Investigation of Service Life Prediction Models for Metallic Organic Coatings Using Full-Range Frequency EIS Data
Abstract
:1. Introduction
2. Materials and Methods
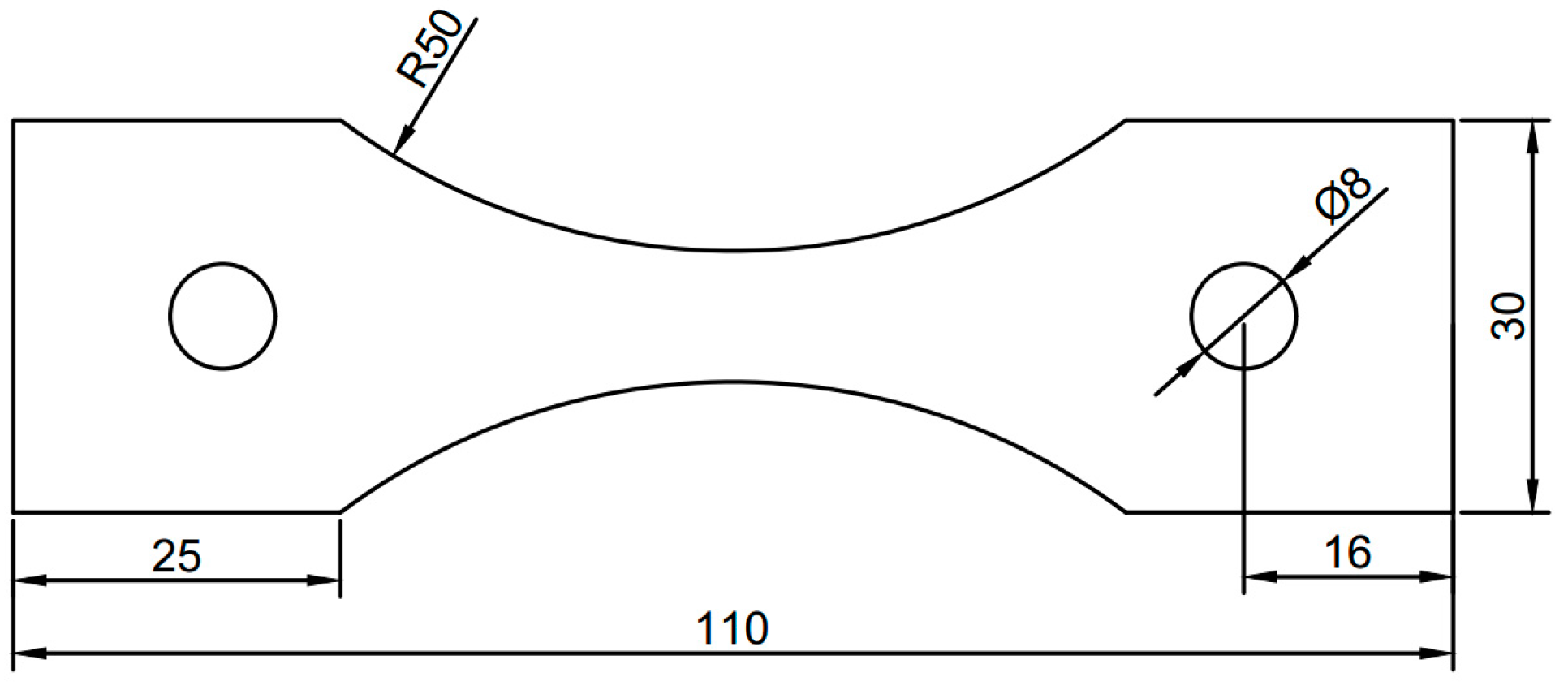
2.1. Preparation of Specimens
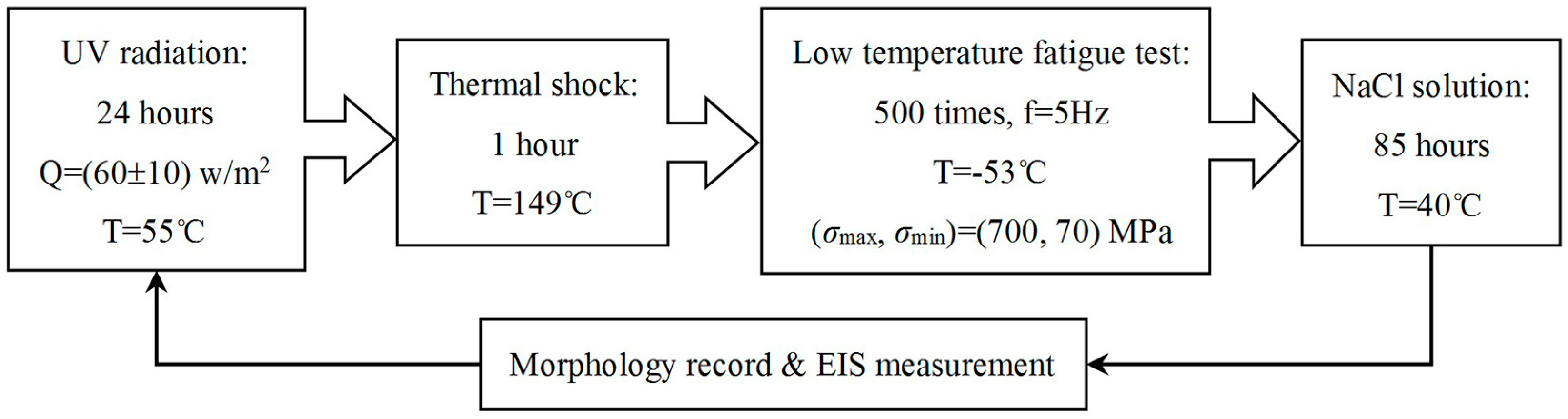
2.2. Accelerated Corrosion Tests
2.3. EIS Measurement
3. Results
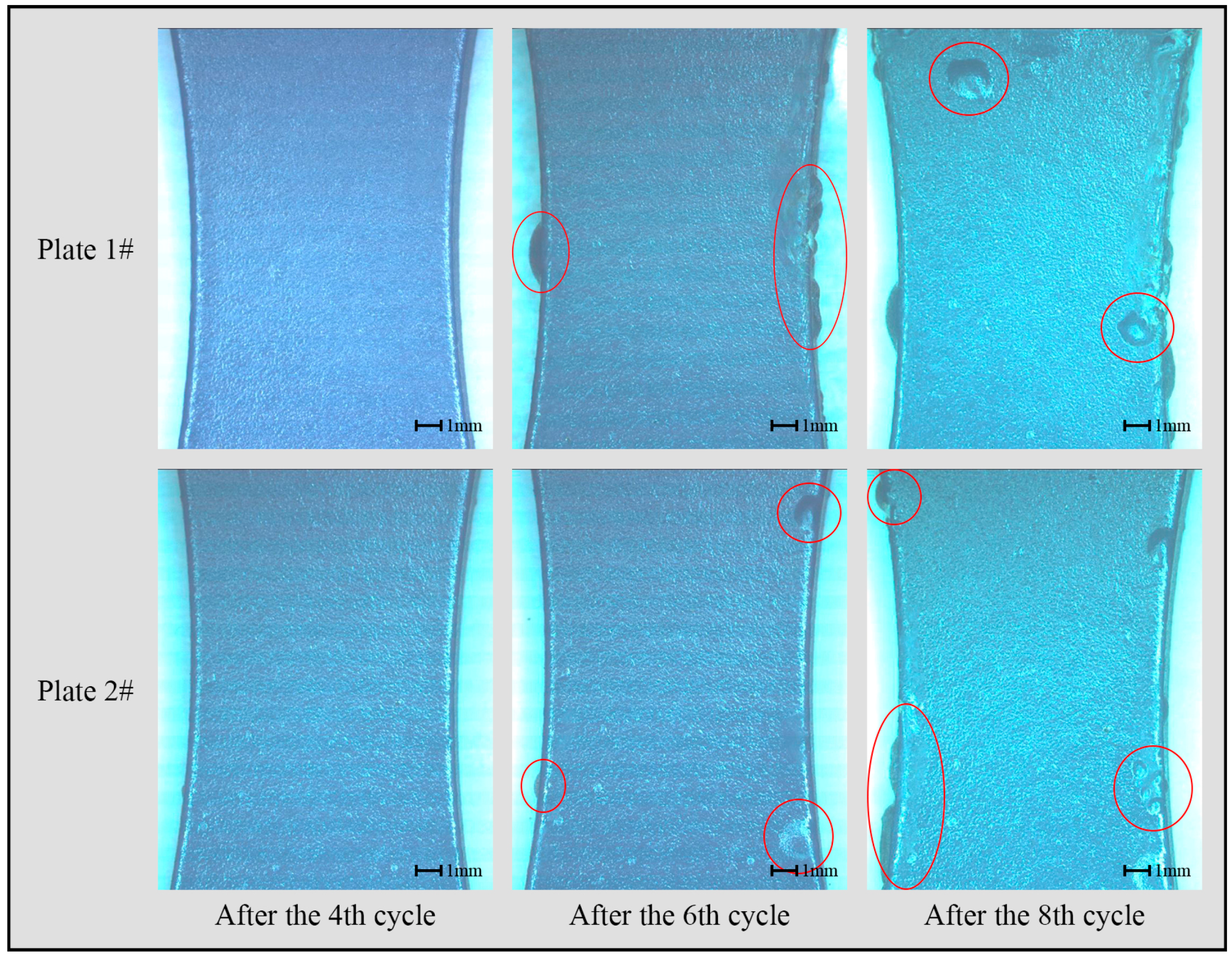
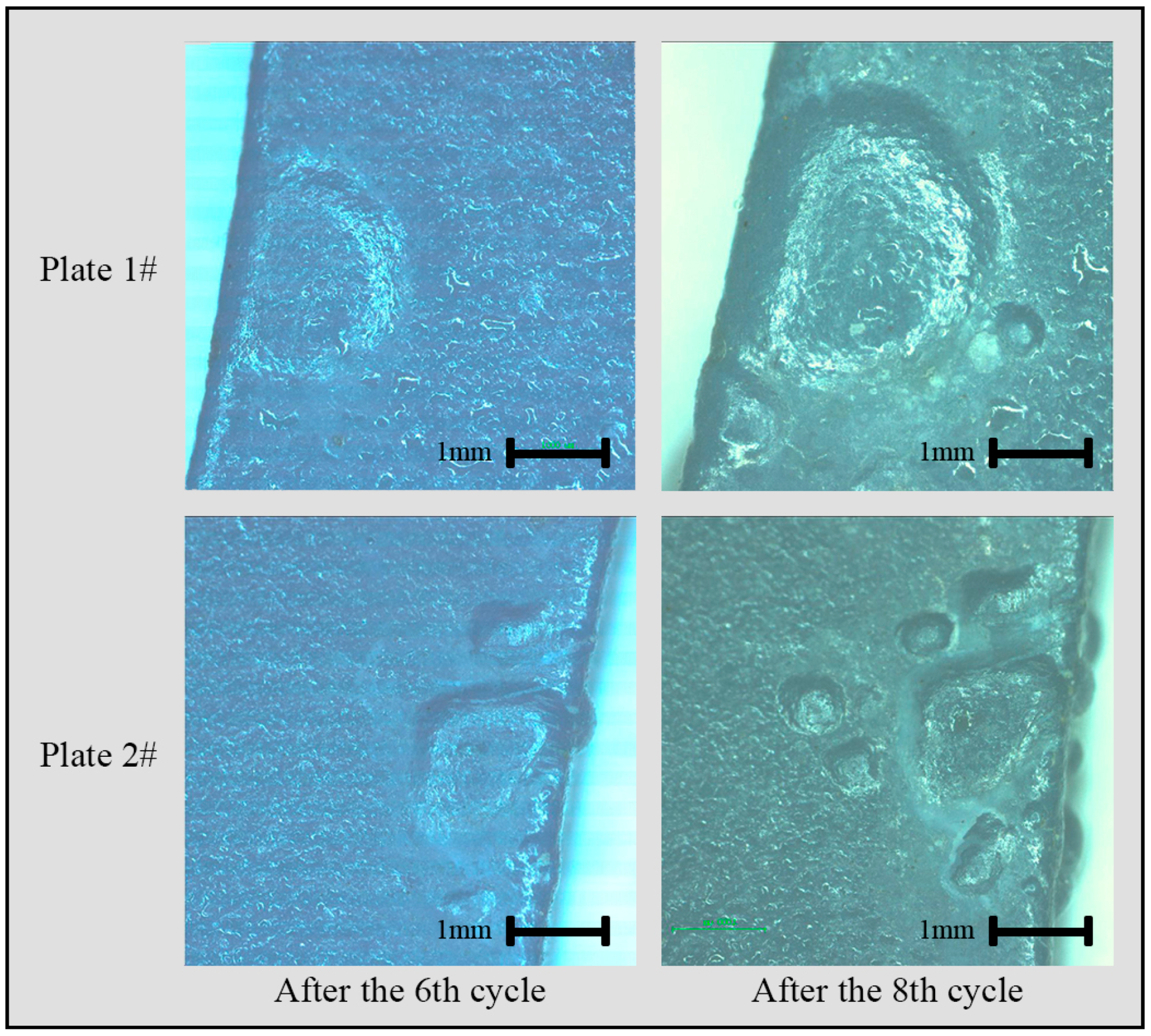
3.1. Analysis of Morphology Change
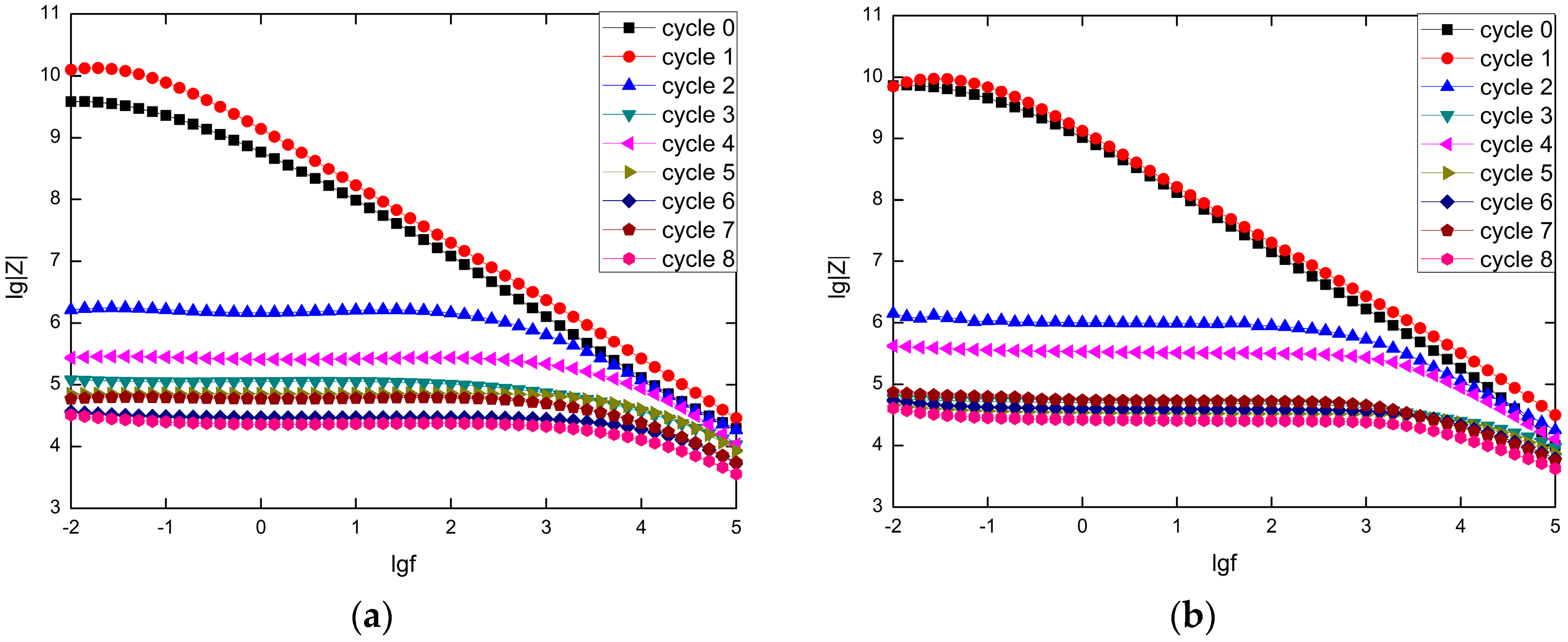
3.2. Analysis of the Electrochemical Impedance Data
4. Life Prediction
4.1. The Brief of Theoretical Life Prediction Model
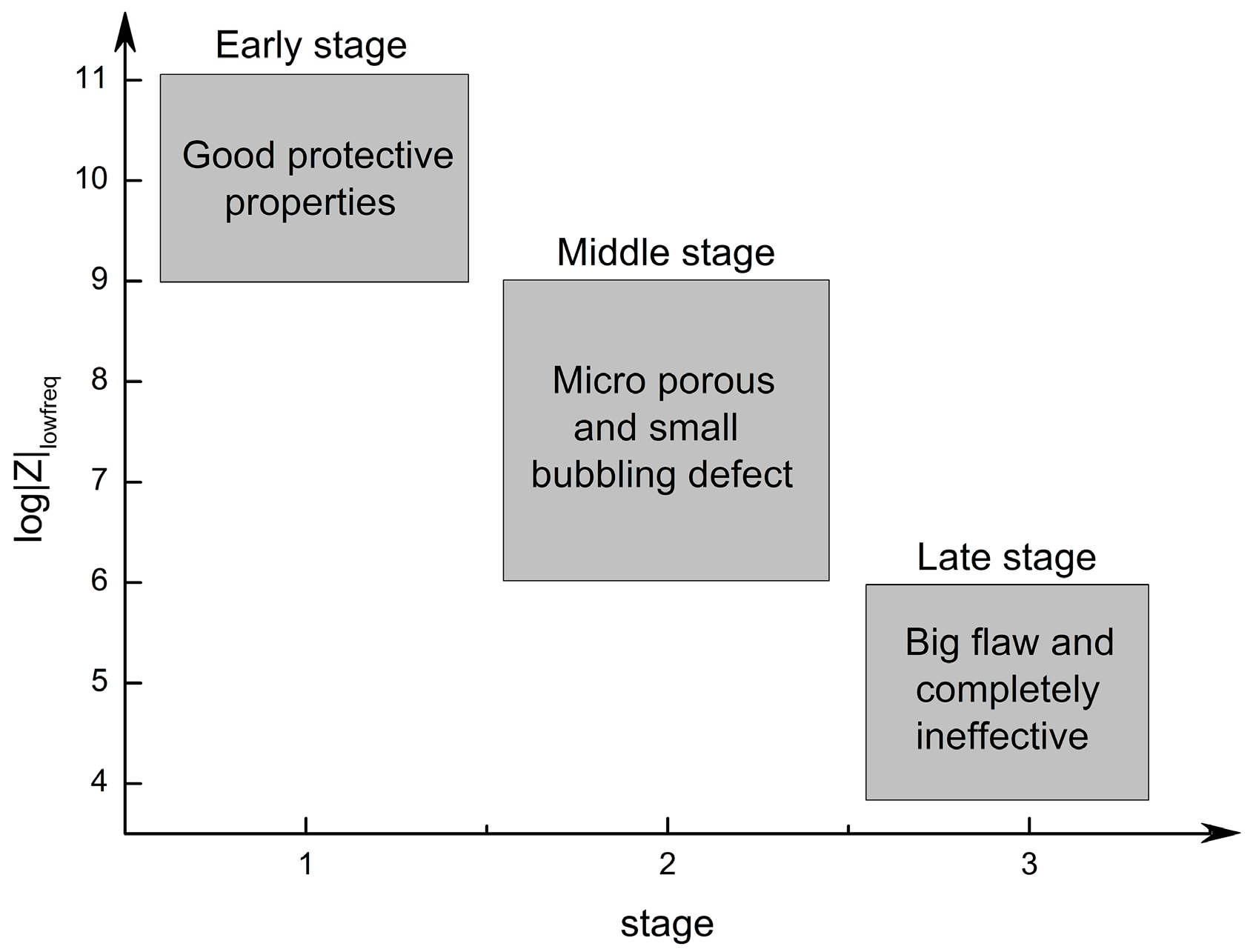
4.2. Prediction from |Z| Data at Low Frequency
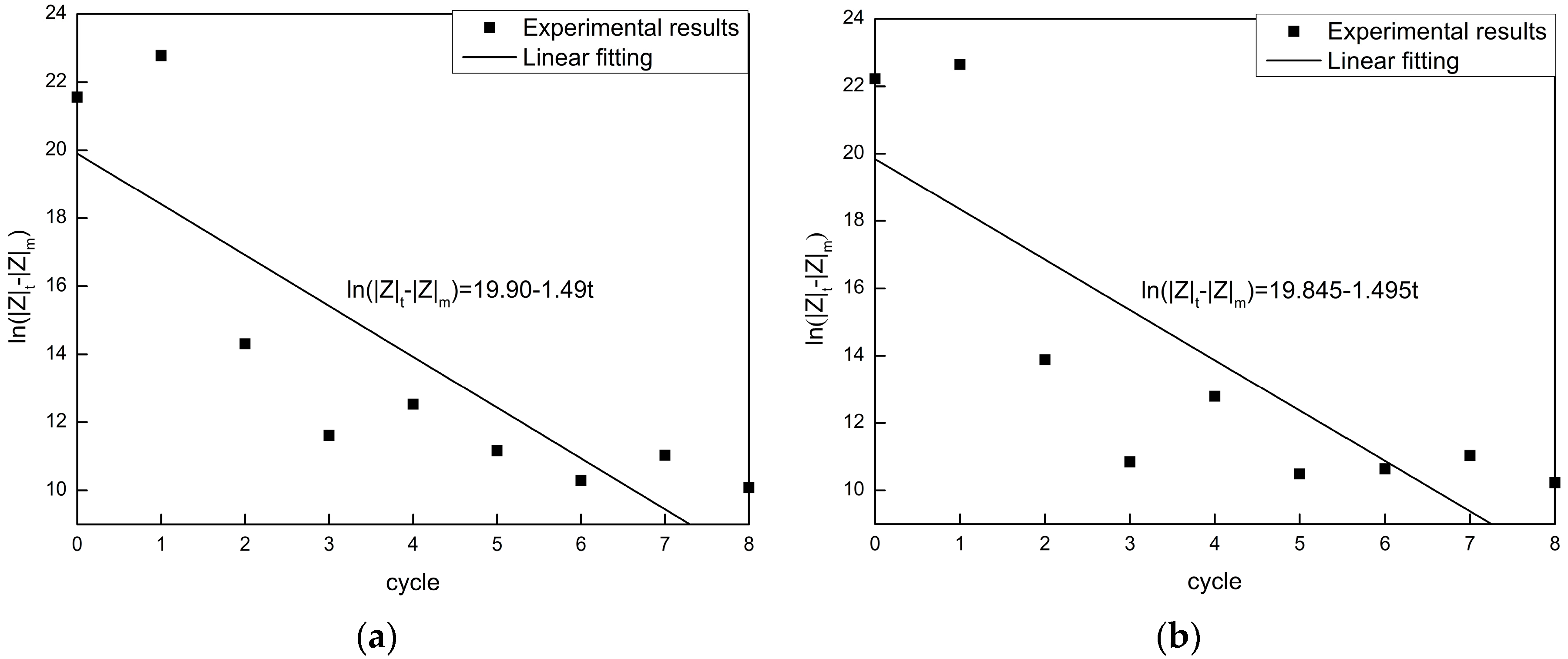
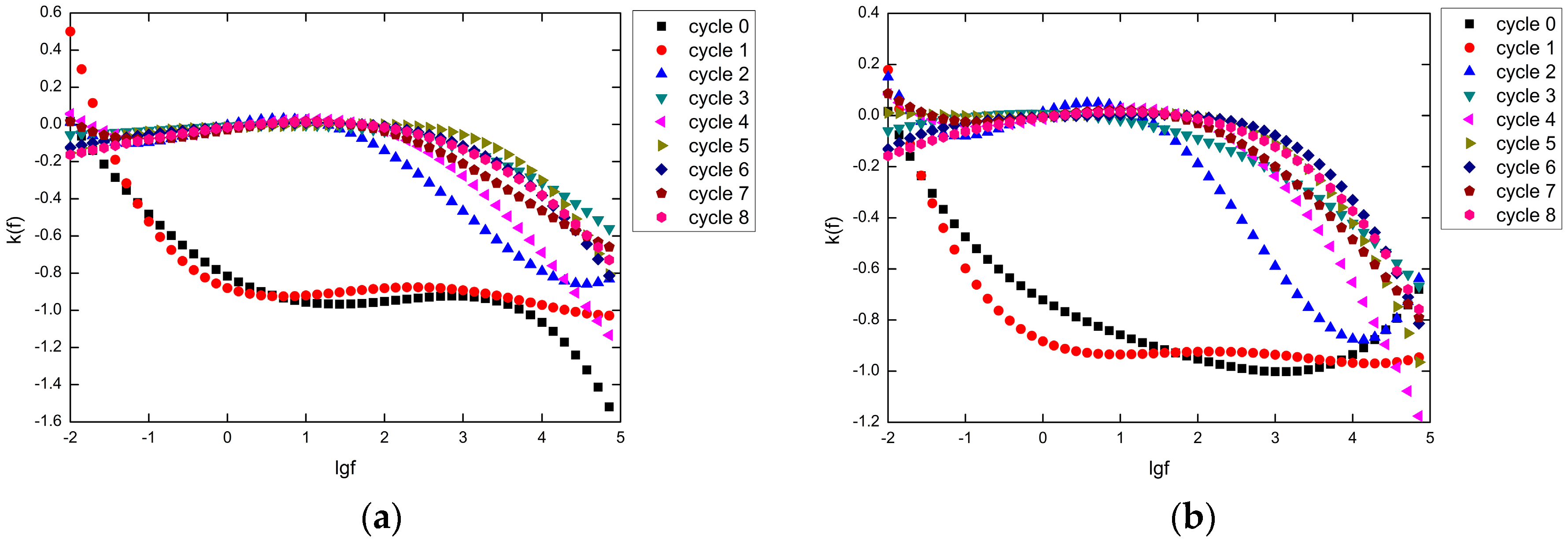
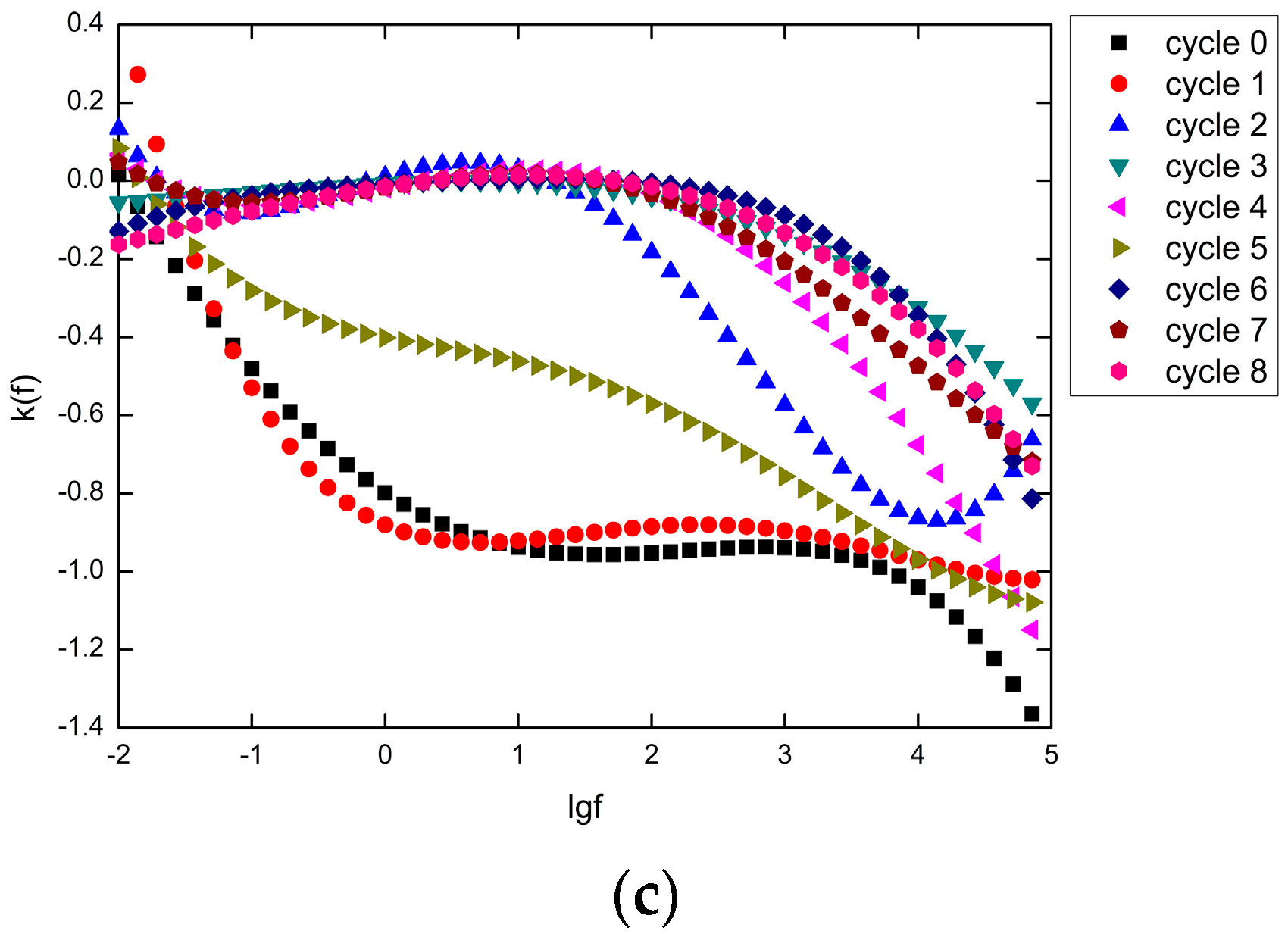
4.3. Degradation Kinetics Model
4.4. Improved Degradation Kinetics Model
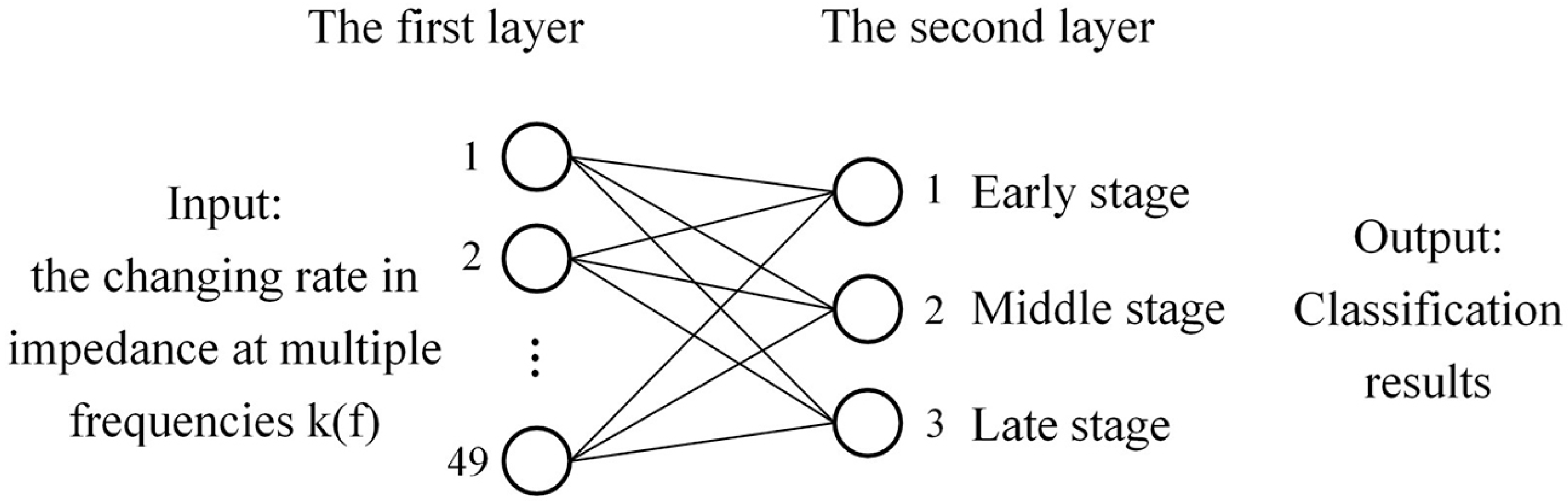
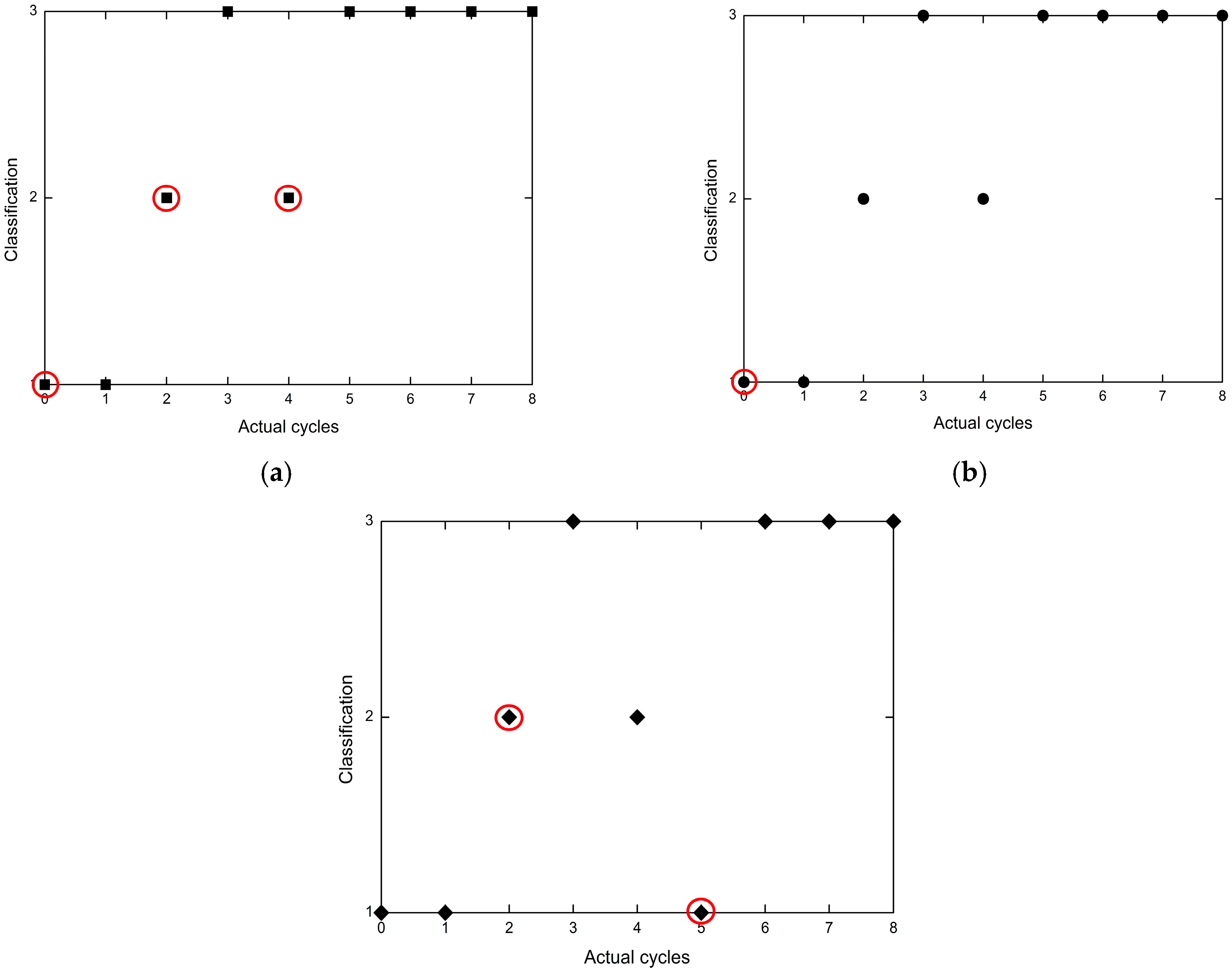
4.5. Neural Network Classification Model Based on Sample Detection
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Croll, S.G.; Hinderliter, B.R.; Liu, S. Statistical approaches for predicting weathering degradation and service life. Prog. Org. Coat. 2006, 55, 75–87. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 2004, 49, 293–301. [Google Scholar] [CrossRef]
- Shreepathi, S.; Guin, A.K.; Naik, S.M.; Vattipalli, M.R. Service life prediction of organic coatings: Electrochemical impedance spectroscopy vs actual service life. J. Coat. Technol. Res. 2011, 8, 191–200. [Google Scholar] [CrossRef]
- Nguyen, A.S.; Musiani, M.; Orazem, M.E.; Pebere, N.; Tribollet, B.; Vivier, V. Impedance study of the influence of chromates on the properties of waterborne coatings deposited on 2024 aluminium alloy. Corros. Sci. 2016, 109, 174–181. [Google Scholar] [CrossRef]
- Cao, C.N.; Zhang, J.Q. An Introduction to Electrochemical Impedance Spectroscopy; Science Press: Beijing, China, 2003. [Google Scholar]
- Scully, J.R.; Silverman, D.C.; Kendig, M.W. Electrochemical Impedance: Analysis and Interpretation, ASTM STP 1188; American Society for Testing and Materials: Philadelphia, PA, USA, 1993. [Google Scholar]
- Bierwagen, G.; Tallman, D.; Li, J.P.; He, L.Y.; Jeffcoate, C. EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 2003, 46, 148–157. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Sun, G.Q.; Cao, C.N. EIS data analysis for evaluation of protective property of organic coatings. Corros. Sci. Prot. Technol. 1994, 6, 318–325. [Google Scholar]
- Zubielewicz, M.; Gnot, W. Mechanisms of non-toxic anticorrosive pigments in organic waterborne coatings. Prog. Org. Coat. 2004, 49, 358–371. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M.M. Investigation on zinc phosphate effectiveness at different pigment volume concentrations via electrochemical impedance spectroscopy. Electrochim. Acta 2005, 50, 4645–4648. [Google Scholar] [CrossRef]
- Amirudin, A.; Thieny, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Zuo, Y.; Pang, R.; Li, W.; Xiong, J.P.; Tang, Y.M. The evaluation of coating performance by the variations of phase angles in middle and high frequency domains of EIS. Corros. Sci. 2008, 50, 3322–3328. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M.M. Another approach in analysis of paint coatings with EIS measurement: Phase angle at high frequencies. Corros. Sci. 2006, 48, 4152–4157. [Google Scholar] [CrossRef]
- Mansfeld, F.; Tsai, C.H. Determination of coating deterioration with EIS: I. Basic relationships. Corrosion 1991, 47, 958–963. [Google Scholar] [CrossRef]
- Fernandez-Perez, B.M.; Gonzalez-Guzman, J.A.; Gonzalez, S.; Souto, R.M. Electrochemical impedance spectroscopy investigation of the corrosion resistance of a waterborne acrylic coating containing active electrochemical pigments for the protection of carbon steel. Int. J. Electrochem. Sci. 2014, 9, 2067–2079. [Google Scholar]
- Zhu, Y.F.; Xiong, J.P.; Tang, Y.M.; Zuo, Y. EIS study on failure process of two polyurethane composite coatings. Prog. Org. Coat. 2010, 69, 7–11. [Google Scholar] [CrossRef]
- Cai, J.P.; Sun, Z.H.; Cui, J.H. Establishment of degradation dynamic model for organic coatings. Mater. Prot. 2012, 45, 8–10. [Google Scholar]
- Lee, C.C.; Mansfeld, F. Automatic classification of polymer coating quality using artificial neural networks. Corros. Sci. 1998, 41, 439–461. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Kong, T.; Zhang, W.; Wang, Y.H. Investigation of deterioration process of organic coating using 1-dimention SOM network combined with EIS. Corros. Sci. Prot. Technol. 2008, 20, 275–278. [Google Scholar]
- Zhen, F. Simple talk about the forecast theory about coating’s life. Coat. Appl. Electr. Plat. 2005, 3, 3–5. [Google Scholar]
- Cai, J.P.; Liu, M.; An, Y.H. Degradation kinetics of protective coating for aluminum alloy. J. Chin. Soc. Corros. Prot. 2012, 32, 256–261. [Google Scholar]
- Evans, M. A statistical degradation model for the service life prediction of aircraft coatings: With a comparison to an existing methodology. Polym. Test. 2012, 31, 46–55. [Google Scholar] [CrossRef]
- Wnag, H.T.; Han, E.H.; Ke, W. Predictive model for atmospheric corrosion of aluminium alloy by artificial neural network. J. Chin. Soc. Corros. Prot. 2006, 26, 272–276. [Google Scholar]
- Akbarinezhad, E.; Bahremandi, M.; Faridi, H.R.; Rezaei, F. Another approach for ranking and evaluating organic paint coatings via electrochemical impedance spectroscopy. Corros. Sci. 2009, 51, 356–363. [Google Scholar] [CrossRef]
- Krahmer, D.M.; Polvorosa, R.; de Lacalle, L.N.L.; Alonso-Pinillos, U.; Abate, G.; Riu, F. Alternatives for specimen manufacturing in tensile testing of steel plates. Exp. Tech. 2016, 40, 1555–1565. [Google Scholar] [CrossRef]
- Rodríguez-Barrero, S.; Fernández-Larrinoa, J.; Azkona, I.; López de Lacalle, L.N.; Polvorosa, R. Enhanced performance of nanostructured coatings for drilling by droplet elimination. Mater. Manuf. Process. 2016, 31, 593–602. [Google Scholar] [CrossRef]
- Zhao, X. Characteristics of Electrochemical Impedance Spectroscopy in Deterioration Process of Organic Coating. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2007. [Google Scholar]
- Cmaitland, C.; Mayne, J.E.O. Factors affecting the electrolytic resistance of polymer films. Off. Dig. 1962, 34, 972. [Google Scholar]
- Fu, D.X.; Xu, B.S.; Zhang, W. Research progress on microscopic mechanism of foaming of organic coatings. J. Mater. Prot. 2007, 40, 42–45. [Google Scholar]
- Ren, B.N. Corrosion and Protection of Highway Steel Bridge; China Communications Press: Beijing, China, 2002. [Google Scholar]
- Geng, G.Q.; Lin, J.; Liu, L.J.; Cui, J.N. Life prediction system for protective coating of steel bridge. J. Chang’an Univ. (Nat. Sci. Ed.) 2006, 26, 43–47. [Google Scholar]
- Lin, J. Study on Protection, Failure Law and Life Prediction of Corrosion Protective Coatings for Bridge Steel Structures. Master’s Thesis, Chang’an University, Xi’an, China, 2006. [Google Scholar]

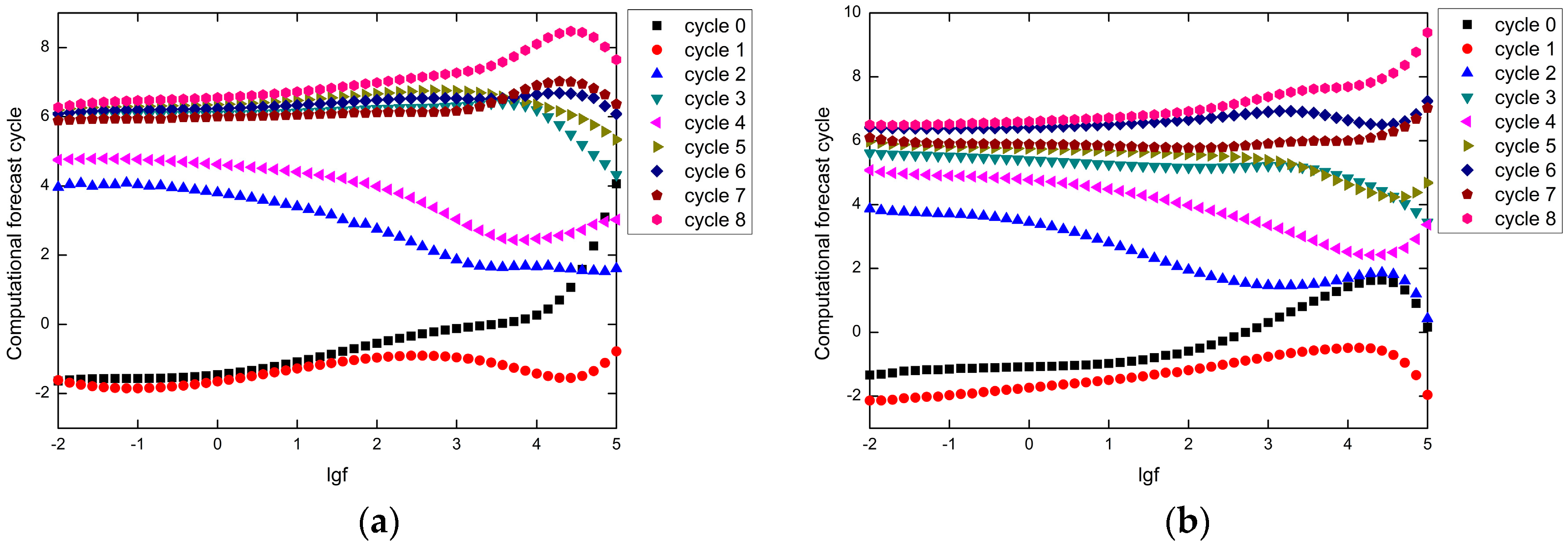
| Real Cycle | Plate 1# | Plate 2# | ||||
|---|---|---|---|---|---|---|
| Computational Forecast Cycle | Rounding Forecast Cycle | Prediction Cycle Error | Computational Forecast Cycle | Rounding Forecast Cycle | Prediction Cycle Error | |
| 0 | −1.56 | / | / | −1.15 | / | / |
| 1 | −1.85 | / | / | −1.96 | / | / |
| 2 | 4.04 | 4 | 2 | 3.70 | 4 | 2 |
| 3 | 6.08 | 6 | 3 | 5.51 | 6 | 3 |
| 4 | 4.77 | 5 | 1 | 4.89 | 5 | 1 |
| 5 | 6.32 | 6 | 1 | 5.81 | 6 | 1 |
| 6 | 6.22 | 6 | 0 | 6.39 | 6 | 0 |
| 7 | 5.95 | 6 | 1 | 5.90 | 6 | 1 |
| 8 | 6.49 | 6 | 2 | 6.53 | 7 | 1 |
| Real Cycle | Plate 1# | Plate 2# | ||||
|---|---|---|---|---|---|---|
| Computational Forecast Cycle | Rounding Forecast Cycle | Prediction Cycle Error | Computational Forecast Cycle | Rounding Forecast Cycle | Prediction Cycle Error | |
| 0 | −0.52 | / | / | −0.31 | 0 | 0 |
| 1 | −1.36 | / | / | −1.34 | / | / |
| 2 | 2.90 | 3 | 1 | 2.52 | 3 | 1 |
| 3 | 6.03 | 6 | 3 | 5.15 | 5 | 2 |
| 4 | 3.88 | 4 | 0 | 4.00 | 4 | 0 |
| 5 | 6.39 | 6 | 1 | 5.42 | 5 | 0 |
| 6 | 6.38 | 6 | 0 | 6.59 | 7 | 1 |
| 7 | 6.21 | 6 | 1 | 5.96 | 6 | 1 |
| 8 | 7.04 | 7 | 1 | 7.07 | 7 | 1 |
| Real Cycle | Plate 1# | Plate 2# | ||
|---|---|---|---|---|
| Forecast Cycle | Prediction Cycle Error | Forecast Cycle | Prediction Cycle Error | |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 1 | 0 |
| 2 | 2 | 0 | 2 | 0 |
| 3 | 3 | 0 | 3 | 0 |
| 4 | 4 | 0 | 4 | 0 |
| 5 | 6 | 1 | 6 | 1 |
| 6 | 5 | 1 | 5 | 1 |
| 7 | 7 | 0 | 7 | 0 |
| 8 | 8 | 0 | 8 | 0 |
| Analytical Methods or Models | The Failure Cycle |
|---|---|
| Morphological analysis | 5 |
| Analysis of |Z| data at low frequency | 3 |
| Degradation kinetics model | 4 |
| Improved degradation kinetics model | 4 |
| Neural network model | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ran, J.; Dai, W.; Zhang, W. Investigation of Service Life Prediction Models for Metallic Organic Coatings Using Full-Range Frequency EIS Data. Metals 2017, 7, 274. https://doi.org/10.3390/met7070274
Xu Y, Ran J, Dai W, Zhang W. Investigation of Service Life Prediction Models for Metallic Organic Coatings Using Full-Range Frequency EIS Data. Metals. 2017; 7(7):274. https://doi.org/10.3390/met7070274
Chicago/Turabian StyleXu, Yuanming, Junshuang Ran, Wei Dai, and Weifang Zhang. 2017. "Investigation of Service Life Prediction Models for Metallic Organic Coatings Using Full-Range Frequency EIS Data" Metals 7, no. 7: 274. https://doi.org/10.3390/met7070274





