Dissolution of Grain Boundary Carbides by the Effect of Solution Annealing Heat Treatment and Aging Treatment on Heat-Resistant Cast Steel HK30
Abstract
:1. Introduction
2. Materials and Methods
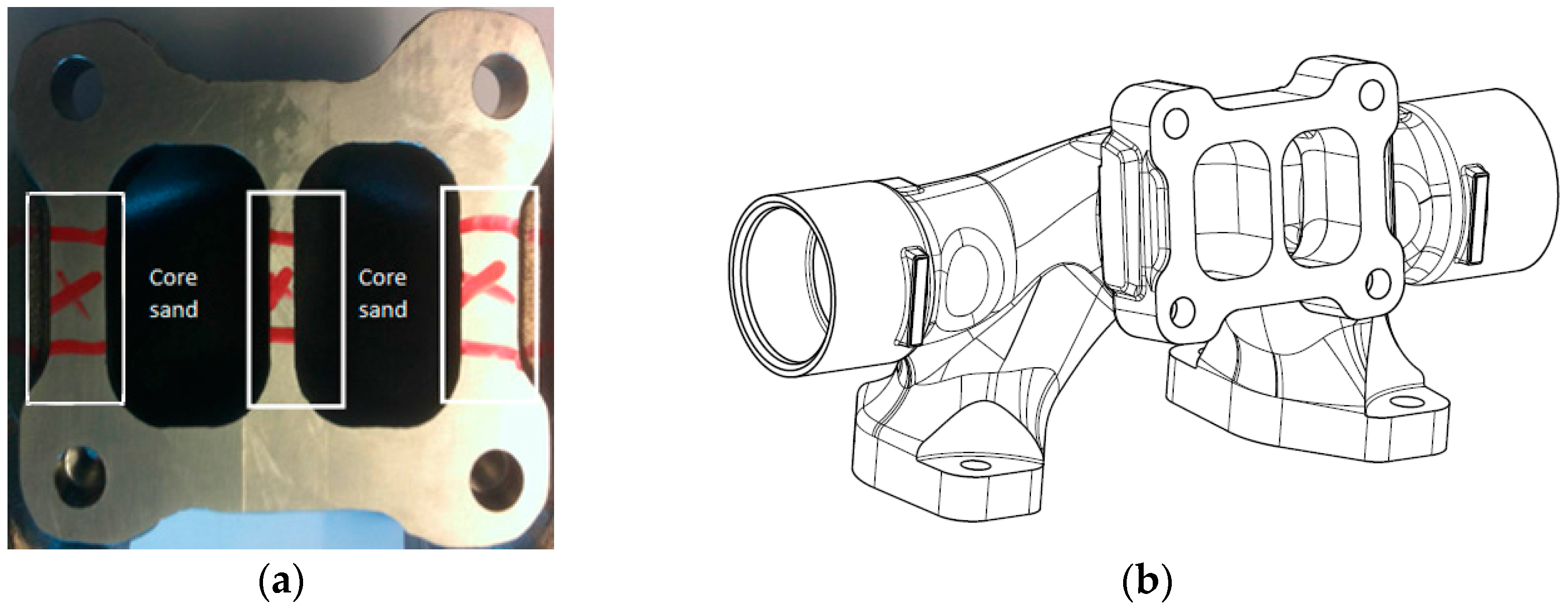
2.1. Materials Characterization
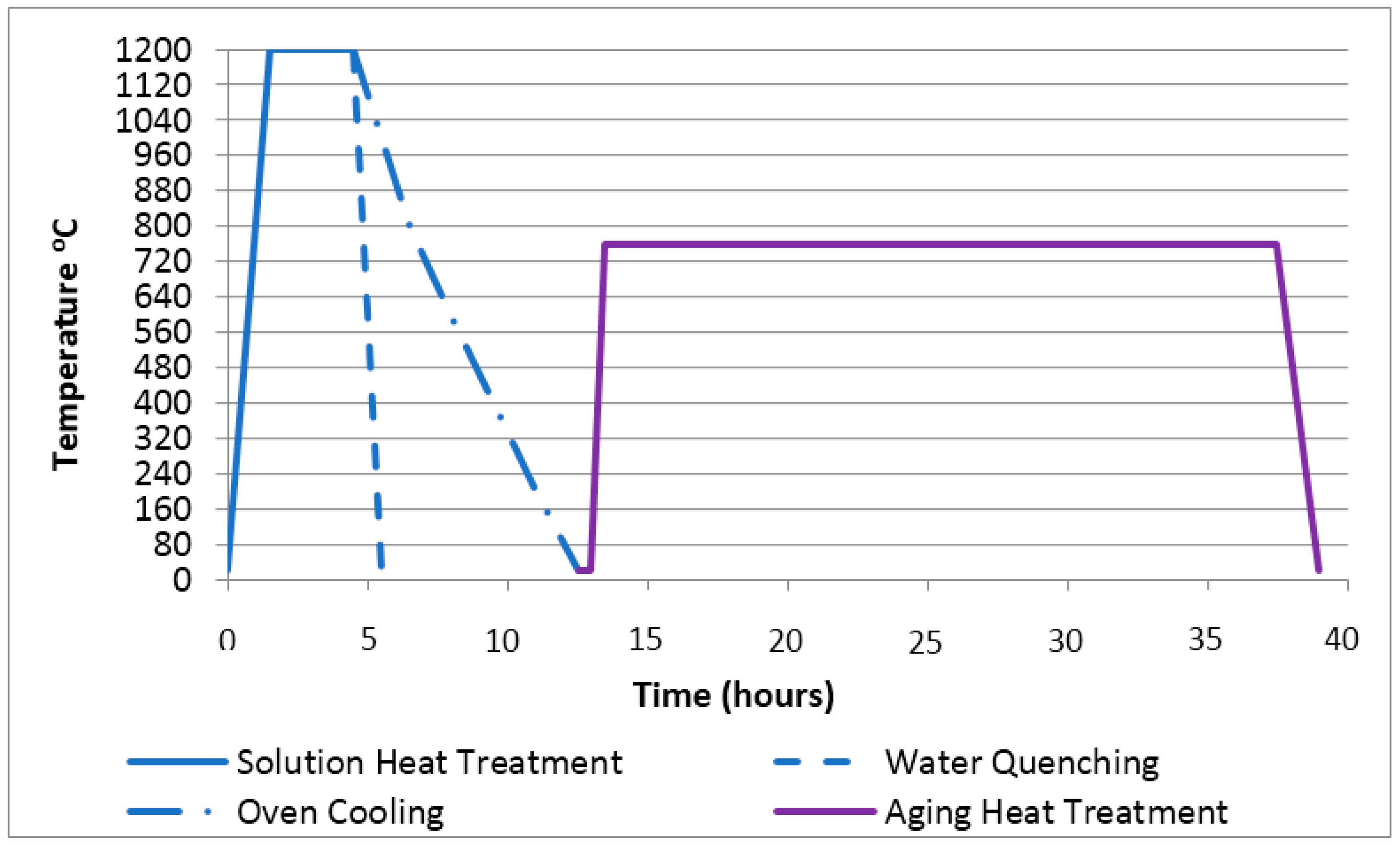
2.2. Heat Treatments
3. Results and Discussion
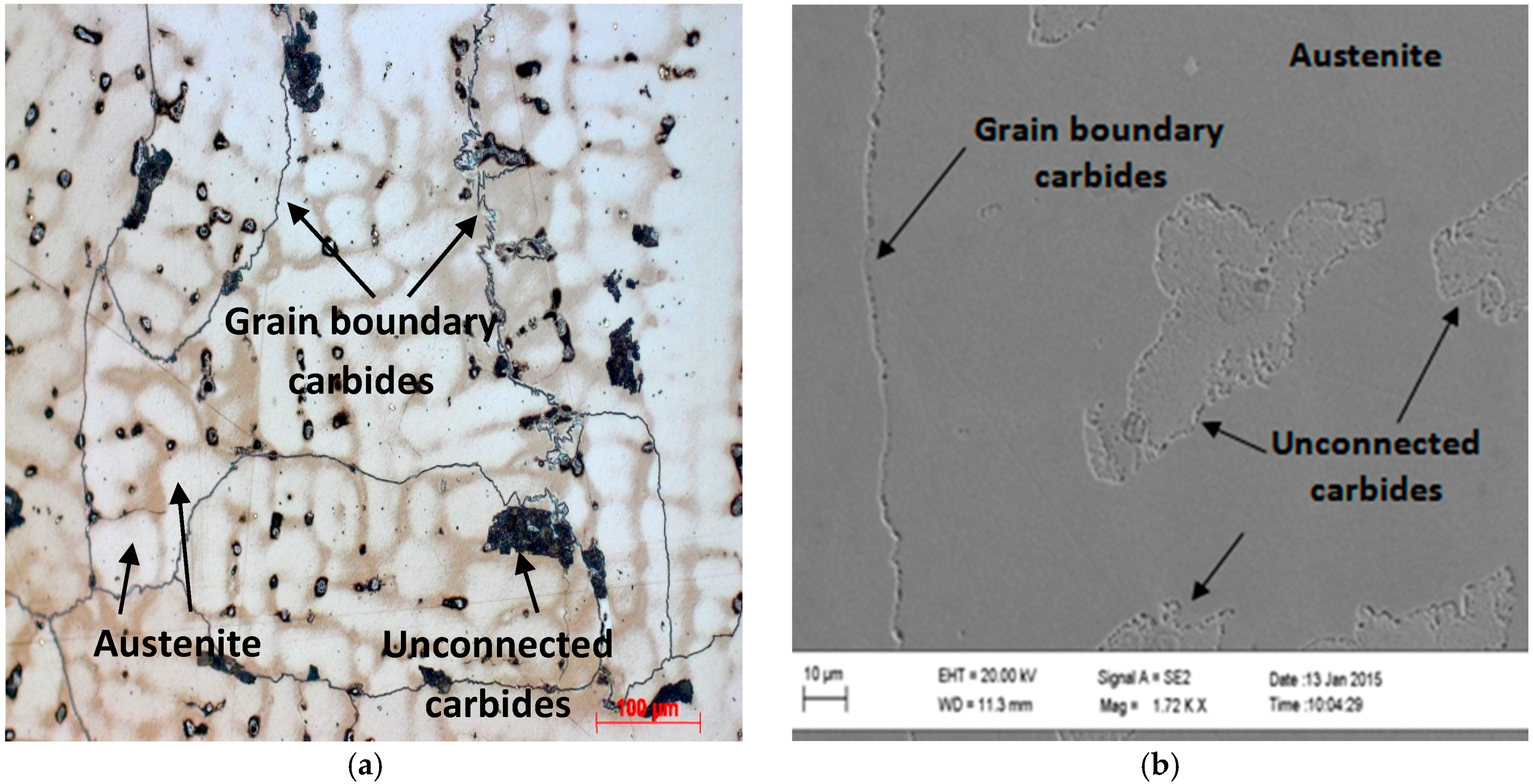
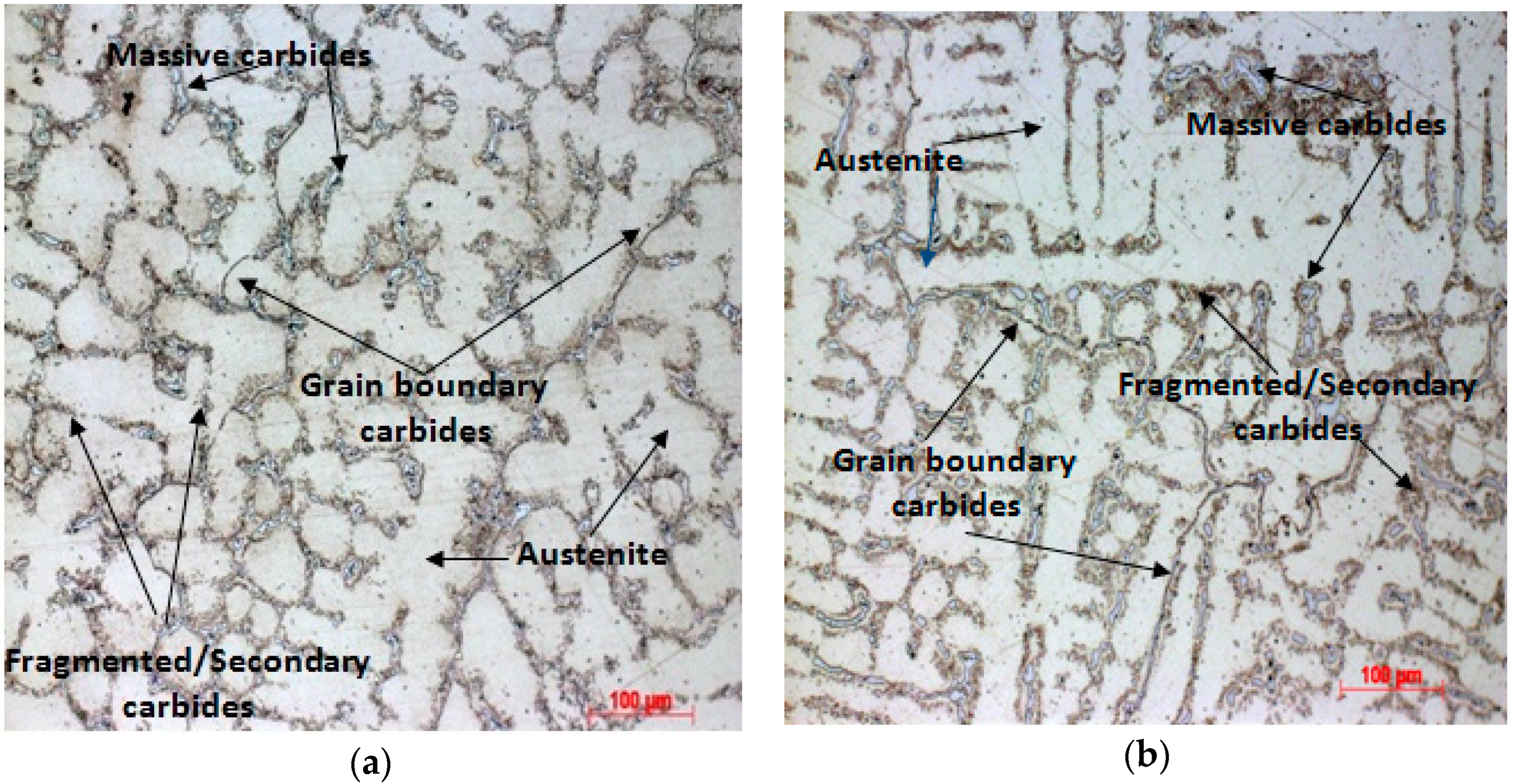
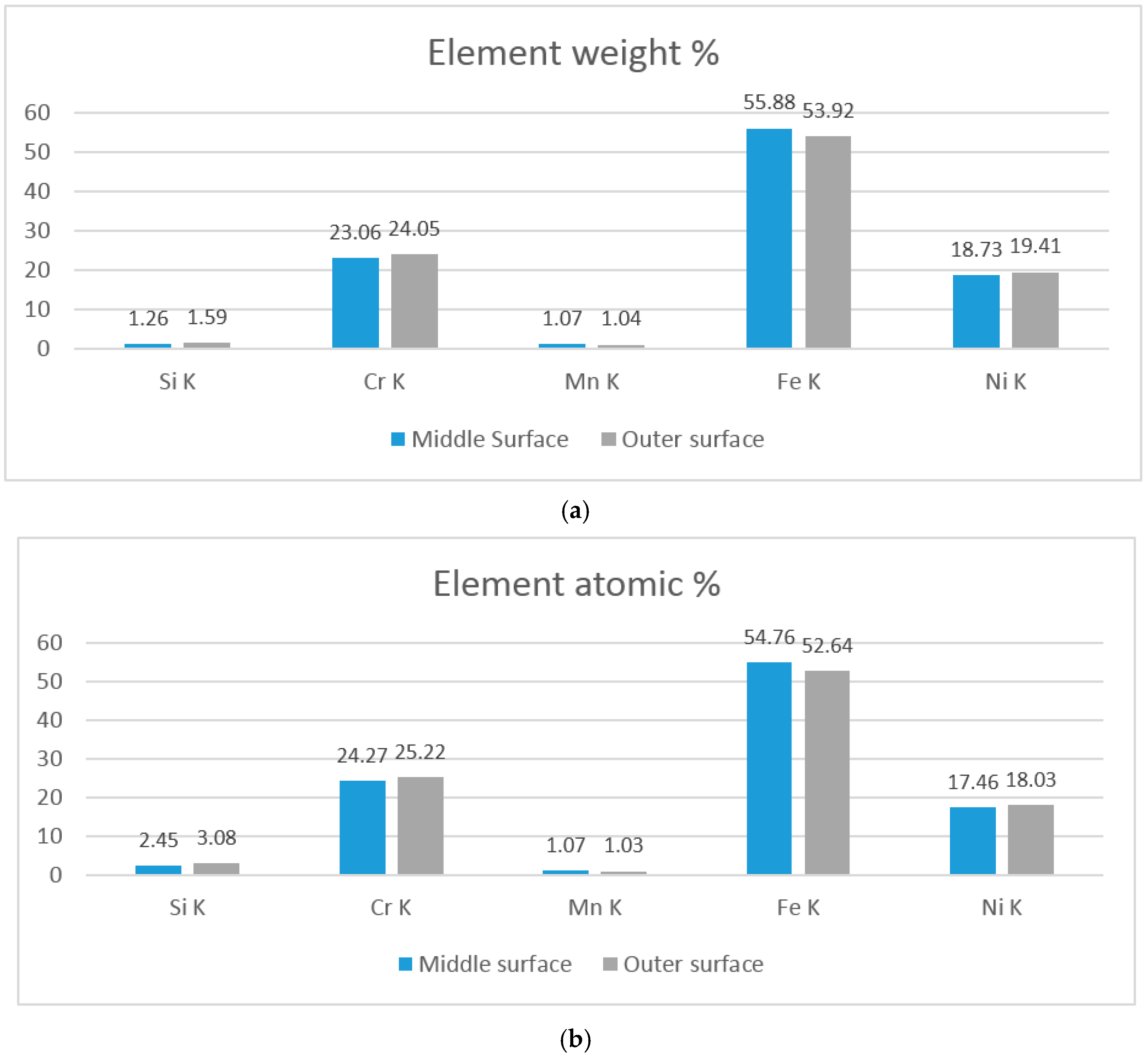
3.1. As-Cast Microstructure
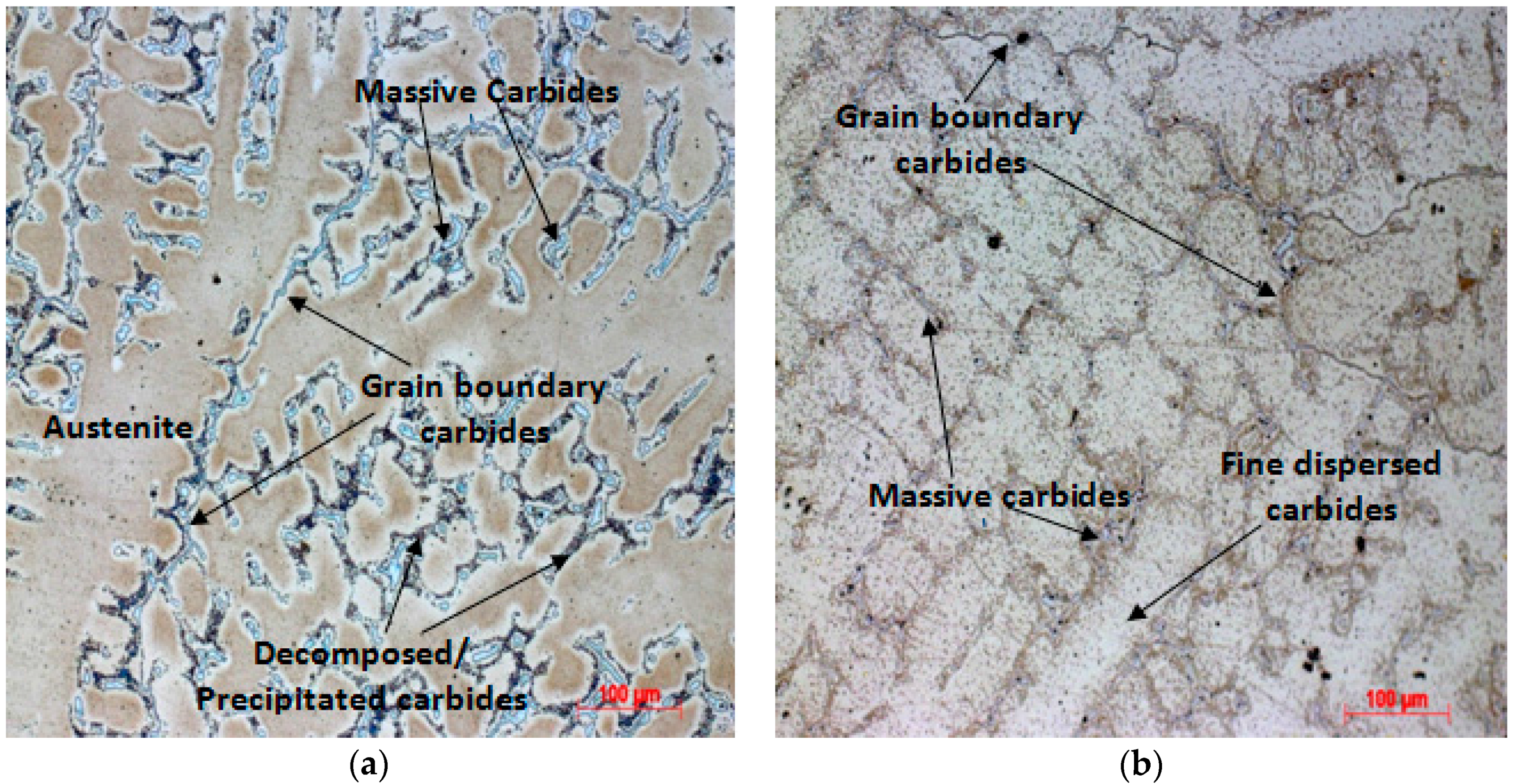
3.2. Solution Annealing Followed by Water Quenching
3.3. Solution Annealing Followed by Oven Cooling
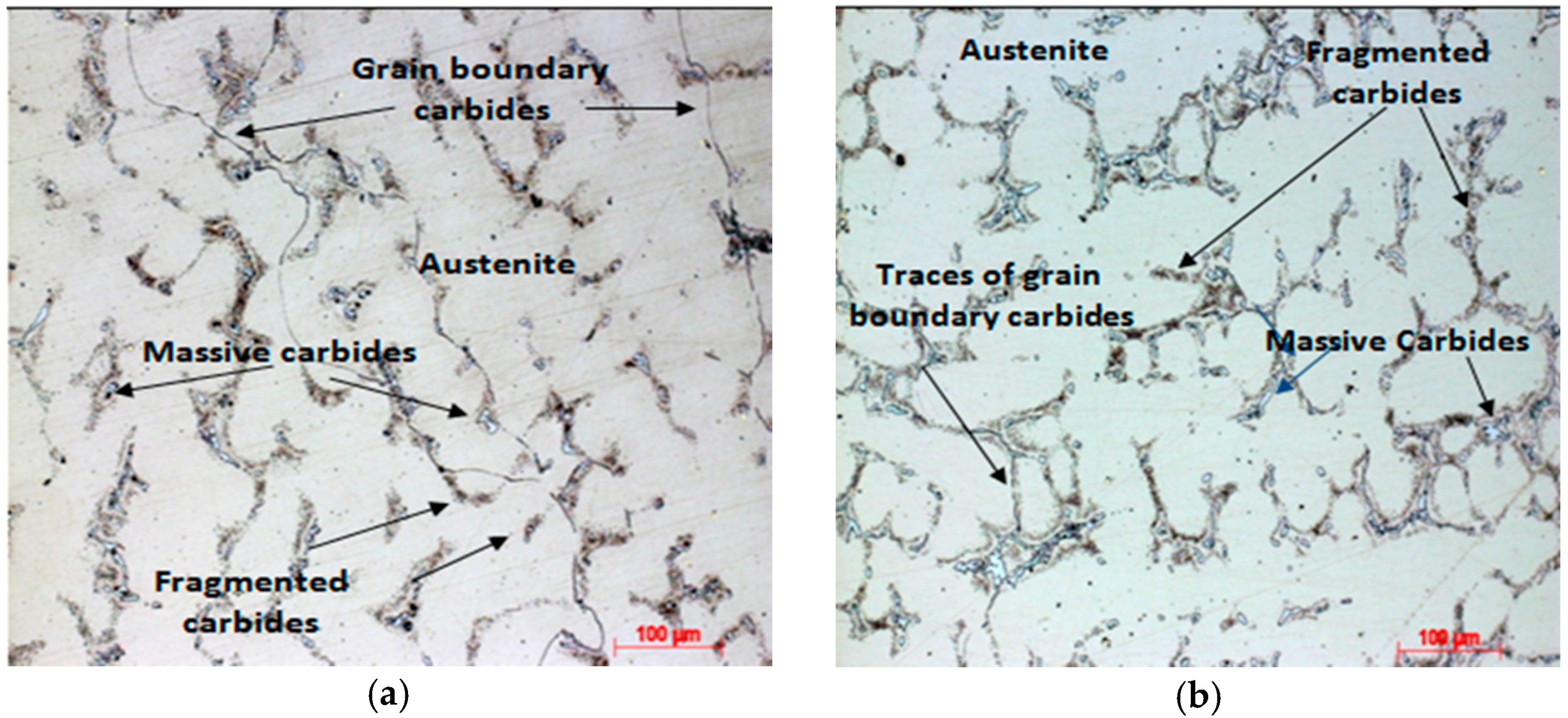
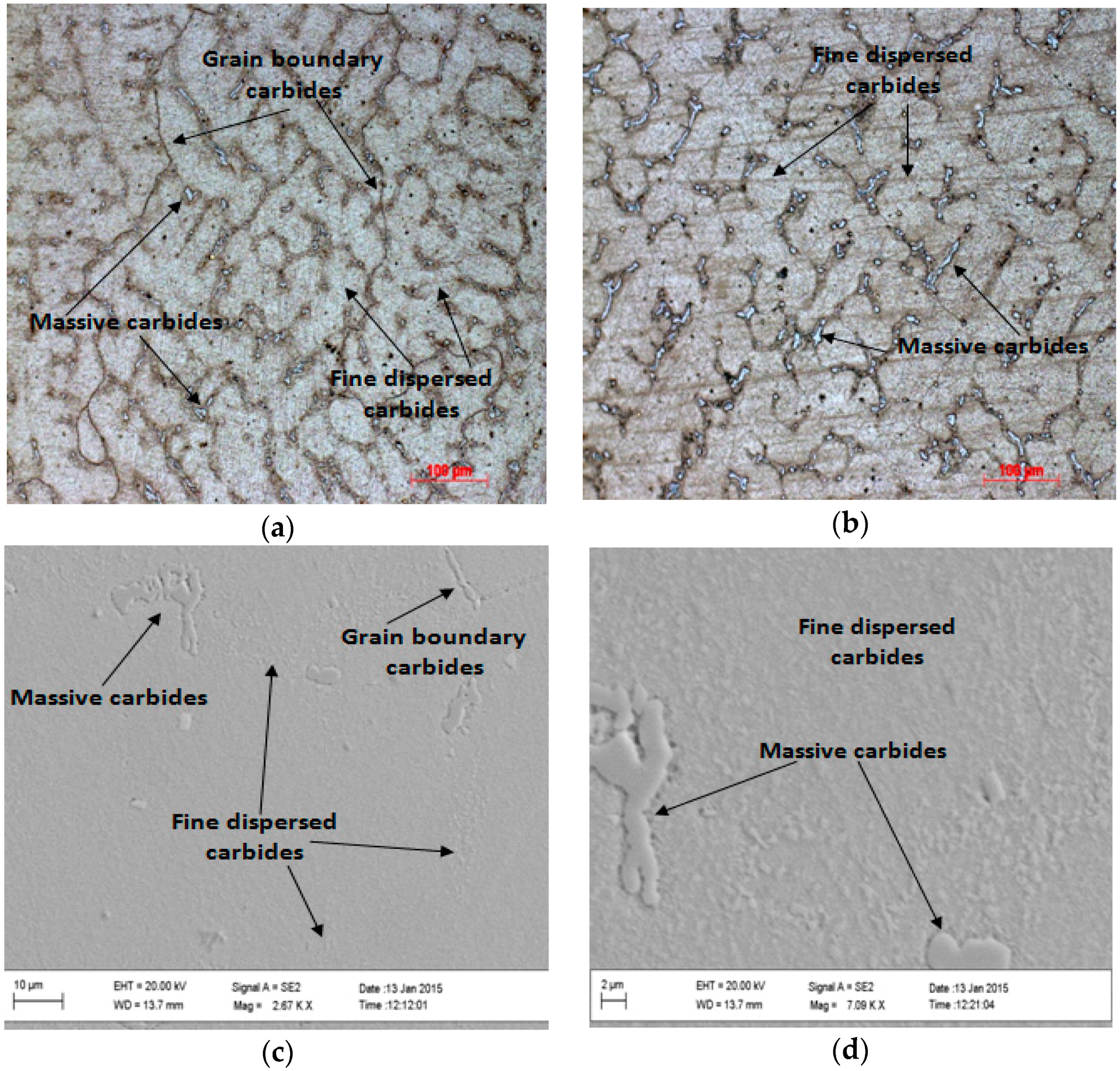
3.4. Aging Heat Treatment—Sample Solutions Annealed Followed by Water Quenching
3.5. Aging Heat Treatment—Sample Solutions Annealed Followed by Oven Cooling
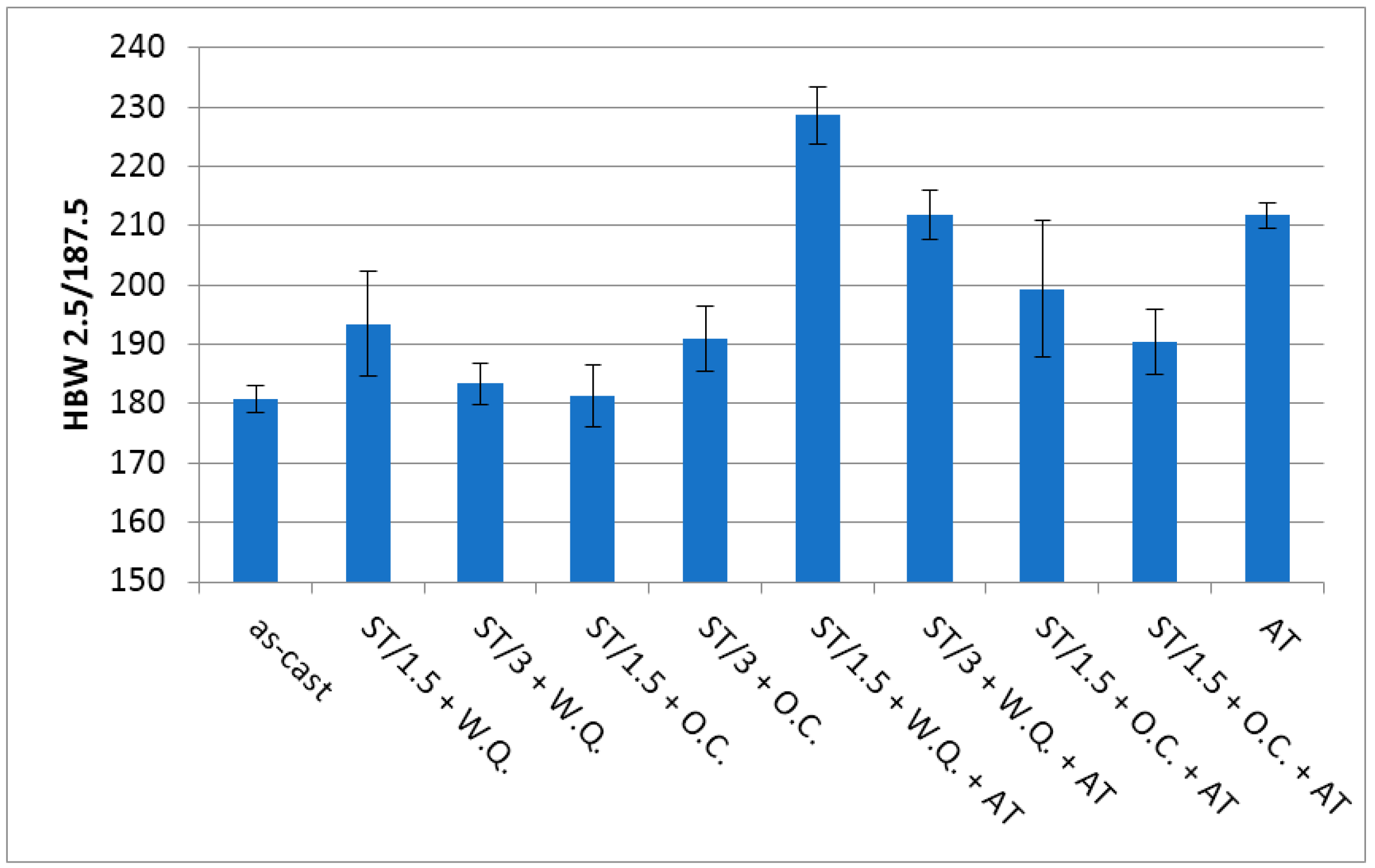
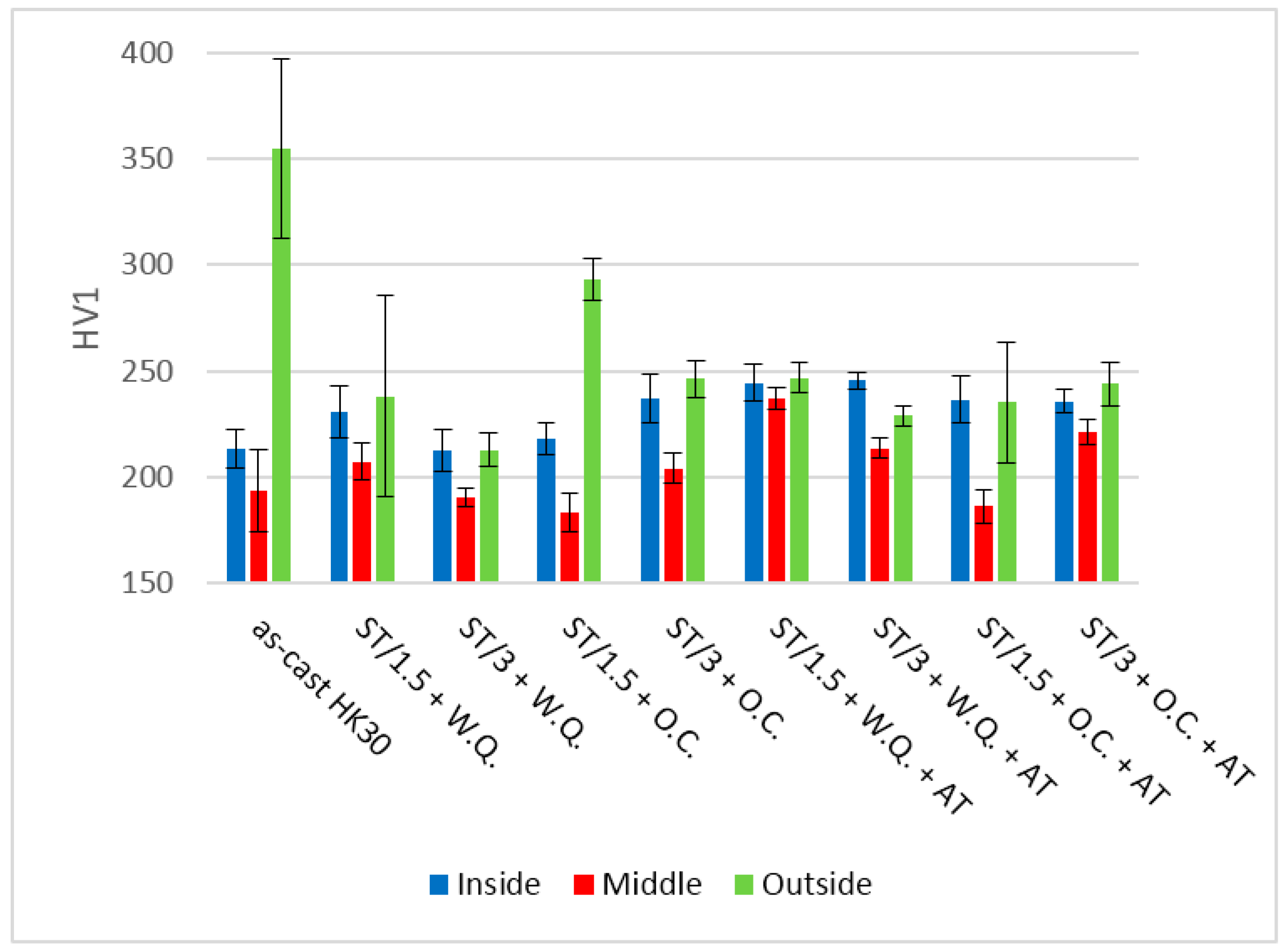
3.6. Hardness Measurements
4. Conclusions
- The formation of grain boundary carbides during alloy processing is possible. This can increase the risk of intergranular corrosion and crack initiation.
- The elimination of the grain boundary carbides can be achieved through the application of a solution annealing heat treatment.
- Aging treatment results in the precipitation of fine dispersed carbides and increase of hardness.
- Slow cooling at high temperature ranges promotes the precipitation of grain boundary carbides.
- The aging treatment will occur naturally in service at high temperatures.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schöggl, J.P.; Baumgartner, R.J.; Hofer, D. Improving sustainability performance in early phases of product design: A checklist for sustainable product development tested in the automotive industry. J. Clean. Prod. 2017, 3, 1602–1617. [Google Scholar] [CrossRef]
- Park, D.B.; Huh, M.Y.; Jung, W.S.; Suh, J.Y.; Shim, J.H.; Lee, S.C. Effect of vanadium addition on the creep resistance of 18Cr9Ni3CuNbN austenitic stainless heat resistant steel. J. Alloys Compd. 2013, 574, 532–538. [Google Scholar] [CrossRef]
- Koa, S.J.; Kim, Y.J. High temperature fatigue behaviors of a cast ferritic stainless steel. Mater. Sci. Eng. A 2012, 534, 7–12. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jang, H.; Oh, Y.J. High temperature low cycle fatigue properties of a HF30-type cast austenitic stainless steel. Mater. Sci. Eng. A 2009, 526, 244–249. [Google Scholar] [CrossRef]
- Shi, S.; Lippold, J.C. Microstructure evolution during service exposure of two cast, heat-resisting stainless steels—HP-Nb modified and 20–32Nb. Mater. Charact. 2008, 59, 1029–1040. [Google Scholar] [CrossRef]
- Nunes, F.C.; Dille, J.; Delplancke, J.L.; De Almeida, L.H. Yttrium addition to heat-resistant cast stainless steel. Scr. Mater. 2006, 54, 1553–1556. [Google Scholar] [CrossRef]
- Ekström, M.; Jonsson, S. High-temperature mechanical- and fatigue properties of cast alloys intended for use in exhaust manifolds. Mater. Sci. Eng. A 2014, 616, 78–87. [Google Scholar] [CrossRef]
- Ekström, M. Development of a Ferritic Ductile Cast Iron for Increased Life in Exhaust Applications. Bachelor’s Thesis, KTH Industrial Engineering Management, Stockholm, Sweden, 7 May 2013. [Google Scholar]
- Li, D.; Sloss, C. Comment on High-temperature mechanical and fatigue properties of cast alloys intended for use in exhaust manifolds by Ekström et al. (Mater. Sci. Eng. A 616 (2014) 78). Mater. Sci. Eng. A 2015, 624, 90–91. [Google Scholar] [CrossRef]
- Davis, J. Heat-Resistant Materials; ASM International: Geauga County, OH, USA, 1997; pp. 247–322. ISBN 978-0-87170-596-9. [Google Scholar]
- Sustaita-Torres, I.A.; Haro-Rodríguez, S.; Guerrero-Mata, M.P.; Garza, M.; Valdés, E.; Deschaux-Beaumed, F.; Colás, R. Aging of a cast 35Cr–45Ni heat resistant alloy. Mater. Chem. Phys. 2012, 133, 1018–1023. [Google Scholar] [CrossRef]
- Laird, R.G. Abrasion-Resistant Cast Iron Handbook; ASM International: Geauga County, OH, USA, 2000; pp. 125–286. ISBN 978-0-87433-224-7. [Google Scholar]
- Santos, H. Aços Inoxidáveis Austeníticos; Lesson #14; Faculdade de Engenharia da Universidade do Porto: Porto, Portugal, 2007. (In Portuguese) [Google Scholar]
- Guimarães, A.A.; Mei, P.R. Precipitation of carbides and sigma phase in AISI type 446 stainless steel under working conditions. J. Mater. Process. Technol. 2004, 155–156, 1681–1689. [Google Scholar] [CrossRef]
- Sourmail, T. Precipitation in creep resistant austenitic stainless steels. Mater. Sci. Technol. 2001, 1, 1–14. [Google Scholar] [CrossRef]
- Ha, V.T.; Jung, W.S. Effects of heat treatment processes on microstructure and creep properties of a high nitrogen 15Cr–15Ni austenitic heat resistant stainless steel. Mater. Sci. Eng. A 2011, 528, 7115–7123. [Google Scholar] [CrossRef]
- Wang, J.Z.; Liu, Z.D.; Bao, H.S.; Cheng, S.C. Evolution of precipitates of S31042 heat-resistant steel during 700 °C aging. J. Iron Steel Res. Int. 2013, 20, 113–121. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Goel, S.K. Effect of heat-treatment on microstructure, mechanical properties and erosion resistance of cast 23-8-N nitronic steel. Mater. Sci. Eng. A 2015, 637, 56–62. [Google Scholar] [CrossRef]
- Davis, J.R. Stainless Steels; ASM International: Geauga County, OH, USA, 1994; pp. 459–474. ISBN 978-0-87170-503-7. [Google Scholar]
- McGuire, M.F. Stainless Steels for Design Engineers; ASM International: Geauga County, OH, USA, 2008; p. 77. ISBN 978-0-87170-717-8. [Google Scholar]
| Composition | Cr | Si | Mn | Cr | Ni | Mo | Cu |
|---|---|---|---|---|---|---|---|
| HK30 | 0.34 | 1.40 | 1.11 | 24.37 | 19.13 | 0.38 | 0.37 |
| Variables | − | + |
|---|---|---|
| ST—Solution annealing temperature holding time | 1.5 h | 3 h |
| QM—Quenching mean | Oven (O.C.) | Water (W.Q.) |
| AT—Aging holding time | 0 h | 24 h |
| Sample Designation | ST | QM | AT |
|---|---|---|---|
| ST/1.5 + O.C. | − | − | − |
| ST/3 + O.C. | + | − | − |
| ST/1.5 + W.Q. | − | + | − |
| ST/3 + W.Q. | + | + | − |
| ST/1.5 + O.C. + AT | − | − | + |
| ST/3 + O.C. + AT | + | − | + |
| ST/1.5 + W.Q. + AT | − | + | + |
| ST/3 + W.Q. + AT | + | + | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.J.G.; Santos, J.; Gouveia, R. Dissolution of Grain Boundary Carbides by the Effect of Solution Annealing Heat Treatment and Aging Treatment on Heat-Resistant Cast Steel HK30. Metals 2017, 7, 251. https://doi.org/10.3390/met7070251
Silva FJG, Santos J, Gouveia R. Dissolution of Grain Boundary Carbides by the Effect of Solution Annealing Heat Treatment and Aging Treatment on Heat-Resistant Cast Steel HK30. Metals. 2017; 7(7):251. https://doi.org/10.3390/met7070251
Chicago/Turabian StyleSilva, Francisco J. G., Jorge Santos, and Ronny Gouveia. 2017. "Dissolution of Grain Boundary Carbides by the Effect of Solution Annealing Heat Treatment and Aging Treatment on Heat-Resistant Cast Steel HK30" Metals 7, no. 7: 251. https://doi.org/10.3390/met7070251





