Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure
Abstract
:1. Introduction
- (1)
- Strength
- (2)
- Fracture toughness
- (3)
- Ductility
- (4)
- Fatigue life
- (5)
- Wear resistance and
- (6)
- Corrosion resistance
2. Corrosion
3. Corrosion Assisted Cracking Phenomena
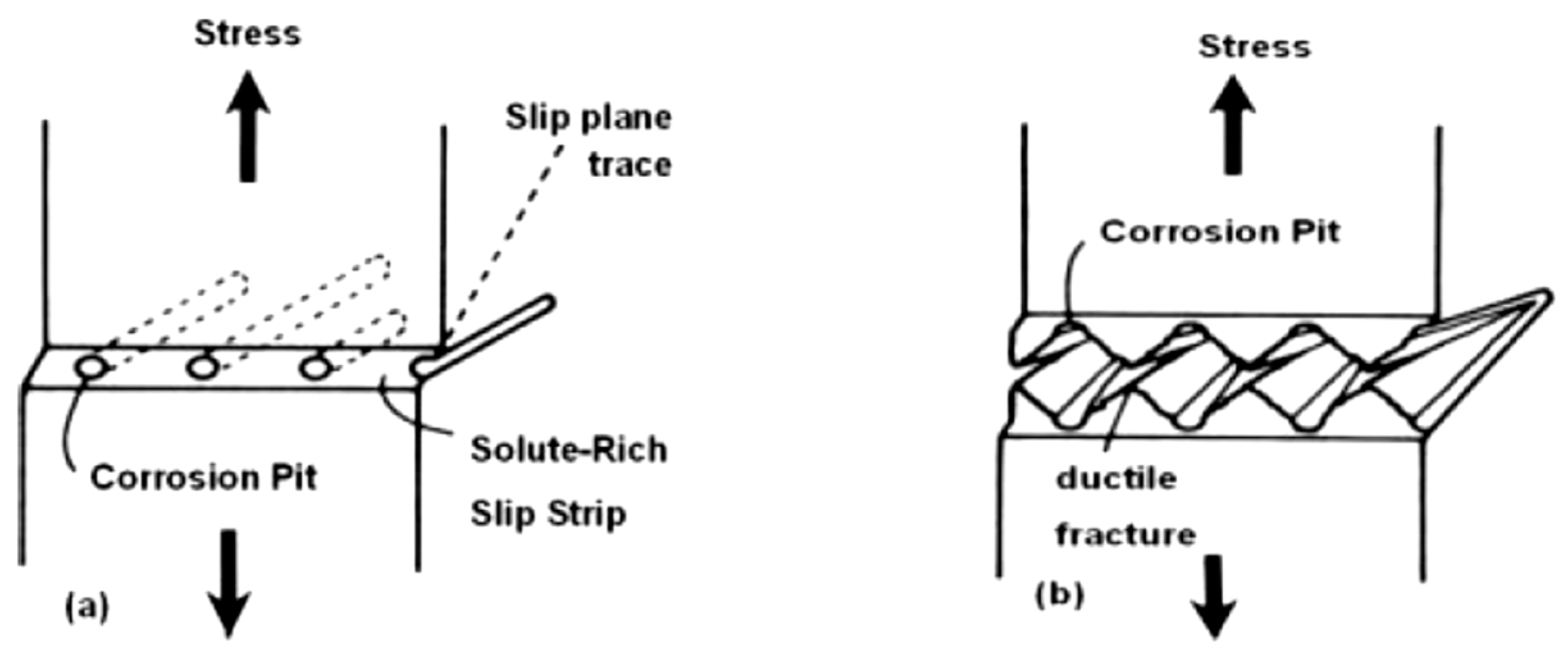
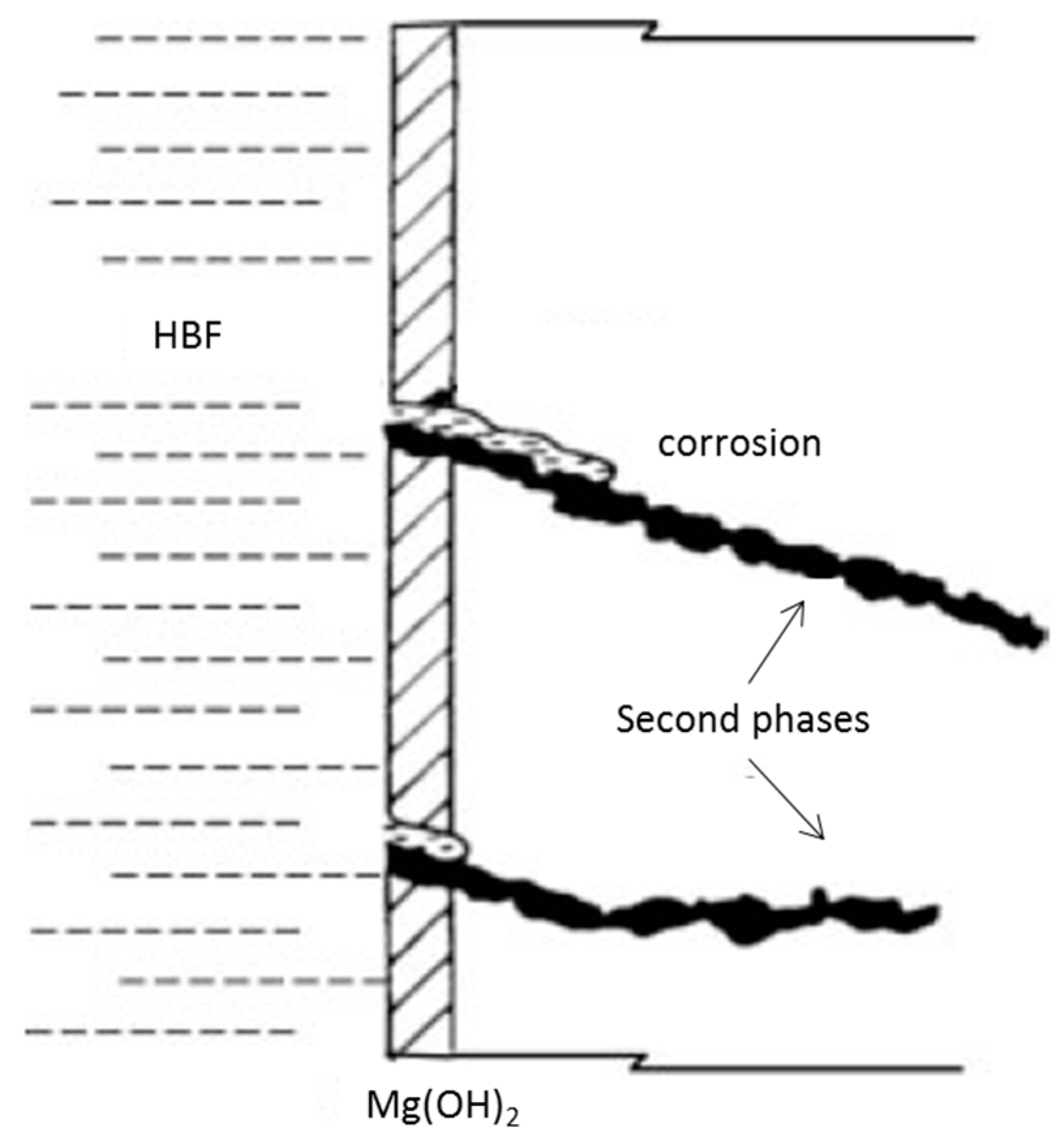
3.1. Stress Corrosion Cracking (SCC)
- Anodic dissolution mechanisms categorized in the: (a) galvanic attack by film rupture model; (b) tunnelling model; (c) preferential attack model.
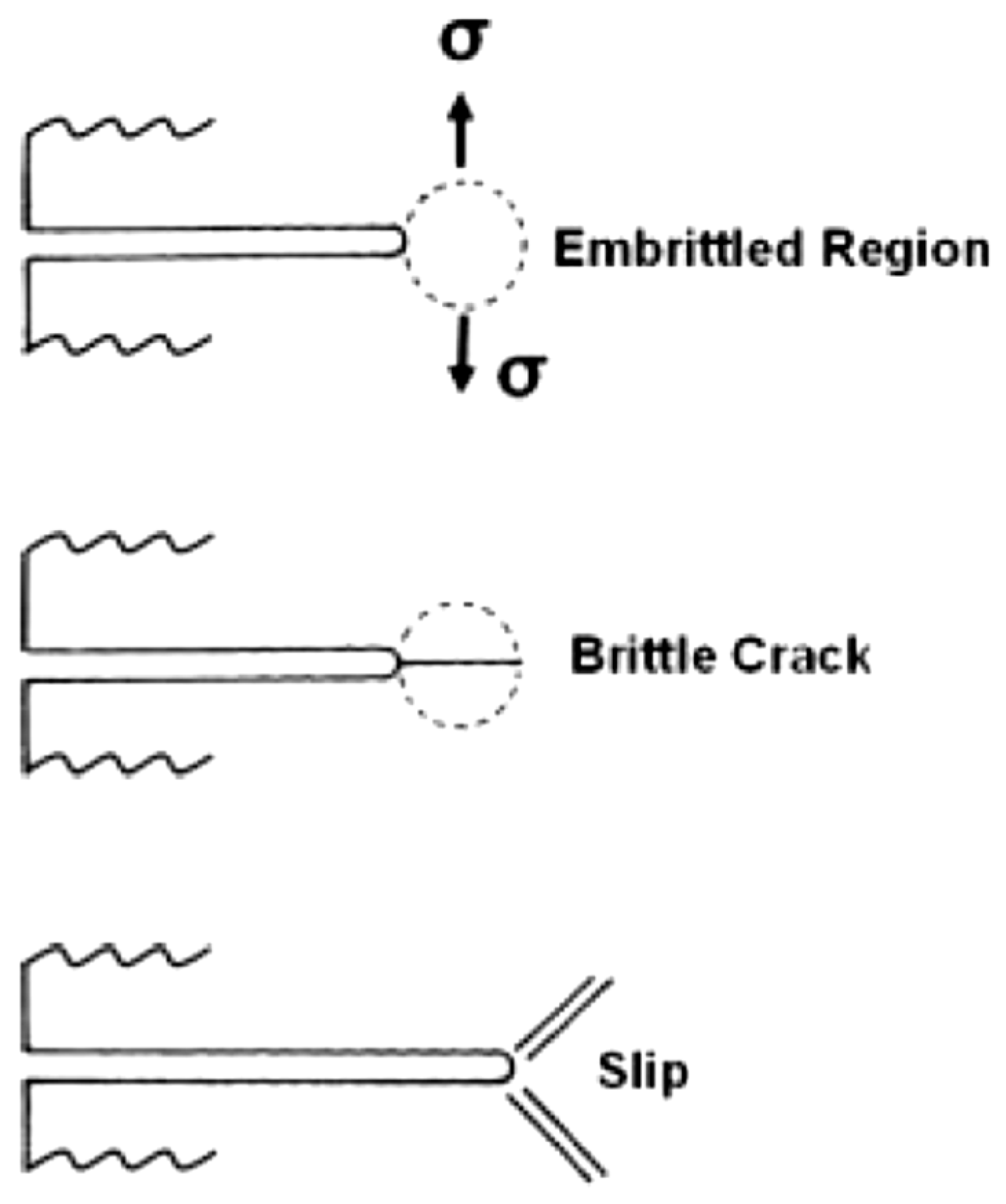
- Mechanical fracture mechanisms or cleavage-like fracture as described in the hydrogen embrittlement model.
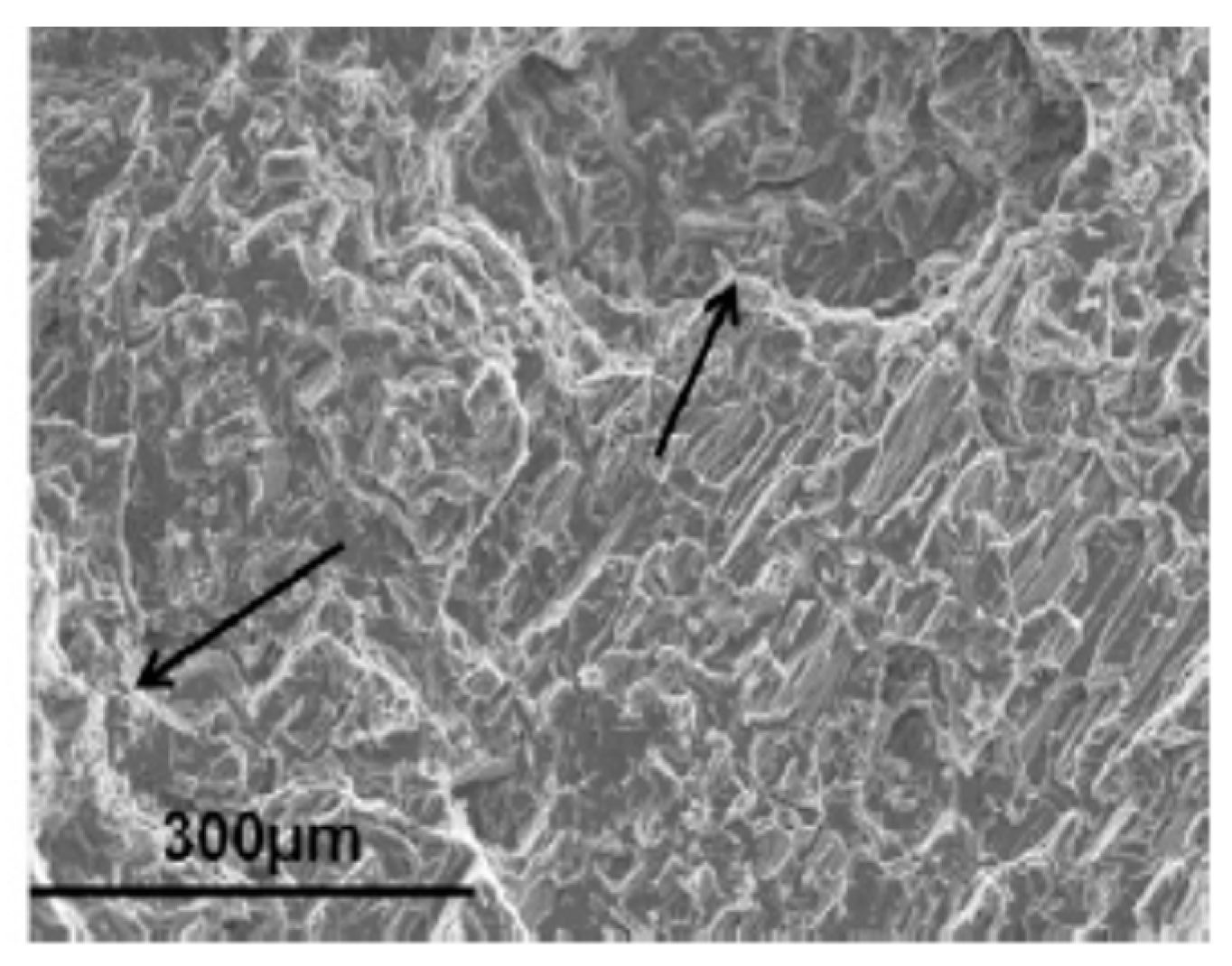
3.2. Corrosion Fatigue (CF)
4. Negative Difference Effect (NDE)
- Partially protective surface film
- Monovalent magnesium ion model
- Particle undermining model
- Magnesium hydride (MgH2) model
4.1. Partially Protective Surface Film
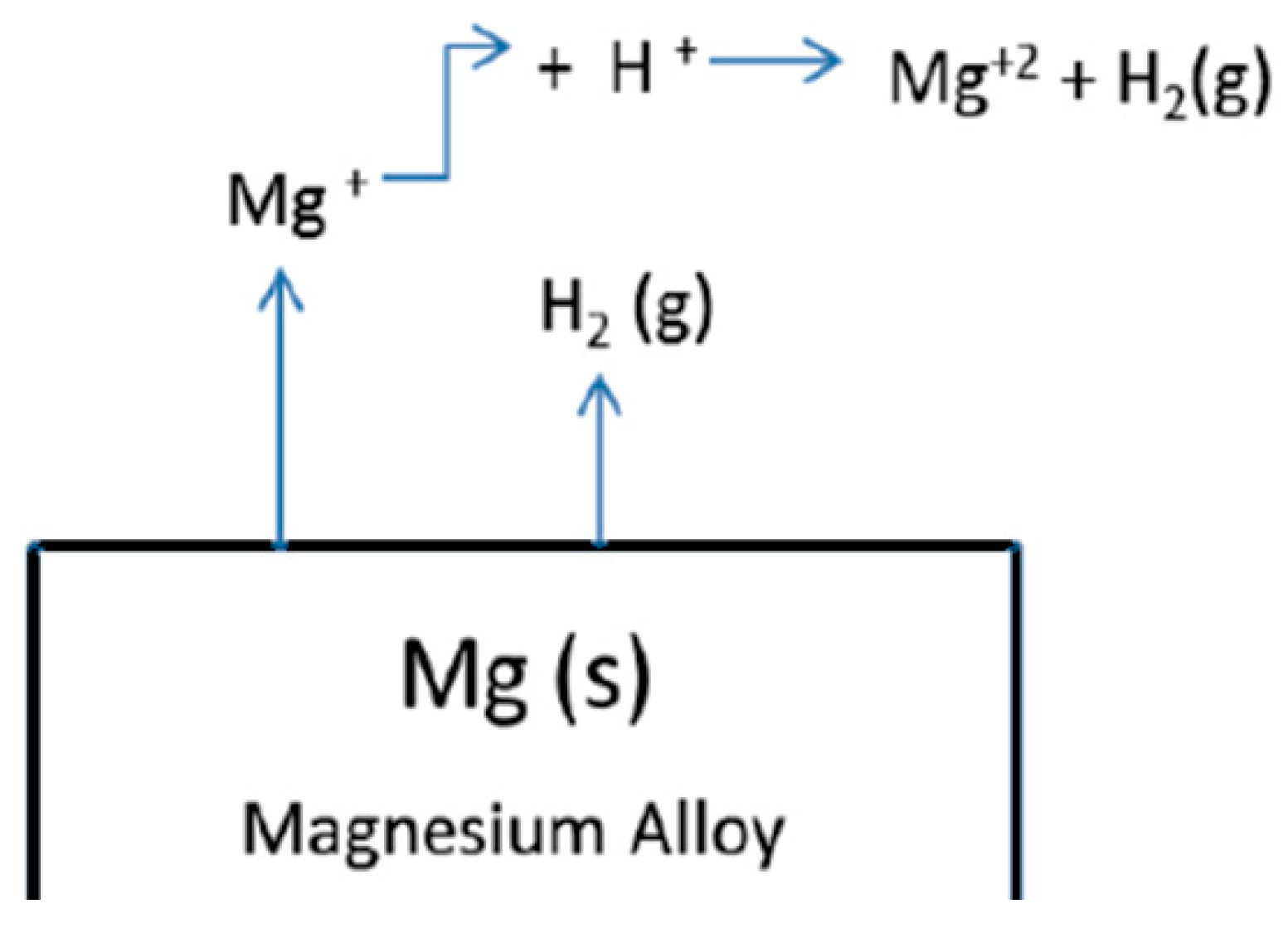
4.2. Monovalent Magnesium Ion Model
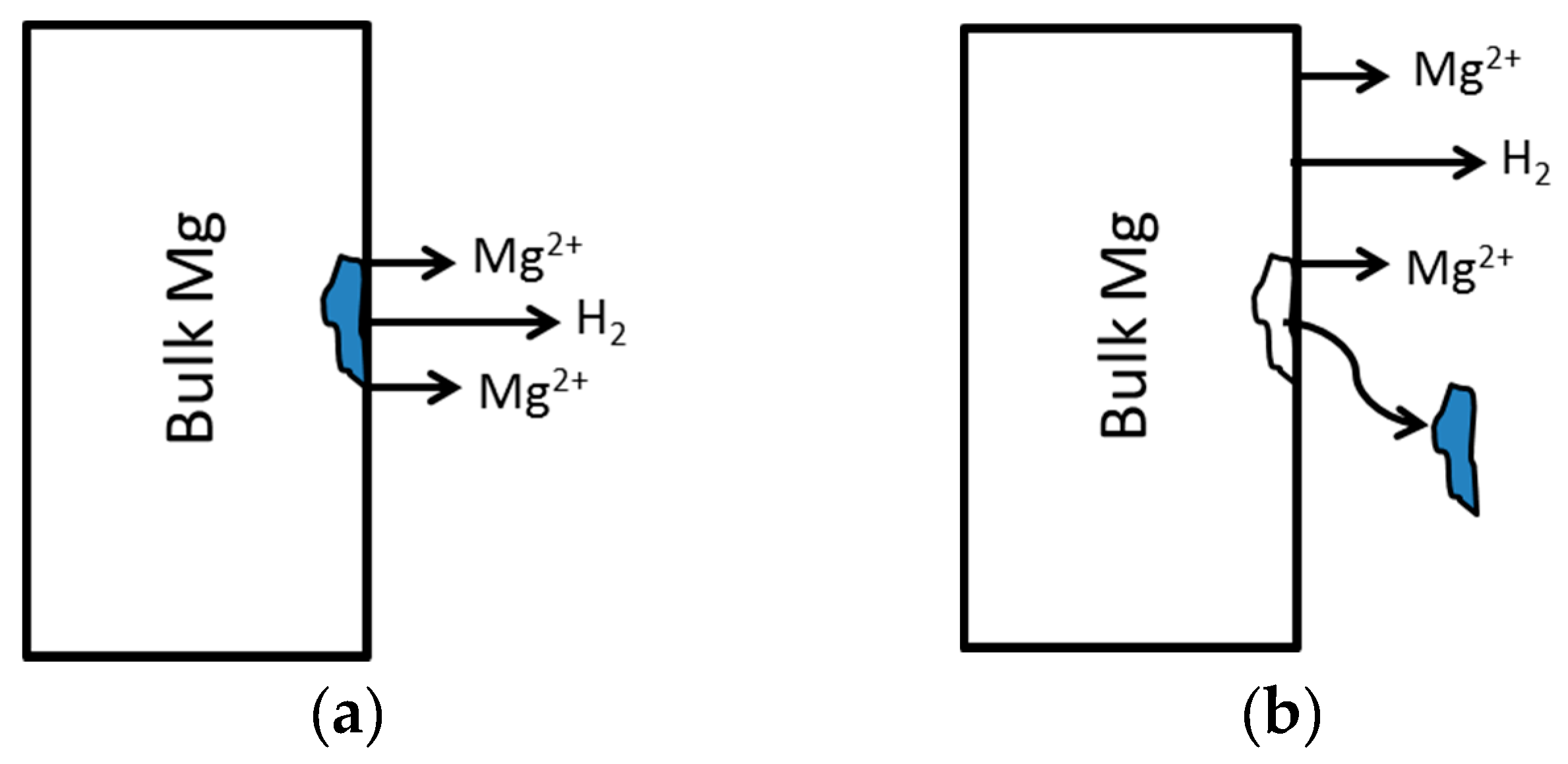
4.3. Particle Undermining Model
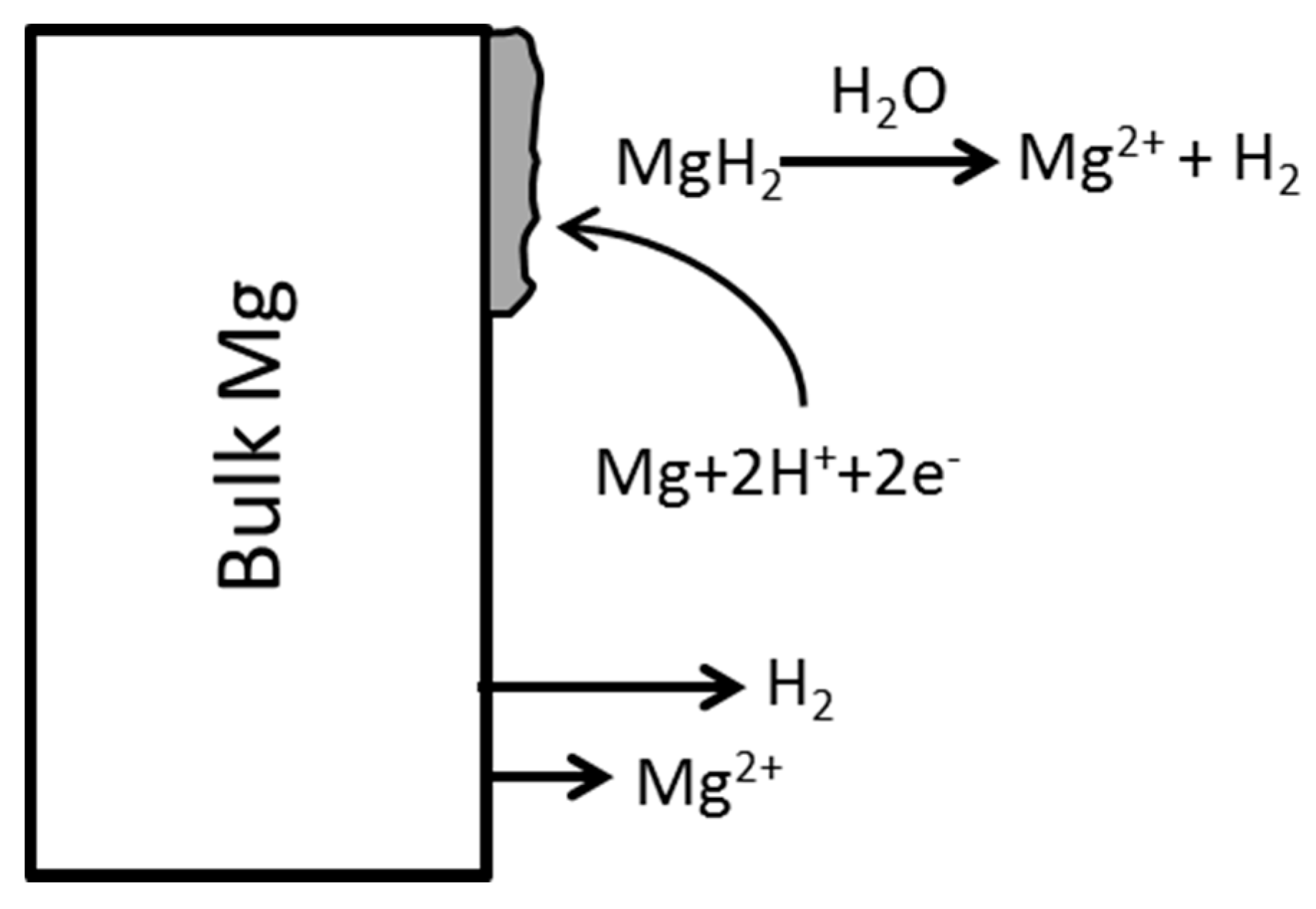
4.4. Magnesium Hydride (MgH2) Model
5. Corrosion Improvements
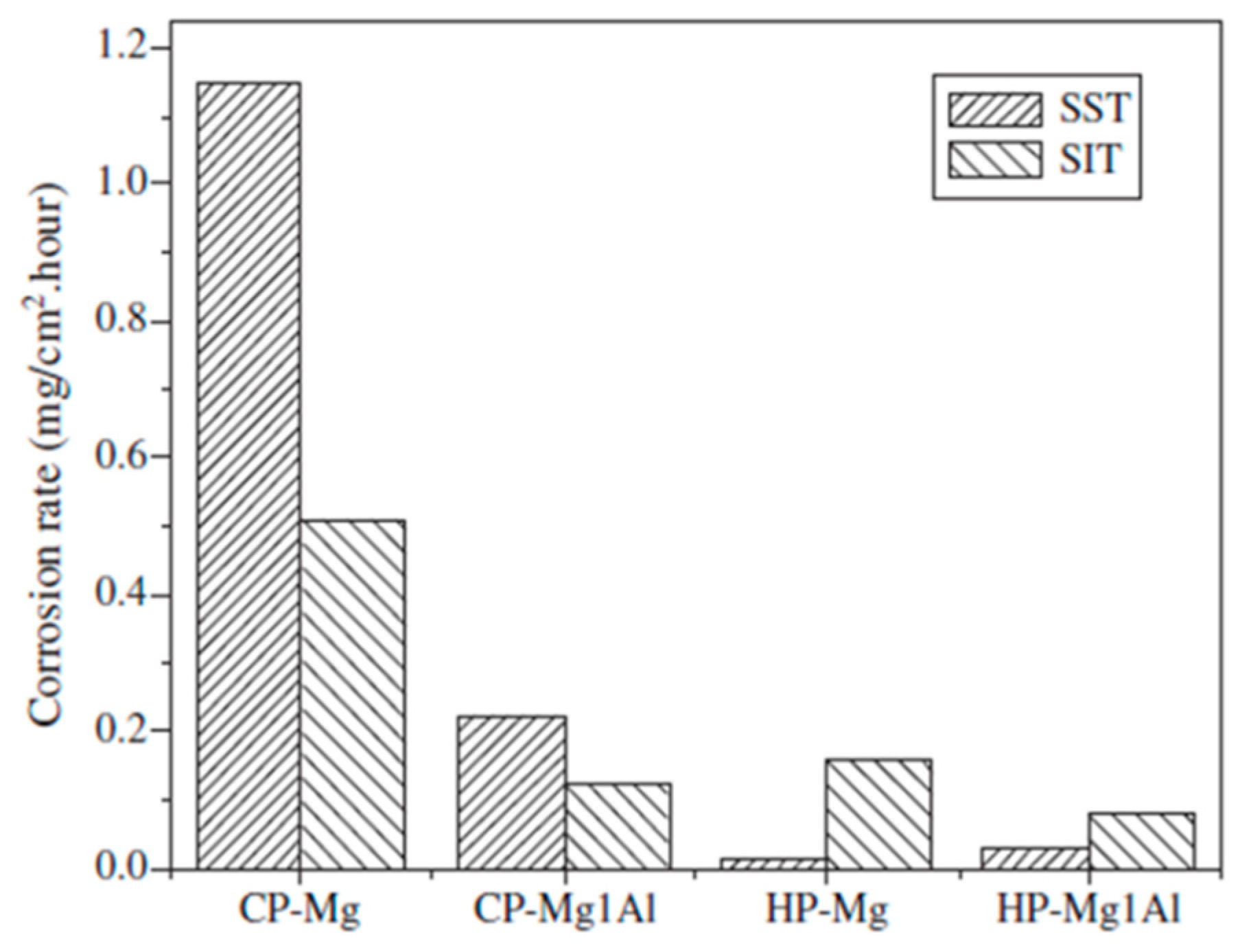
5.1. High-Purity Mg and Mg-Alloys
Tolerance Level
5.2. Alloying
- Refining the grain size through alloying. Since grain boundaries are characterized by higher imperfection and higher internal energy compared to the Mg matrix, any corrosive attack acts preferentially on grain boundaries. Segregation of alloying elements and second phases occur on these boundaries leading to an accelerated cathodic activity of the surrounding Mg matrix. This would normally favour coarse grains, however, such segregations are continuously distributed in Mg alloys with finer grains leading to a more homogeneous corrosion behaviour acting as a corrosion barrier [60,68]. Furthermore, fine grain sizes improve the corrosion assisted cracking resistance since they inhibit crack initiation and dislocation motion and lead to an increase in the number of barriers to crack propagation.
- Surrounding the Mg matrix continuously with passivating second phases allows the development of an oxidative film protecting the Mg matrix and acts as barrier to hamper corrosion.
- Adding elements reduces precipitation of second phases at grain boundaries or balances the potential difference between matrix and second phases to decrease microgalvanic corrosion.
5.2.1. Aluminium
5.2.2. Manganese
5.2.3. Zinc
5.2.4. Zirconium
5.2.5. Calcium
5.2.6. Rare Earth (RE) Elements
5.3. Surface Treatment
- (1)
- Tailor composition and microstructure;
- (2)
- Treat the surface/apply coatings.
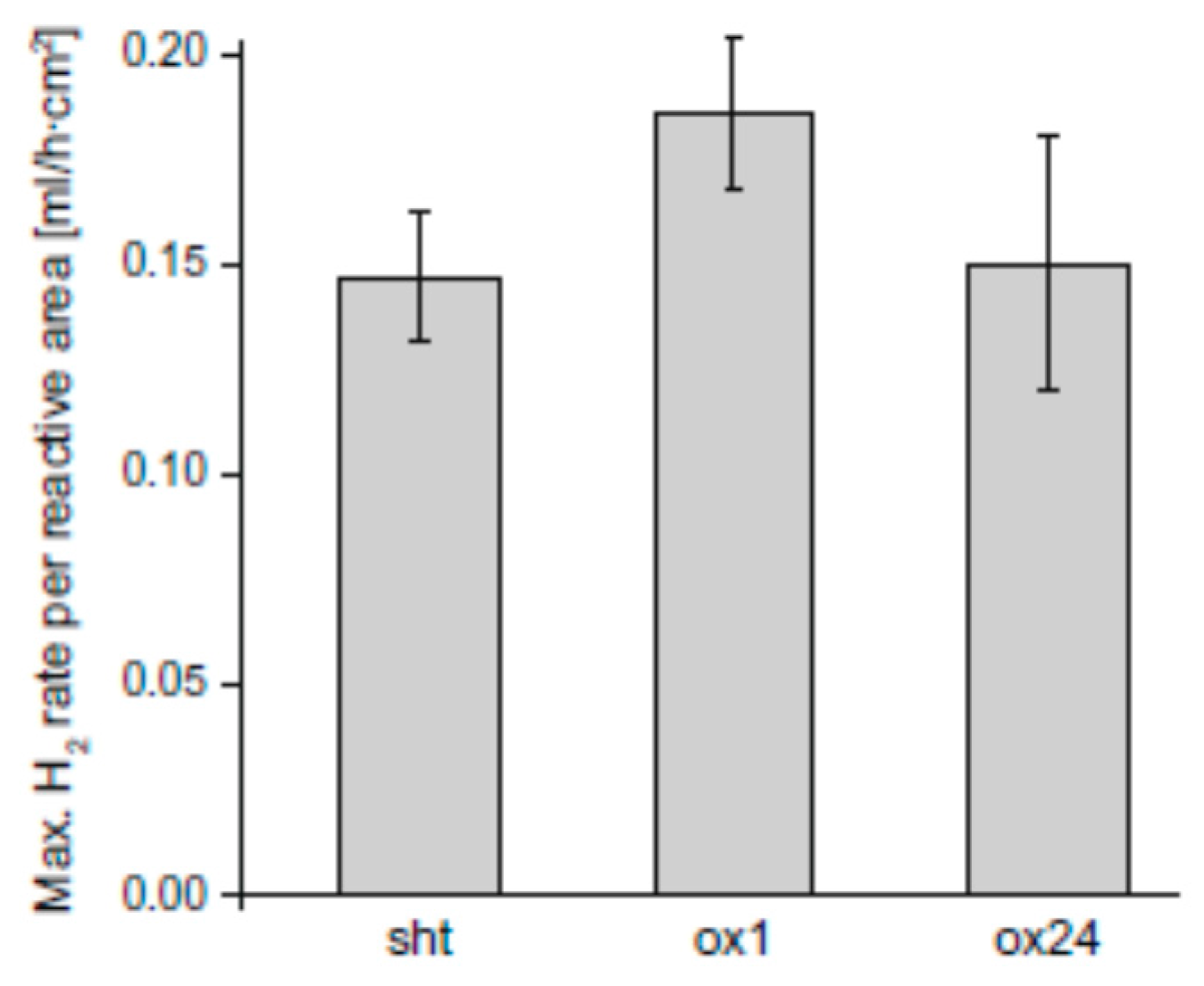
- Samples were first heat-treated at 525 °C for 6 h and then they were grinded and polished (labelled as SHT);
- Samples were first heat-treated at 525 °C for 6 h and then they were artificially aged at 250 °C for 16 h before being grinded and polished (T6);
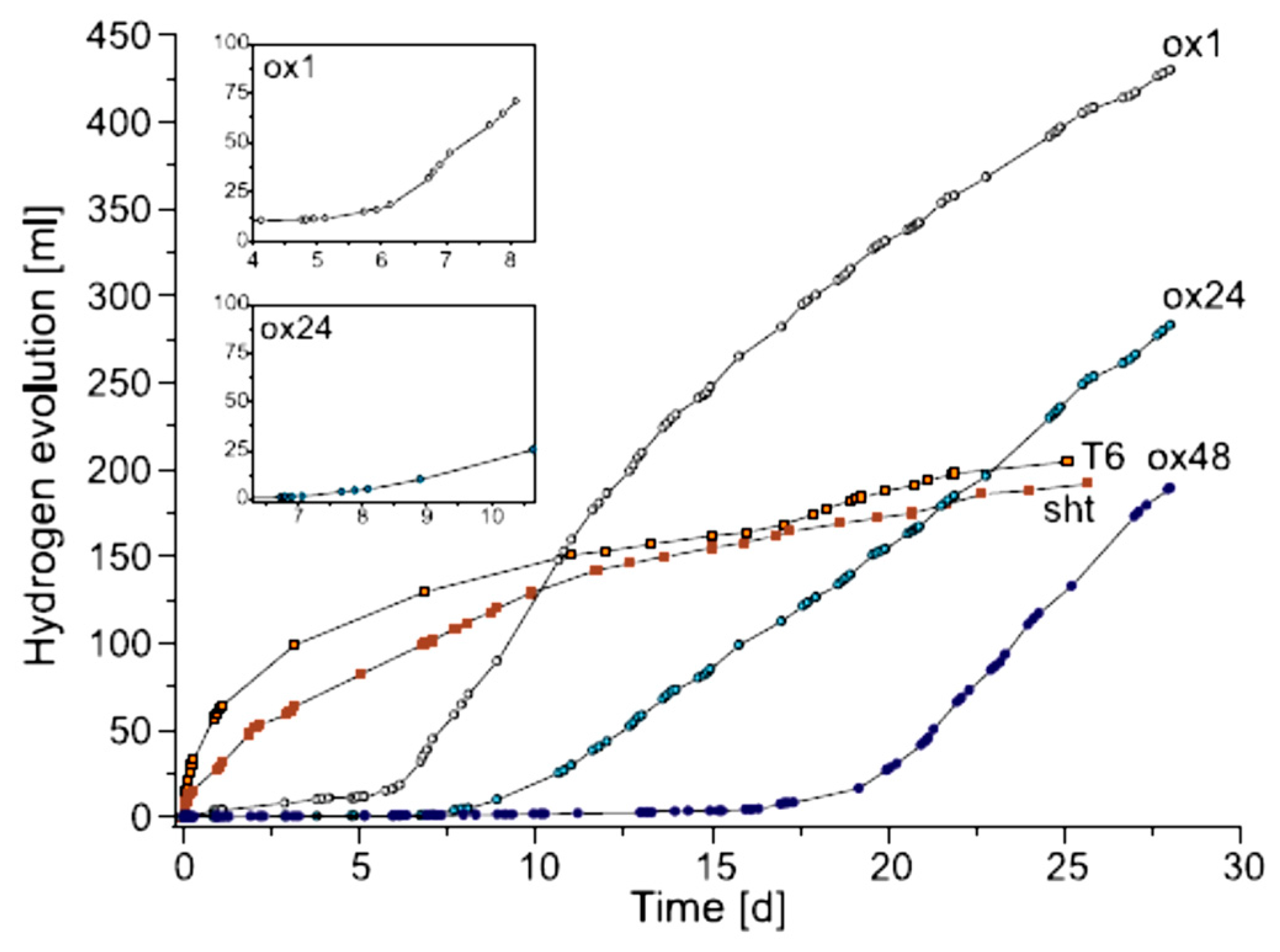
- Specimens were first polished, then annealed at 500 °C, being covered by an oxide layer during the annealing. The oxidation in air during the heat treatment was carried out for various times, i.e., 1 (ox1), 8 (ox8), 24 (ox24), 48 (ox48), 168 h (ox168).
6. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Regar, E.; Sianos, G.; Serruys, P.W. Stent development and local drug delivery. Br. Med. Bull. 2001, 59, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Greatbatch, W.; Holmes, C.F. History of implantable devices. IEEE Eng. Med. Biol. Mag. 1991, 10, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Long, P.H. Medical Devices in Orthopedic Applications. Toxicol. Pathol. 2008, 36, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Muntimadugu, E.; Jaffe, M.; Domb, A.J. Implantable medical devices. In Focal Controlled Drug Delivery; Springer: New York, NY, USA, 2014; pp. 33–59. [Google Scholar]
- Long, M.; Rack, H. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Gultepe, E.; Nagesha, D.; Sridhar, S.; Amiji, M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Deliv. Rev. 2010, 62, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.P. Towards New Biocompatible Materials: From Biological Polyesters to Synthetic Copoly (ester-urethanes) for Biomedical Applications. Ph.D. Thesis, University of Bern, Bern, Switzerland, January 2003. [Google Scholar]
- Lutz, J.-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Hou, Z.-T.; Ye, X.; Xu, Z.-B.; Bai, X.-L.; Shang, P. The effect of selected alloying element additions on properties of Mg-based alloy as bioimplants: A literature review. Front. Mater. Sci. 2013, 7, 227–236. [Google Scholar] [CrossRef]
- Dubok, V.A. Bioceramics—Yesterday, Today, Tomorrow. Powder Metall. Met. Ceram. 2000, 39, 381–394. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B. Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Hanawa, T. Overview of metals and applications. In Metals for Biomedical Devices; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–24. [Google Scholar]
- Lendlein, A.; Rehahn, M.; Buchmeiser, M.R.; Haag, R. Polymers in Biomedicine and Electronics. Macromol. Rapid Commun. 2010, 31, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, L.; Furmanski, J. Polymeric biomaterials for load-bearing medical devices. JOM J. Miner. Met. Mater. Soc. 2009, 61, 14–20. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Wang, M. Bioactive ceramic-polymer composites for tissue replacement. In Engineering Materials for Biomedical Applications; World Scientific: Singapore, 2004; pp. 8-1–8-29. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Thamaraiselvi, T.V.; Rajeswari, S. Biological evaluation of bioceramic materials—A review. Carbon 2004, 24, 172. [Google Scholar]
- Pound, B.G. Corrosion behavior of metallic materials in biomedical applications. II. Stainless steels and Co-Cr alloys. Corros. Rev. 2014, 32, 21–41. [Google Scholar] [CrossRef]
- Pound, B.G. Corrosion behavior of metallic materials in biomedical applications. I. Ti and its alloys. Corros. Rev. 2014, 32, 1–20. [Google Scholar] [CrossRef]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bauer, T.W.; Schils, J. The pathology of total joint arthroplasty. II. Mechanisms of implant failure. Skelet. Radiol. 1999, 28, 483–497. [Google Scholar] [CrossRef]
- Dujovne, A.R.; Bobyn, J.D.; Krygier, J.J.; Miller, J.E.; Brooks, C.E. Mechanical compatibility of noncemented hip prostheses with the human femur. J. Arthroplast. 1993, 8, 7–22. [Google Scholar] [CrossRef]
- Engh, C.; Bobyn, J.; Glassman, A. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. Bone Jt. J. 1987, 69, 45–55. [Google Scholar]
- Engh, C.A.; Bobyn, J.D. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin. Orthop. Relat. Res. 1988, 231, 7–28. [Google Scholar] [CrossRef]
- Kerner, J.; Huiskes, R.; van Lenthe, G.H.; Weinans, H.; van Rietbergen, B.; Engh, C.A.; Amis, A.A. Correlation between pre-operative periprosthetic bone density and post-operative bone loss in THA can be explained by strain-adaptive remodelling. J. Biomech. 1999, 32, 695–703. [Google Scholar] [CrossRef]
- Sumner, D.R.; Galante, J.O. Determinants of stress shielding: Design versus materials versus interface. Clin. Orthop. Relat. Res. 1992, 274, 202–212. [Google Scholar] [CrossRef]
- Sumner, D.R.; Turner, T.M.; Igloria, R.; Urban, R.M.; Galante, J.O. Functional adaptation and ingrowth of bone vary as a function of hip implant stiffness. J. Biomech. 1998, 31, 909–917. [Google Scholar] [CrossRef]
- Turner, T.M.; Sumner, D.R.; Urban, R.M.; Igloria, R.; Galante, J.O. Maintenance of proximal cortical bone with use of a less stiff femoral component in hemiarthroplasty of the hip without cement. An investigation in a canine model at six months and two years. J. Bone Jt. Surg. Am. 1997, 79, 1381–1390. [Google Scholar] [CrossRef]
- Van Rietbergen, B.; Huiskes, R.; Weinans, H.; Sumner, D.R.; Turner, T.M.; Galante, J.O. The mechanism of bone remodeling and resorption around press-fitted THA stems. J. Biomech. 1993, 26, 369–382. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Joint-IMplant-Surgeons-Hip-Replacement-Imgae.png (215 × 360). Available online: http://www.jointimplantsurgeons.com/wp-content/uploads/2015/09/Joint-IMplant-Surgeons-Hip-replacement-imgae.png (accessed on 5 May 2017).
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 2000, 405, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Mullender, M.G.; Huiskes, R. Proposal for the regulatory mechanism of Wolff’s law. J. Orthop. Res. 1995, 13, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lanyon, L.E. Using functional loading to influence bone mass and architecture: Objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone 1996, 18, S37–S43. [Google Scholar] [CrossRef]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in bone—Role of the lacuno-canalicular network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [PubMed]
- Noble, B.S.; Stevens, H.; Loveridge, N.; Reeve, J. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone 1997, 20, 273–282. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- DeGarmo, E.P.; Black, J.T.; Kohser, R.A. DeGarmo’s Materials and Processes in Manufacturing; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 1184. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure & Properties; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
- Hänzi, A.C.; Sologubenko, A.S.; Uggowitzer, P.J. Design strategy for new biodegradable Mg–Y–Zn alloys for medical applications. Int. J. Mater. Res. 2009, 100, 1127–1136. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, R.J.; Helder, M.N.; Govaert, L.E.; Smit, T.H. Biodegradable Polymers in Bone Tissue Engineering. Materials 2009, 2, 833–856. [Google Scholar] [CrossRef]
- Gunde, P.; Hänzi, A.C.; Sologubenko, A.S.; Uggowitzer, P.J. High-strength magnesium alloys for degradable implant applications. Mater. Sci. Eng. A 2011, 528, 1047–1054. [Google Scholar] [CrossRef]
- Granchi, D.; Ciapetti, G.; Stea, S.; Savarino, L.; Filippini, F.; Sudanese, A.; Zinghi, G.; Montanaro, L. Cytokine release in mononuclear cells of patients with Co}Cr hip prosthesis. Biomaterials 1999, 20, 1079–1086. [Google Scholar] [CrossRef]
- Niki, Y.; Matsumoto, H.; Suda, Y.; Otani, T.; Fujikawa, K.; Toyama, Y.; Hisamori, N.; Nozue, A. Metal ions induce bone-resorbing cytokine production through the redox pathway in synoviocytes and bone marrow macrophages. Biomaterials 2003, 24, 1447–1457. [Google Scholar] [CrossRef]
- Haynes, D.R.; Boyle, S.J.; Rogers, S.D.; Howie, D.W.; Vernon-Roberts, B. Variation in cytokines induced by particles from different prosthetic materials. Clin. Orthop. Relat. Res. 1998, 352, 223–230. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wicklund, B.H.; Gustilo, R.B.; Tsukayarna, D.T. Titaniurn, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomoterials 1996, 17, 2233–2240. [Google Scholar] [CrossRef]
- Bi, Y.; van de Motter, R.R.; Ragab, A.A.; Goldberg, V.M.; Anderson, J.M.; Greenfield, E.M. Titanium particles stimulate bone resorption by inducing differentiation of murine osteoclasts. J. Bone Jt. Surg. Am. 2001, 83, 501–508. [Google Scholar] [CrossRef]
- Allen, M.J.; Myer, B.J.; Millett, P.J.; Rushton, N. The effects of particulate cobalt, chromium and cobalt-chromium alloy on human osteoblast-like cells in vitro. J. Bone Jt. Surg. Br. 1997, 79, 475–482. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Corrosion of metal orthopaedic implants. J. Bone Jt. Surg. Am. 1998, 80, 268–282. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Hallab, N.J.; Skipor, A.K.; Urban, R.M. Metal degradation products: A cause for concern in metal-metal bearings? Clin. Orthop. Relat. Res. 2003, 417, 139–147. [Google Scholar]
- Beech, I.B.; Sunner, J.A.; Arciola, C.R.; Cristiani, P. Microbially-influenced corrosion: Damage to prostheses, delight for bacteria. Int. J. Artif. Organs 2006, 29, 443–452. [Google Scholar] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.K.S.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Sanderson, G.; Scully, J.C. Room Temperature Stress Corrosion Cracking of Titanium Alloys. Nature 1966, 211, 179. [Google Scholar] [CrossRef]
- Mori, K.; Takamura, A.; Shimose, T. Stress Corrosion Cracking off Ti and Zr in HCl-Methanol Solutions. Corrosion 1966, 22, 29–31. [Google Scholar] [CrossRef]
- Sutcliffe, J.M.; Fessler, R.R.; Boyd, W.K.; Parkins, R.N. Stress Corrosion Cracking of Carbon Steel in Carbonate Solutions. Corrosion 1972, 28, 313–320. [Google Scholar] [CrossRef]
- Mueller, W.-D.; Nascimento, M.L.; de Mele, M.F.L. Critical discussion of the results from different corrosion studies of Mg and Mg alloys for biomaterial applications. Acta Biomater. 2010, 6, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dai, J.; Zhang, X. Improvement of corrosion resistance of magnesium alloys for biomedical applications. Corros. Rev. 2015, 33, 101–117. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Shaw, B.A. Corrosion Resistance of Magnesium Alloys. In ASM Handbook; ASM International Handbook Committee: Metals Park, OH, USA, 2003; Volume 13A. [Google Scholar]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Xu, L.; Yu, G.; Zhang, E.; Pan, F.; Yang, K. In vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant application. J. Biomed. Mater. Res. Part A 2007, 83, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Ghali, E.; Dietzel, W.; Kainer, K.-U. General and Localized Corrosion of Magnesium Alloys: A Critical Review. J. Mater. Eng. Perform. 2004, 13, 7–23. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Manivasagam, G.; Suwas, S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater. Sci. Technol. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A critical review of the Stress Corrosion Cracking (SCC) of magnesium alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1998, 41, 249–273. [Google Scholar] [CrossRef]
- Song, G.-L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.E.; Raman, R.K.S. A review of stress-corrosion cracking and corrosion fatigue of magnesium alloys for biodegradable implant applications. JOM 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Matias, T.B.; Asato, G.H.; Ramasco, B.T.; Botta, W.J.; Kiminami, C.S.; Bolfarini, C. Processing and characterization of amorphous magnesium based alloy for application in biomedical implants. J. Mater. Res. Technol. 2014, 3, 203–209. [Google Scholar] [CrossRef]
- Kirkland, N.T. Magnesium biomaterials: Past, present and future. Corros. Eng. Sci. Technol. 2012, 47, 322–328. [Google Scholar] [CrossRef]
- Choudhary, L.; Raman, R.K.S. Acta Biomaterialia Magnesium alloys as body implants: Fracture mechanism under dynamic and static loadings in a physiological environment. Acta Biomater. 2012, 8, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, J.; Martinelli, E.; Weinberg, A.M.; Becker, M.; Mingler, B.; Uggowitzer, P.J.; Löffler, J.F. Assessing the degradation performance of ultrahigh-purity magnesium in vitro and in vivo. Corros. Sci. 2015, 91, 29–36. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; McGoron, A. Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J. Biomim. Biomater. Tissue Eng. 2012, 12, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Raman, R.K.S.; Davies, C.H.J. Corrosion fatigue of a magnesium alloy in modified simulated body fluid. Eng. Fract. Mech. 2015, 137, 2–11. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Potzies, C.; Kainer, K.U. Fatigue of magnesium alloys. Adv. Eng. Mater. 2004, 6, 281–289. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Maragoni, L.; Carraro, P.A.; Peron, M.; Quaresimin, M. Fatigue behaviour of glass/epoxy laminates in the presence of voids. Int. J. Fatigue 2017, 95, 18–28. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion fatigue behaviors of two biomedical Mg alloys—AZ91D and WE43—In simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Logan, H.L. Mechanism of Stress-Corrosion Cracking in the AZ31B Magnesium Alloy. J. Res. Natl. Bur. Stand. (1934) 1958, 61, 503–508. [Google Scholar] [CrossRef]
- Logan, H.L. Film-Rupture Mechanism of Stress Corrosion. J. Res. Natl. Bur. Stand. (1934) 1952, 48, 99–105. [Google Scholar] [CrossRef]
- Ebtehaj, K.; Hardie, D.; Parkins, R.N. The influence of chloride-chromate solution composition on the stress corrosion cracking of a Mg-Al alloy. Corros. Sci. 1988, 28, 811–821. [Google Scholar] [CrossRef]
- Wearmouth, W.R.; Dean, G.P.; Parkins, R.N. Role of Stress in the Stress Corrosion Cracking of a Mg-Al Alloy. Corrosion 1973, 29, 251–260. [Google Scholar] [CrossRef]
- Pugh, E.N.; Green, J.A.S.; Slattery, P.W. On the propagation of stress-corrosion cracks in magnesium-aluminum alloy. In Proceedings of the 2nd International Conference on Fracture, London, UK, April 1969; Chapman & Hall Limited: London, UK, 1969. [Google Scholar]
- Pickering, H.W.; Swann, P.R. Electron Metallography of Chemical Attack Upon Some Alloys Susceptible to Stress Corrosion Cracking. Corrosion 1963, 19, 373t–389t. [Google Scholar] [CrossRef]
- Fairman, L.; Bray, H.J. Intergranular Stress-Corrosion Crack Propagation in Magnesium Aluminium Alloys. Br. Corros. J. 1971, 6, 170–174. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Wu, X.; Zhang, B. Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride. Corros. Sci. 1998, 40, 1769–1791. [Google Scholar] [CrossRef]
- Pardue, W.M.; Beck, F.H.; Fontana, M.G. Propagation of stress-corrosion cracking in a magnesium-base alloy as determined by several techniques. Trans. Am. Soc. Met. 1961, 54, 539–548. [Google Scholar]
- Raman, R.K.S.; Harandi, S.E. Understanding corrosion-assisted cracking of magnesium alloys for bioimplant applications. In Magnesium Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 343–346. [Google Scholar]
- Uematsu, Y.; Kakiuchi, T.; Nakajima, M.; Nakamura, Y.; Miyazaki, S.; Makino, H. Fatigue crack propagation of AZ61 magnesium alloy under controlled humidity and visualization of hydrogen diffusion along the crack wake. Int. J. Fatigue 2014, 59, 234–243. [Google Scholar] [CrossRef]
- Chakrapani, D.G.; Pugh, E.N. The transgranular SCC of a Mg-Al alloy: Crystallographic, fractographic and acoustic-emission studies. Metall. Trans. A 1975, 6, 1155–1163. [Google Scholar] [CrossRef]
- Lynch, S.P.; Trevena, P. Stress Corrosion Cracking and Liquid Metal Embrittlement in Pure Magnesium. Corrosion 1988, 44, 113–124. [Google Scholar] [CrossRef]
- Bursle, A.; Pugh, E. On the Mechanism of transgranular stress-corrosion cracking. In Mechanisms of Environment Sensitive Cracking of Materials; Materials Society: London, UK, 1977. [Google Scholar]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Fairman, L.; West, J.M. Stress corrosion cracking of a magnesium aluminium alloy. Corros. Sci. 1965, 5, 711–716. [Google Scholar] [CrossRef]
- Gross, B.; Mendelson, A. Plane elastostatic analysis of V-notched plates. Int. J. Fract. Mech. 1972, 8, 267–276. [Google Scholar] [CrossRef]
- Taylor, D. Fatigue of bone and bones: An analysis based on stressed volume. J. Orthop. Res. 1998, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.; Mutoh, Y.; Murai, T.; Iwakami, S. Corrosion fatigue behavior of extruded magnesium alloy AZ61 under three different corrosive environments. Int. J. Fatigue 2008, 30, 1756–1765. [Google Scholar] [CrossRef]
- Sivakumar, M.; Rajeswari, S. Investigation of failures in stainless steel orthopaedic implant devices: Pit-induced stress corrosion cracking. J. Mater. Sci. Lett. 1992, 11, 1039–1042. [Google Scholar] [CrossRef]
- Antunes, R.A.; de Oliveira, M.C.L. Corrosion fatigue of biomedical metallic alloys: Mechanisms and mitigation. Acta Biomater. 2012, 8, 937–962. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.C.; Han, E.H.; Ke, W. Effect of Temperature and Relative Humidity on Fatigue Crack Propagation Behavior of AZ61 Magnesium Alloy. Mater. Sci. Forum 2007, 546–549, 409–412. [Google Scholar] [CrossRef]
- Shiplov, S.A. Mechanisms for corrosion fatigue crack propagation. Fatigue Fract. Eng. Mater. Struct. 2002, 25, 243–259. [Google Scholar] [CrossRef]
- Vasudevan, A.K.; Sadananda, K. Classification of environmentally assisted fatigue crack growth behavior. Int. J. Fatigue 2009, 31, 1696–1708. [Google Scholar] [CrossRef]
- Rozali, S.; Mutoh, Y.; Nagata, K. Effect of frequency on fatigue crack growth behavior of magnesium alloy AZ61 under immersed 3.5 mass% NaCl environment. Mater. Sci. Eng. A 2010, 528, 2509–2516. [Google Scholar] [CrossRef]
- Chakrapani, D.G.; Pugh, E.N. Hydrogen Embrittlement in a Mg-Al Alloy. Metall. Trans. A 1976, 7, 173–178. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Stjohn, D.; Nairn, J.; Li, Y. The electrochemical corrosion of pure magnesium in 1 N NaCl. Corros. Sci. 1997, 39, 855–875. [Google Scholar] [CrossRef]
- Perrault, G.G. Potentiostatic study of the magnesium electrode in aqueous solution. J. Electroanal. Chem. Interfac. Electrochem. 1970, 27, 47–58. [Google Scholar] [CrossRef]
- Murray, R.W.; Hillis, J.E. Magnesium Finishing: Chemical Treatment and Coating Practices. SAE Technical Paper 900791, 1990. [Google Scholar]
- Hillis, J.E. The Effects of Heavy Metal Contamination on Magnesium Corrosion Performance. SAE Technical Paper 830523, 1983. [Google Scholar]
- Ahamed, M. Toxic response of nickel nanoparticles in human lung epithelial A549 cells. Toxicol. In Vitro 2011, 25, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Milavec-Puretić, V.; Orlić, D.; Marusić, A. Sensitivity to metals in 40 patients with failed hip endoprosthesis. Arch. Orthop. Trauma Surg. 1998, 117, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Hillis, J.; Murray, R. Finishing alternatives for high purity magnesium alloys. In Proceedings of the SDCE 14th International Die Casting Congress and Exposition, Toronto, ON, Canada, 11–14 May 1987. [Google Scholar]
- Pelensky, M.A.; Gallaccio, A. Stress corrosion of magnesium alloys—Environmental factors. In Stress Corrosion Testing, ASTM STP425; American Society for Testing and Materials: West Conshohocken, PA, USA, 1967. [Google Scholar]
- Shi, Z.; Song, G.; Atrens, A. Corrosion resistance of anodised single-phase Mg alloys. Surf. Coat. Technol. 2006, 201, 492–503. [Google Scholar] [CrossRef]
- Hofstetter, J.; Becker, M.; Martinelli, E.; Weinberg, A.M.; Mingler, B.; Kilian, H.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. High-strength low-alloy (HSLA) Mg-Zn-Ca alloys with excellent biodegradation performance. JOM 2014, 66, 566–572. [Google Scholar] [CrossRef]
- Li, L.; Gao, J.; Wang, Y. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf. Coat. Technol. 2004, 185, 92–98. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Nelson, C.E.; Peloubet, J.A. Corrosion studies of magnesium and its alloys. Trans AIME 1942, 47, 273–299. [Google Scholar]
- Reichek, K.N.; Clark, K.J.; Hillis, J.E. Controlling the Salt Water Corrosion Performance of Magnesium AZ91 Alloy. SAE Technical Paper 850417, 1985. [Google Scholar]
- Lunder, O.; Aune, T.K.; Nisancioglu, K. Effect of Mn Additions on the Corrosion Behavior of Mould-Cast Magnesium ASTM AZ91. Corrosion 1987, 43, 291–295. [Google Scholar] [CrossRef]
- Mercer, W.E.; Hillis, J.E. The Critical Contaminant Limits and Salt Water Corrosion Performance of Magnesium AE42 Alloy. SAE Technical Paper 920073, 1992. [Google Scholar]
- Avedesian, M.M.; Baker, H. Magnesium and Magnesium Alloys; ASM International Handbook Committee: Novelty, OH, USA, 1999. [Google Scholar]
- Kainer, K.U.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Corrosion of magnesium and its alloys. Shreir’s Corros. 2010, 3, 2011–2041. [Google Scholar]
- Mezbahul-Islam, M.; Mostafa, A.O.; Medraj, M. Essential Magnesium Alloys Binary Phase Diagrams and Their Thermochemical Data. J. Mater. 2014, 2014, 704283. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Hermann, F.; Sommer, F.; Jones, H.; Edyvean, R.G.J. Corrosion inhibition in magnesium-aluminium-based alloys induced by rapid solidification processing. J. Mater. Sci. 1989, 24, 2369–2379. [Google Scholar] [CrossRef]
- Lunder, O.; Lein, J.E.; Aune, T.K.; Nisancioglu, K. The Role of Mg 17 Al 12 Phase in the Corrosion of Mg Alloy AZ91. Corrosion 1989, 45, 741–748. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, W.J. Enhancement of mechanical properties and corrosion resistance of Mg–Ca alloys through microstructural refinement by indirect extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Domingo, J.L. Reproductive and developmental toxicity of aluminum: A review. Neurotoxicol. Teratol. 1995, 17, 515–521. [Google Scholar] [CrossRef]
- Venugopal, B.; Luckey, T.D. Metal Toxicity in Mammals. Volume 2. Chemical Toxicity of Metals and Metalloids; Plenum Press: Berlin, Germany, 1978. [Google Scholar]
- Flaten, T.P. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar] [CrossRef]
- El-Rahman, S.S.A. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacol. Res. 2003, 47, 189–194. [Google Scholar] [CrossRef]
- Robinson, H.A.; George, P.F. Effect of Alloying and Impurity Elements In Magnesium Alloy Cast Anodes. Corrosion 1954, 10, 182–188. [Google Scholar] [CrossRef]
- Culotta, V.C.; Yang, M.; Hall, M.D. Manganese Transport and Trafficking: Lessons Learned from Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.G.; Hellgren, M.; Brinck, T.; Bergman, T.; Edholm, O. Molecular dynamics study of zinc binding to cysteines in a peptide mimic of the alcohol dehydrogenase structural zinc site. Phys. Chem. Chem. Phys. 2009, 11, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, D.N.; Mair, E.A.; Cable, B.B. Chronic ingestion of a zinc-based penny. Pediatrics 2003, 111, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, C.; Mushahary, D.; Sravanthi, R.; Harishankar, N.; Pande, G.; Hodgson, P. Mg–Zr–Sr alloys as biodegradable implant materials. Acta Biomater. 2012, 8, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; StJohn, D. The effect of zirconium grain refinement on the corrosion behaviour of magnesium-rare earth alloy MEZ. J. Light Met. 2002, 2, 1–16. [Google Scholar] [CrossRef]
- Jiao, W.; Li, H.F.; Zhao, K.; Bai, H.Y.; Whang, Y.B.; Zheng, Y.F.; Wang, W.H. Development of CaZn based glassy alloys as potential biodegradable bone graft substitute. J. Non-Cryst. Solids 2011, 357, 3830–3840. [Google Scholar] [CrossRef]
- Rad, H.R.B.; Idris, M.H.; Kadir, M.R.A.; Farahany, S. Microstructure analysis and corrosion behavior of biodegradable Mg–Ca implant alloys. Mater. Des. 2012, 33, 88–97. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L. Microstructure, mechanical properties and bio-corrosion properties of Mg–Zn–Mn–Ca alloy for biomedical application. Mater. Sci. Eng. A 2008, 497, 111–118. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Walker, J.; Woodfield, T.; Dias, G.J.; Staiger, M.P. In-vitro dissolution of magnesium-calcium binary alloys: Clarifying the unique role of calcium additions in bioresorbable magnesium implant alloys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Bornapour, M.; Celikin, M.; Cerruti, M.; Pekguleryuz, M. Magnesium implant alloy with low levels of strontium and calcium: The third element effect and phase selection improve bio-corrosion resistance and mechanical performance. Mater. Sci. Eng. C 2014, 35, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Lazar, L.L.; Rokhlin, L. Magnesium Alloys Containing Rare Earth Metals: Structure and Properties; Taylor & Francis: Abingdon, UK, 2003. [Google Scholar]
- Kannan, M.B.; Dietzel, W.; Blawert, C.; Atrens, A.; Lyon, P. Stress corrosion cracking of rare-earth containing magnesium alloys ZE41, QE22 and Elektron 21 (EV31A) compared with AZ80. Mater. Sci. Eng. A 2008, 480, 529–539. [Google Scholar] [CrossRef]
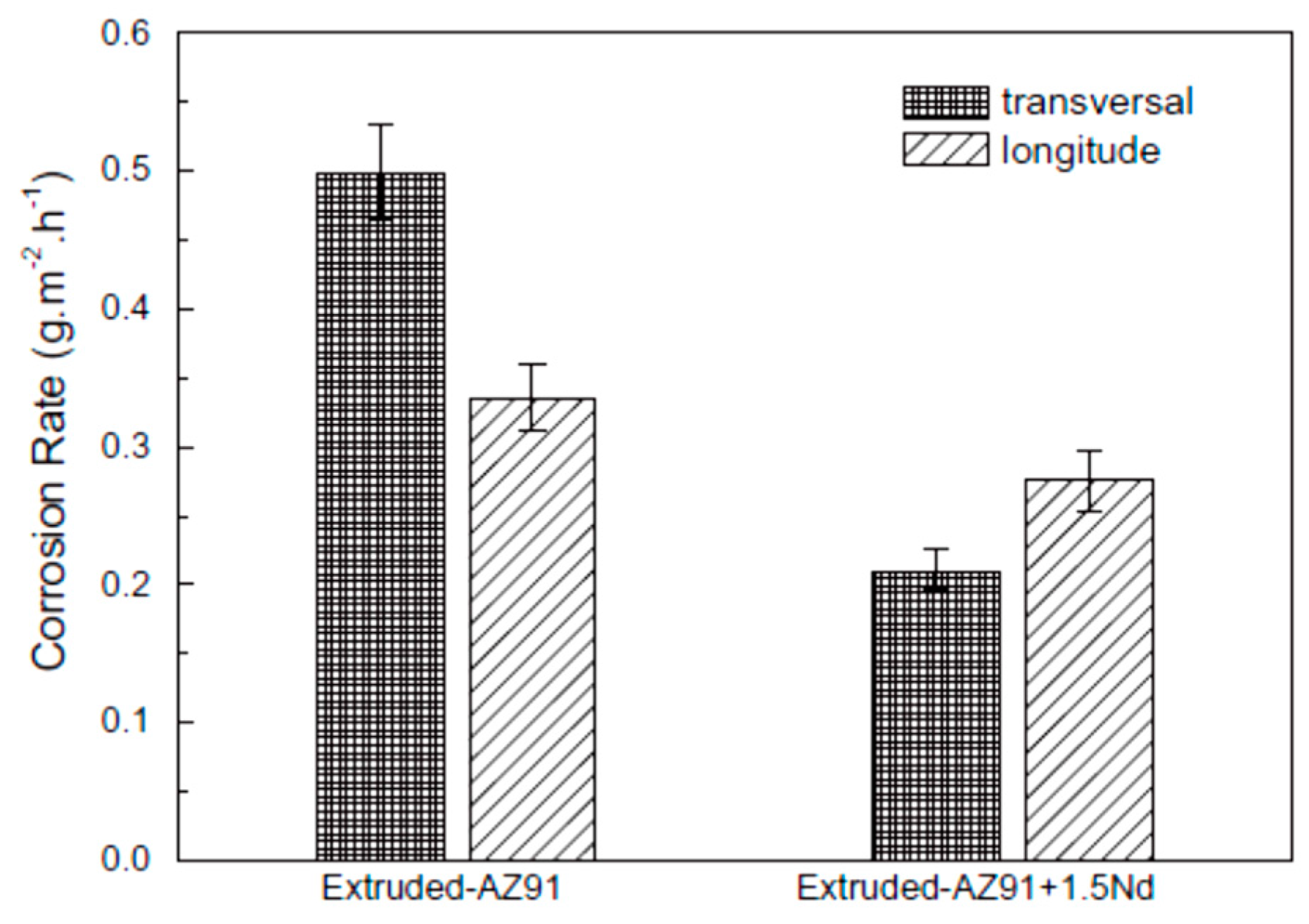
- Zhang, T.; Meng, G.; Shao, Y.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy. Part II: Effect of rare earth element neodymium (Nd) on the corrosion behavior of extruded alloy. Corros. Sci. 2011, 53, 2934–2942. [Google Scholar] [CrossRef]
- Li, W.; Cao, F.; Zhong, L.; Zheng, L.; Jia, B.; Zhang, Z.; Zhang, J. Influence of rare earth element Ce and La addition on corrosion behavior of AZ91 magnesium alloy. Mater. Corros. 2009, 60, 795–803. [Google Scholar] [CrossRef]
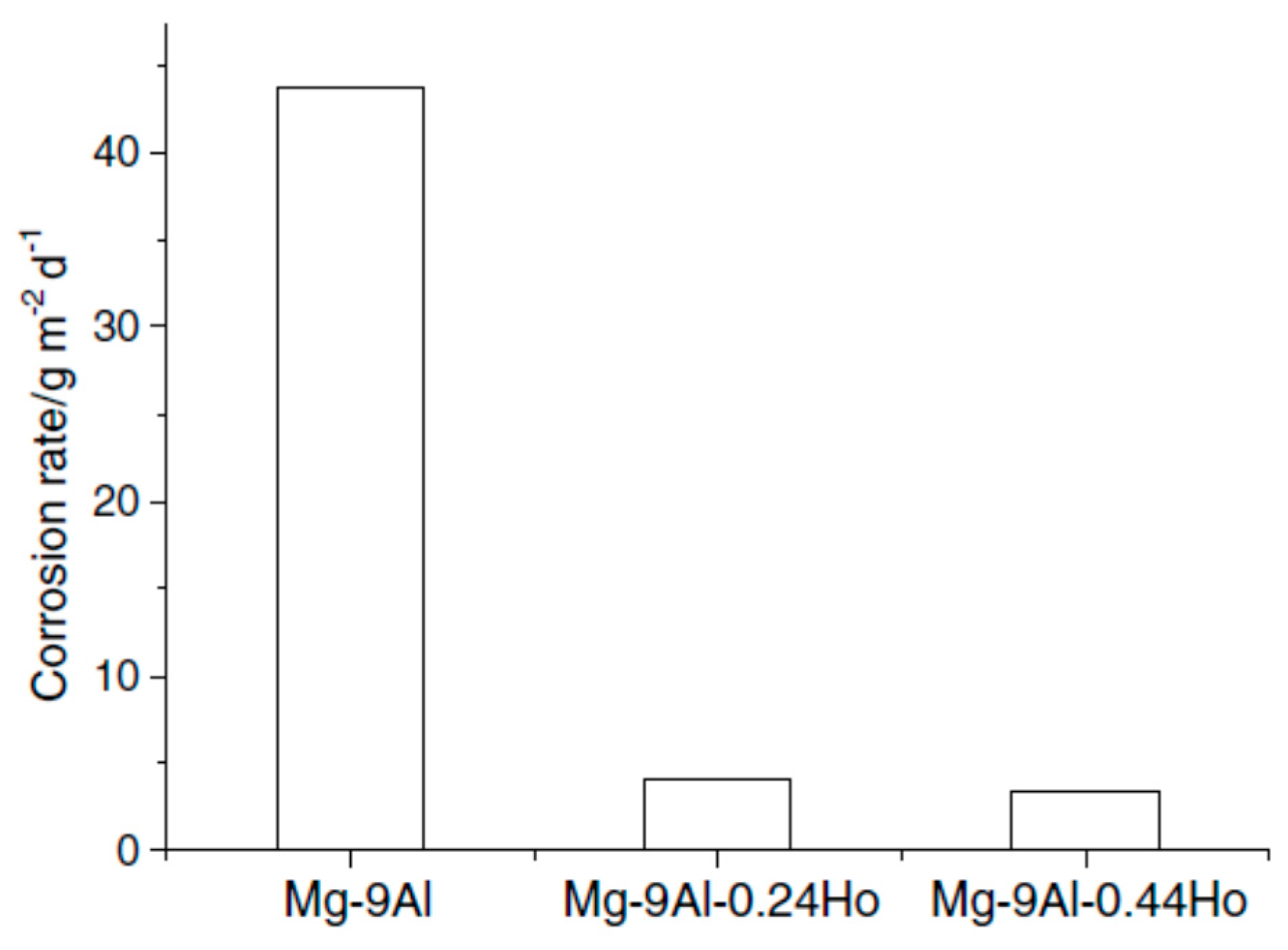
- Zhou, X.; Huang, Y.; Wei, Z.; Chen, Q.; Gan, F. Improvement of corrosion resistance of AZ91D magnesium alloy by holmium addition. Corros. Sci. 2006, 48, 4223–4233. [Google Scholar] [CrossRef]
- Yao, S.J.; Yi, D.Q.; Yang, S.; Cang, X.H.; Li, W.X. Effect of Sc on Microstructures and Corrosion Properties of AZ91. Mater. Sci. Forum 2007, 546–549, 139–142. [Google Scholar] [CrossRef]
- Santamaria, M.; di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef]
- Hänzi, A.C.; Gunde, P.; Schinhammer, M.; Uggowitzer, P.J. On the biodegradation performance of an Mg–Y–RE alloy with various surface conditions in simulated body fluid. Acta Biomater. 2009, 5, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca–P coatings on biodegradable Mg alloy: In vitro biomineralization behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Guan, S.K.; Wang, X.; Ren, C.X.; Wang, L.G. In vitro degradation and mechanical integrity of Mg–Zn–Ca alloy coated with Ca-deficient hydroxyapatite by the pulse electrodeposition process. Acta Biomater. 2010, 6, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xu, Y.; Sun, J.; Yang, L.; Guo, C.; Liang, J.; Cao, B. Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy. Metals 2017, 7, 214. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.Z.; Lee, I.; Zhang, Y.; Yin, Q.S.; Xia, H.; Yang, S.X. In vivo biocompatibility and degradation behavior of Mg alloy coated by calcium phosphate in a rabbit model. J. Biomater. Appl. 2012, 27, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y.; Wong, M.H.; Cheng, F.T.; Man, H.C. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Cao, P.; Zhang, X.N.; Zhang, S.X.; He, Y.H. In vitro degradation and cell attachment of a PLGA coated biodegradable Mg–6Zn based alloy. J. Mater. Sci. 2010, 45, 6038–6045. [Google Scholar] [CrossRef]
- Albayrak, O.; El-Atwani, O.; Altintas, S. Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: Effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf. Coat. Technol. 2008, 202, 2482–2487. [Google Scholar] [CrossRef]
- Glocker, D.A.; Ranade, S.V. Medical Coatings and Deposition Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
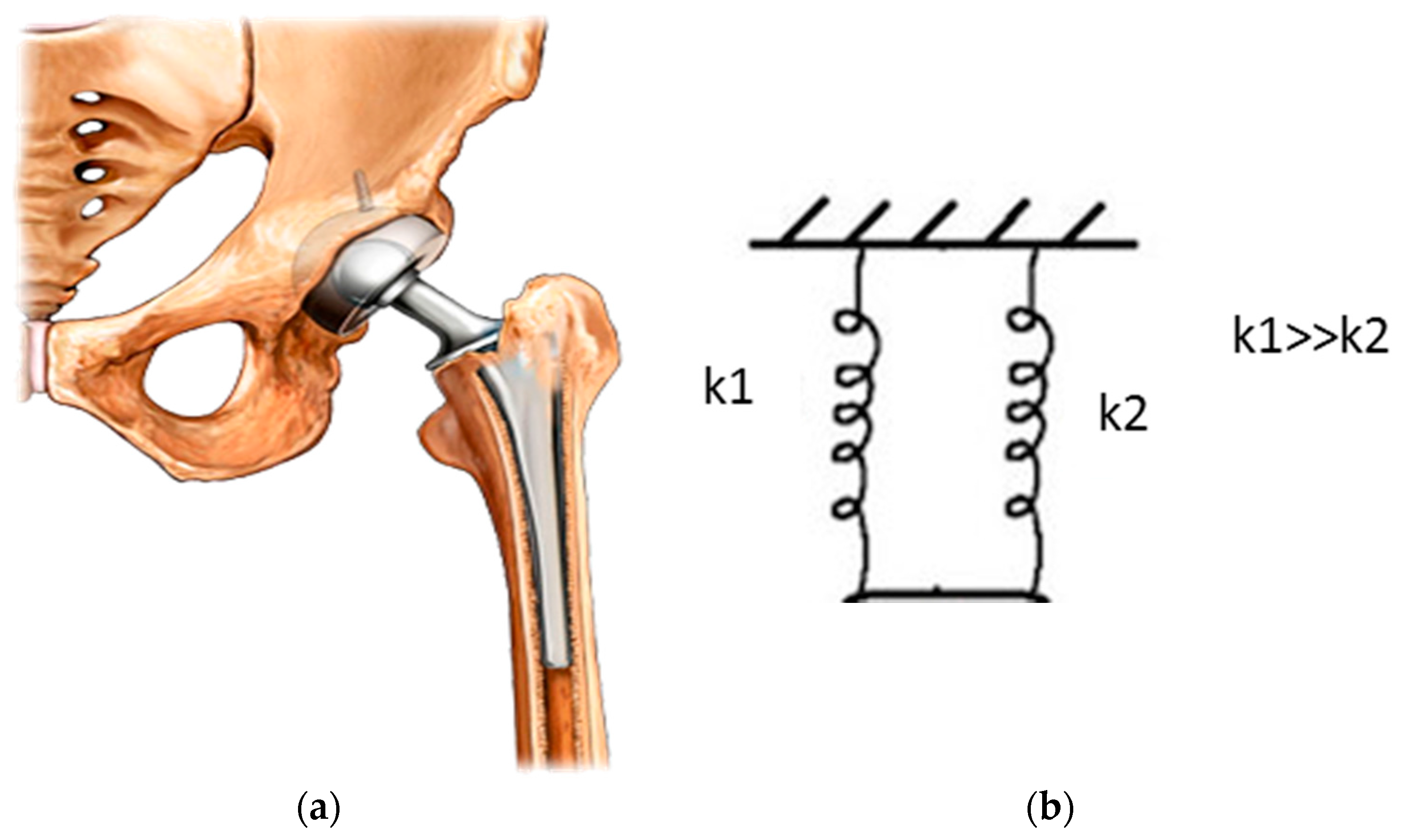
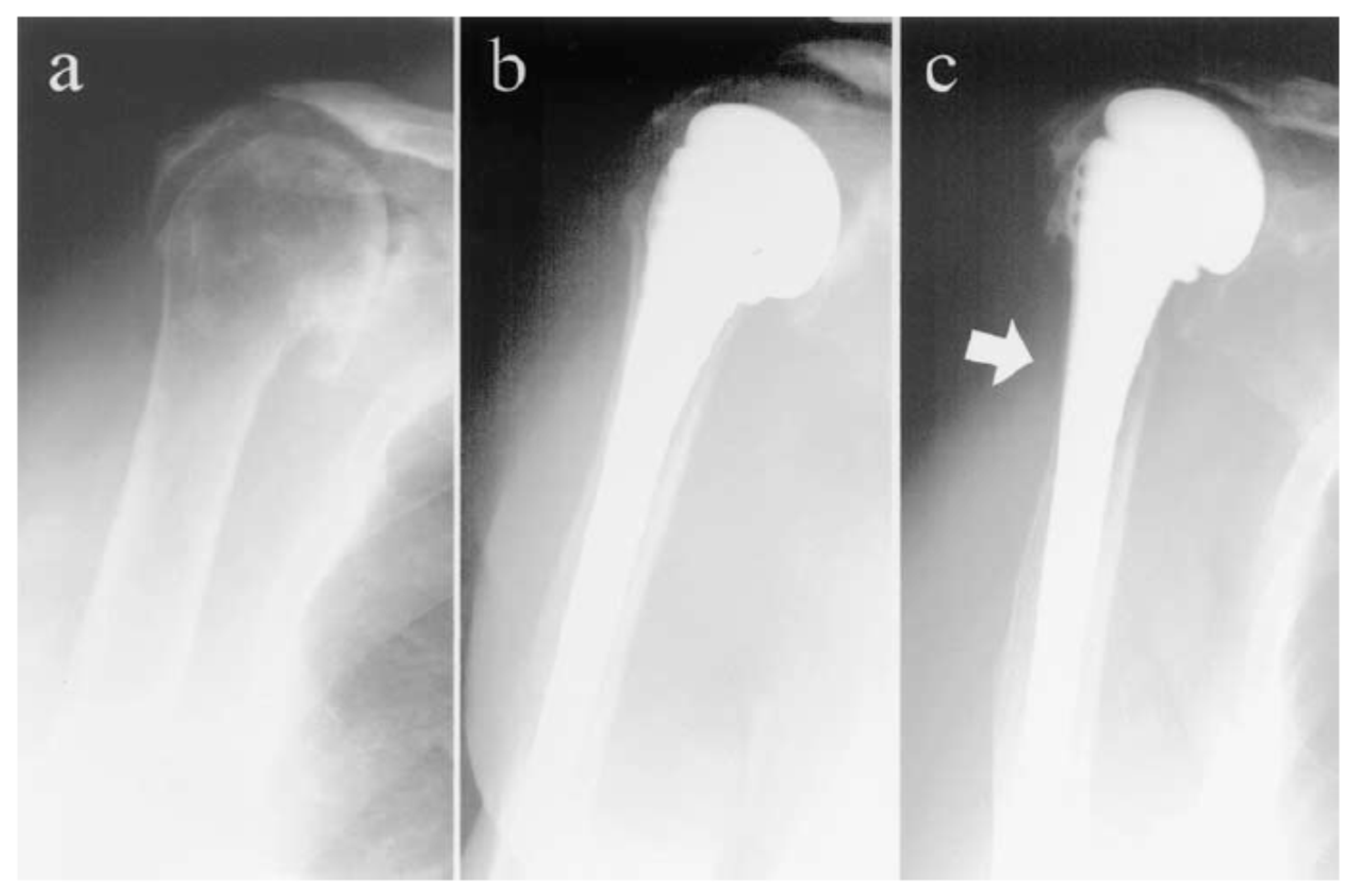
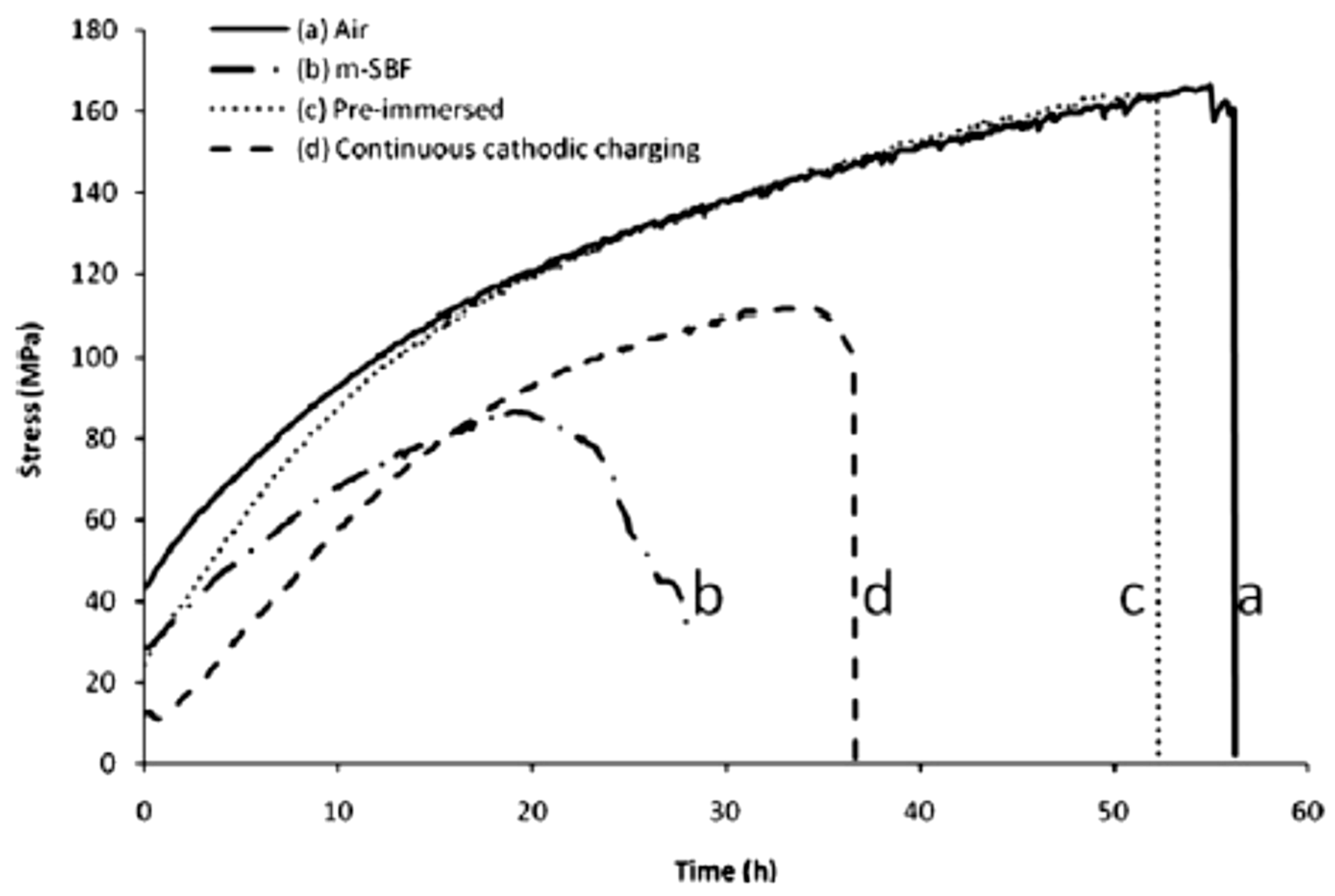
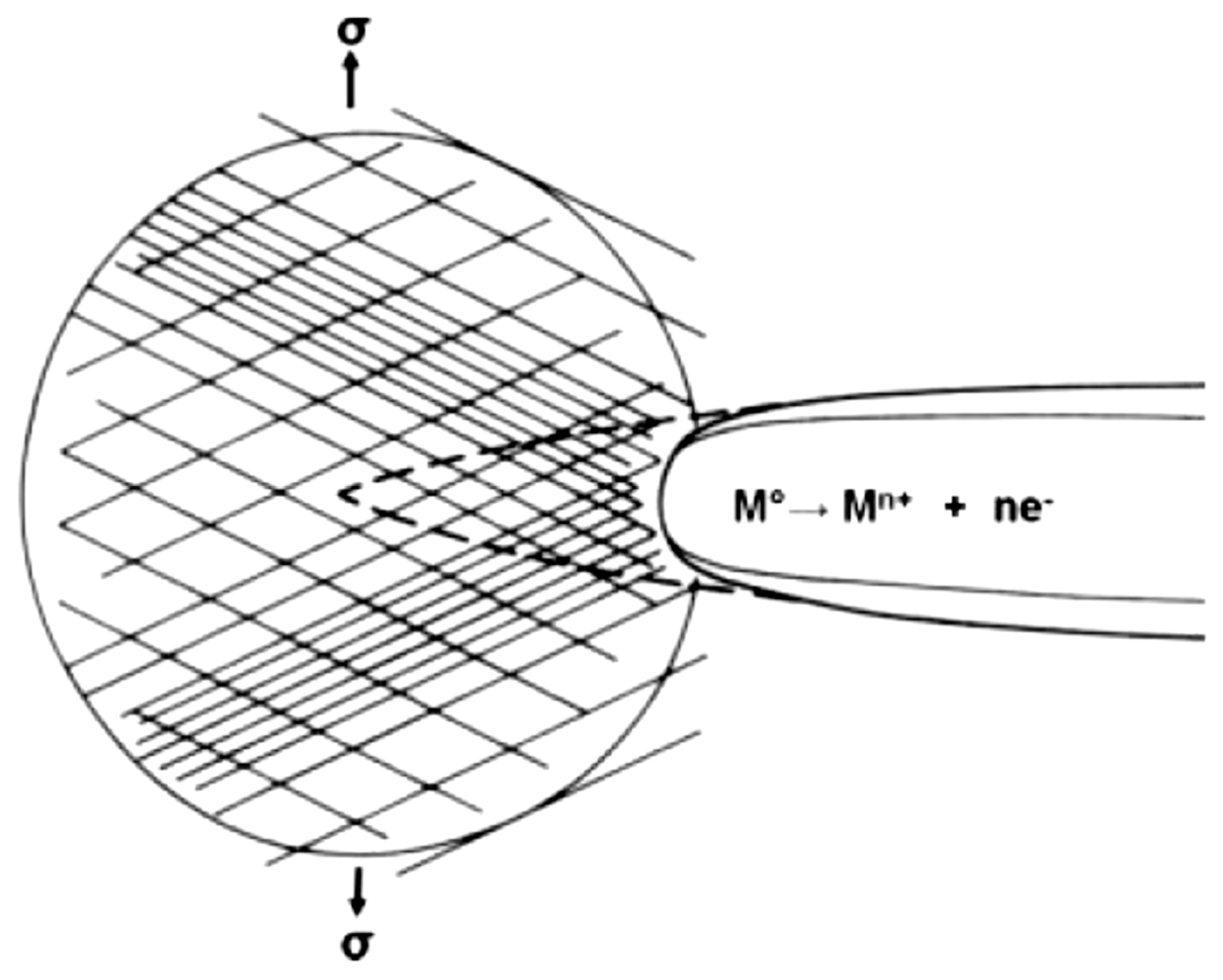
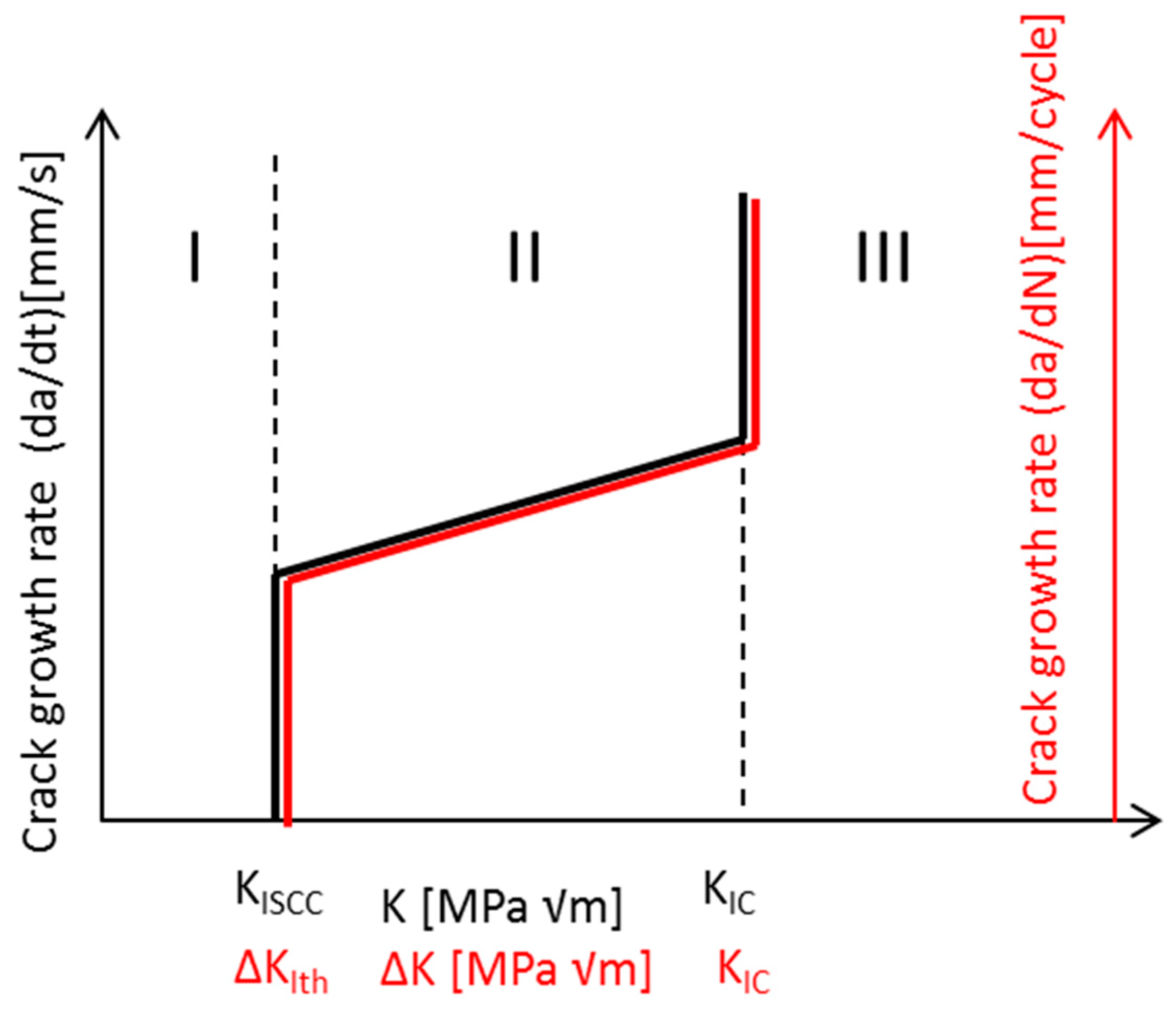
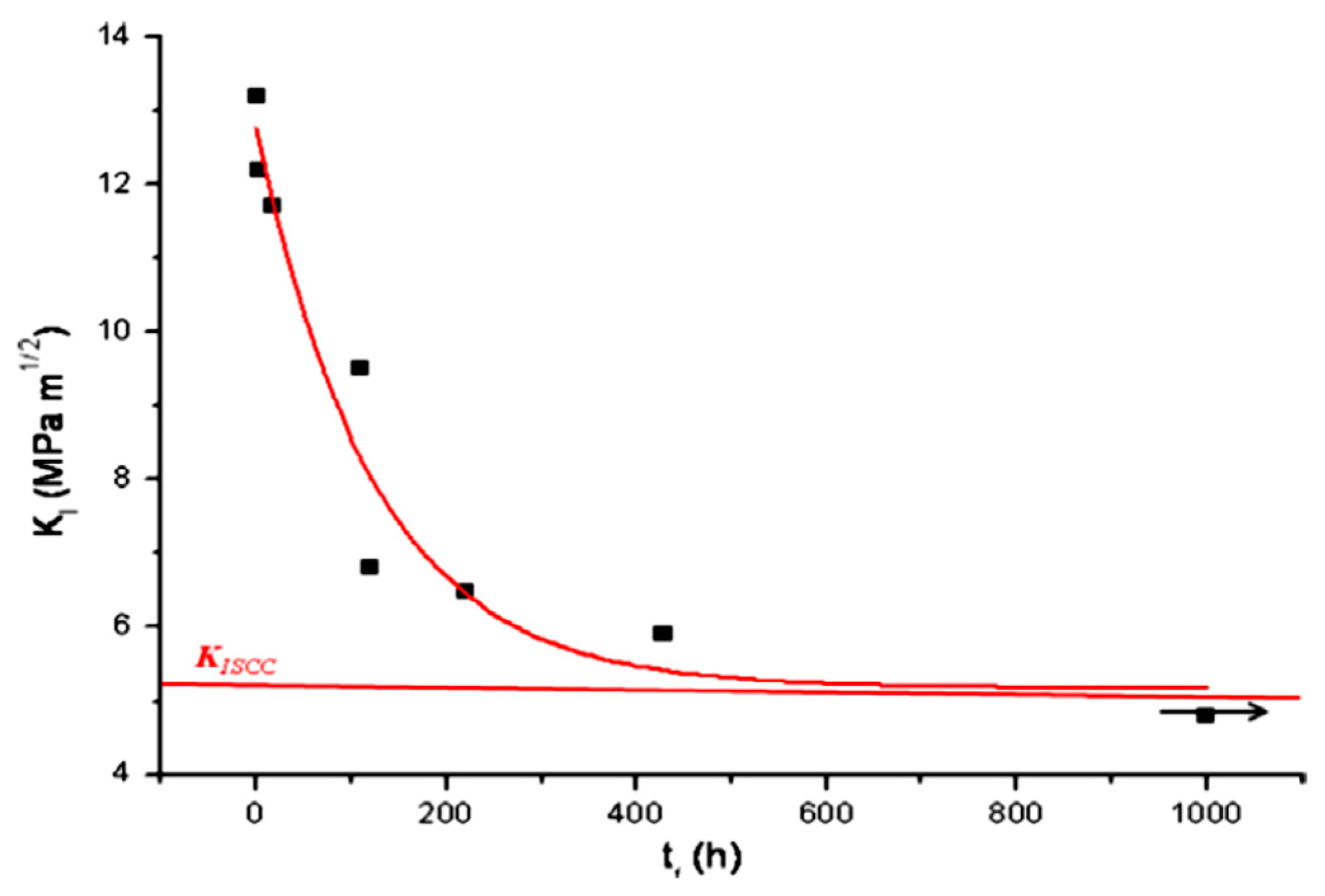
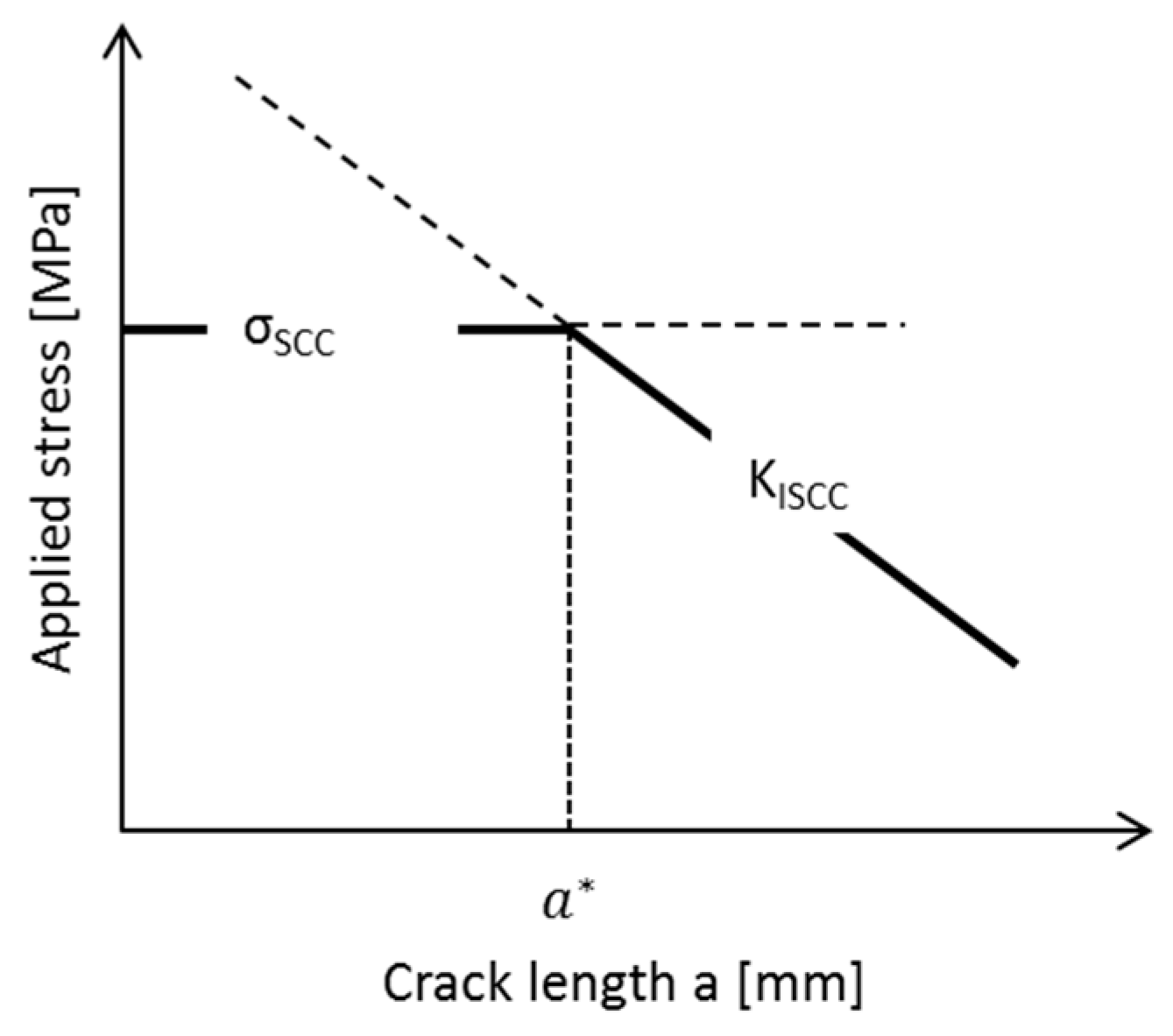
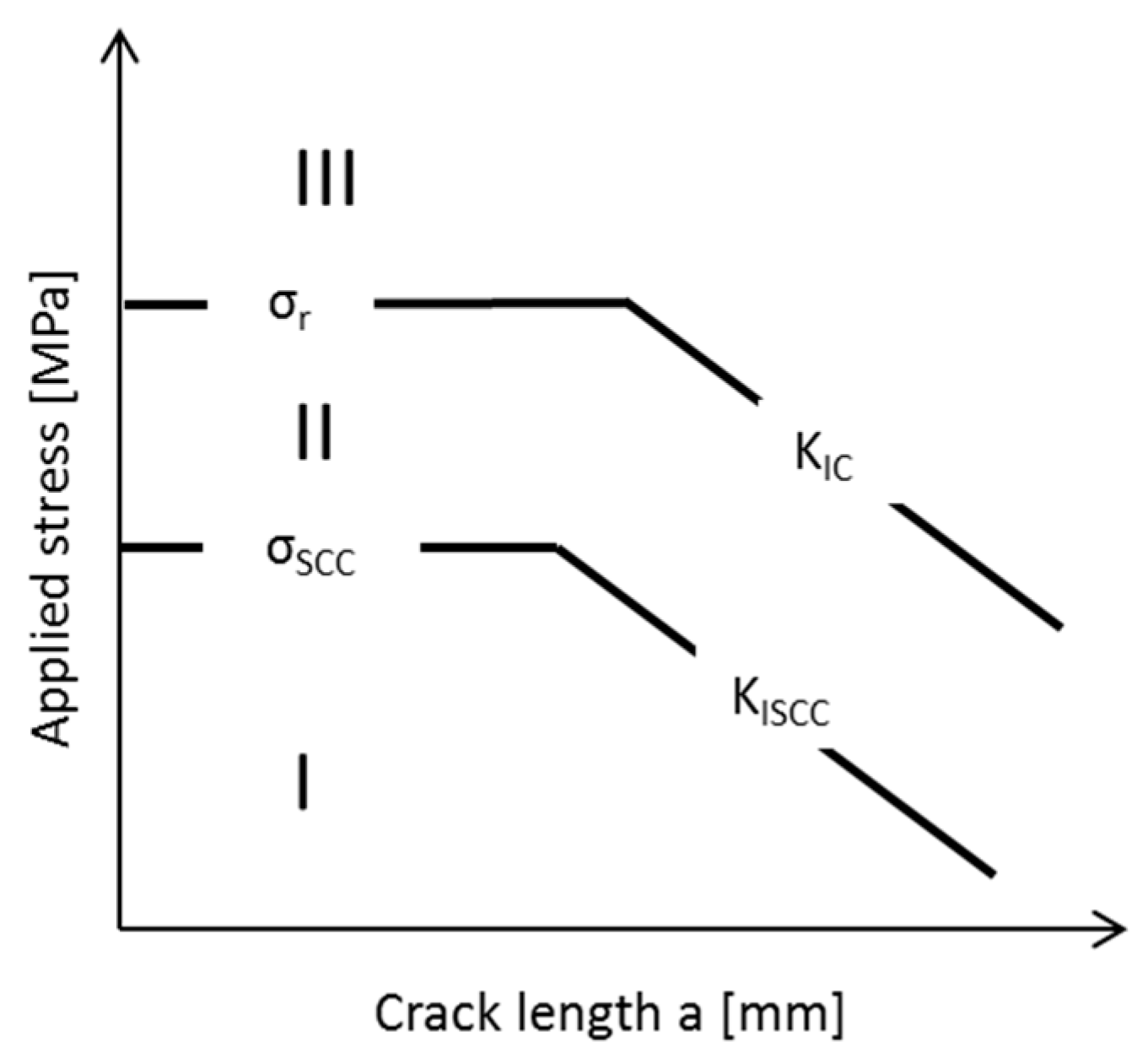
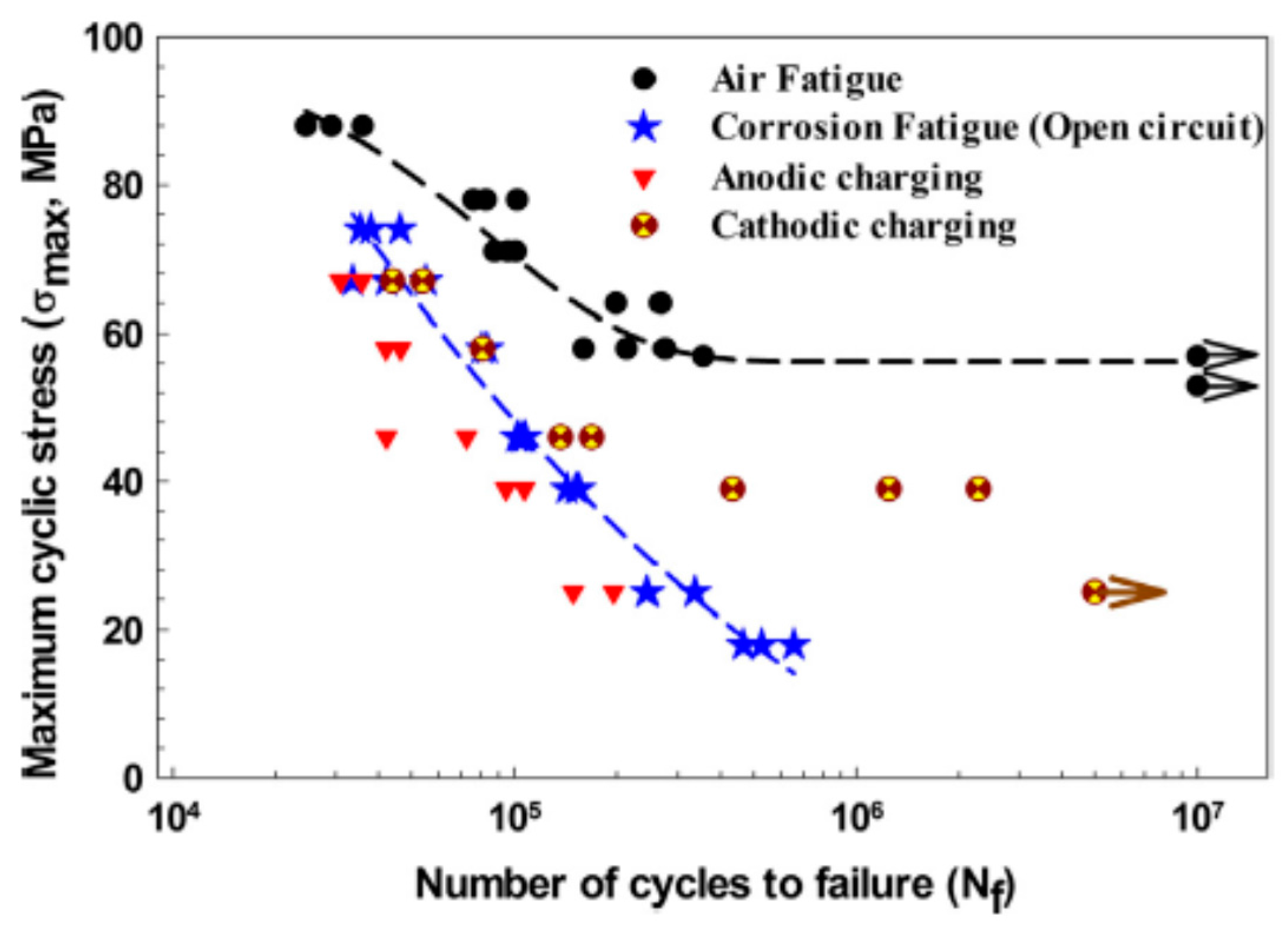
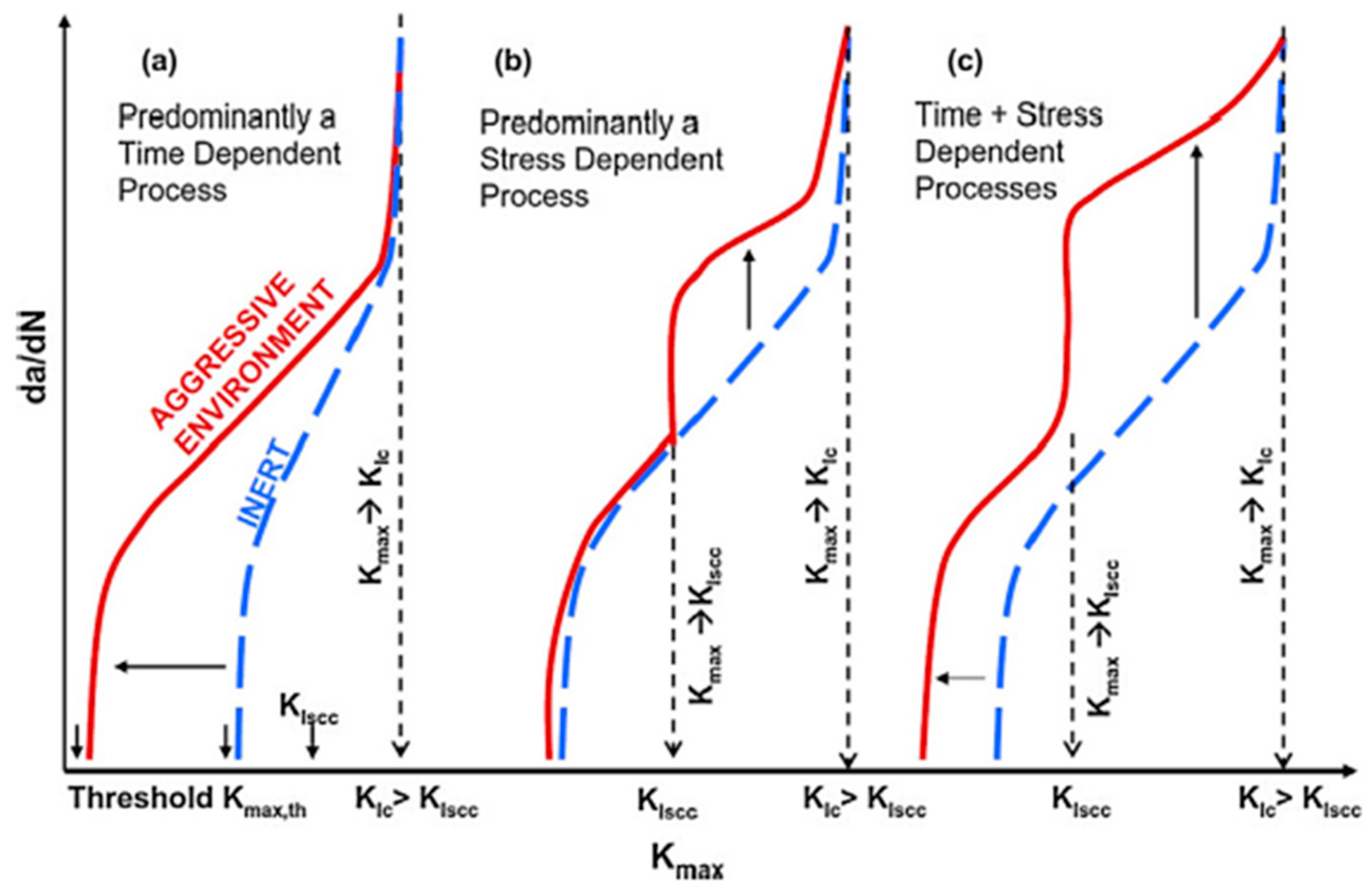
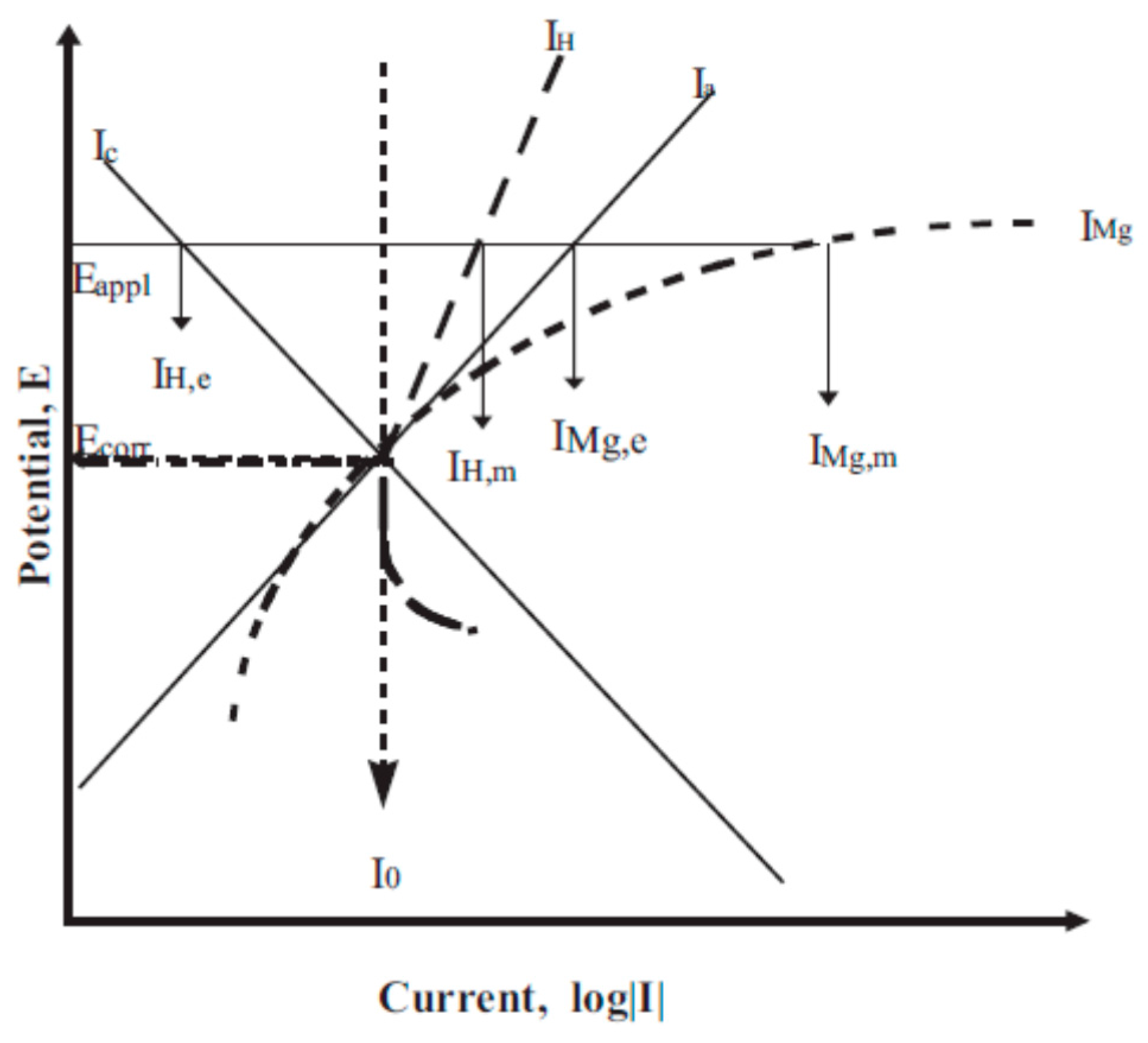
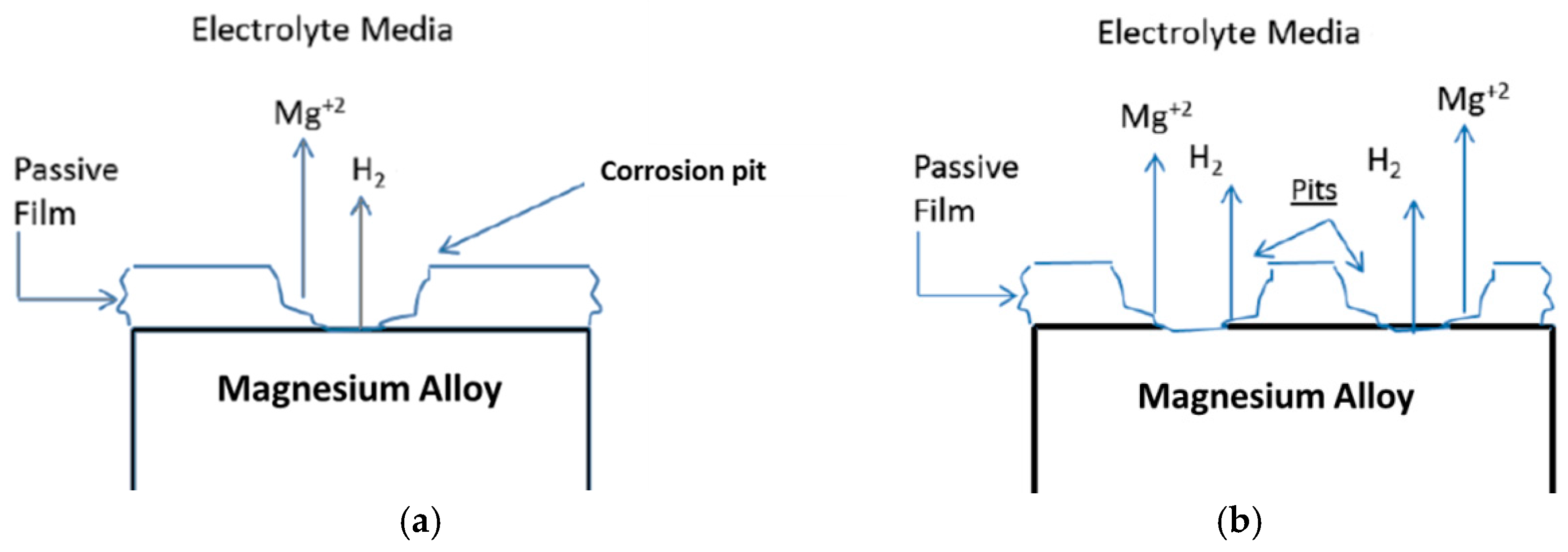
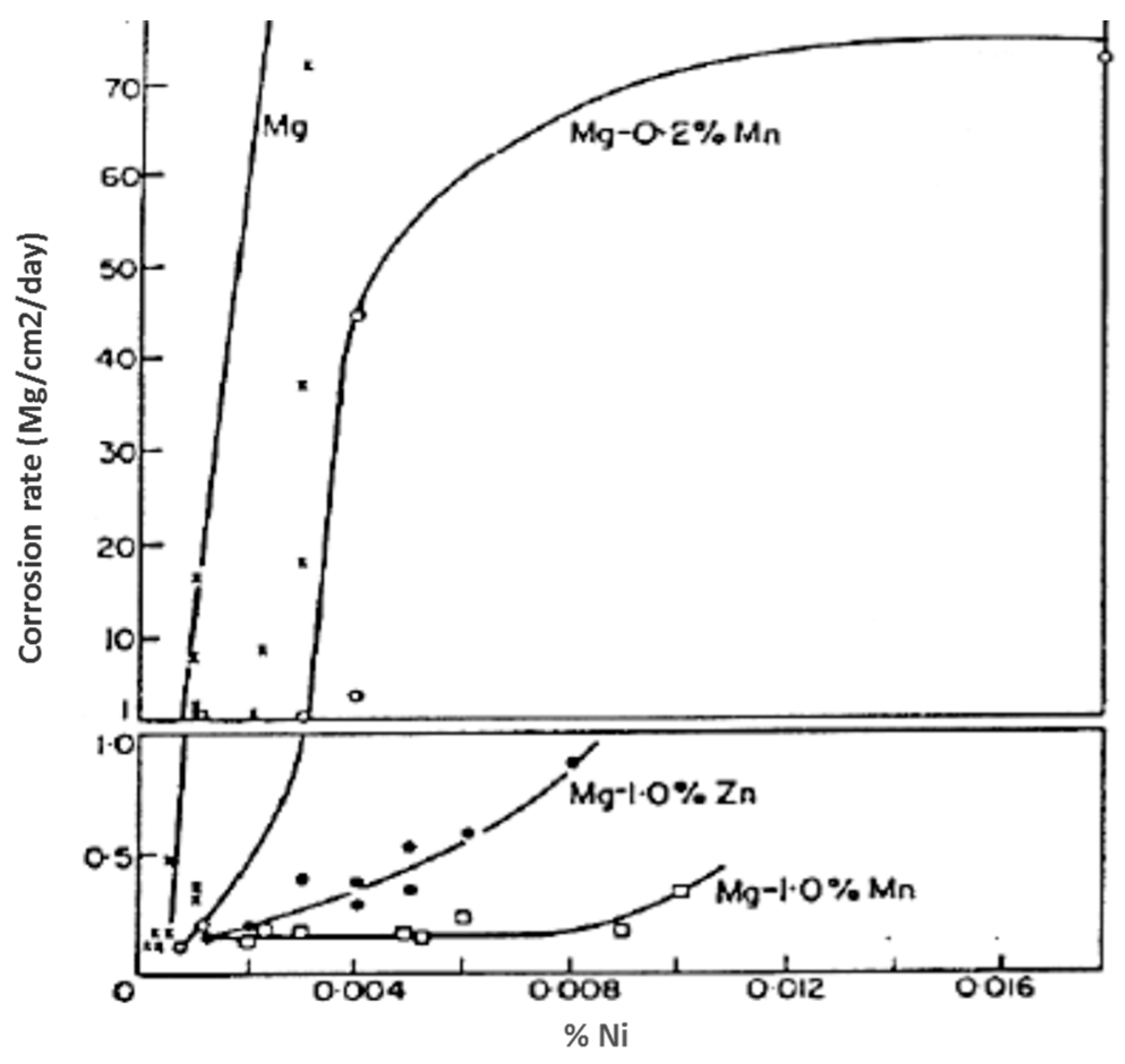
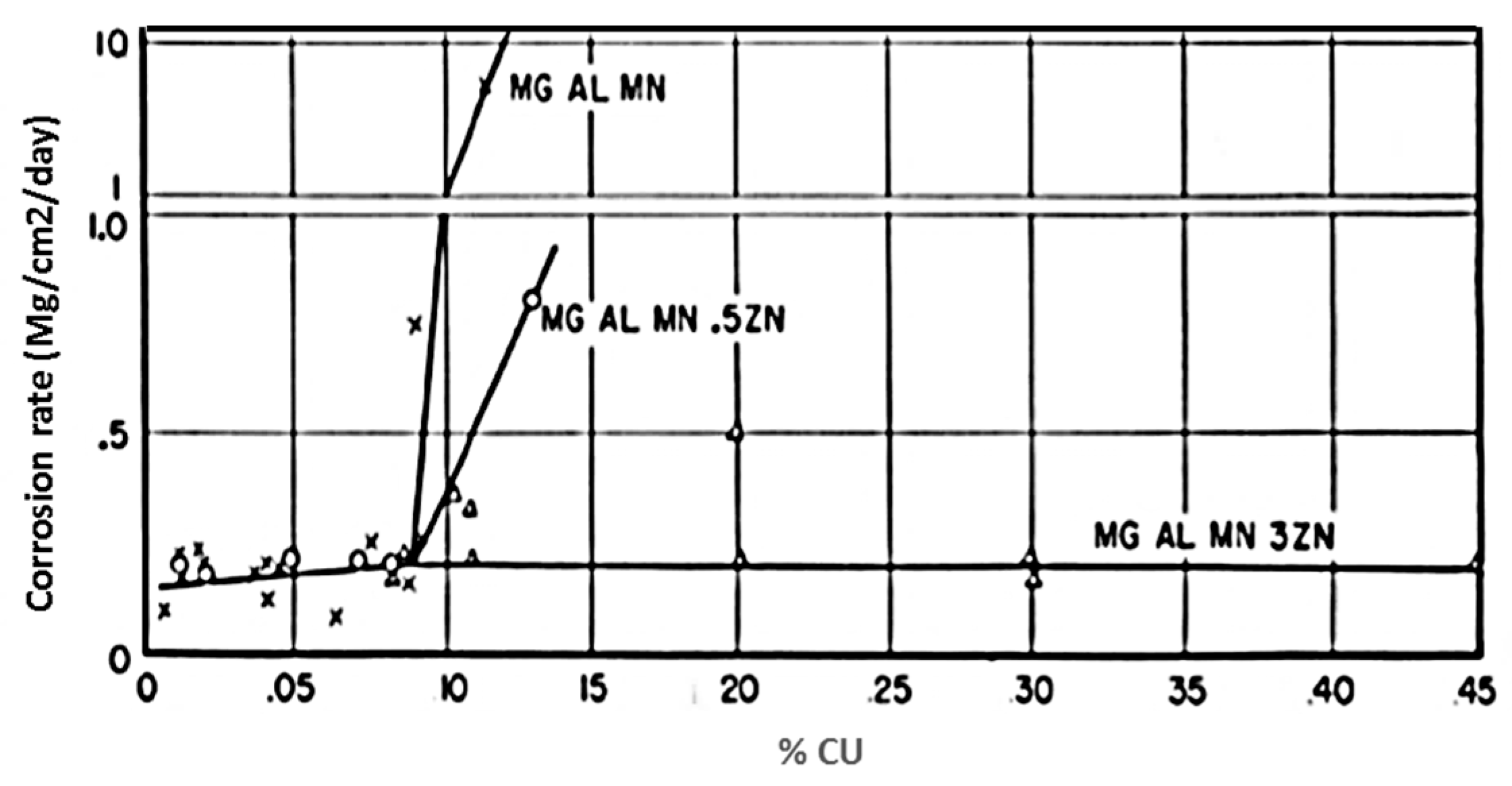
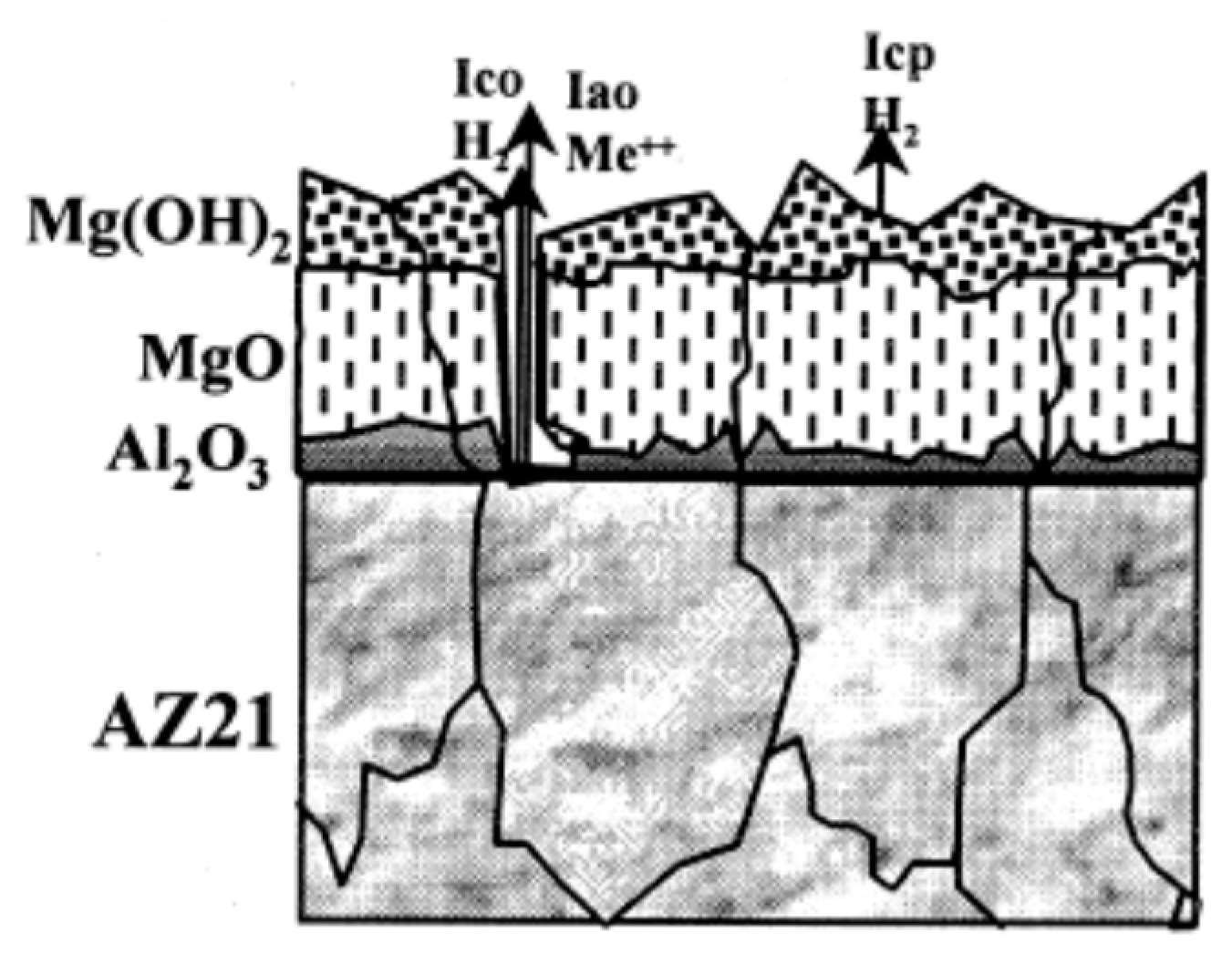
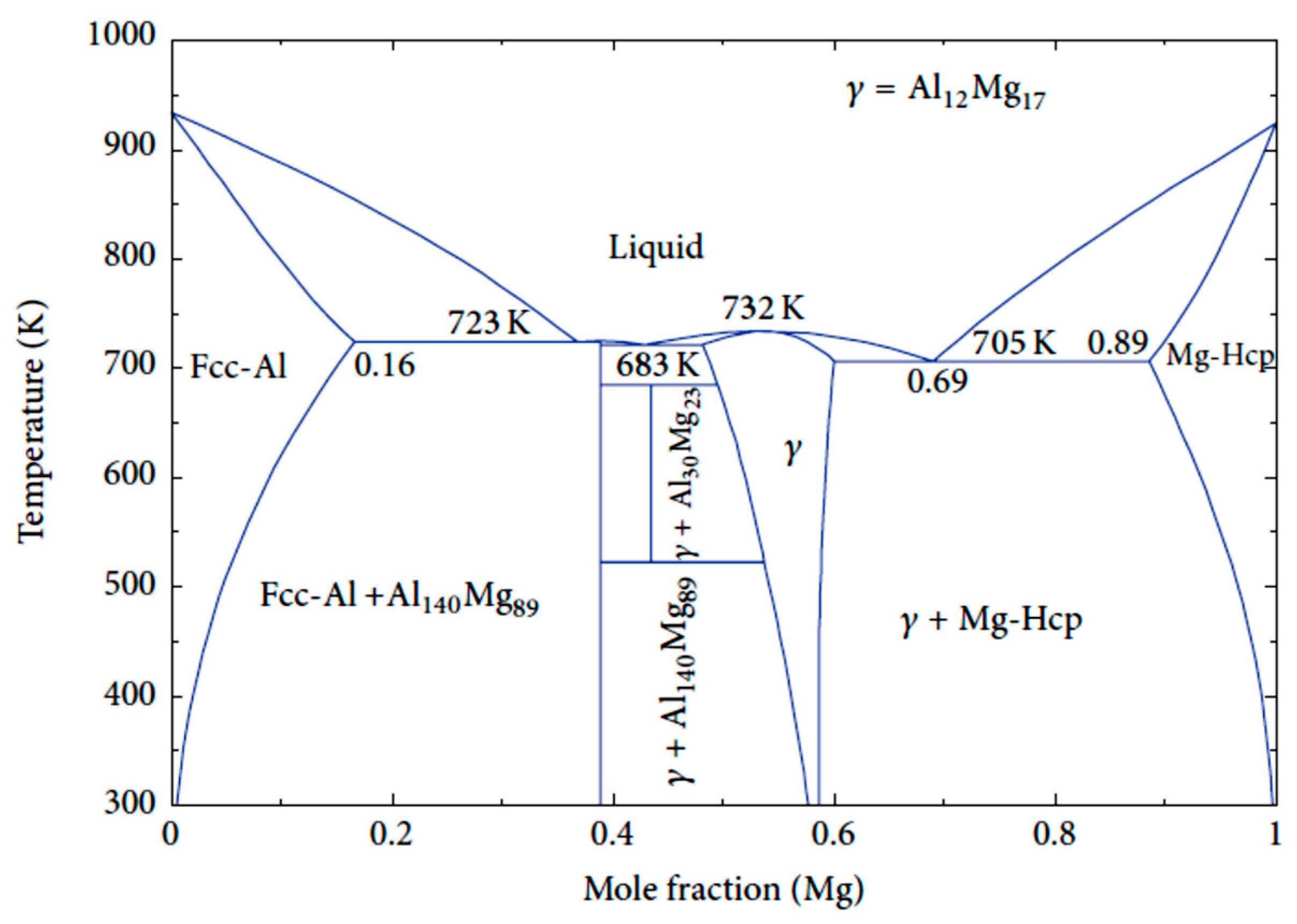

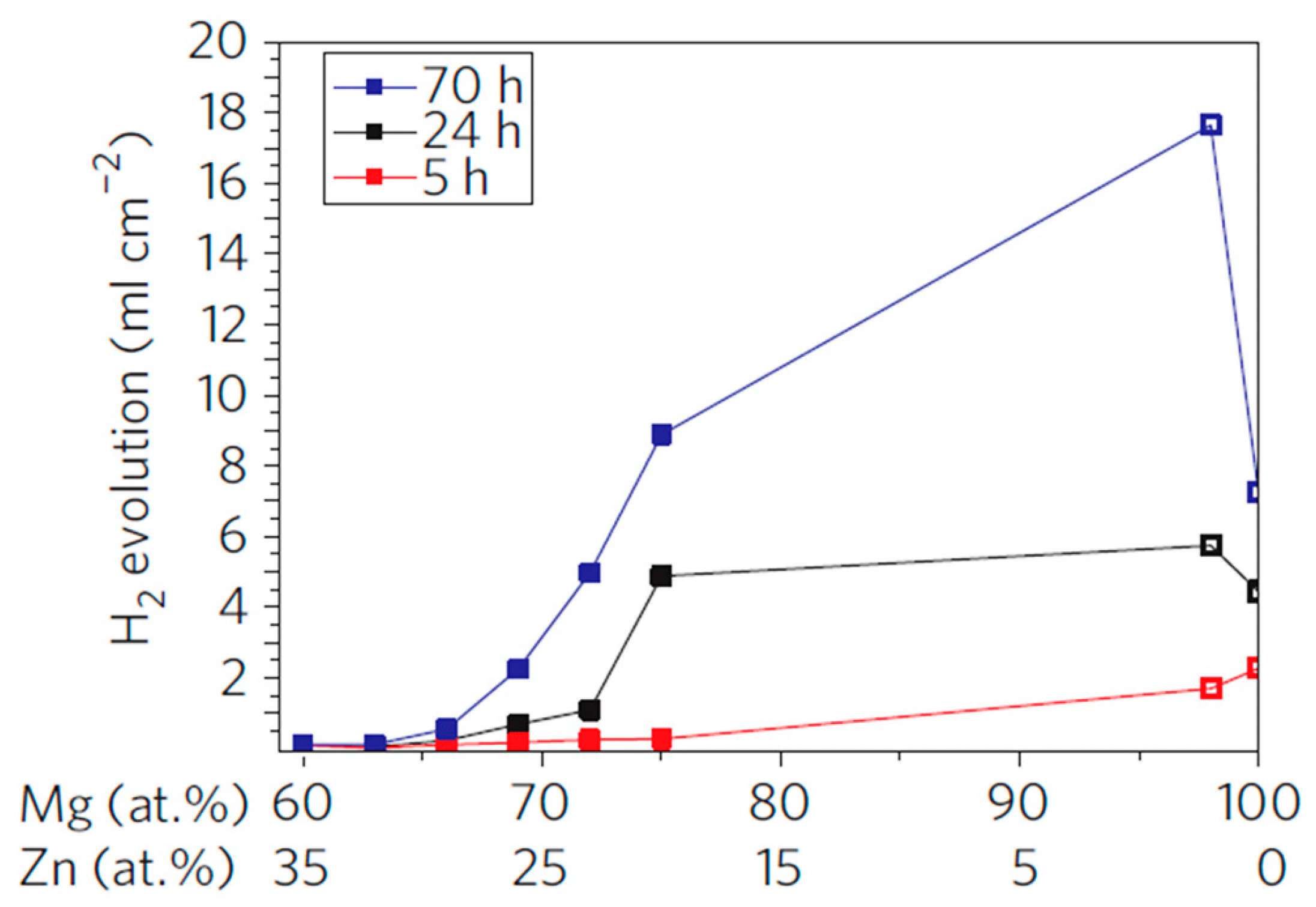
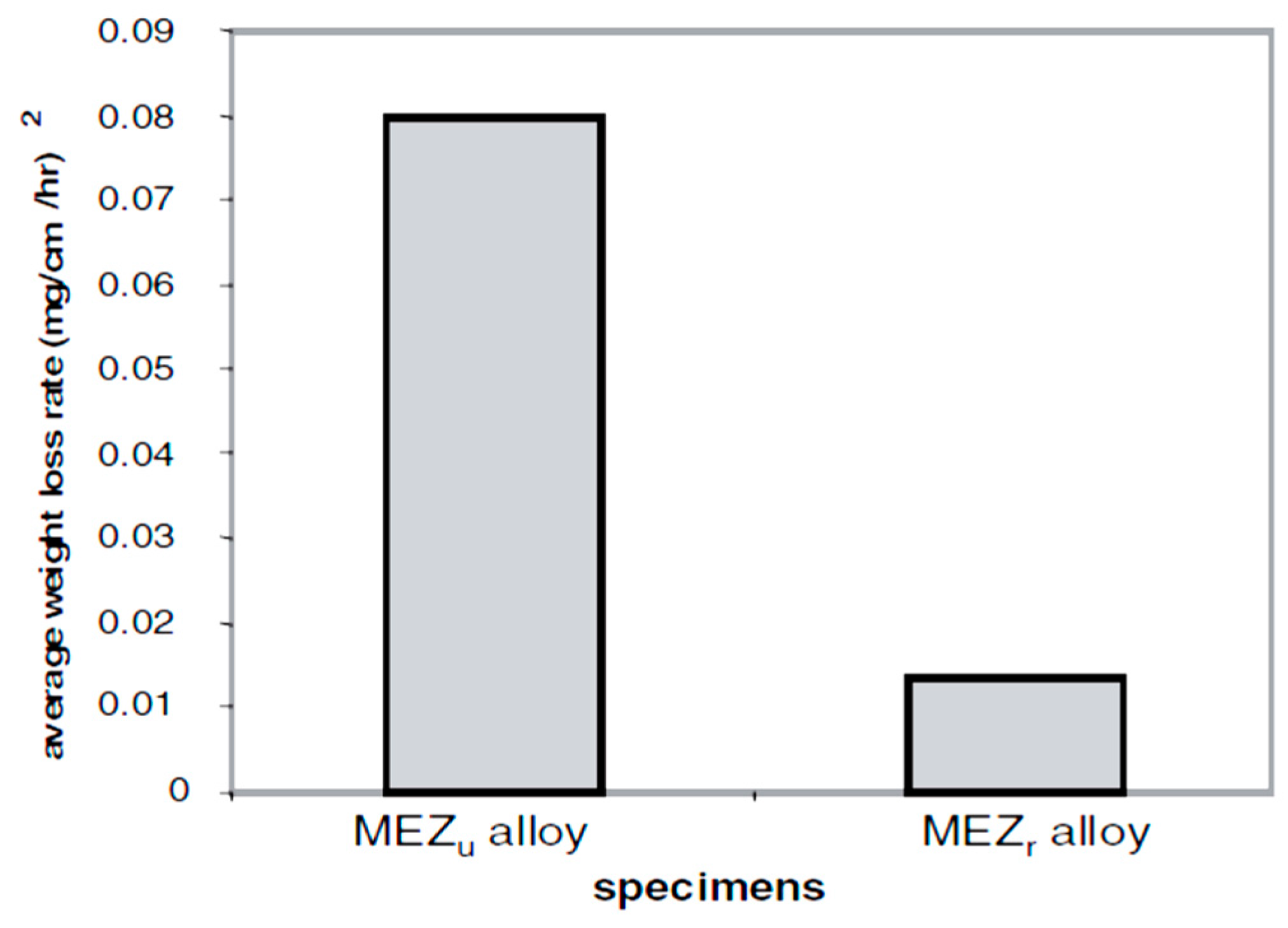
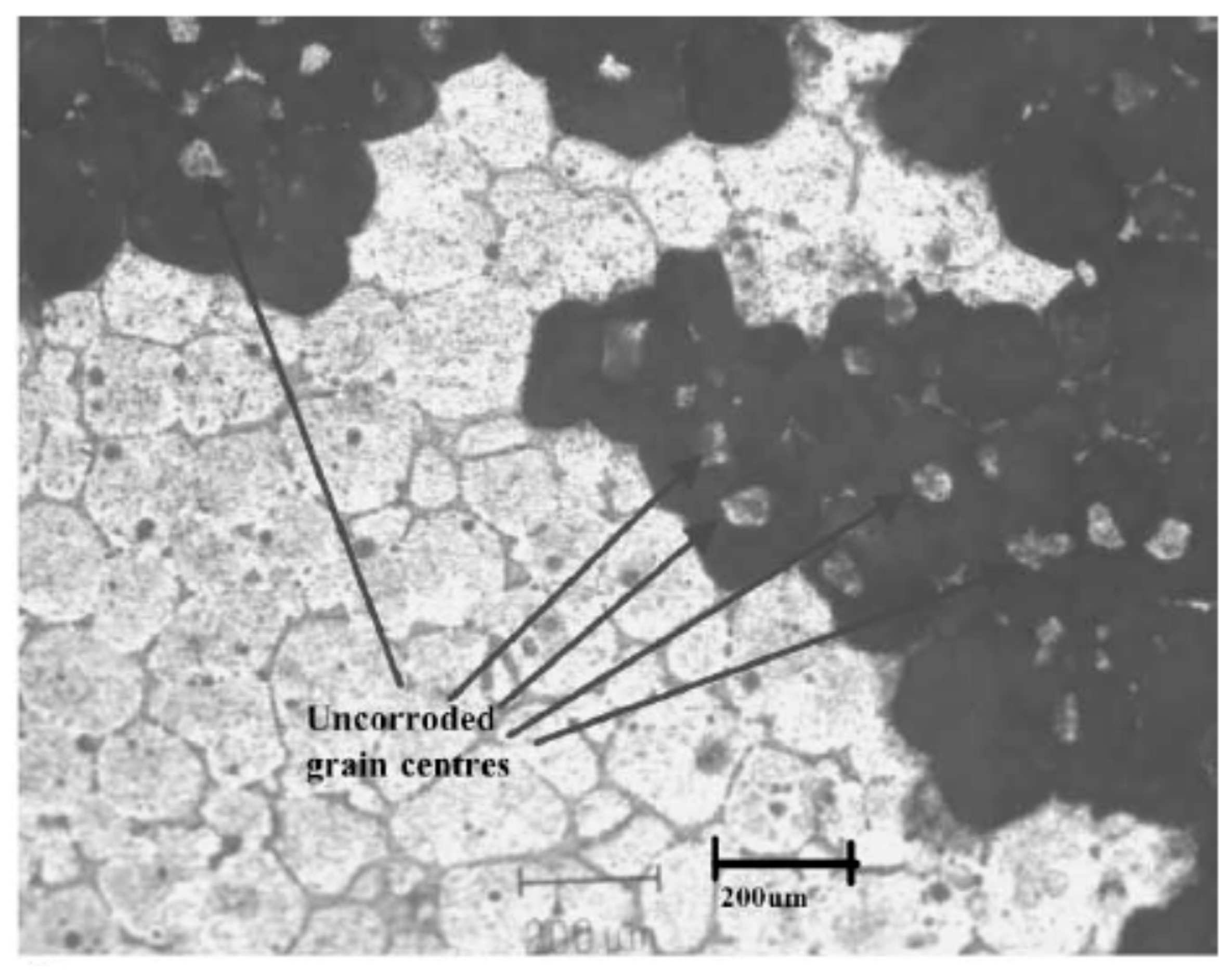
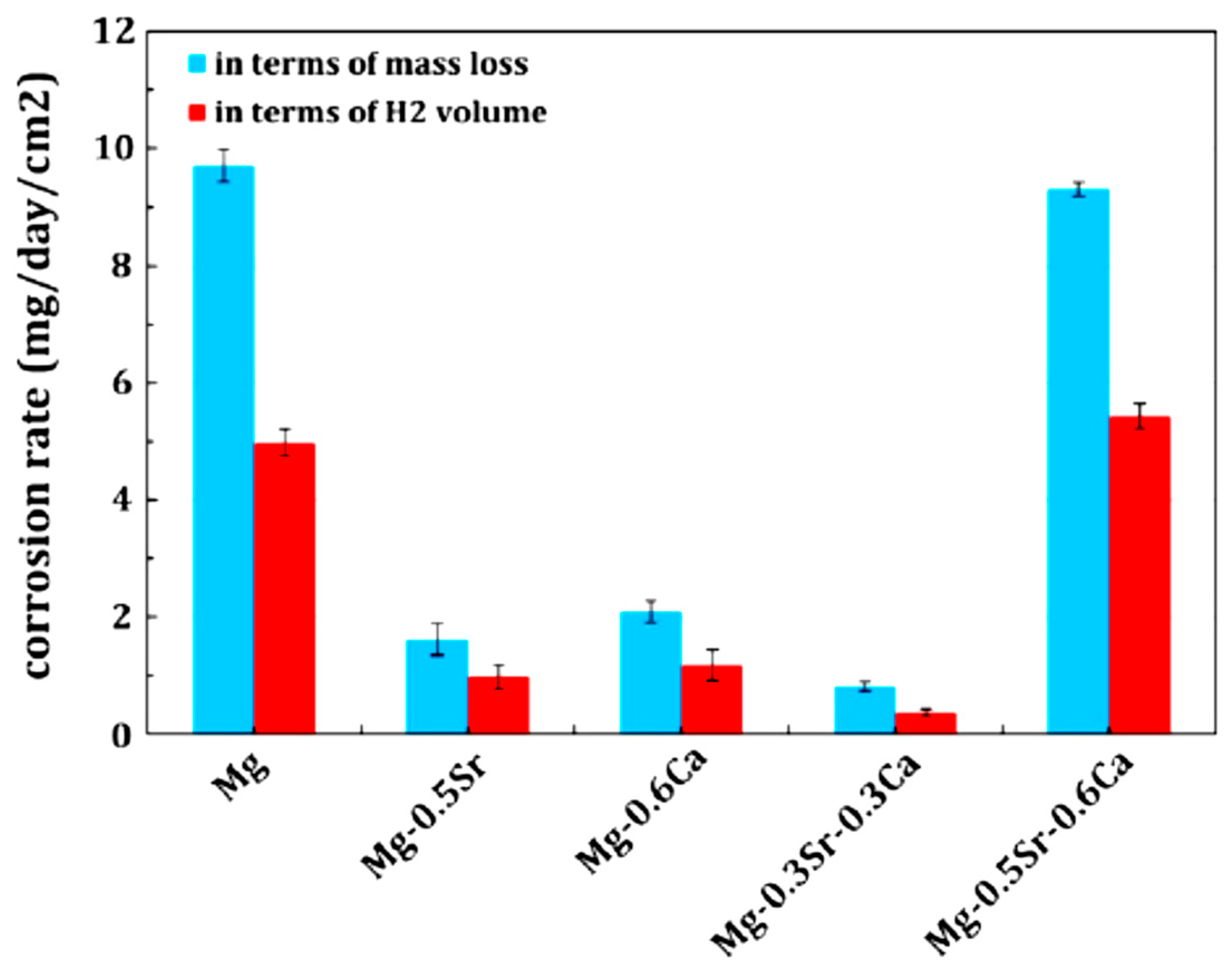

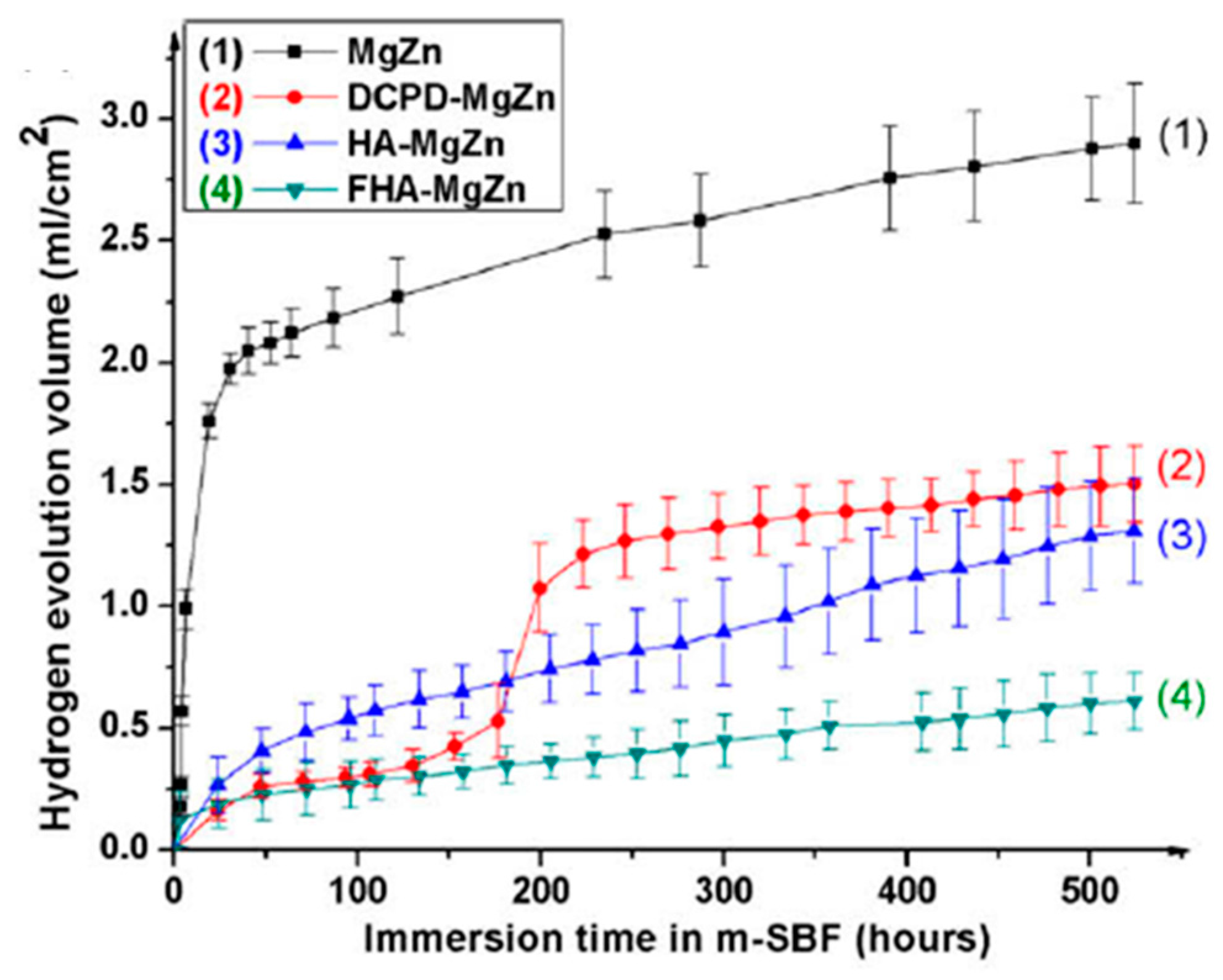
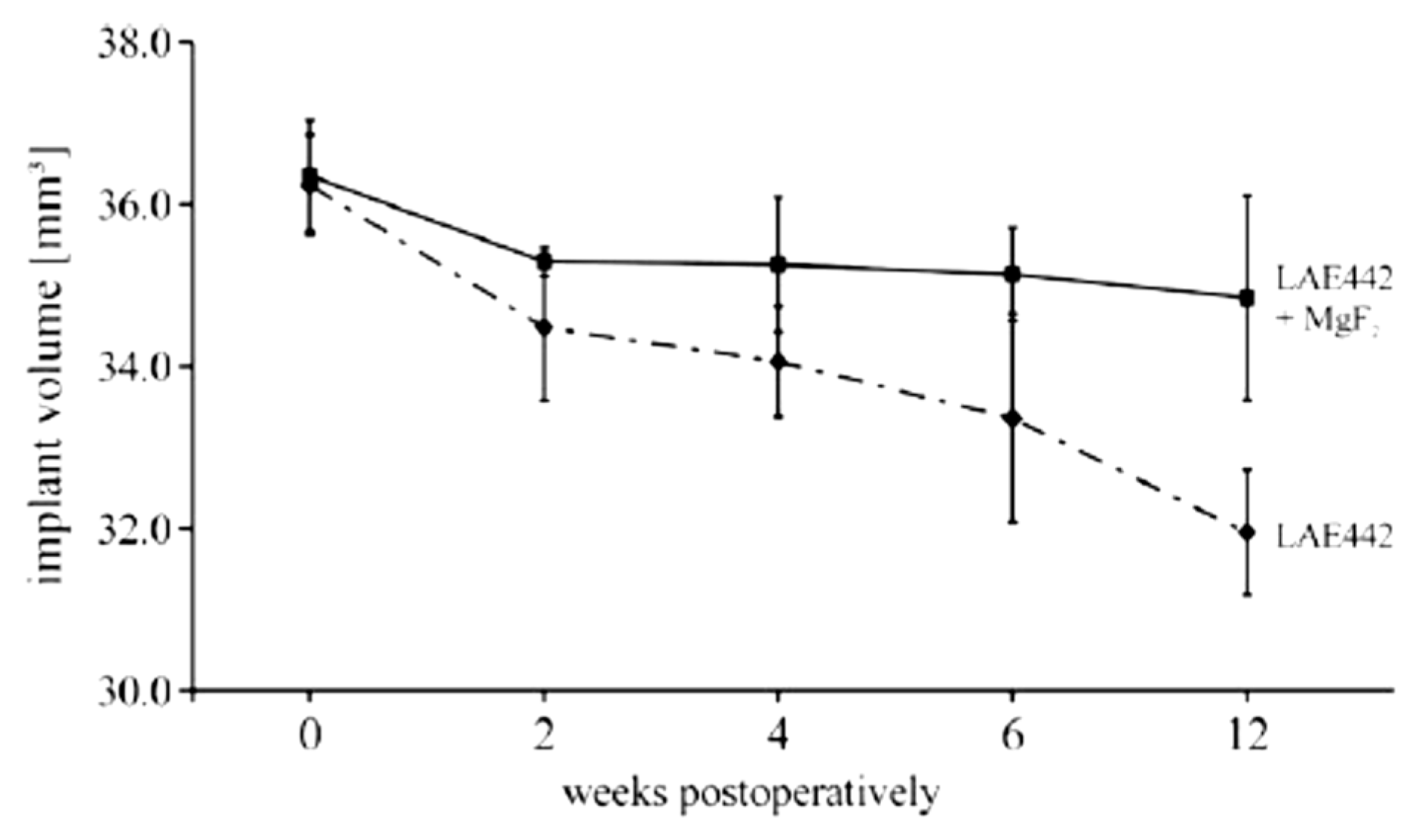
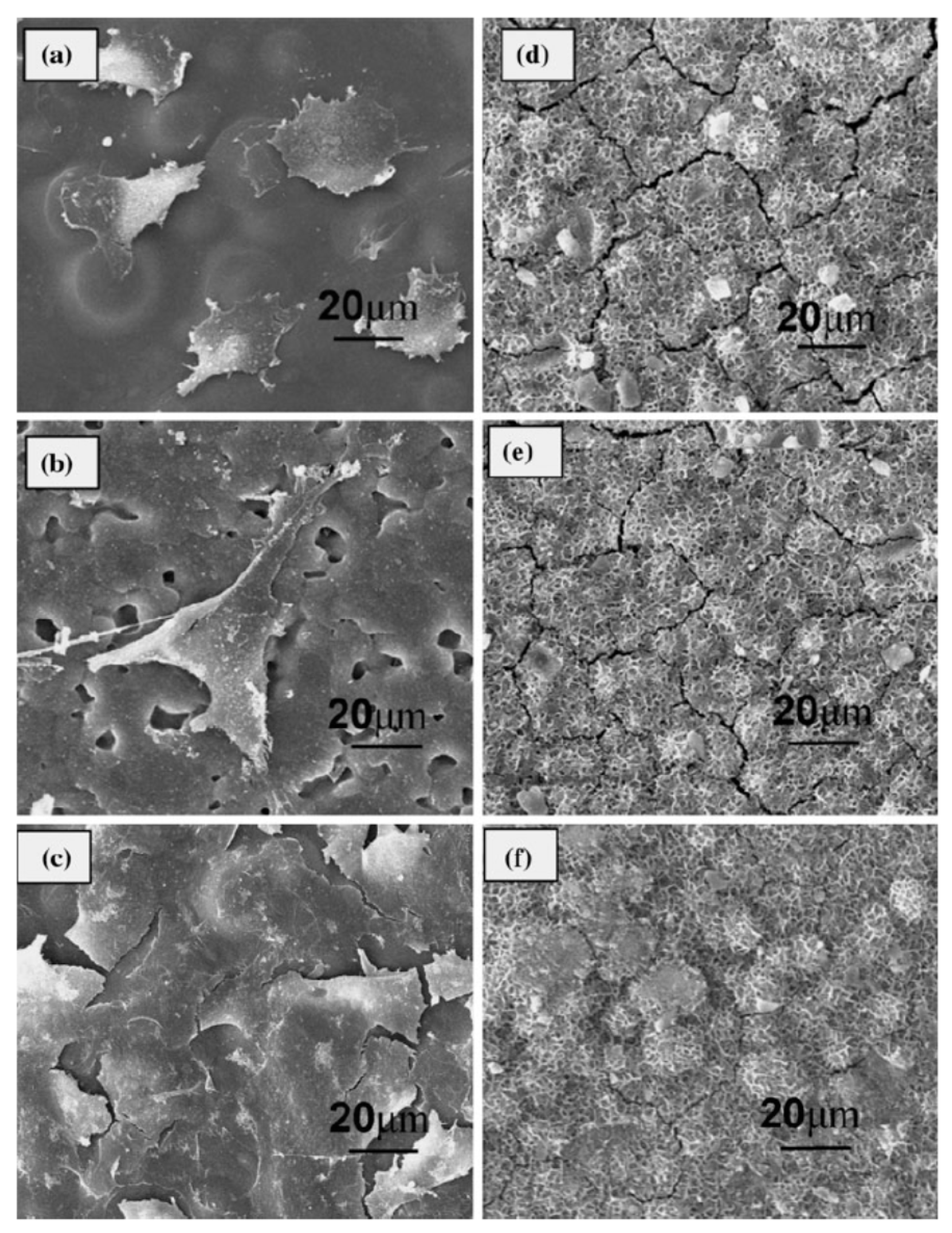
| Application | Material |
|---|---|
| Spinal fixation | stainless steel; Ti, Ti alloys |
| Bone fixation (bone plate, screw, wire, bone nail, etc.) | stainless steel; Ti, Ti alloys; PMMA; PS; CF/PEEK; CF/Epoxy; CF/PS; |
| Bio-Glass/PU; Bio-Glass/PS; PET/SR; PET/Hydrogel; CF/LCP; GF/PEEK; | |
| Bone particles/PMMA; Ti/PMMA; UHMWPE/PMMA; UHMWPE; | |
| GF/PMMA; CF/PMMA; KF/PMMA; PMMA/PMMA; Bio-Glass/Bis-GMA; | |
| CF/PP; CF/PS; CF/PLLA; CF/PLA; KF/PC; HA/PE; PLLA/PLDLA; | |
| PGA/PGA; Alumina; Zirconia; Tricalcium phosphate; | |
| Bio-Glass-Metal fiber composite; Bio-Glass | |
| Artificial joint | Co-Cr-Mo alloy; Ti alloys; Alumina; Zirconia; PET/SR;CF/UHMWPE; |
| PET/Hydrogel; CF/Epoxy; CF/C; CF/PS; CF/PEEK; CF/PTFE; CF/UHMWPE; | |
| CF/PE; UHMWPE/UHMWPE; HA/HDPE; Metal Bio-Glass coatings | |
| Spinal spacer | stainless steel; Ti alloys |
| Dental implant and bridge | Ti; Ti alloys; Au; CF/C; SiC/C; Alumina; Bio-Glass; HA; |
| Bio-Glass coated alumina; Bio-Glass coated vitallium; Au-Cu-Ag; | |
| Au-Cu-Ag-Pt-Pd; UHMWPE/PMMA; CF/PMMA; GF/PMMA; KF/PMMA; | |
| Tendon/Ligament/Cartilage Replacement | PET/PU; PTFE/PU; CF/PTFE; CF/C; PET/PHEMA; KF/PMA; KF/PE; |
| CF/PTFE; CF/PLLA; GF/PU; PP; ePTFE; PET/PET; PA; PU; PLGA |
| Properties | Natural Bone | Stainless Steel | Ti Alloy | Co-Cr Alloy | Magnesium |
|---|---|---|---|---|---|
| Density (g/cm3) | 1.7–2.0 | 7.9–8.1 | 4.4–4.5 | 8.3–9.2 | 1.74–2.0 |
| Elastic modulus (MPa) | 3–20 | 189–205 | 110–117 | 230 | 41–45 |
| Tensile strength (MPa) | 80–150 | 480–620 | 930–1140 | 900–1540 | 170–270 |
| Compressive yield strength (MPa) | 130–180 | 170–310 | 758–1117 | 450–1000 | 65–100 |
| Elongation at failure (%) | 1–7 | 30–40 | 8–15 | 30–45 | 6–20 |
| Fracture toughness (MPa m1/2) | 3–6 | 50–200 | 55–115 | 100 | 15–40 |
| Implants | Frequency (Hz) | Activity |
|---|---|---|
| Orthopaedic | 1–3 | Normal walking (vertical direction) |
| Orthopaedic | 0.5–1.5 | Normal walking (lateral direction) |
| Alloy | 10−6 s−1 Strain Rate | 10−7 s−1 Strain Rate | ||
|---|---|---|---|---|
| ISCC | ISCC | |||
| εf | UTS | εf | UTS | |
| AZ80 | 0.35 | 0.83 | 0.09 | 0.62 |
| ZE41 | 0.35 | 0.80 | 0.17 | 0.66 |
| QE22 | 0.12 | 0.82 | 0.05 | 0.65 |
| EV31A | 0.70 | 0.85 | 0.67 | 0.85 |
| Immersion Time (h) | Average Degradation Rate (mg/cm2/h) | |
|---|---|---|
| Uncoated | Coated | |
| 72 | 0.063 | 2.44 × 10−4 |
| 144 | 0.161 | 3.23 × 10−4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peron, M.; Torgersen, J.; Berto, F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals 2017, 7, 252. https://doi.org/10.3390/met7070252
Peron M, Torgersen J, Berto F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals. 2017; 7(7):252. https://doi.org/10.3390/met7070252
Chicago/Turabian StylePeron, Mirco, Jan Torgersen, and Filippo Berto. 2017. "Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure" Metals 7, no. 7: 252. https://doi.org/10.3390/met7070252






