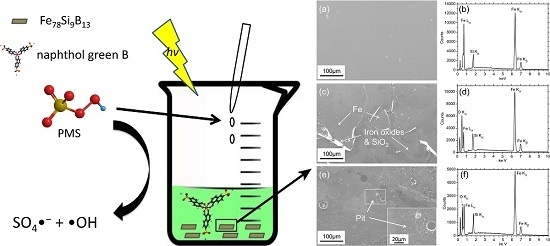
Excellent Performance of Fe78Si9B13 Metallic Glass for Activating Peroxymonosulfate in Degradation of Naphthol Green B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Methods
3. Results and Discussion
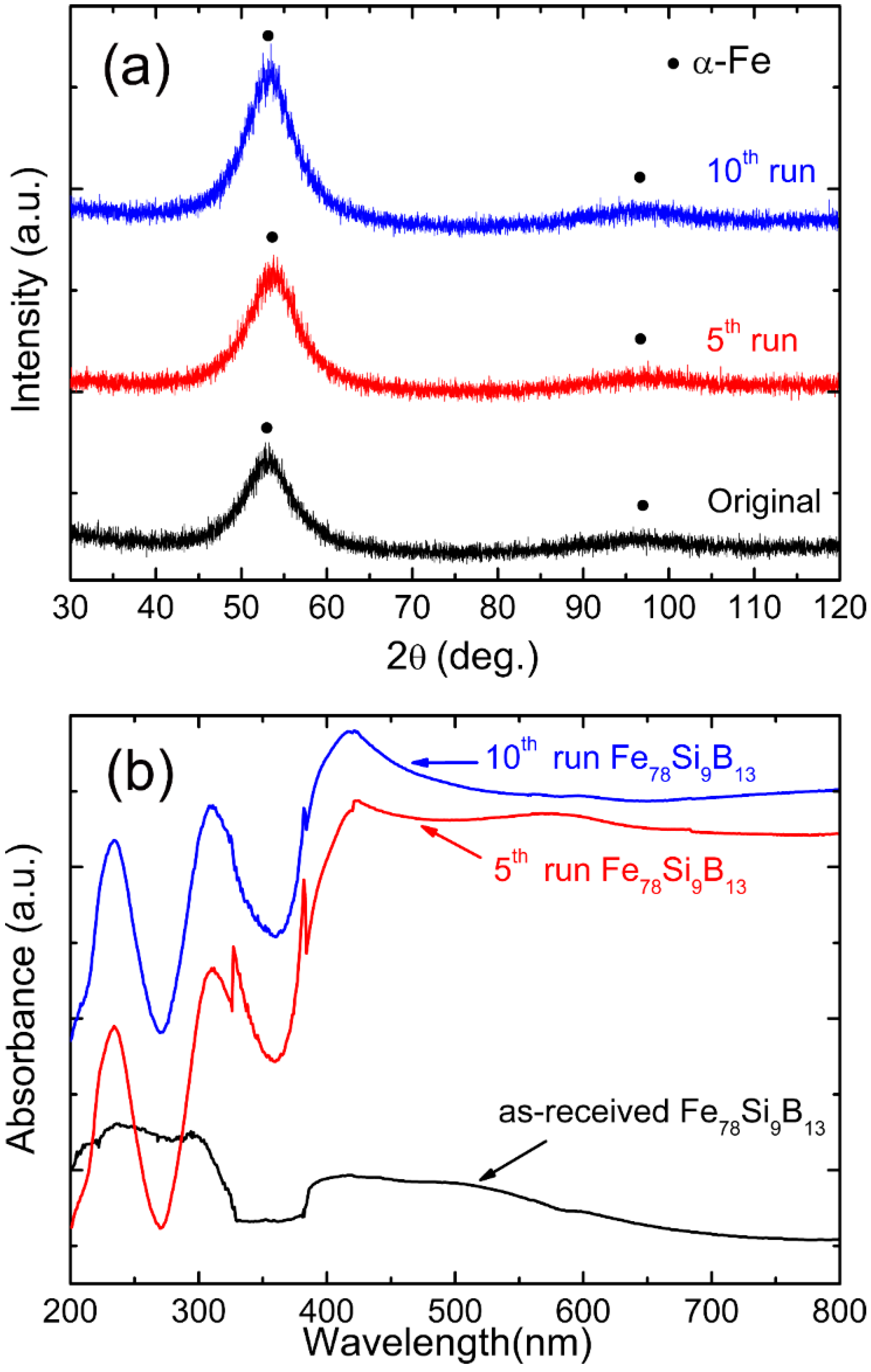
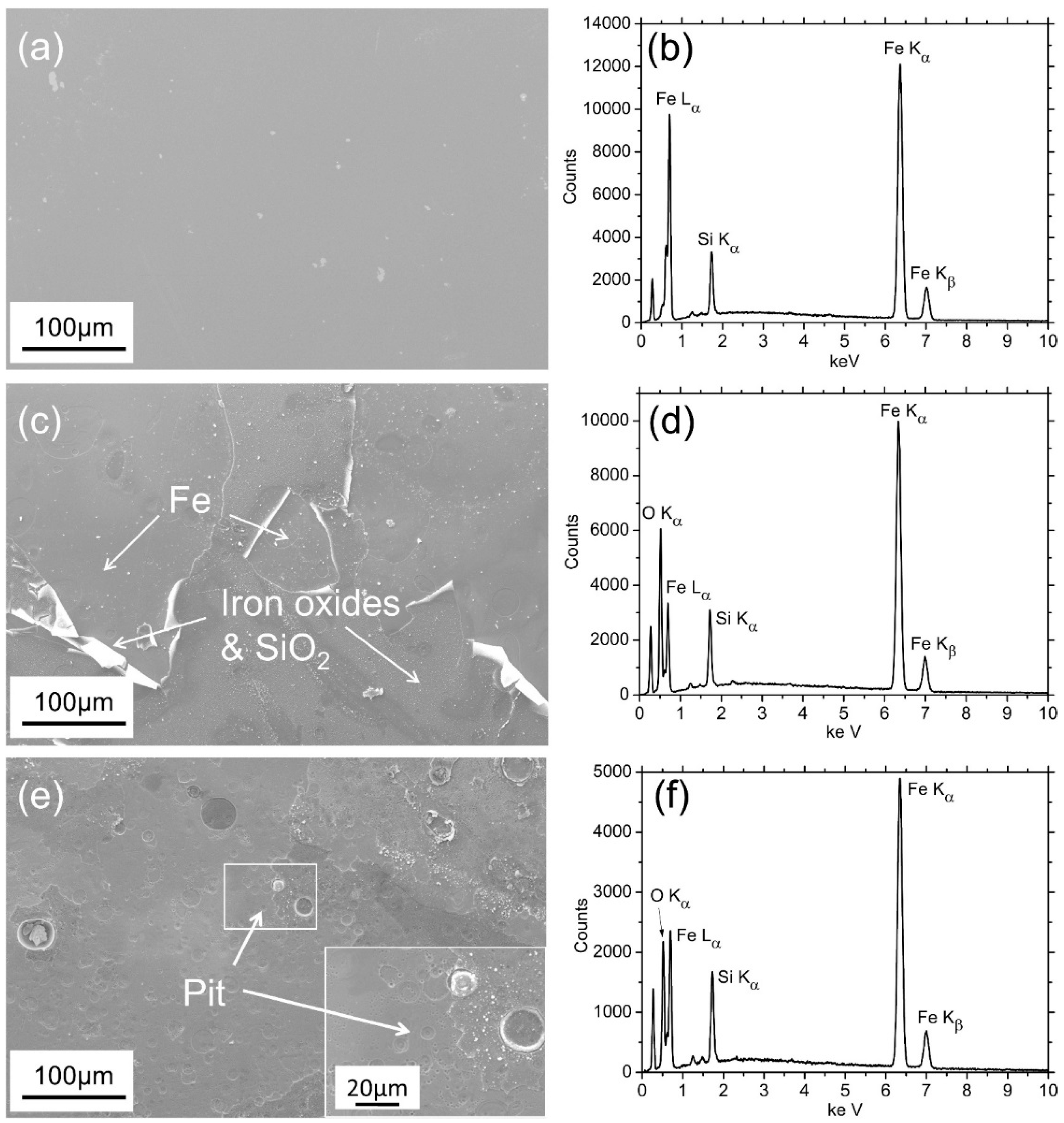
3.1. Characterizations of Fe78Si9B13
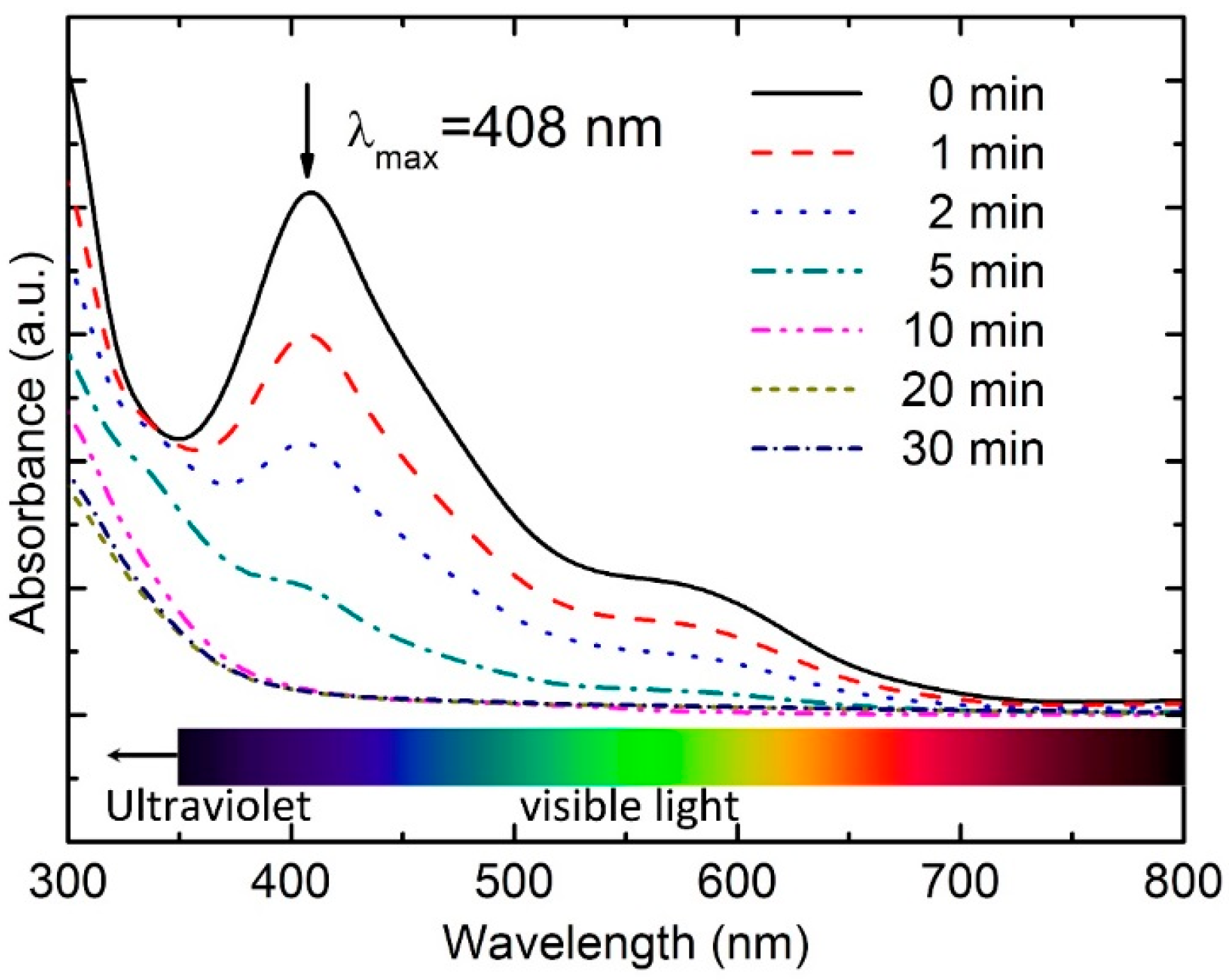
3.2. UV–Vis Spectra
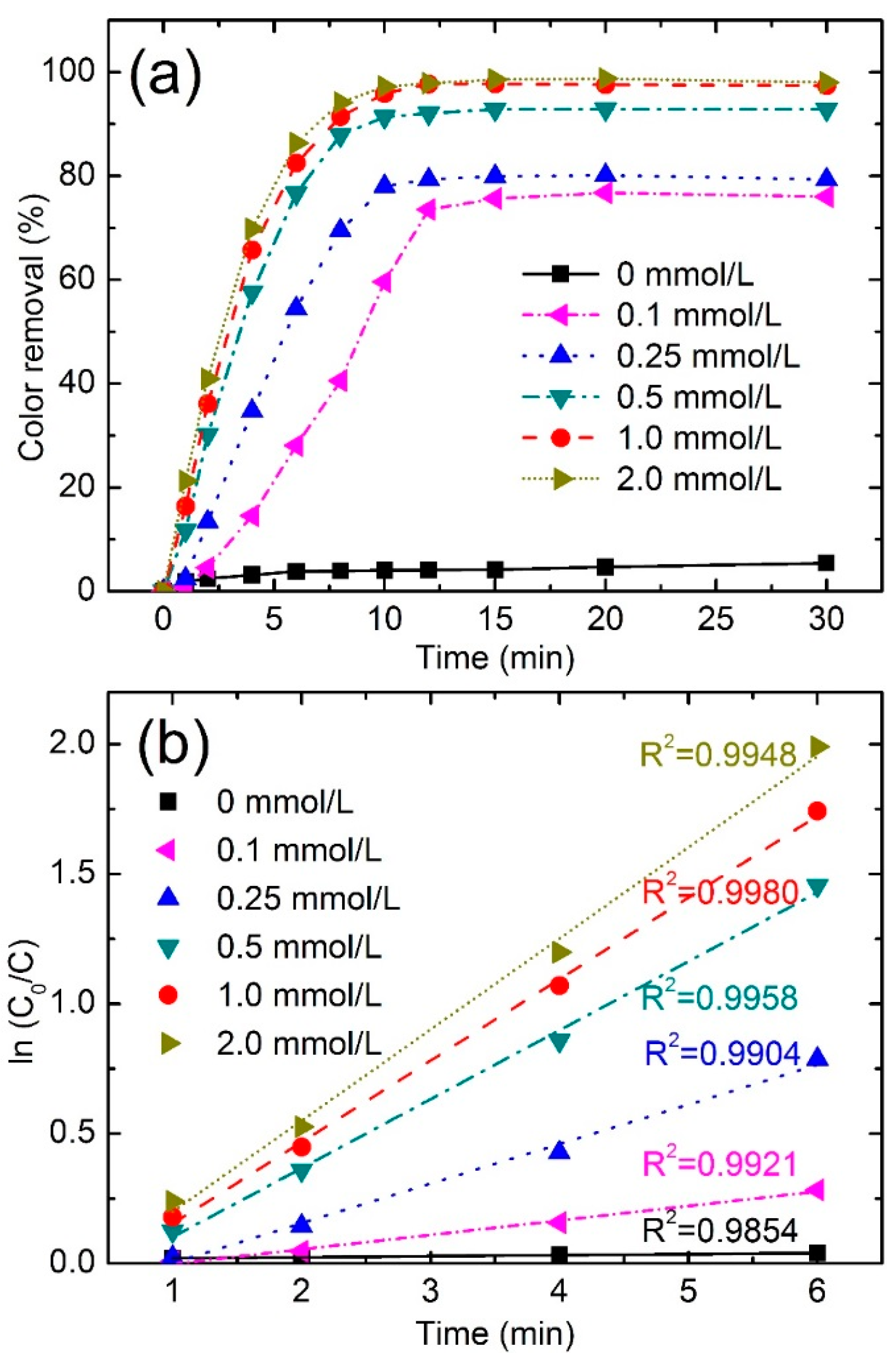
3.3. Single Parameter Effect on Dye Degradation
3.3.1. Effect of PMS Concentration
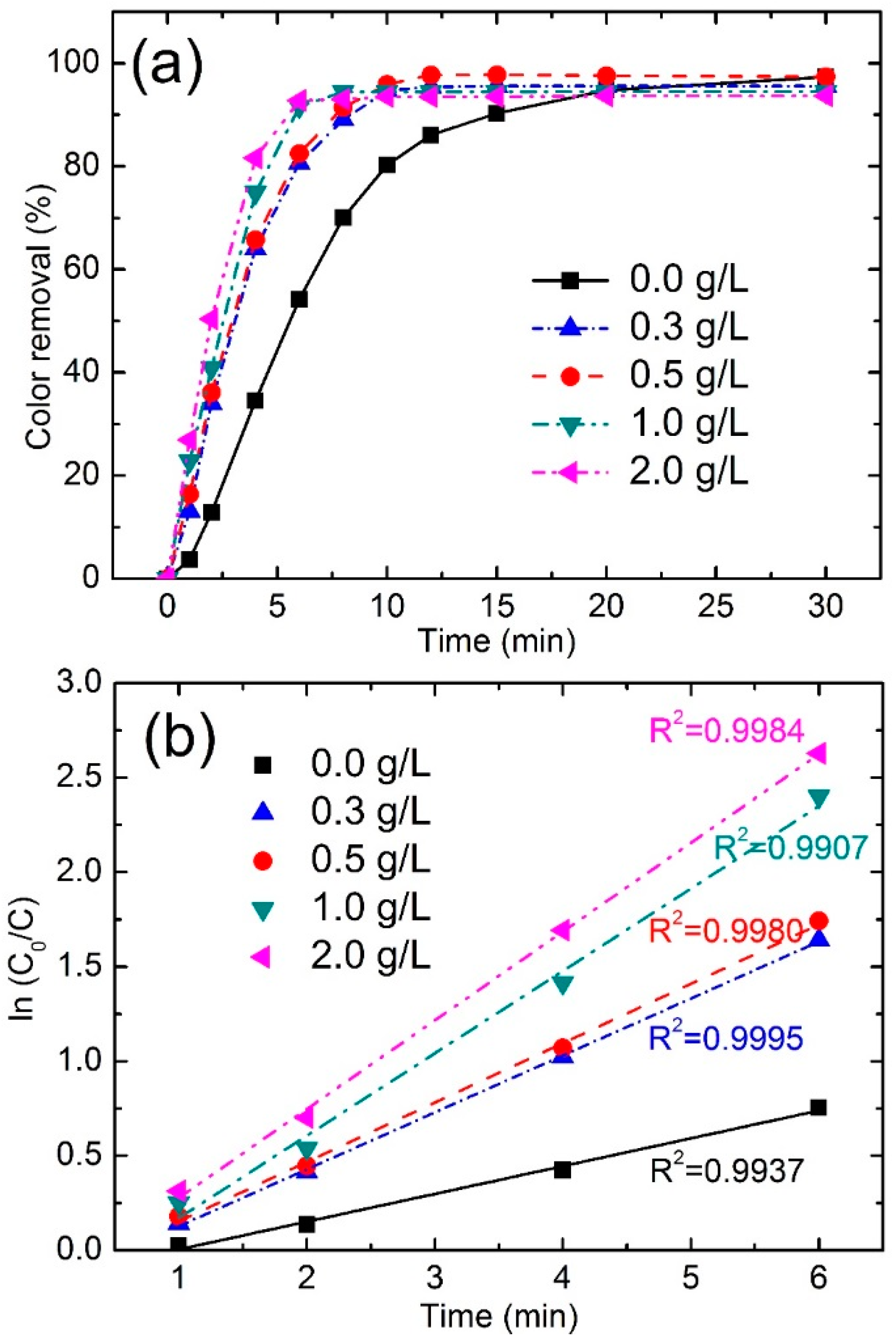
3.3.2. Effect of Fe78Si9B13 Dosage
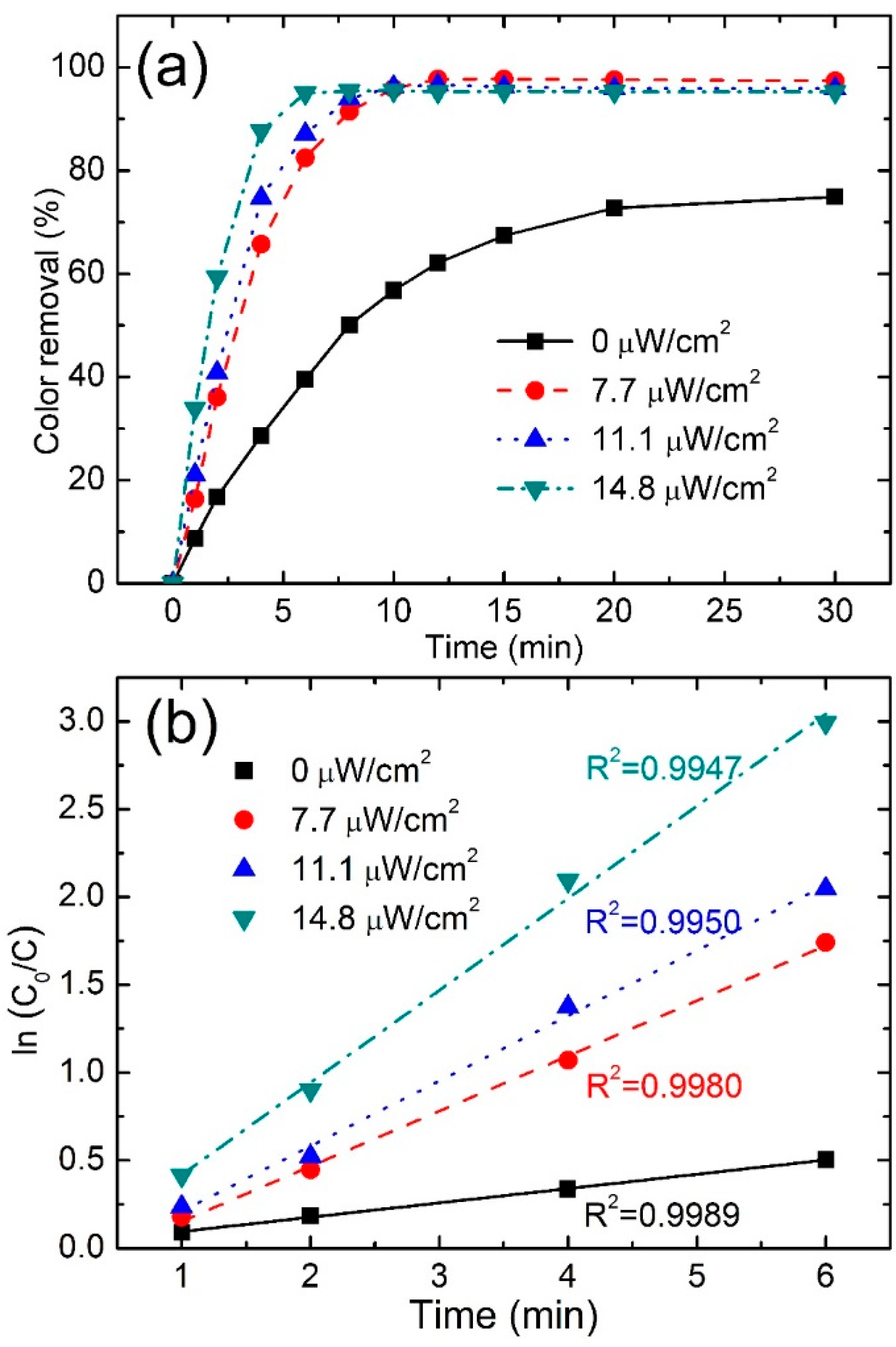
3.3.3. Effect of Irradiation Intensity
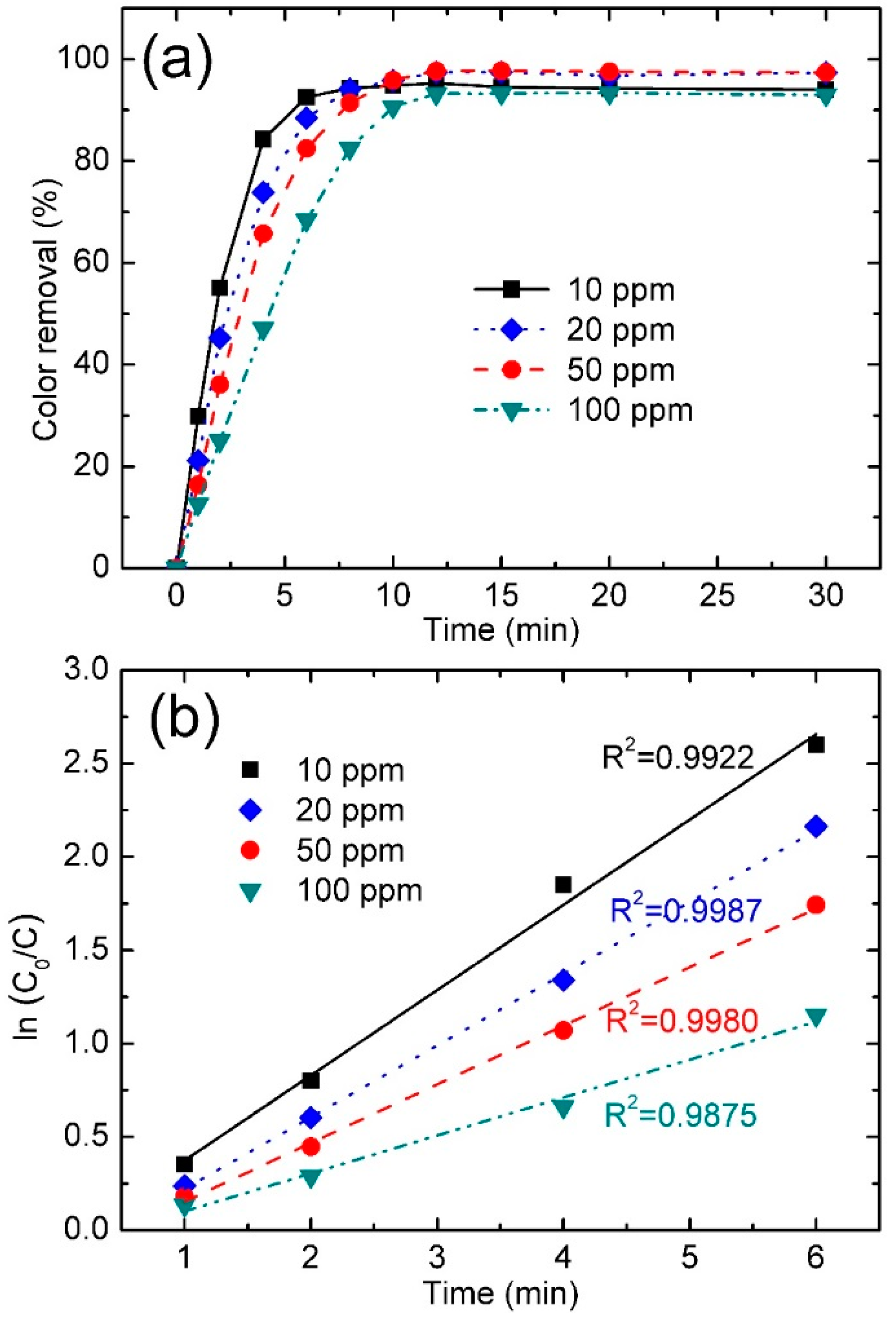
3.3.4. Effect of Dye Concentration
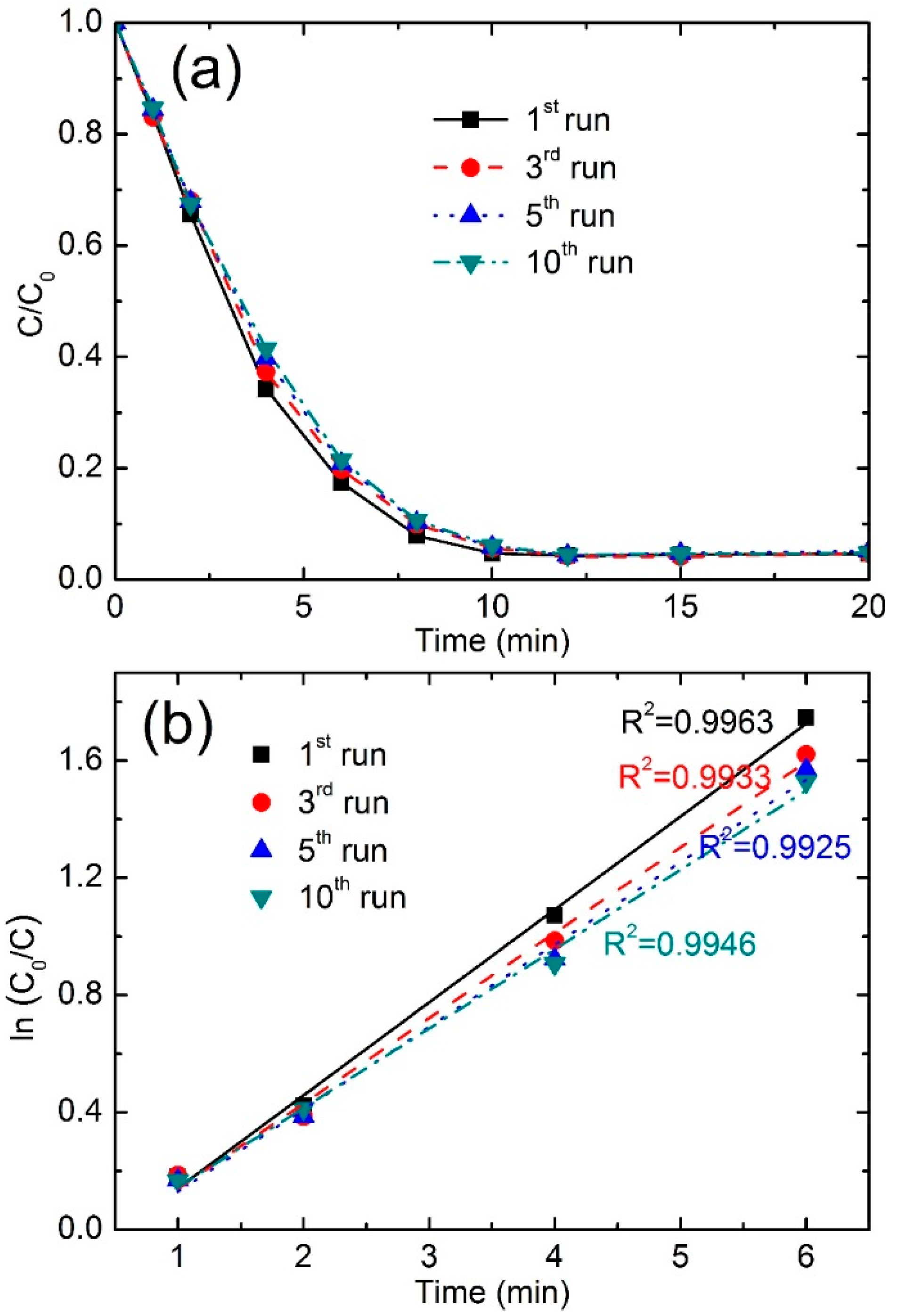
3.3.5. Stability and Reusability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zberg, B.; Uggowitzer, P.J.; Loffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Henao, J.; Concustell, A.; Cano, I.G.; Dosta, S.; Cinca, N.; Guilemany, J.M.; Suhonen, T. Novel Al-based metallic glass coatings by cold gas spray. Mater. Des. 2016, 94, 253–261. [Google Scholar] [CrossRef]
- Mariano, N.A.; Souza, C.A.C.; May, J.E.; Kuri, S.E. Influence of Nb content on the corrosion resistance and saturation magnetic density of FeCuNbSiB alloys. Mater. Sci. Eng. A 2003, 354, 1–5. [Google Scholar] [CrossRef]
- Ghidelli, M.; Idrissi, H.; Gravier, S.; Blandin, J.J.; Raskin, J.P.; Schryvers, D.; Pardoen, T. Homogeneous flow and size dependent mechanical behavior in highly ductile Zr65Ni35 metallic glass films. Acta Mater. 2017, 131, 246–259. [Google Scholar] [CrossRef]
- Ghidelli, M.; Gravier, S.; Blandin, J.J.; Raskin, J.P.; Lani, F.; Pardoen, T. Size-dependent failure mechanisms in ZrNi thin metallic glass films. Scr. Mater. 2014, 89, 9–12. [Google Scholar] [CrossRef]
- Ghidelli, M.; Gravier, S.; Blandin, J.J.; Djemia, P.; Mompiou, F.; Abadias, G.; Raskin, J.P.; Pardoen, T. Extrinsic mechanical size effects in thin ZrNi metallic glass films. Acta Mater. 2015, 90, 232–241. [Google Scholar] [CrossRef]
- Ghidelli, M.; Gravier, S.; Blandin, J.J.; Pardoen, T.; Raskin, J.P.; Mompiou, F. Compositional-induced structural change in ZrxNi100−x thin film metallic glasses. J. Alloy. Compd. 2014, 615, S348–S351. [Google Scholar] [CrossRef]
- Ghidelli, M.; Volland, A.; Blandin, J.J.; Pardoen, T.; Raskin, J.P.; Mompiou, F.; Djemia, P.; Gravier, S. Exploring the mechanical size effects in Zr65Ni35 thin film metallic glasses. J. Alloy. Compd. 2014, 615, S90–S92. [Google Scholar] [CrossRef]
- Qin, X.D.; Zhu, Z.W.; Liu, G.; Fu, H.M.; Zhang, H.W.; Wang, A.M.; Li, H.; Zhang, H.F. Ultrafast degradation of azo dyes catalyzed by cobalt-based metallic glass. Sci. Rep. 2015, 5, 18226. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Excellent capability in degrading azo dyes by MgZn-based metallic glass powders. Sci. Rep. 2012, 2, 418. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.Q.; Li, H.; Yang, H.; Huo, J.; Wang, J.; Chang, C.; Wang, X.; Li, R.W.; Wang, G. Fast decolorization of azo dyes in both alkaline and acidic solutions by Al-based metallic glasses. J. Alloy. Compd. 2017, 701, 759–767. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, W.C.; Wang, W.M.; Habibi, D.; Zhang, L.C. Amorphous Fe78Si9B13 alloy: An efficient and reusable photo-enhanced Fenton-like catalyst in degradation of cibacron brilliant red 3B-A dye under UV–vis light. Appl. Catal. B Environ. 2016, 192, 46–56. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Liu, X.; Chen, S.Q.; Yao, K.F. Insight into the high reactivity of commercial Fe-Si-B amorphous zero-valent iron in degrading azo dye solutions. RSC Adv. 2015, 5, 34032–34039. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Ma, H.; Jiang, K.; Chang, C.; Wang, J.Q.; Song, Z.; Wang, X.; Li, R.W. Improved corrosion resistance of novel Fe-based amorphous alloys. Mater. Des. 2016, 95, 225–230. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, X.H.; Chan, K.C.; Liu, L.; Li, T. Fe-based metallic glass catalyst with nanoporous surface for azo dye degradation. Chemosphere 2017, 174, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q.; Louzguine Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Rapid degradation of azo dye by Fe-based metallic glass powder. Adv. Funct. Mater. 2012, 22, 2567–2570. [Google Scholar] [CrossRef]
- Yang, J.F.; Bian, X.F.; Yuan, M.L.; Bai, Y.W.; Liu, Y.; Fan, J.P.; Lu, X.Q.; Song, K.K. Excellent degradation performance of azo dye by metallic glass/titanium dioxide composite powders. J. Sol-Gel Sci. Technol. 2013, 67, 362–367. [Google Scholar] [CrossRef]
- Wang, P.P.; Wang, J.Q.; Huo, J.T.; Xu, W.; Wang, X.M.; Wang, G. Fast degradation of azo dye by nanocrystallized Fe-based alloys. Sci. China Phys. Mech. Astron. 2017, 60, 076112. [Google Scholar] [CrossRef]
- Chen, S.Q.; Yang, G.N.; Luo, S.T.; Yin, S.J.; Jia, J.L.; Li, Z.; Gao, S.H.; Shao, Y.; Yao, K.F. Unexpected high performance of Fe-based nanocrystallized ribbons for azo dye decomposition. J. Mater. Chem. A 2017, 5, 14230–14240. [Google Scholar] [CrossRef]
- Chen, S.Q.; Chen, N.; Cheng, M.T.; Luo, S.T.; Shao, Y.; Yao, K.F. Multi-phase nanocrystallization induced fast degradation of methyl orange by annealing Fe-based amorphous ribbons. Intermetallics 2017, 90, 30–35. [Google Scholar] [CrossRef]
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 2016, 6, 21947. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, J.L.; Zha, M.Q.; Shek, C.H. Synthesis of an Fe rich amorphous structure with a catalytic effect to rapidly decolorize azo dye at room temperature. ACS Appl. Mater. Interfaces 2014, 6, 5500–5505. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Duan, X.; Zhang, W.; Wang, W.; Sun, H.; Wang, S.; Zhang, L.C. Ultra-sustainable Fe78Si9B13 metallic glass as a catalyst for activation of persulfate on methylene blue degradation under UV–vis light. Sci. Rep. 2016, 6, 38520. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B Environ. 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Liu, F.J.; Yao, K.F.; Ding, H.Y. Fe-based glassy alloys with high iron content and high saturation magnetization. Intermetallics 2011, 19, 1674–1677. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Wang, W.M.; Zhang, L.C. Rapid malachite green degradation using Fe73.5Si13.5B9Cu1Nb3 metallic glass for activation of persulfate under UV–vis light. Mater. Des. 2017, 119, 244–253. [Google Scholar] [CrossRef]
- Mousavi Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative effects of ZnO nanoparticles, ZnO bulk particles, and Zn2+ on brassica napus after long-term exposure: Changes in growth, biochemical compounds, antioxidant enzyme activities, and Zn bioaccumulation. Water Air Soil Pollut. 2015, 226, 364. [Google Scholar] [CrossRef]
- Jia, Z.; La, L.B.T.; Zhang, W.C.; Liang, S.X.; Jiang, B.; Xie, S.K.; Habibi, D.; Zhang, L.C. Strong enhancement on dye photocatalytic degradation by ball-milled TiO2: A study of cationic and anionic dyes. J. Mater. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Miranda, B.S.; Jacintho, G.V.M. Interaction of indigo carmine dye with silica modified with humic acids at solid/liquid interface. Surf. Sci. 2003, 542, 276–282. [Google Scholar] [CrossRef]
- Karcher, S.; Kornmüller, A.; Jekel, M. Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res. 2002, 36, 4717–4724. [Google Scholar] [CrossRef]
- Silva, G.C.; Ciminelli, V.S.T.; Ferreira, A.M.; Pissolati, N.C.; Paiva, P.R.P.; López, J.L. A facile synthesis of Mn3O4/Fe3O4 superparamagnetic nanocomposites by chemical precipitation: Characterization and application in dye degradation. Mater. Res. Bull. 2014, 49, 544–551. [Google Scholar] [CrossRef]
- Jia, Z.; Miao, J.; Lu, H.B.; Habibi, D.; Zhang, W.C.; Zhang, L.C. Photocatalytic degradation and absorption kinetics of cibacron brilliant yellow 3G-P by nanosized ZnO catalyst under simulated solar light. J. Taiwan Inst. Chem. Eng. 2016, 60, 267–274. [Google Scholar] [CrossRef]
- Miao, J.; Jia, Z.; Lu, H.B.; Habibi, D.; Zhang, L.C. Heterogeneous photocatalytic degradation of mordant black 11 with ZnO nanoparticles under UV–Vis light. J. Taiwan Inst. Chem. Eng. 2014, 45, 1636–1641. [Google Scholar] [CrossRef]
- Jin, X.; Peldszus, S.; Huck, P.M. Reaction kinetics of selected micropollutants in ozonation and advanced oxidation processes. Water Res. 2012, 46, 6519–6530. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Ma, C.W. Quantitative prediction of direct and indirect dye ozonation kinetics. Water Res. 2000, 34, 3153–3160. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Alvaro, M.; Garcia, H. Metal nanoparticles as heterogeneous Fenton catalysts. ChemSusChem 2012, 5, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Liang, S.X.; Zhang, W.C.; Wang, W.M.; Yang, C.; Zhang, L.C. Heterogeneous photo Fenton-like degradation of cibacron brilliant red 3B-A dye using amorphous Fe78Si9B13 and Fe73.5Si13.5B9Cu1Nb3 alloys: The influence of adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 128–136. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.M.; Dionysiou, D.D.; Kumar, V. Oxidative removal of Bisphenol A by UV–C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J. 2015, 276, 193–204. [Google Scholar] [CrossRef]
- Attallah, M.F.; Ahmed, I.M.; Hamed, M.M. Treatment of industrial wastewater containing congo red and naphthol green B using low-cost adsorbent. Environ. Sci. Pollut. Res. 2013, 20, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Riahi-Madvaar, R.; Taher, M.A.; Fazelirad, H. Synthesis and characterization of magnetic halloysite-iron oxide nanocomposite and its application for naphthol green B removal. Appl. Clay Sci. 2017, 137, 101–106. [Google Scholar] [CrossRef]
- Saber, O.; El-Brolossy, T.A.; Al Jaafari, A.A. Improvement of photocatalytic degradation of naphthol green B under solar light using aluminum doping of Zinc oxide nanoparticles. Water Air Soil Pollut. 2012, 223, 4615–4626. [Google Scholar] [CrossRef]
- Ameta, R.; Punjabi, P.B.; Ameta, S.C. Photodegradation of Naphthol green B in the presence of semiconducting antimony trisulphide. J. Serbian Chem. Soc. 2011, 76, 1049–1055. [Google Scholar] [CrossRef]
- Kunkely, H.; Vogler, A. Photolysis of naphthol green B in aqueous solution. Photoreduction of Fe(III) induced by ligand-to-metal charge transfer excitation. Z. Naturforsch. B 2003, 58, 922–924. [Google Scholar]
- Kumar, A.; Paliwal, M.; Ameta, R.; Ameta, S.C. A novel route for waste water treatment: Photo-assisted fenton degradation of naphthol green B. Collect. Czechoslov. Chem. Commun. 2008, 73, 679–689. [Google Scholar] [CrossRef]
- Calin, M.; Zhang, L.C.; Eckert, J. Tailoring of microstructure and mechanical properties of a Ti-based bulk metallic glass-forming alloy. Scr. Mater. 2007, 57, 1101–1104. [Google Scholar] [CrossRef]
- Zhang, L.C.; Shen, Z.Q.; Xu, J. Glass formation in a (Ti,Zr,Hf)-(Cu,Ni,Ag)-Al high-order alloy system by mechanical alloying. J. Mater. Res. 2003, 18, 2141–2149. [Google Scholar] [CrossRef]
- Zhang, L.C.; Calin, M.; Branzei, M.; Schultz, L.; Eckert, J. Phase stability and consolidation of glassy/nanostructured Al85Ni9Nd4Co2 alloys. J. Mater. Res. 2007, 22, 1145–1155. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Yao, K.F. Rapid decomposition of direct blue 6 in neutral solution by Fe-B amorphous alloys. RSC Adv. 2015, 5, 6215–6221. [Google Scholar] [CrossRef]
- Zhang, L.C.; Shen, Z.Q.; Xu, J. Mechanically milling-induced amorphization in Sn-containing Ti-based multicomponent alloy systems. Mater. Sci. Eng. A 2005, 394, 204–209. [Google Scholar] [CrossRef]
- Zhang, L.C.; Kim, K.B.; Yu, P.; Zhang, W.Y.; Kunz, U.; Eckert, J. Amorphization in mechanically alloyed (Ti, Zr, Nb)–(Cu, Ni)–Al equiatomic alloys. J. Alloy. Compd. 2007, 428, 157–163. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, J. Glass-forming ability of melt-spun multicomponent (Ti, Zr, Hf)-(Cu, Ni, Co)-Al alloys with equiatomic substitution. J. Non-Cryst. Solids 2004, 347, 166–172. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, J.; Ma, E. Mechanically alloyed amorphous Ti50(Cu0.45Ni0.55)44-xAlxSi4B2 alloys with supercooled liquid region. J. Mater. Res. 2002, 17, 1743–1749. [Google Scholar] [CrossRef]
- Ma, Y.; Rheingans, B.; Liu, F.; Mittemeijer, E.J. Isochronal crystallization kinetics of Fe40Ni40B20 amorphous alloy. J. Mater. Sci. 2013, 48, 5596–5606. [Google Scholar] [CrossRef]
- Huang, C.M.; Pan, G.T.; Li, Y.C.M.; Li, M.H.; Yang, T.C.K. Crystalline phases and photocatalytic activities of hydrothermal synthesis Ag3VO4 and Ag4V2O7 under visible light irradiation. Appl. Catal. A Gen. 2009, 358, 164–172. [Google Scholar] [CrossRef]
- Perezramirez, J. Active iron sites associated with the reaction mechanism of N2O conversions over steam-activated FeMFI zeolites. J. Catal. 2004, 227, 512–522. [Google Scholar] [CrossRef]
- Sun, K. Chemistry of N2O decomposition on active sites with different nature: Effect of high-temperature treatment of Fe/ZSM-5. J. Catal. 2006, 238, 186–195. [Google Scholar] [CrossRef]
- Koli, P. Study of enhanced photogalvanic effect of naphthol green B in natural sunlight. J. Power Sources 2015, 285, 310–317. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, G.; Liu, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Nano-Fe0 encapsulated in microcarbon spheres: Synthesis, characterization, and environmental applications. ACS Appl. Mater. Interfaces 2012, 4, 6235–6241. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Bian, X.; Wang, P.; Luo, G. Application of Fe-based metallic glasses in wastewater treatment. Mater. Sci. Eng. B 2012, 177, 92–95. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Si, J.J.; Song, J.G.; Yang, Q.; Hui, X.D. Synthesis of Mg-Zn-Ca metallic glasses by gas-atomization and their excellent capability in degrading azo dyes. Mater. Sci. Eng. B 2014, 181, 46–55. [Google Scholar] [CrossRef]
| Structure | Empirical Formula | Molar Mass (g/mol) | λmax (nm) |
|---|---|---|---|
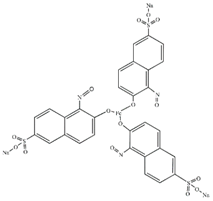 | C30H15FeN3Na3O15S3 | 878.45 | 714 |
| Method | Material | Dosage (g/L) | Light Source | Complete Removal Time (min) | kobs (min−1) | Reference |
|---|---|---|---|---|---|---|
| Photocatalysis | 10% Al-doped ZnO particles | 1.0 | Solar light | 360 | 5.95 × 10−3 | [45] |
| Photocatalysis | Semiconducting Sb2S3 | 2.0 | Visible light | 60 | 5.37 × 10−2 | [46] |
| Photo-Fenton process | FeCl3 | 0.11 | Visible light | >20 | 1.01 × 10−1 | [48] |
| Photo-enhanced sulfate-based oxidation | Fe78Si9B13 ribbons | 0.5 | UV–vis light | 10 | 3.14 × 10−1 | This work |
| PMS Concentration (mmol/L) | Color Removal (%) at 4 min | Color Removal (%) at 8 min | Reaction Kinetics k (min−1) | R2 |
|---|---|---|---|---|
| 0 | 3.2 | 3.9 | 0.004 | 0.9854 |
| 0.1 | 14.56 | 40.6 | 0.0557 | 0.9921 |
| 0.25 | 34.7 | 69.5 | 0.1526 | 0.9904 |
| 0.5 | 57.6 | 87.9 | 0.2654 | 0.9958 |
| 1.0 | 65.7 | 91.4 | 0.3140 | 0.9980 |
| 2.0 | 69.8 | 94.2 | 0.3509 | 0.9948 |
| Dosage (g/L) | Color Removal (%) at 4 min | Color Removal (%) at 8 min | Reaction Kinetics k (min−1) | R2 |
|---|---|---|---|---|
| 0 | 34.5 | 70.0 | 0.1489 | 0.9937 |
| 0.3 | 64.0 | 89.1 | 0.3011 | 0.9995 |
| 0.5 | 65.7 | 91.4 | 0.3140 | 0.9980 |
| 1.0 | 75.7 | 94.5 | 0.4357 | 0.9907 |
| 2.0 | 81.6 | 93.0 | 0.4691 | 0.9984 |
| Irradiation Intensity (μW/cm2) | Color Removal (%) at 4 min | Color Removal (%) at 8 min | Reaction Kinetics k (min−1) | R2 |
|---|---|---|---|---|
| 0.0 | 28.7 | 50.1 | 0.0816 | 0.9989 |
| 7.7 | 65.7 | 91.4 | 0.2736 | 0.9980 |
| 11.1 | 74.7 | 93.9 | 0.314 | 0.9950 |
| 14.8 | 87.7 | 95.4 | 0.5247 | 0.9947 |
| NGB Concentration (ppm) | Color Removal (%) at 4 min | Color Removal (%) at 8 min | Reaction Kinetics k (min−1) | R2 |
|---|---|---|---|---|
| 10 | 84.3 | 94.3 | 0.4566 | 0.9922 |
| 20 | 73.8 | 94.2 | 0.3841 | 0.9987 |
| 50 | 65.7 | 91.4 | 0.3140 | 0.9980 |
| 100 | 48.3 | 82.5 | 0.2032 | 0.9875 |
| Reused Times | Color Removal (%) at 4 min | Color Removal (%) at 8 min | Reaction Kinetics k (min−1) | R2 |
|---|---|---|---|---|
| first run | 65.7 | 92.1 | 0.3170 | 0.9963 |
| third run | 62.7 | 90.1 | 0.2913 | 0.9933 |
| fifth run | 60.2 | 89.8 | 0.2811 | 0.9925 |
| tenth run | 58.6 | 89.4 | 0.2708 | 0.9946 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-F.; Liang, S.-X.; Xi, X.-W.; Jia, Z.; Xie, S.-K.; Lin, H.-C.; Hu, J.-P.; Zhang, L.-C. Excellent Performance of Fe78Si9B13 Metallic Glass for Activating Peroxymonosulfate in Degradation of Naphthol Green B. Metals 2017, 7, 273. https://doi.org/10.3390/met7070273
Li X-F, Liang S-X, Xi X-W, Jia Z, Xie S-K, Lin H-C, Hu J-P, Zhang L-C. Excellent Performance of Fe78Si9B13 Metallic Glass for Activating Peroxymonosulfate in Degradation of Naphthol Green B. Metals. 2017; 7(7):273. https://doi.org/10.3390/met7070273
Chicago/Turabian StyleLi, Xue-Fen, Shun-Xing Liang, Xiao-Wang Xi, Zhe Jia, Shi-Kun Xie, He-Chun Lin, Jun-Ping Hu, and Lai-Chang Zhang. 2017. "Excellent Performance of Fe78Si9B13 Metallic Glass for Activating Peroxymonosulfate in Degradation of Naphthol Green B" Metals 7, no. 7: 273. https://doi.org/10.3390/met7070273