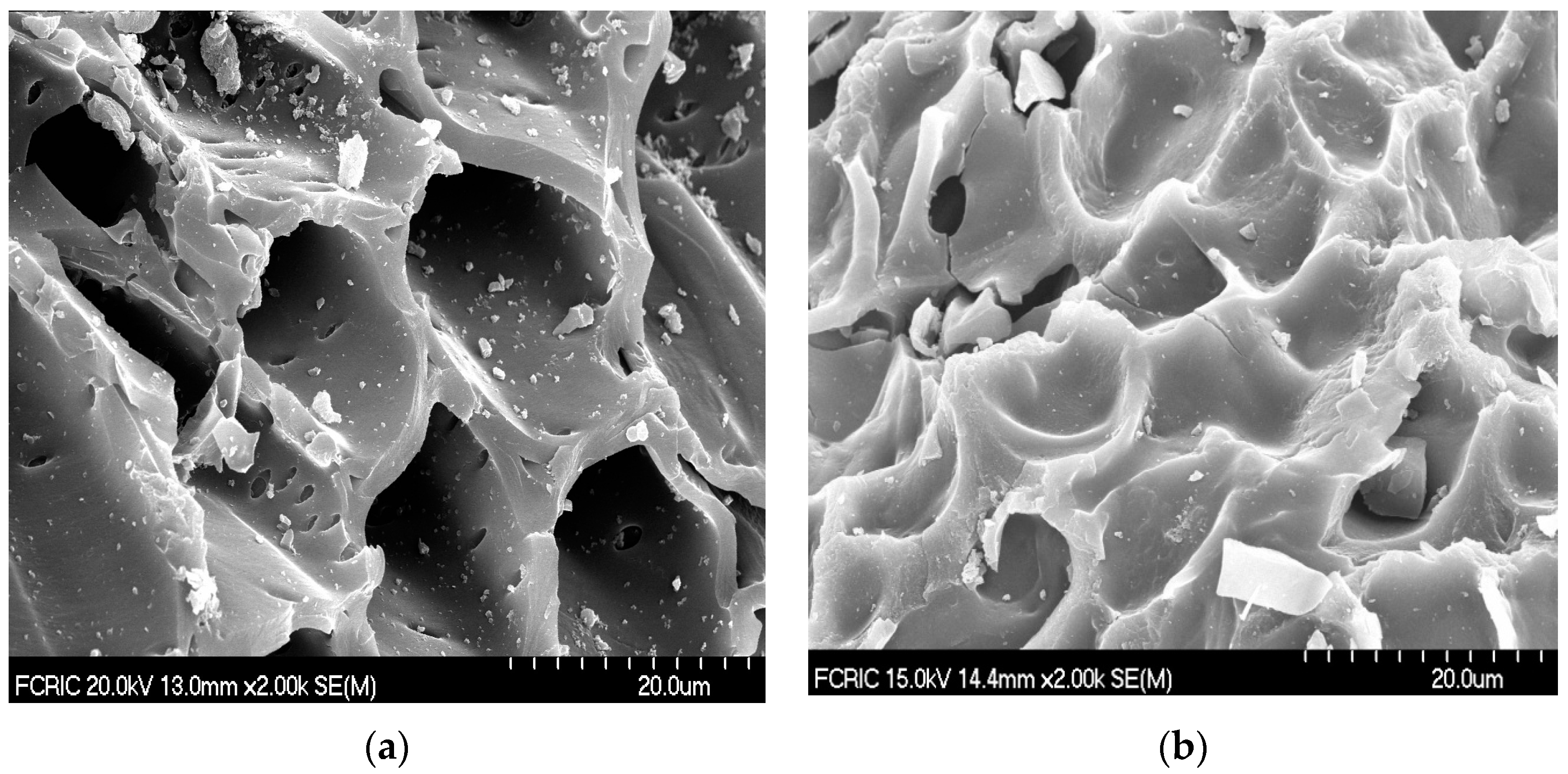
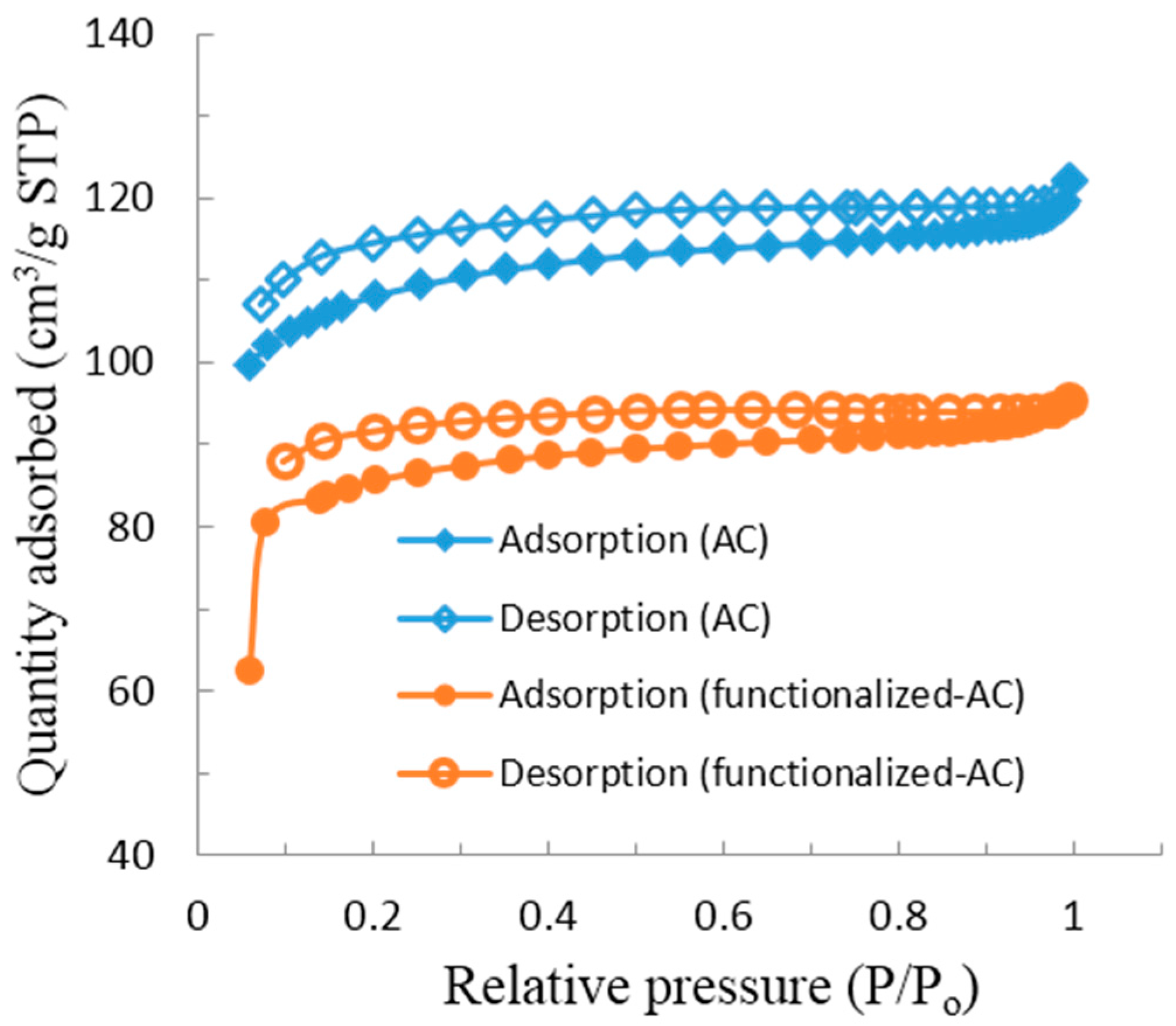
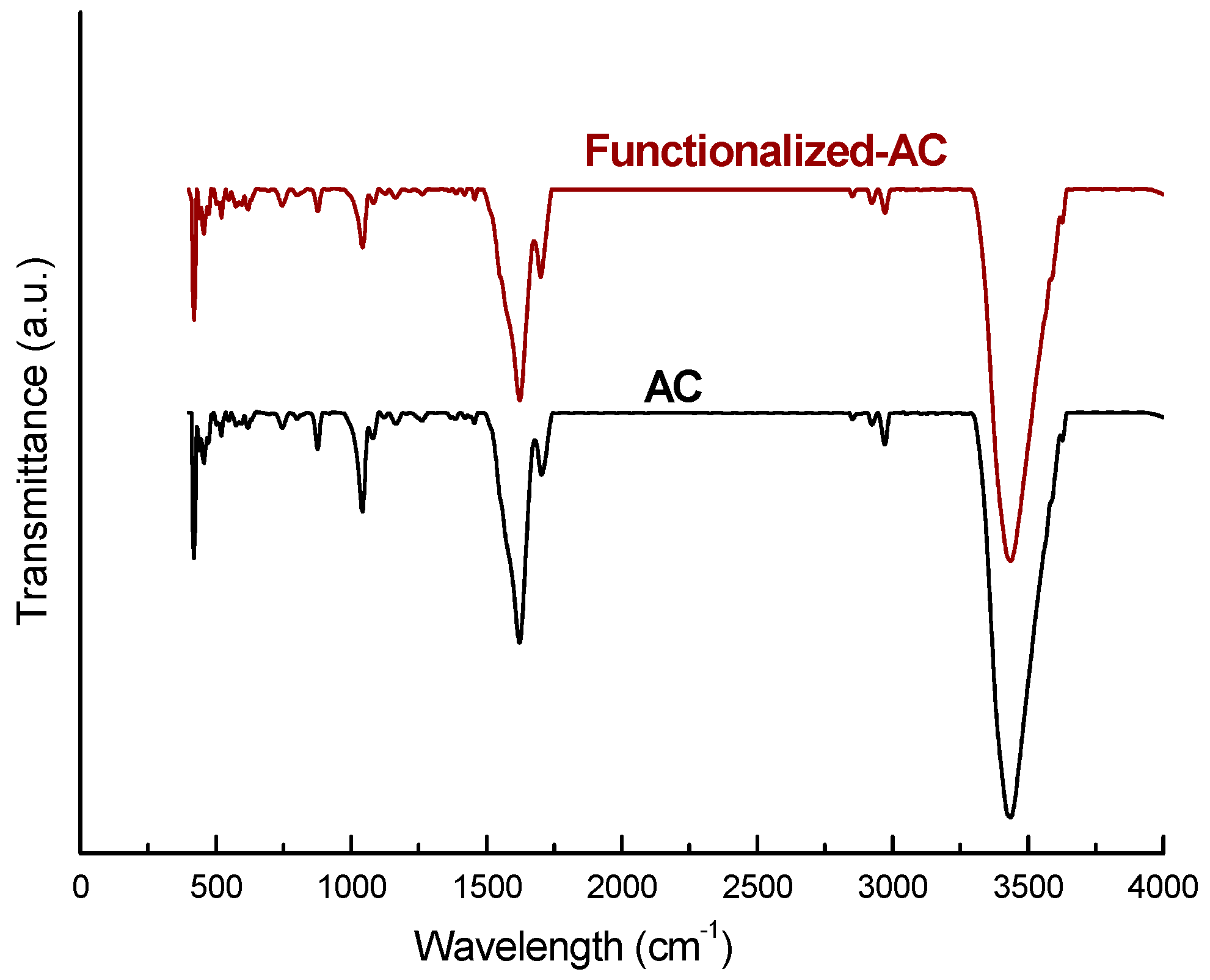
3.1. Characterization of Materials
3.2. Batch Adsorption Experiments
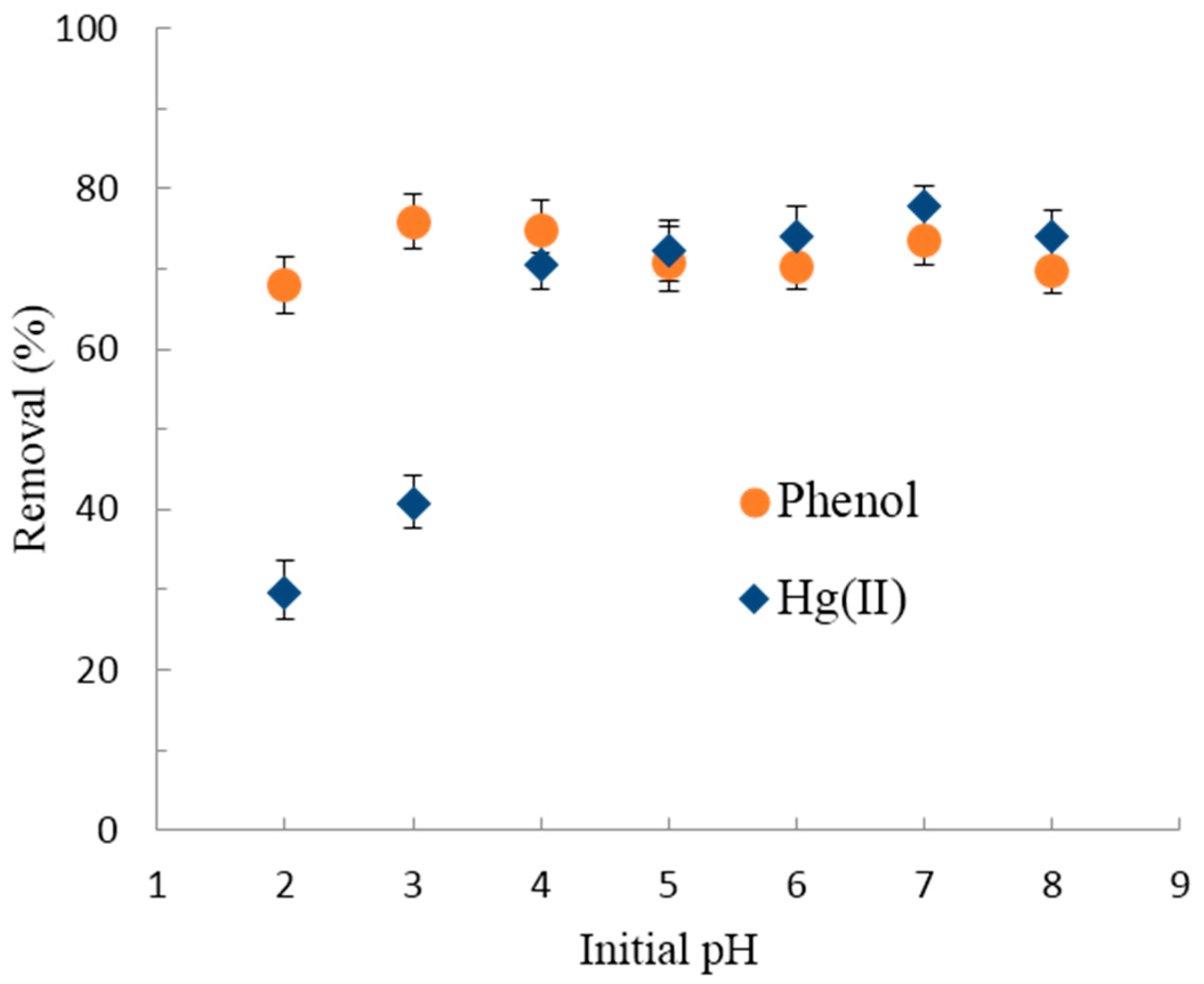
3.2.1. Effect of pH on Hg(II)/Phenol Adsorption
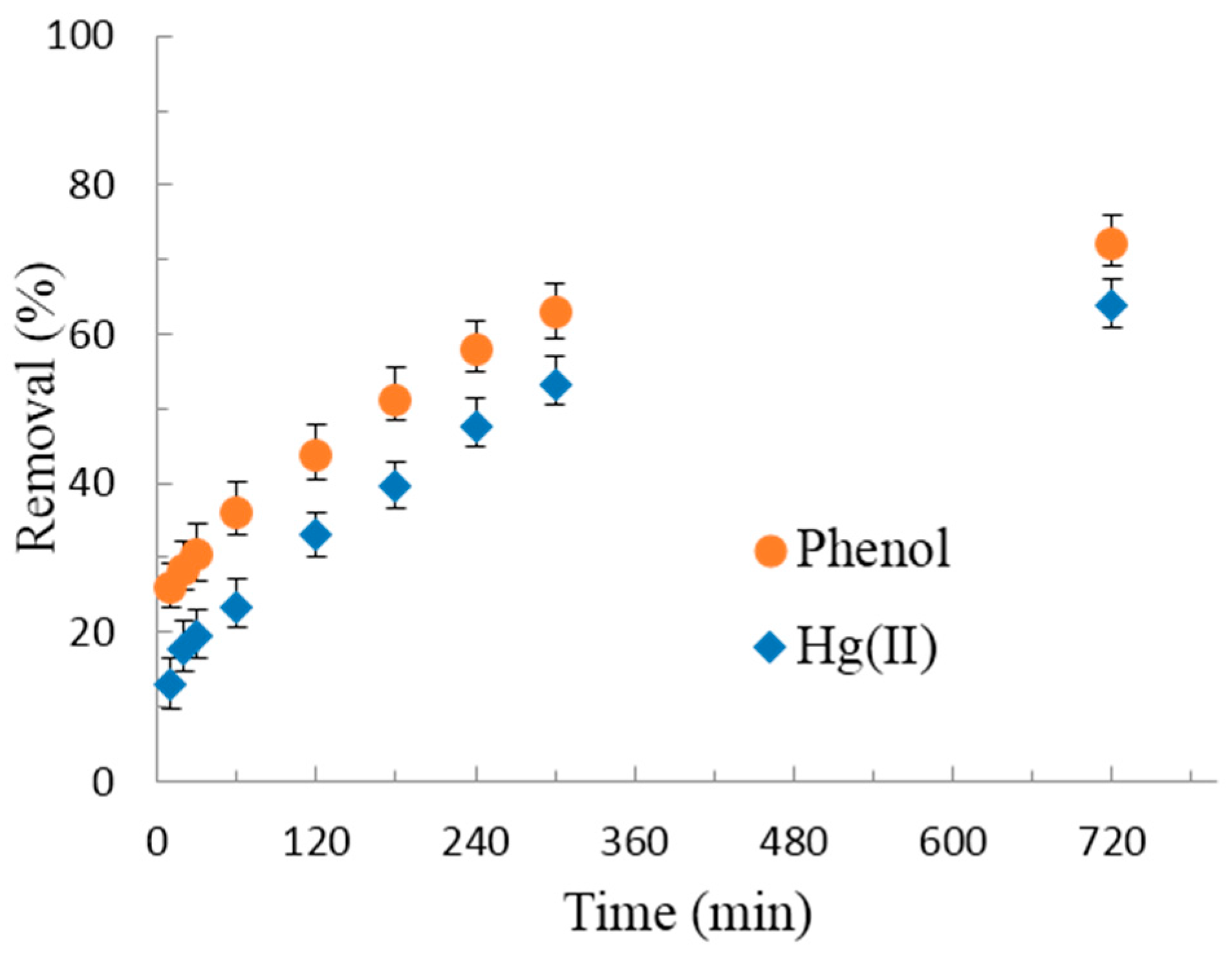
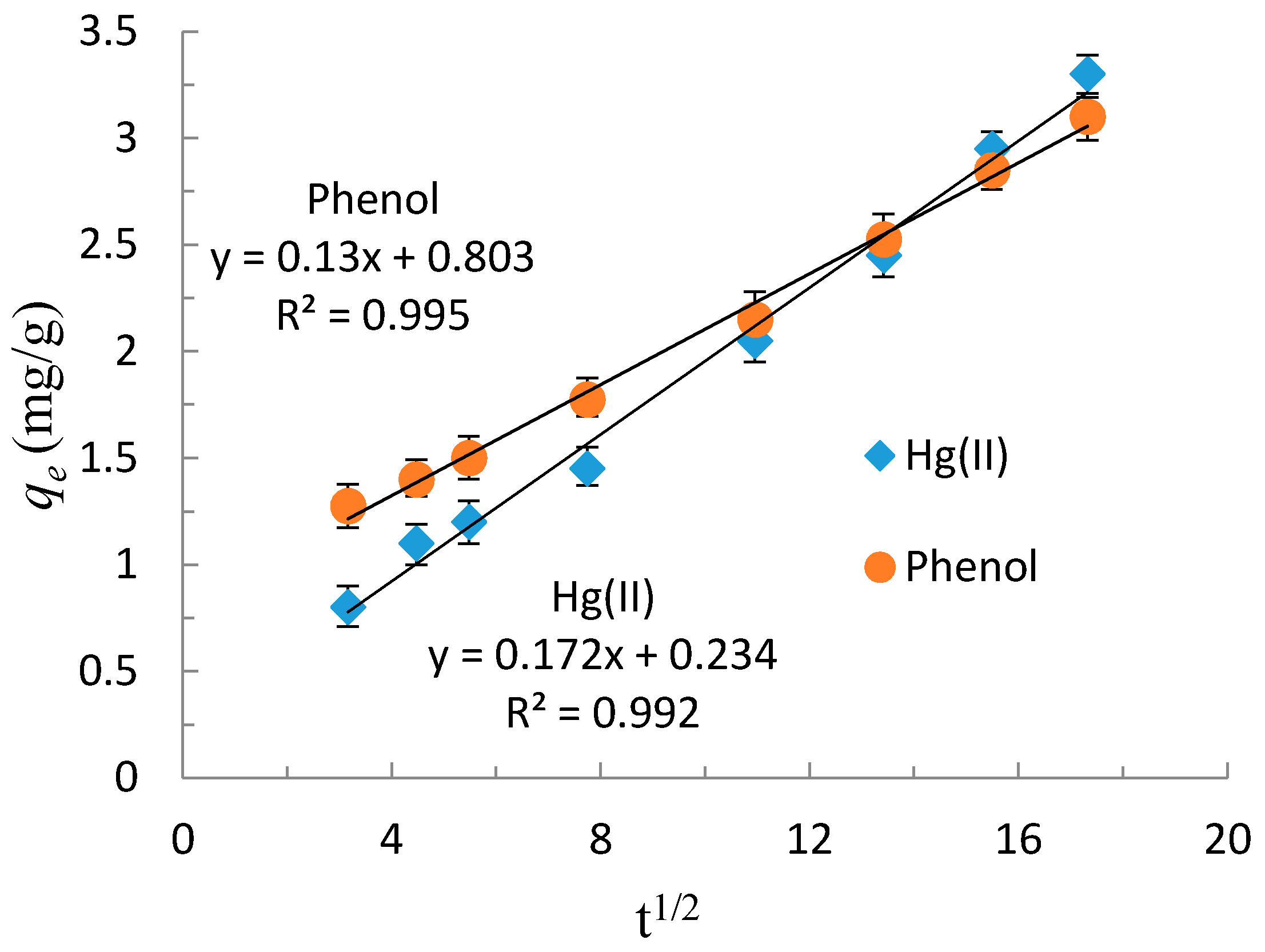
3.2.2. Adsorption Kinetics of Hg(II) and Phenol
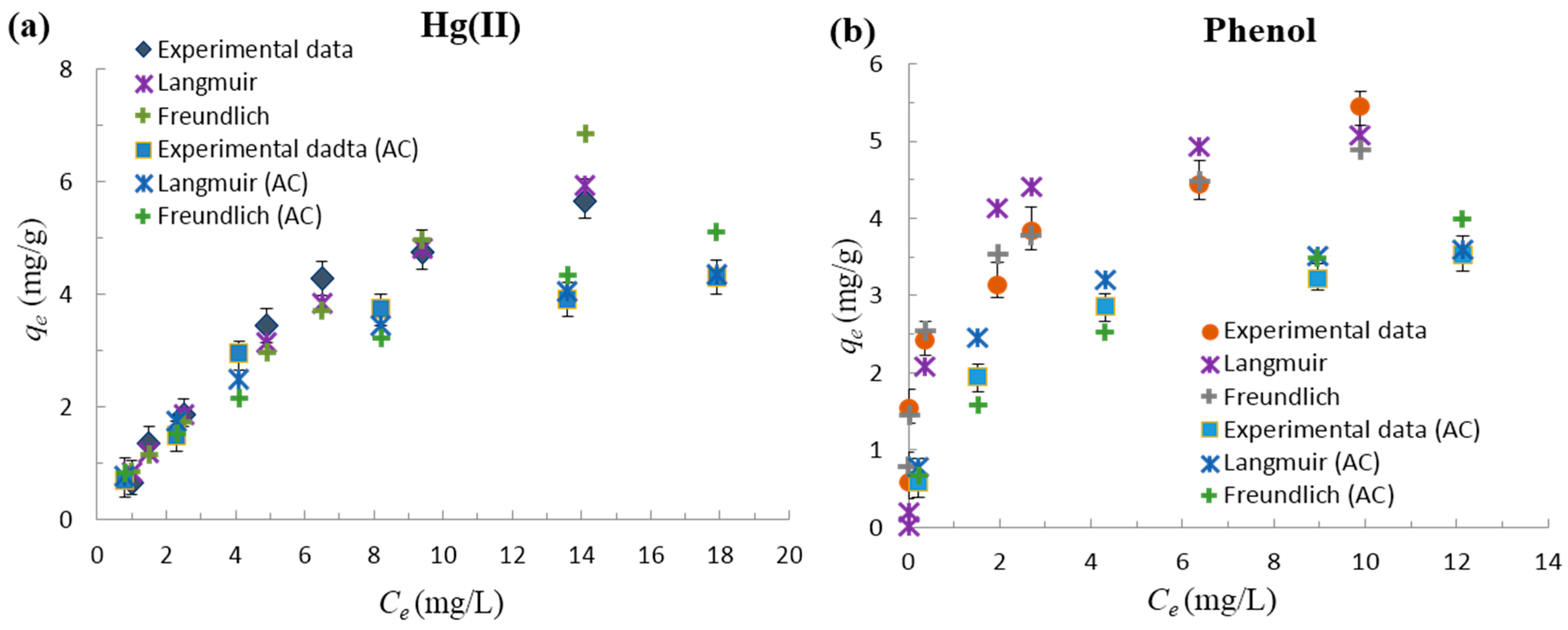
3.2.3. Adsorption Isotherm Studies
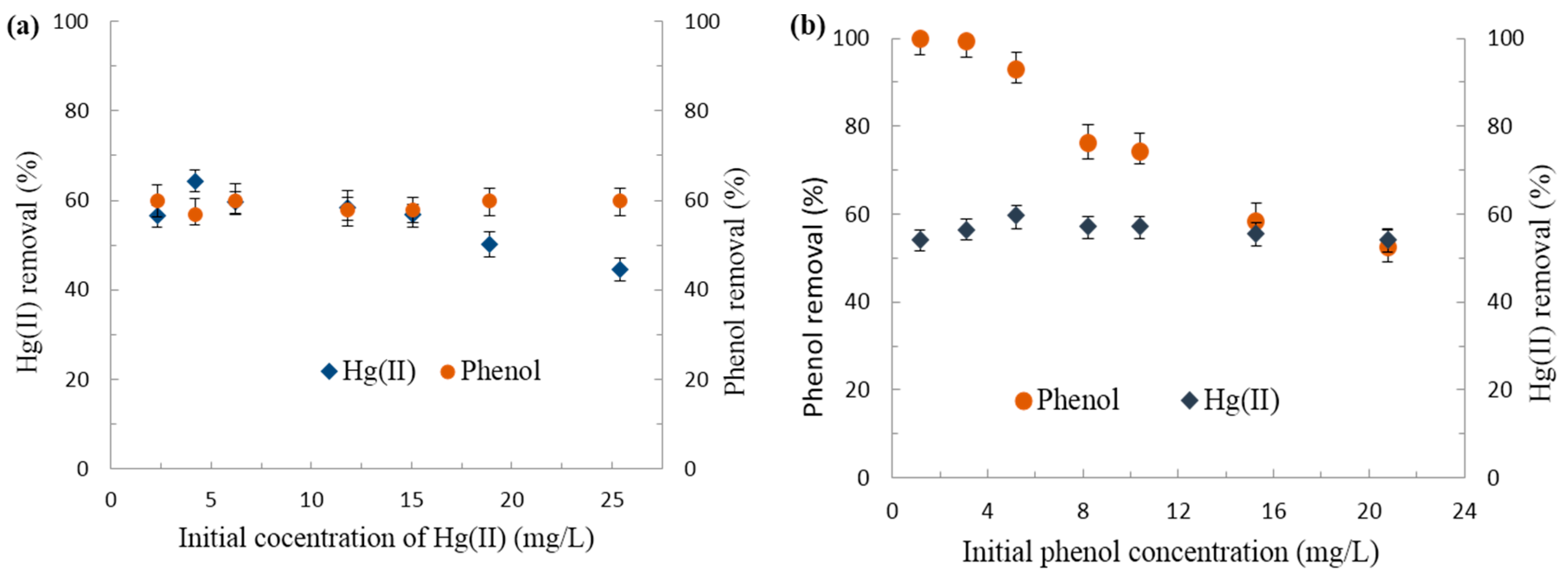
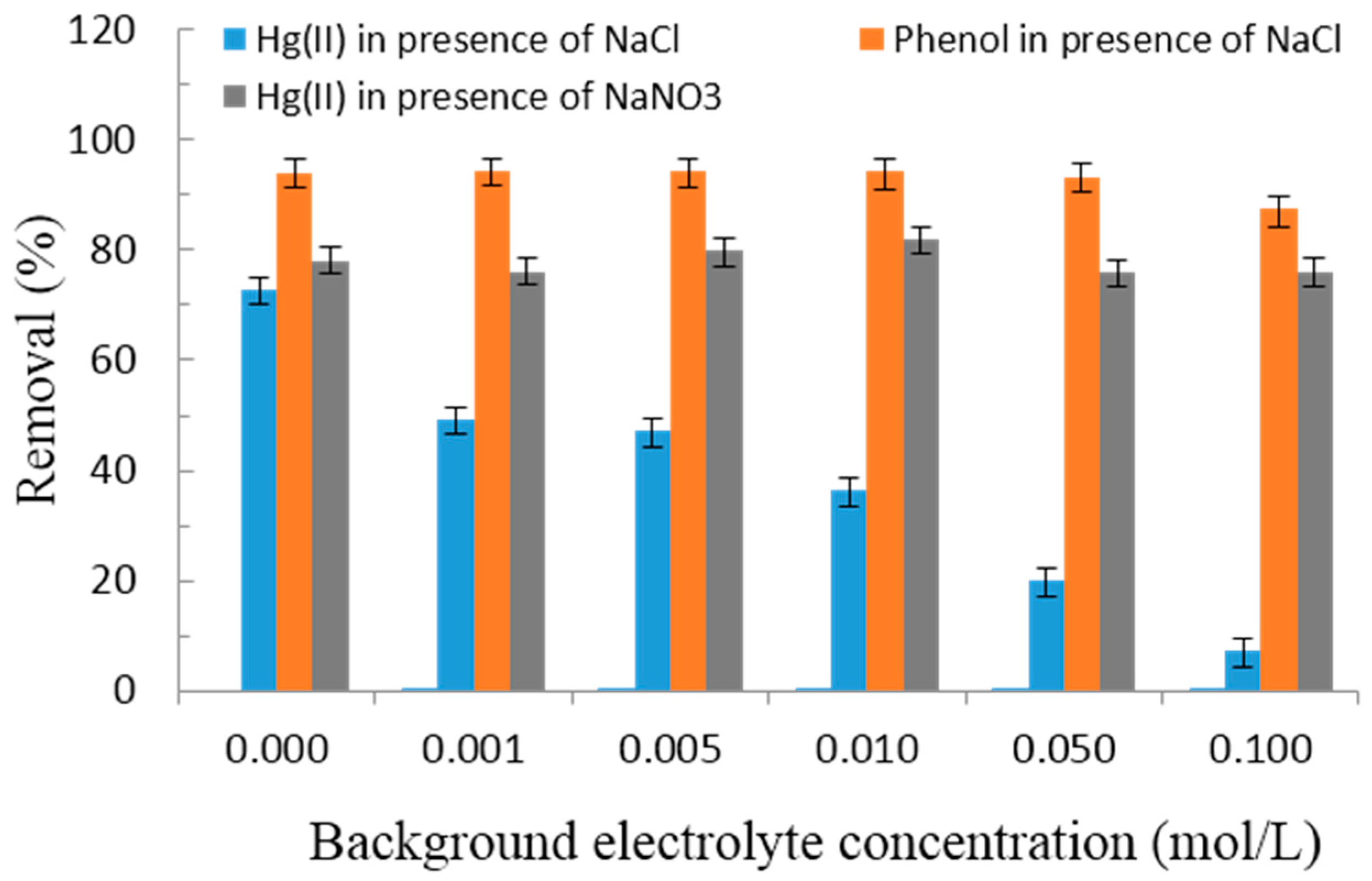
3.2.4. Effect of Background Electrolyte Concentrations
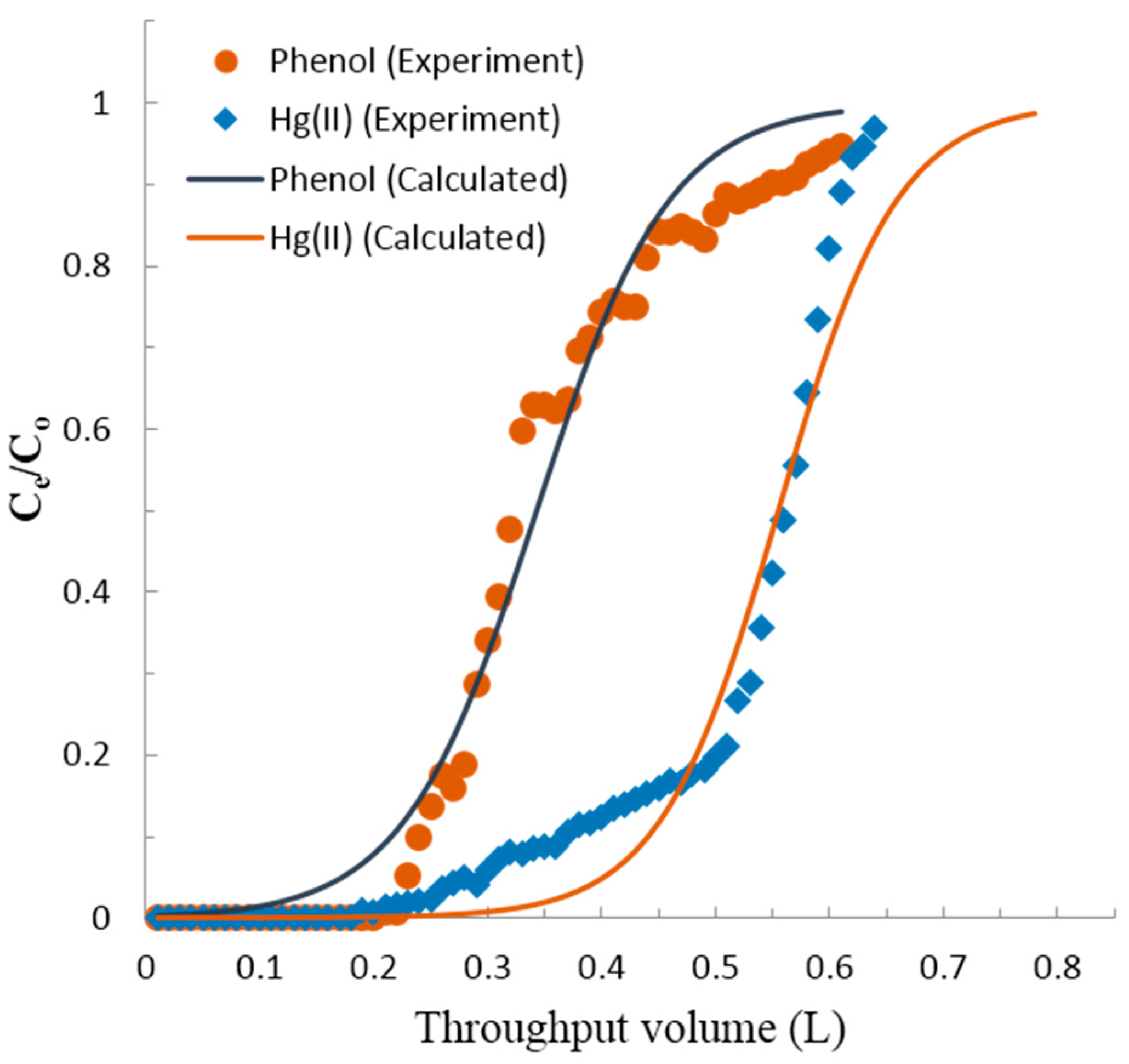
3.3. Fixed-Bed Column Adsorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Han, J.; Du, Z.; Zou, W.; Li, H.; Zhang, C. In-situ improved phenol adsorption at ions-enrichment interface of porous adsorbent for simultaneous removal of copper ions and phenol. Chem. Eng. J. 2015, 262, 571–578. [Google Scholar] [CrossRef]
- Shim, J.; Lim, J.-M.; Shea, P.J.; Oh, B.-T. Simultaneous removal of phenol, Cu and Cd from water with corn cob silica-alginate beads. J. Hazard. Mater. 2014, 272, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.P.; Gao, W.; Cui, X.; Ma, J.H.; Li, R.F. Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J. Taiwan Inst. Chem. Eng. 2016, 62, 192–198. [Google Scholar] [CrossRef]
- Fadzil, F.; Ibrahim, S.; Hanafiah, M.A.K.M. Adsorption of lead(II) onto organic acid modified rubber leaf powder: Batch and column studies. Process. Saf. Environ. Prot. 2016, 100, 1–8. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Sundseth, K.; Munthe, J.; Kindbom, K.; Wilson, S.; Steenhuisen, F.; Maxson, P. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 2010, 44, 2487–2499. [Google Scholar] [CrossRef]
- Han, C.; Wang, W.; Xie, F. Study on the leaching of mercuric oxide with thiosulfate solutions. Metals 2016, 6, 206. [Google Scholar] [CrossRef]
- Solis, K.L.; Nam, G.-U.; Hong, Y. Effectiveness of gold nanoparticle-coated silica in the removal of inorganic mercury in aqueous systems: Equilibrium and kinetic studies. Environ. Eng. Res. 2016, 21, 99–107. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Lemos, V.A.; Silva, L.O.B.; Queiroz, A.F.S.; Souza, A.S.; Silva, E.G.P.; Santos, W.N.L.; Virgens, C.F. Analytical strategies of sample preparation for the determination of mercury in food matrices—A review. Microchem. J. 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Crespo-López, M.E.; Macêdo, G.L.; Pereira, S.I.D.; Arrifano, G.P.F.; Picanço-Diniz, D.L.W.; Nascimento, J.L.M.; Herculano, A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Yang, J.-K.; Chang, Y.-Y. Rapid degradation of phenol by ultrasound-dispersed nano-metallic particles (NMPs) in the presence of hydrogen peroxide: A possible mechanism for phenol degradation in water. J. Environ. Manag. 2016, 175, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Păcurariu, C.; Mihoc, G.; Popa, A.; Muntean, S.G.; Ianoş, R. Adsorption of phenol and p-chlorophenol from aqueous solutions on poly (styrene-co-divinylbenzene) functionalized materials. Chem. Eng. J. 2013, 222, 218–227. [Google Scholar] [CrossRef]
- Cheng, Z.; Fu, F.; Pang, Y.; Tang, B.; Lu, J. Removal of phenol by acid-washed zero-valentaluminium in the presence of H2O2. Chem. Eng. J. 2015, 260, 284–290. [Google Scholar] [CrossRef]
- Huber, J.; Leopold, K. Nanomaterial-based strategies for enhanced mercury trace analysis in environmental and drinking waters. TrAC Trends Anal. Chem. 2016, 80, 280–292. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; To, M.-H.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-S.; Nriagu, J.O.; Itoh, H. Mercury removal from water using activated carbons derived from organic sewage sludge. Water Res. 2005, 39, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Thielke, M.W.; Bultema, L.A.; Brauer, D.D.; Richter, B.; Fischer, M.; Theato, P. Rapid mercury(II) removal by electrospun sulfur copolymers. Polymers 2016, 8, 266. [Google Scholar] [CrossRef]
- Arias, F.E.A.; Beneduci, A.; Chidichimo, F.; Furia, E.; Straface, S. Study of the adsorption of mercury(II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017, 180, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Luo, L.; Zhang, J.; Christie, P.; Zhang, S. Adsorption of mercury on lignin: Combined surface complexation modeling and X-ray absorption spectroscopy studies. Environ. Pollut. 2012, 162, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Ma, D.; Shi, Z.; Ma, S. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Juang, R.-S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Abussaud, B.; Asmaly, H.A.; Ihsanullah; Saleh, T.A.; Gupta, V.K.; laoui, T.; Atieh, M.A. Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide and titanium oxide. J. Mol. Liq. 2016, 213, 351–359. [Google Scholar] [CrossRef]
- Feng, J.; Qiao, K.; Pei, L.; Lv, J.; Xie, S. Using activated carbon prepared from TyphaorientalisPresl to remove phenol from aqueous solutions. Ecol. Eng. 2015, 84, 209–217. [Google Scholar] [CrossRef]
- Dalai, C.; Jha, R.; Desai, V.R. Rice husk and sugarcane baggase based activated carbon for iron and manganese removal. Aquat. Procedia 2015, 4, 1126–1133. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- AL-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Johari, K.; Saman, N.; Song, S.T.; Chin, C.S.; Kong, H.; Mat, H. Adsorption enhancement of elemental mercury by various surface modified coconut husk as eco-friendly low-cost adsorbents. Int. Biodeterior. Biodegrad. 2016, 109, 45–52. [Google Scholar] [CrossRef]
- Sasmal, S.; Goud, V.V.; Mohanty, K. Characterization of biomasses available in the region of North-East India for production of biofuels. Biomass Bioenergy 2012, 45, 212–220. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Mohanraj, G.T. Synthesis and characterization of chemically activated carbon derived from areca nut shell. Carbon Sci. Technol. 2016, 8, 32–39. [Google Scholar]
- Ranugadevi, N.; Anitha, G.; Lalitha, P. Kinetics of the removal of hexavalent chromium using a lowcost activated carbon adsorbent. Adv. Appl. Sci. Res. 2010, 1, 102–105. [Google Scholar]
- Renugadevi, N.; Anitha, G.; Lalitha, P. Hexavalent chromium removal using a low-cost activated carbon adsorbent from areca catechu. Indian J. Environ. Prot. 2011, 31, 52–58. [Google Scholar]
- Sundaram, M.M.; Sivakumar, S. Effective role of areca nut shell carbon and cashew nut shell carbon in the removal of congo red dye for the application towards effluent treatment. Ind. J. Environ. Prot. 2013, 33, 546–553. [Google Scholar]
- Gopalswami, P.; Sivakumar, N.; Ponnuswamy, S.; Venkateswaren, V.; Kavitha, G. Adsorption of direct dye onto activated carbon prepared from areca nut pod—An agricultural waste. J. Environ. Sci. Eng. 2010, 52, 367–372. [Google Scholar] [PubMed]
- Geetha, A.; Sivakumar, P.; Sujatha, M.; Palanisamy, P.N.; Somasundaram, V. Adsorption of acid blue from an aqueous solution onto activated areca nut shell carbon: Equilibrium, kinetic and thermodynamic studies. Res. J. Chem. Environ. 2009, 13, 52–58. [Google Scholar]
- Guo, H.; Zhang, S.; Kou, Z.; Zhai, S.; Ma, W.; Yang, Y. Removal of cadmium(II) from aqueous solutions by chemically modified maize straw. Carbohydr. Polym. 2015, 115, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Elandaloussi, E.H.; Belhalfaoui, B.; Ouali, M.S.; De Ménorval, L.C. Efficiency of succinylated-olive stone biosorbent on the removal of cadmium ions from aqueous solutions. Colloids Surf. B Biointerfaces 2009, 73, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Clave, E.; François, J.; Billon, L.; Sèbe, G.; Jéso, B.D.; Guimon, M.F. Crude and modified corncobs as complexing agents for water decontamination. J. Appl. Polym. Sci. 2004, 91, 820–826. [Google Scholar] [CrossRef]
- Gurgel, L.V.A.; Freitas, R.P.D.; Gil, L.F. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by sugarcane bagasse and mercerized sugarcane bagasse chemically modified with succinic anhydride. Carbohydr. Polym. 2008, 74, 922–929. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, J.; Shen, D.; Xiao, R.; Gu, S.; Zhao, M.; Liang, J. Removal of Pb(II) from water by the activated carbon modified by nitric acid under microwave heating. J. Colloid Interface Sci. 2016, 463, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Lalhmunsiama; Choi, S.-I.; Tiwari, D. Manganese and iron oxide immobilized activated carbons precursor to dead biomasses in the remediation of cadmium-contaminated waters. Environ. Sci. Pollut. Res. 2013, 20, 7464–7477. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, L.V.A.; Júnior, O.K.; Gil, R.P.d.F.; Gil, L.F. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Bioresour. Technol. 2008, 99, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Chadlia, A.; Mohamed, K.; Najah, L.; Farouk, M.M. Preparation and characterization of new succinic anhydride grafted Posidonia for the removal of organic and inorganic pollutants. J. Hazard. Mater. 2009, 172, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Kim, W.; Kim, M.; Prasad, S.K.; Lee, S.-M. Organo-modified sericite in the remediation of phenol-contaminated waters. Desalin. Water Treat. 2015, 53, 446–451. [Google Scholar] [CrossRef]
- Mudasir, M.; Karelius, K.; Aprilita, N.H.; Wahyuni, E.T. Adsorption of mercury(II) on dithizone-immobilized natural zeolite. J. Environ. Chem. Eng. 2016, 4, 1839–1849. [Google Scholar] [CrossRef]
- Singanan, M. Biosorption of Hg(II) ions from synthetic wastewater using a novel biocarbon technology. Environ. Eng. Res. 2015, 20, 33–39. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.-P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Lalhmunsiama; Tiwari, D.; Lee, S.-M. Surface-functionalized activated sericite for the simultaneous removal of cadmium and phenol from aqueous solutions: Mechanistic insights. Chem. Eng. J. 2016, 283, 1414–1423. [Google Scholar]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ. Technol. 1996, 17, 71–77. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Rao, M.M.; Wang, M.C. Biosorption of Pb2+ from aqueous solutions by Moringaoleifera bark: Equilibrium and kinetic studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, M.; Ajithkumar, P.S.; Singh, R.P.; Natarajan, N. Mass transfer kinetics using two-site interface model for removal of Cr(VI) from aqueous solution with cassava peel and rubber tree bark as adsorbents. Environ. Eng. Res. 2016, 21, 152–163. [Google Scholar] [CrossRef]
- Hayes, K.F.; Papelis, C.; Leckie, J.O. Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interfaces. J. Colloid Interface Sci. 1988, 125, 717–726. [Google Scholar] [CrossRef]
- El-Bayaa, A.A.; Badawy, N.A.; Alkhalik, E.A. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Terzyk, A.P. Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption. J. Colloid Interface Sci. 2003, 268, 301–329. [Google Scholar] [CrossRef]
- Zhang, D.; Huo, P.; Liu, W. Behavior of phenol adsorption on thermal modified activated carbon. Chin. J. Chem. Eng. 2016, 24, 446–452. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Tiwari, D.; Laldanwngliana, C.; Choi, C.-H.; Lee, S.M. Manganese-modified natural sand in the remediation of aquatic environment contaminated with heavy metal toxic ions. Chem. Eng. J. 2011, 171, 958–966. [Google Scholar] [CrossRef]
| Sample | Pseudo-First Order Kinetic | Pseudo-Second Order Kinetic | Intra-Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (1/min) | qe (mg/g) | R2 | k2 (g/mg/min) | qe (mg/g) | R2 | Kid (mg/g/min1/2) | Z | R2 | |
| Hg(II) | 5.0 × 10−3 | 1.72 | 0.95 | 1.0 × 10−2 | 3.19 | 0.97 | 0.12 | 0.23 | 0.99 |
| Phenol | 5.0 × 10−3 | 1.48 | 0.90 | 6.0 × 10−3 | 3.27 | 0.95 | 0.13 | 0.80 | 0.99 |
| Material | Pollutant | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qo (mg/g) | KL (L/g) | R2 | 1/n | KF(mg/g) | R2 | ||
| Functionalized-AC | Hg(II) | 11.235 | 0.079 | 0.848 | 0.792 | 0.843 | 0.953 |
| Activated carbon (AC) | Hg(II) | 5.555 | 0.199 | 0.977 | 0.586 | 0.942 | 0.925 |
| Functionalized-AC | Phenol | 5.376 | 1.706 | 0.970 | 0.198 | 3.104 | 0.968 |
| AC | Phenol | 3.846 | 0.116 | 0.997 | 0.443 | 1.320 | 0.954 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalhmunsiama; Lee, S.M.; Choi, S.S.; Tiwari, D. Simultaneous Removal of Hg(II) and Phenol Using Functionalized Activated Carbon Derived from Areca Nut Waste. Metals 2017, 7, 248. https://doi.org/10.3390/met7070248
Lalhmunsiama, Lee SM, Choi SS, Tiwari D. Simultaneous Removal of Hg(II) and Phenol Using Functionalized Activated Carbon Derived from Areca Nut Waste. Metals. 2017; 7(7):248. https://doi.org/10.3390/met7070248
Chicago/Turabian StyleLalhmunsiama, Seung Mok Lee, Suk Soon Choi, and Diwakar Tiwari. 2017. "Simultaneous Removal of Hg(II) and Phenol Using Functionalized Activated Carbon Derived from Areca Nut Waste" Metals 7, no. 7: 248. https://doi.org/10.3390/met7070248