Effect of Pyrite on Thiosulfate Leaching of Gold and the Role of Ammonium Alcohol Polyvinyl Phosphate (AAPP)
Abstract
:1. Introduction
- (i)
- Catalytic effect of pyrite on thiosulfate decomposition. The catalyzed decomposition of thiosulfate by pyrite has been reported by a number of researchers [8,23,28,29,30,31]. It is believed that the strong affinity of pyrite for aqueous sulfur species and the semiconducting properties of pyrite lead to the catalysis of pyrite in the degradation of thiosulfate in aqueous solutions of pH 2.9–8.6 [29]. An interfacial intermediate complex was considered to be formed by pyrite, thiosulfate and oxygen, of which thiosulfate is the aqueous electron donor on anodic sites and oxygen is the terminal electron acceptor on cathodic sites [24,29].
- (ii)
- Dissolution behavior of pyrite. It has been shown that iron-containing sulfide minerals such as pyrite, pyrrhotite, arsenopyrite, and chalcopyrite can be partially dissolved in ammoniacal thiosulfate liquors [8,28]. During the dissolution of pyrite, copper(II) and oxygen were found to be consumed and simultaneously a lower slurry potential was caused [24]. Scanning Electron Microscopy (SEM), Raman spectroscopy and X-ray Photoelectron Spectroscopy (XPS) analyses indicated that the leaching of pyrite largely took place at high-energy crystal boundaries and defect sites whilst iron oxide (hematite) was found to be formed at the pyrite surface after leaching [23,28].
- (iii)
- Gold adsorption and precipitation on pyrite surfaces. In thiosulfate-deficient solutions, dissolved gold can adsorb and precipitate on sulfide minerals, resulting in a lower gold recovery. The adsorption and precipitation of gold as the metal tended to occur at defect sites on the surface of sulfide mineral [31,32,33,34]. It was reported that gold precipitated on pyrite was difficult to be recovered unless undergoing the treatment with cyanide [31].
- (iv)
- Passivation of gold by iron species coating on gold surfaces. In the presence of pyrite, some iron-containing species can also be formed and may be responsible for the reduced dissolution of gold due to the surface passivation. The likely passivating products were iron hydroxides/oxides such as FeO·OH, Fe2O3 or Fe2O3·nH2O [8,23,24,28].
2. Experimental Section
2.1. Minerals and Reagents
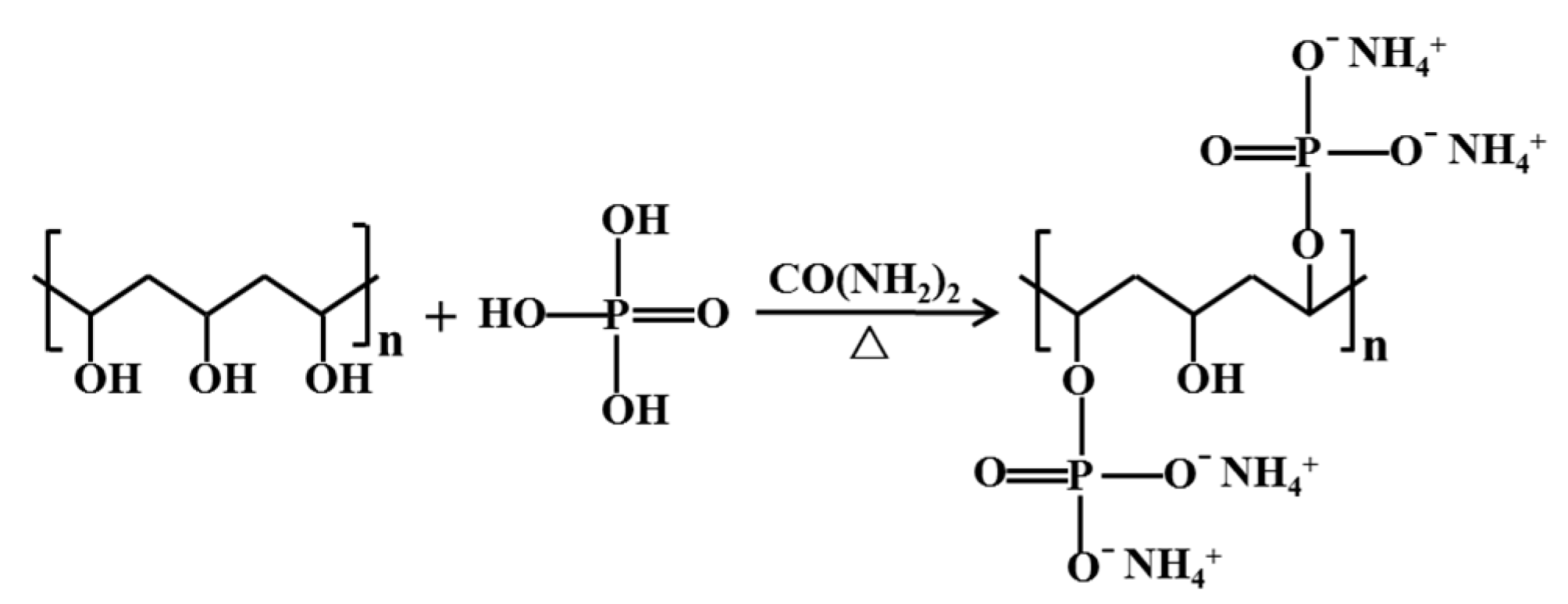
2.2. Preparation of Ammonium Alcohol Polyvinyl Phosphate (AAPP)
2.3. Leaching Experiments
2.4. Analytical Methods
3. Results
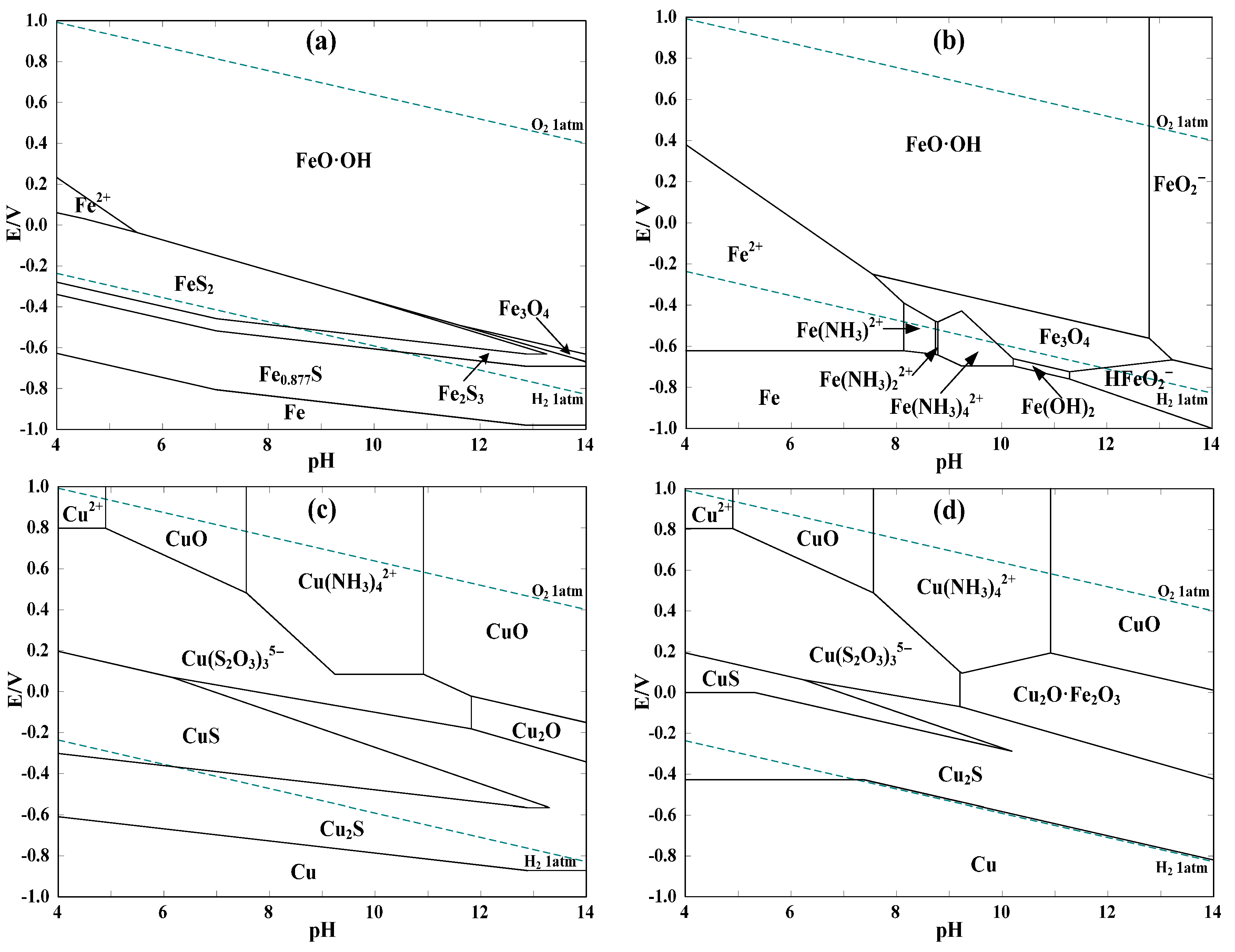
3.1. Thermodynamic Analysis
4 Cu(S2O3)35− + 8 NH3 + 4 H+ (ΔGo = −616.1 kcal/mol)
3.2. Effect of Pyrite on Thiosulfate Leaching of Gold
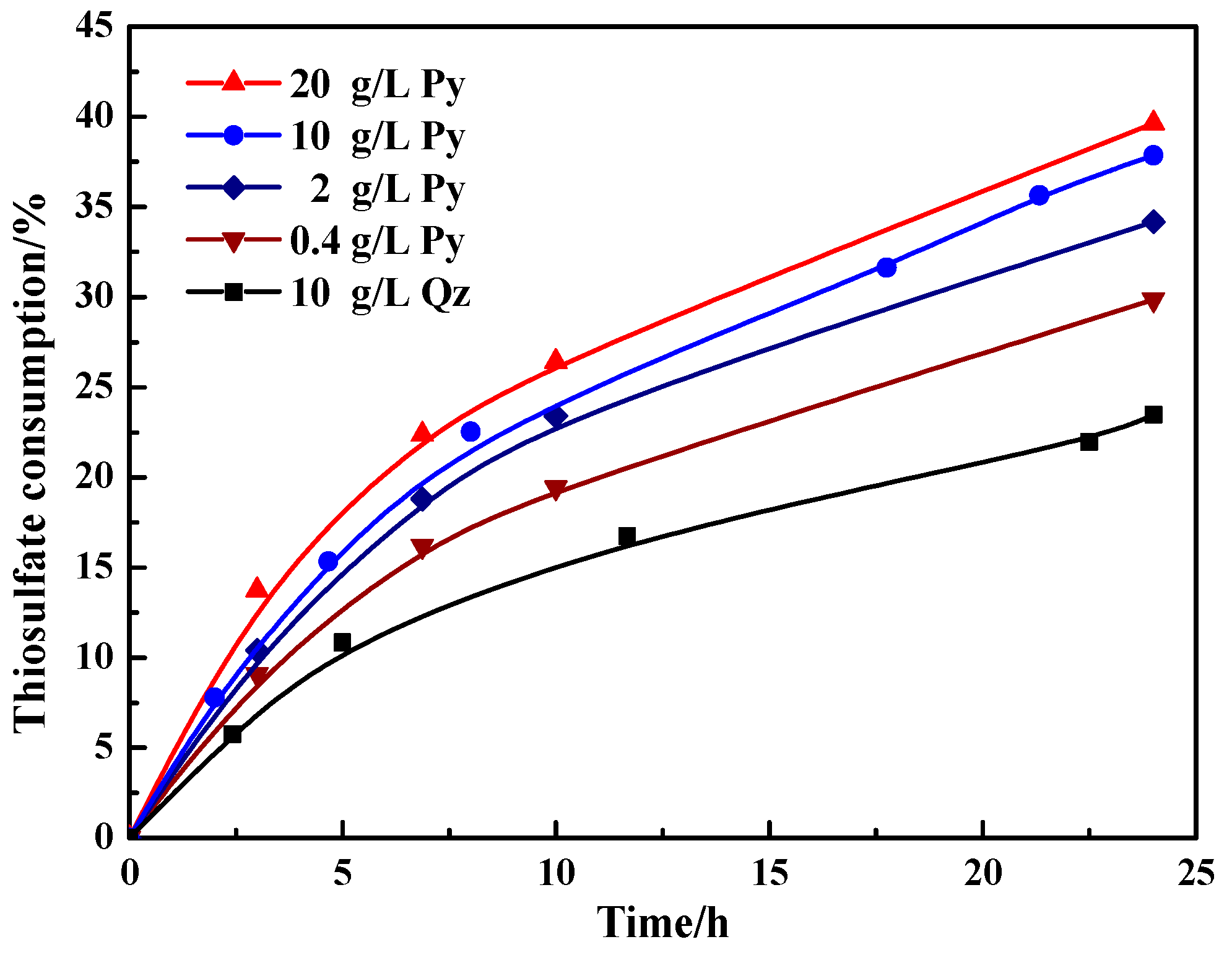
3.2.1. Effect of Pyrite on Thiosulfate Decomposition
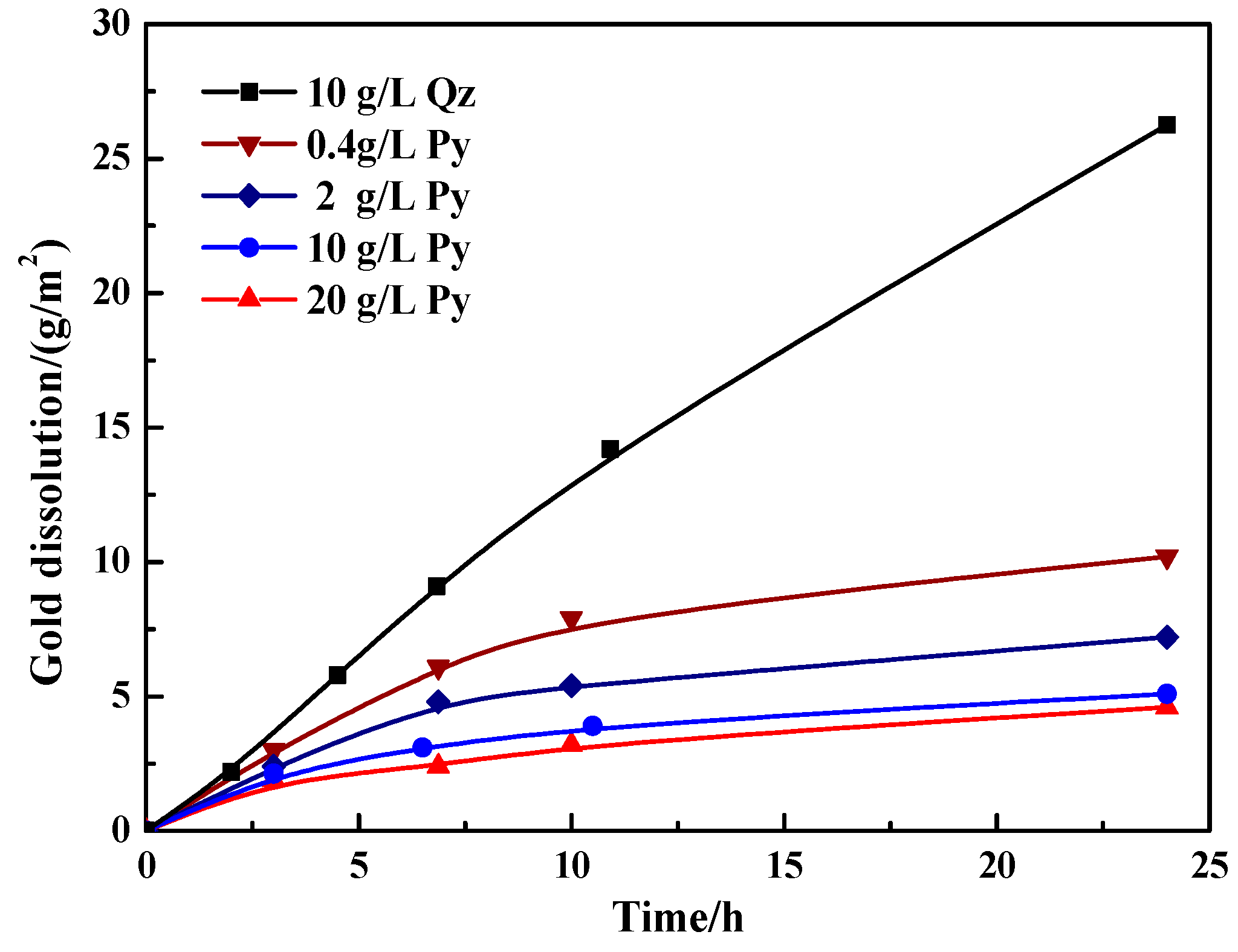
3.2.2. Effect of Pyrite on Gold Dissolution
3.3. Role of AAPP Additive
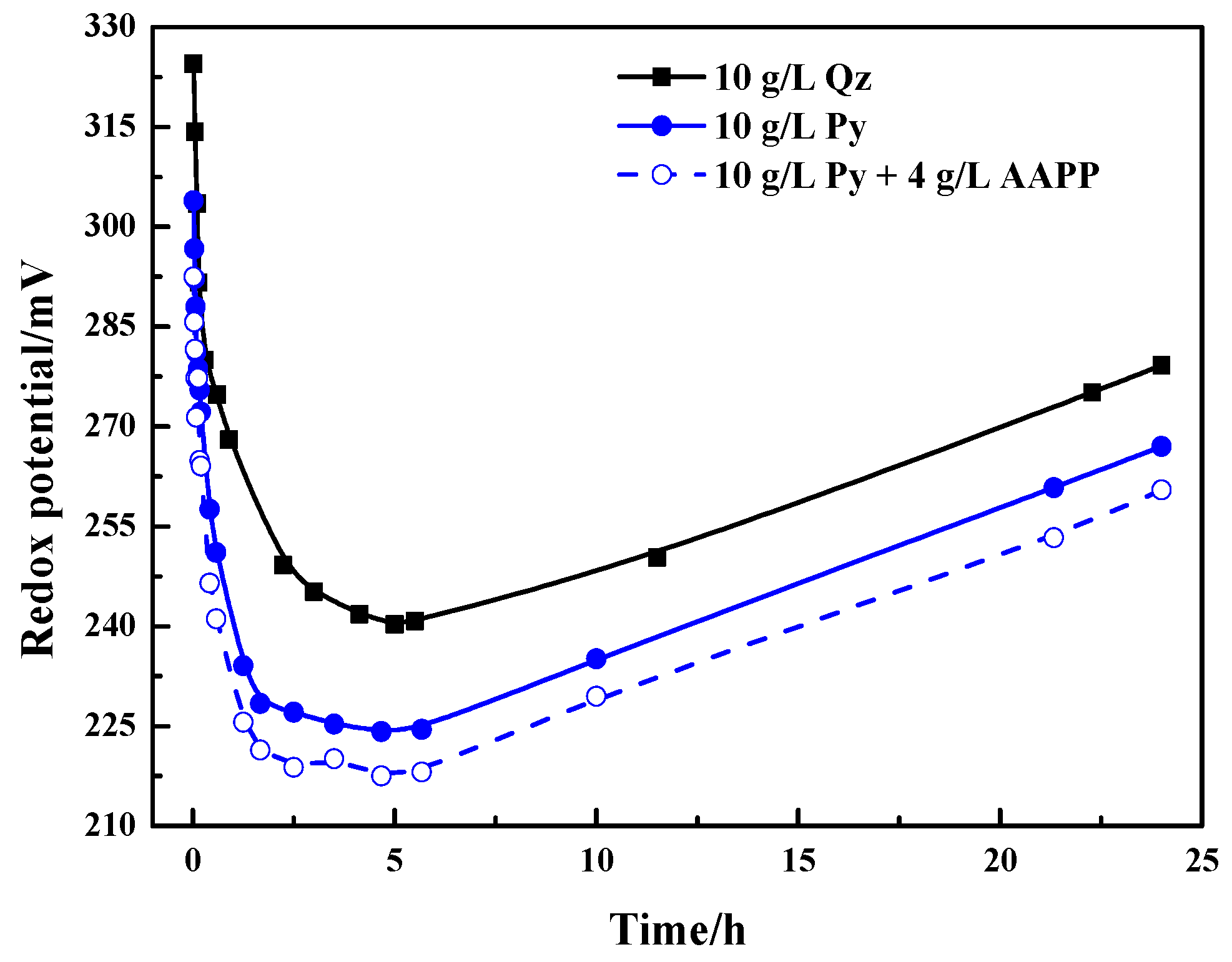
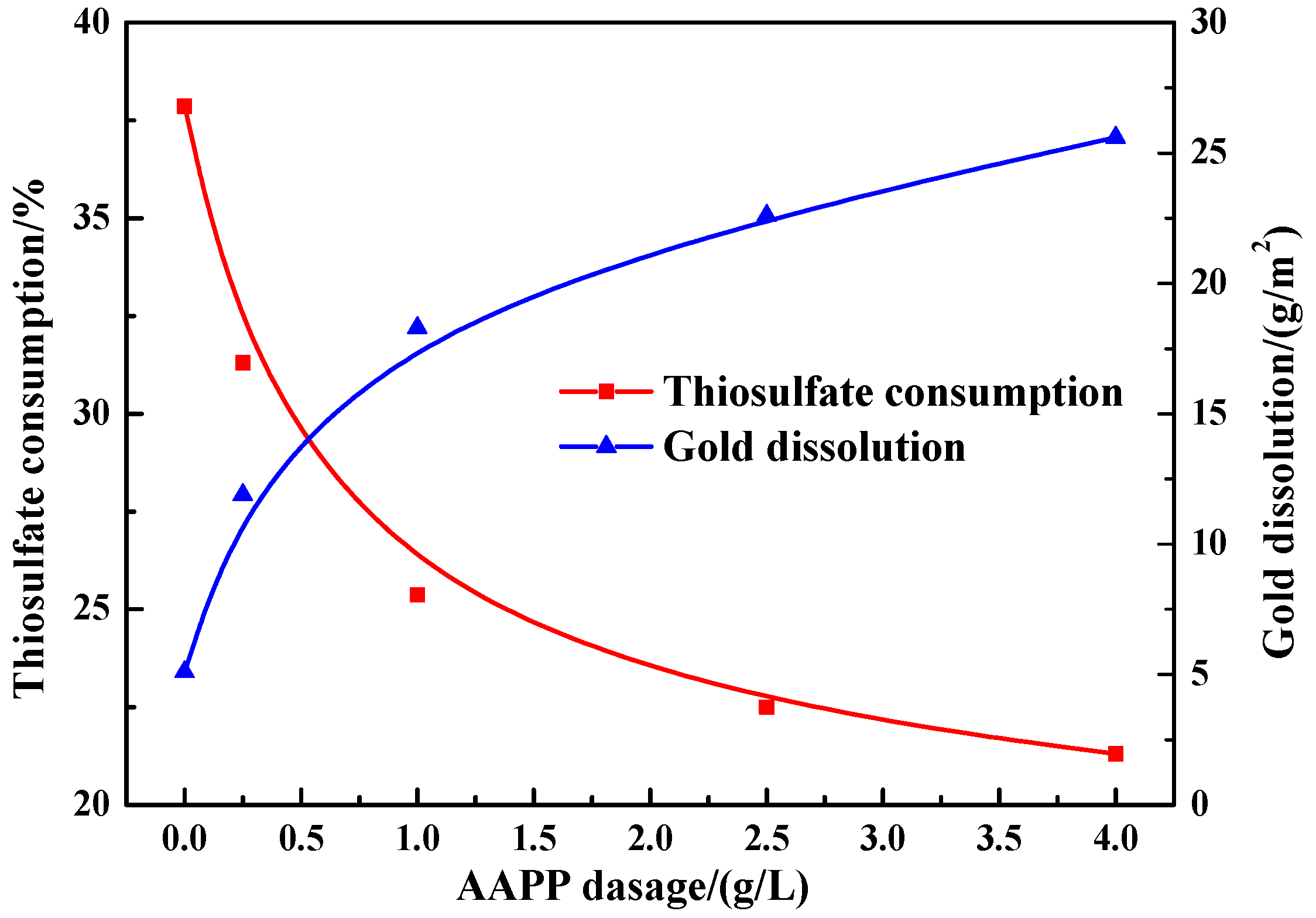
3.3.1. Role of AAPP in Thiosulfate Decomposition
3.3.2. Role of AAPP in Gold Dissolution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Species | ΔGo298/ (kcal/mol) | Species | ΔGo298/ (kcal/mol) | Species | ΔGo298/ (kcal/mol) | Species | ΔGo298/ (kcal/mol) |
|---|---|---|---|---|---|---|---|
| Fe | 0 | Fe(OH)O(aq) | −105.700 | Cu(NH3)42+(aq) | −28.011 | H2S(aq) | −6.607 |
| Fe0.877S | −25.533 | HFeO2(aq) | −105.700 | Cu(NH3)52+(aq) | −33.759 | HS−(aq) | 2.973 |
| FeS | −24.369 | HFeO2−(aq) | −95.353 | Cu(NH3)+(aq) | −2.516 | HSO3−(aq) | −126.103 |
| FeS2 | −38.247 | Fe(NH3)2+(aq) | −30.159 | Cu(NH3)2+(aq) | −15.614 | HSO4−(aq) | −180.524 |
| Fe2S3 | −67.03 | Fe(NH3)22+(aq) | −37.625 | Cu(S2O3)−(aq) | −126.885 | HS2O3−(aq) | −127.183 |
| FeO | −58.729 | Fe(NH3)42+(aq) | −52.421 | Cu(S2O3)23−(aq) | −254.368 | HS2O4−(aq) | −146.862 |
| Fe2O3 | −177.114 | Fe(S2O3)+(aq) | −131.632 | Cu(S2O3)35−(aq) | −381.404 | S2−(aq) | 20.548 |
| Fe3O4 | −241.956 | Fe(S2O3)(aq) | −147.928 | CuO22−(aq) | −41.220 | S22−(aq) | 19.055 |
| Fe(OH)2 | −117.578 | Cu | 0.000 | Cu(OH)+(aq) | −30.210 | S32−(aq) | 17.657 |
| Fe(OH)3 | −168.638 | CuS | −13.530 | Cu(OH)3−(aq) | −119.865 | S42−(aq) | 16.589 |
| Fe2O3·H2O | 5.776 | Cu2S | −20.607 | Cu(OH)42−(aq) | −156.970 | S52−(aq) | 15.821 |
| FeO·OH | −116.928 | CuO | −30.387 | Cu2(OH)3+(aq) | −16.424 | S62−(aq) | 15.827 |
| Fe3+(aq) | −4.107 | Cu2O | −35.335 | Cu2(OH)22+(aq) | −67.825 | SO32−(aq) | −116.287 |
| Fe2+(aq) | −21.875 | CuO·Fe2O3 | −205.969 | Cu3(OH)42+(aq) | −151.420 | SO42−(aq) | −177.907 |
| FeO(aq) | −50.715 | Cu2O·Fe2O3 | −220.015 | Cu(OH)O−(aq) | −60.096 | S2O32−(aq) | −124.825 |
| FeO+(aq) | −53.093 | Cu(OH)2 | −85.262 | H+(aq) | 0 | S2O42−(aq) | −143.539 |
| FeO2−(aq) | −92.642 | Cu2+(aq) | 15.545 | O2(aq) | 3.899 | S2O62−(aq) | −231.605 |
| FeOH2+(aq) | −57.830 | Cu+(aq) | 11.946 | H2O(aq) | −56.678 | S2O82−(aq) | −266.46 |
| FeOH+(aq) | −65.845 | Cu(NH3)2+(aq) | 3.400 | NH3(aq) | −6.375 | S3O62−(aq) | −228.815 |
| Fe(OH)2+(aq) | −108.077 | Cu(NH3)22+(aq) | −7.979 | NH4+(aq) | −18.977 | S4O62−(aq) | −248.627 |
| Fe2(OH)24+(aq) | −111.743 | Cu(NH3)32+(aq) | −18.500 | S | 0 | S5O62−(aq) | −228.331 |
References
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Kerley, B.J. Recovery of Precious Metals from Difficult Ores. U.S. Patent 4,269,622, 26 May 1981. [Google Scholar]
- Kerley, B.J. Recovery of Precious Metals from Difficult Ores. U.S. Patent 4,369,061, 18 January 1983. [Google Scholar]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Vegliò, F.; Ubaldini, S. Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Marchbank, A.R.; Thomas, K.G.; Dreisinger, D.; Fleming, C. Gold Recovery from Refractory Carbonaceous Ores by Pressure Oxidation and Thiosulphate Leaching. U.S. Patent 5,536,297, 16 July 1996. [Google Scholar]
- Wan, R.Y.; Brierley, J.A. Thiosulfate leaching following biooxidation pretreatment for gold recovery from refractory carbonaceous-sulfidic ore. Miner. Eng. 1997, 76, 76–80. [Google Scholar]
- Xia, C. Associated Sulfide Minerals in Thiosulfate Leaching of Gold: Problems and Solutions. Ph.D. Thesis, Department of Mining Engineering, Queen’s University, Kingston, ON, Canada, 2008. [Google Scholar]
- Feng, D.; van Deventer, J.S.J. The effect of sulphur species on thiosulphate leaching of gold. Miner. Eng. 2007, 20, 273–281. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. The role of oxygen in thiosulphate leaching of gold. Hydrometallurgy 2007, 85, 193–202. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Effect of hematite on thiosulphate leaching of gold. Int. J. Miner. Process. 2007, 82, 138–147. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Effect of thiosulphate salts on ammoniacal thiosulphate leaching of gold. Hydrometallurgy 2010, 105, 120–126. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Oxidative pre-treatment in thiosulphate leaching of sulphide gold ores. Int. J. Miner. Process. 2010, 94, 28–34. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Liu, X.; Xu, B.; Yang, Y.; Jiang, T. Improvement of Gold Leaching from a Refractory Gold Concentrate Calcine by Separate Pretreatment of Coarse and Fine Size Fractions. Minerals 2017, 7, 80. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Jiang, T.; Li, Q.; Zhang, X.; Wang, D. Improved thiosulfate leaching of a refractory gold concentrate calcine with additives. Hydrometallurgy 2015, 152, 214–222. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Li, G. Stage leaching of a complex polymetallic sulfide concentrate: Focus on the extraction of Ag and Au. Hydrometallurgy 2015, 159, 87–94. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Yin, W.; Jiang, T.; Li, G. Thiosulfate leaching of Au, Ag and Pd from a high Sn, Pb and Sb bearing decopperized anode slime. Hydrometallurgy 2016, 164, 278–287. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Liu, S.; Li, G. The development of an environmentally friendly leaching process of a high C, As and Sb bearing sulfide gold concentrate. Miner. Eng. 2016, 89, 138–147. [Google Scholar] [CrossRef]
- Pedraza, A.M.; Villegas, I.; Freund, P.L.; Chornik, B. Electro-oxidation of thiosulphate ion on gold: Study by means of cyclic voltammetry and Auger electron spectroscopy. J. Electroanal. Chem. 1988, 250, 443–449. [Google Scholar] [CrossRef]
- Wan, R.-Y.; LeVier, K.M. Solution chemistry factors for gold thiosulfate heap leaching. Int. J. Miner. Process. 2003, 72, 311–322. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Watling, K.; Hope, G.A.; Woods, R. Identification of surface species that inhibit and passivate thiosulfate leaching of gold. Miner. Eng. 2008, 21, 443–452. [Google Scholar] [CrossRef]
- Mirza, J.; Smith, S.R.; Baron, J.Y.; Choi, Y.; Lipkowski, J. A SERS characterization of the stability of polythionates at the gold-electrolyte interface. Surf. Sci. 2015, 631, 196–206. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Ammoniacal thiosulphate leaching of gold in the presence of pyrite. Hydrometallurgy 2006, 82, 126–132. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Effect of sulfides on gold dissolution in ammoniacal thiosulfate medium. Metall. Mater. Trans. B 2003, 34, 5–13. [Google Scholar] [CrossRef]
- Yang, Y.B.; Zhang, X.; Xu, B.; Li, Q.; Jiang, T.; Wang, Y.X. Effect of arsenopyrite on thiosulfate leaching of gold. Trans. Nonferr. Met. Soc. 2015, 25, 3454–3460. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Zhang, Y. Effect of galena on thiosulfate leaching of gold. Hydrometallurgy 2017, 171, 157–164. [Google Scholar] [CrossRef]
- Marsden, J.O.; House, C.I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2006. [Google Scholar]
- Feng, D.; van Deventer, J.S.J. Leaching behaviour of sulfides in ammoniacal thiosulphate systems. Hydrometallurgy 2002, 63, 189–200. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The stability of thiosulfate in the presence of pyrite in low-temperature aqueous solutions. Geochim. Cosmochim. Acta 1995, 59, 4605–4622. [Google Scholar] [CrossRef]
- Zhang, H.; Jeffrey, M.I. A study of pyrite catalysed oxidation of thiosulfate. In Proceedings of the Sixth International Symposium Hydrometallurgy 2008, Phoenix, AZ, USA, 17–20 August 2008; Young, C.A., Talor, P.R., Anderson, C.G., Choi, Y., Eds.; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2008; pp. 769–778. [Google Scholar]
- Aylmore, M.G.; Muir, D.M.; Staunton, W.P. Effect of minerals on the stability of gold in copper ammoniacal thiosulfate solutions—The role of copper, silver and polythionates. Hydrometallurgy 2014, 143, 12–22. [Google Scholar] [CrossRef]
- Jean, G.E.; Michael, B.G. An XPS and SEM study of gold deposition at low temperatures on sulphide mineral surfaces: Concentration of gold by adsorption/reduction. Geochim. Cosmochim. Acta 1985, 49, 979–987. [Google Scholar] [CrossRef]
- Schoonen, M.A.A.; Fisher, N.S.; Wente, M. Gold sorption onto pyrite and goethite: A radiotracer study. Geochim. Cosmochim. Acta 1992, 56, 1801–1814. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Preg-robbing phenomena in the thiosulphate leaching of gold ores. Miner. Eng. 2001, 14, 1387–1402. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A review of ammoniacal thiosulfate leaching of gold: An update useful for further research in non-cyanide gold lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Senanayake, G.; Zhang, X.M. Gold leaching by copper(II) in ammoniacal thiosulphate solutions in the presence of additives. Part II: Effect of residual Cu(II), pH and redox potentials on reactivity of colloidal gold. Hydrometallurgy 2012, 115–116, 21–29. [Google Scholar] [CrossRef]
- Senanayake, G. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulfate oxidation by ammoniacal copper(II) solutions. Hydrometallurgy 2004, 75, 55–75. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper(II) in ammoniacal thiosulphate solutions in the presence of additives. Part I: A review of the effect of hard–soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115–116, 1–20. [Google Scholar] [CrossRef]
- Xia, C.; Yen, W.T.; Deschenes, G. Improvement of thiosulfate stability in gold leaching. Miner. Metall. Process. 2003, 20, 68–72. [Google Scholar]
- Feng, D.; van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of orthophosphate and polyphosphate. Hydrometallurgy 2011, 106, 38–45. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of ethylenediaminetetraacetic acid (EDTA). Miner. Eng. 2010, 23, 143–150. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of carboxymethyl cellulose (CMC). Miner. Eng. 2011, 24, 115–121. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. The role of amino acids in the thiosulphate leaching of gold. Miner. Eng. 2011, 24, 1022–1024. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Li, G. Effect of common associated sulfide minerals on thiosulfate leaching of gold and the role of humic acid additive. Hydrometallurgy 2017, 171, 44–52. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, Y.; Dai, X.; Zhao, D.; Zhou, X.; Qi, Y.; Koo, J.H. Ammonium alcohol polyvinyl phosphate intercalated LDHs/epoxy nanocomposites. J. Therm. Anal. Calorim. 2015, 122, 135–144. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Zou, J.; Duan, H.; Li, J.; Chen, C.; Meng, P. Preparation and membrane separation performances of quarternized ammonium cationic polyvinyl alcohol. J. Appl. Polym. Sci. 2011, 119, 2584–2594. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thermodynamic analysis of gold leaching by ammoniacal thiosulfate using Eh/pH and speciation diagrams. Miner. Metall. Proc. 2001, 18, 221–227. [Google Scholar]
- Hiskey, B.J.; Atluri, V.P. Dissolution chemistry of gold and silver in different lixiviants. Miner. Process. Extr. Met. Rev. 1988, 4, 95–134. [Google Scholar] [CrossRef]
- Weiss, R.F. The solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Res. 1970, 17, 721–735. [Google Scholar] [CrossRef]
- Luther, G.W. The frontier-molecular-orbital theory approach in geochemical process. In Aquatic Chemical Kinetics; Stumm, W., Ed.; John Wiley & Sons: Toronto, ON, Canada, 1990; Chapter 6; pp. 173–198. [Google Scholar]
- Zhang, X. Research on the Effect of Sulfide Minerals on Thiosulfate Leaching of Gold and the Intensification Method. Master’s Thesis, School of Minerals Processing and Bioengineering, Central South University, Changsha, China, 2015. [Google Scholar]
- Ouyang, S. Research on Quantum Chemistry of Effects of Impurities and Flotation Separation Process of Pb-Zn Sulfide Containing High Sulfur in Huanjiang Guangxi. Master’s Thesis, Department of Mining Engineering, Guangxi University, Nanning, China, 2010. [Google Scholar]
- Zhang, H.; Dreisinger, D.B. The adsorption of gold and copper onto ion-exchange resins from ammoniacal thiosulphate solutions. Hydrometallurgy 2002, 66, 67–76. [Google Scholar] [CrossRef]
- Miura, Y.; Koh, T. Spectroscopic determination of tetrathionate by means of its alkaline decomposition. Nippon Kagaku Kaishi 1983, 11, 1597–1601. [Google Scholar] [CrossRef]
- Langer, D.W.; Vesely, C.J. Electronic core levels of zinc chalcogenides. Phys. Rev. B 1970, 2, 4885–4892. [Google Scholar] [CrossRef]
- Manocha, A.S.; Park, R.L. Flotation related ESCA studies on PbS surfaces. Appl. Surf. Sci. 1977, 1, 129–141. [Google Scholar] [CrossRef]
- Laajalehto, K.; Kartio, I.; Nowak, P. XPS study of clean metal sulfide surfaces. Appl. Surf. Sci. 1994, 81, 11–15. [Google Scholar] [CrossRef]
- Perry, D.L.; Taylor, J.A. X-ray photoelectron and Auger spectroscopic studies of Cu2S and CuS. J. Mater. Sci. Lett. 1986, 5, 384–386. [Google Scholar] [CrossRef]
- Jolley, J.G.; Geesey, G.G.; Hankins, M.R.; Wright, R.B.; Wichlacz, P.L. Auger electron and X-ray photoelectron spectroscopic study of the biocorrosion of copper by alginic acid polysaccharide. Appl. Surf. Sci. 1989, 37, 469–480. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Losev, A.; Rostov, K.; Tyuliev, G. Electron beam induced reduction of CuO in the presence of a surface carbonaceous layer: An XPS/HREELS study. Surf. Sci. 1989, 213, 564–579. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 2002, 2, 186–191. [Google Scholar] [CrossRef]
- Oscarson, D.W.; Huang, P.M.; Defosse, C.; Herbillon, A. Oxidative power of Mn(IV) and Fe(III) oxides with respect to As(III) in terrestrial and aquatic environments. Nature 1981, 291, 50–51. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Escudey, M.; Vyhmeister, E.; Higueras, P.; Godoy-Faúndez, A.; Salazar, J.L.; Valdés-González, H.; Wolf-Sepúlveda, G.; Herrera-Urbina, R. Adsorption of biosolids and their main components on chalcopyrite, molybdenite and pyrite: Zeta potential and FTIR spectroscopy studies. Miner. Eng. 2015, 78, 128–135. [Google Scholar] [CrossRef]
- Byerley, J.J.; Fouda, S.A.; Rempel, G.L. Kinetics and mechanism of the oxidation of thiosulphate ions by copper(II) ions in aqueous ammonia solution. J. Chem. Soc. Dalton Trans. 1973, 8, 889–893. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M.I. The reduction of copper(II) and the oxidation of thiosulfate and oxysulfur anions in gold leaching solutions. Hydrometallurgy 2003, 70, 163–173. [Google Scholar] [CrossRef]



| Reactant | EHOMO/eV | ELUMO/eV | |ΔE1|/eV a | |ΔE2|/eV b |
|---|---|---|---|---|
| FeS2 | −6.295 [51] | −5.732 [51] | 1.780 | 3.231 |
| S2O32− | −7.703 [52] | −3.064 [52] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, B.; Min, X.; Li, Q.; Yang, Y.; Jiang, T.; He, Y.; Zhang, X. Effect of Pyrite on Thiosulfate Leaching of Gold and the Role of Ammonium Alcohol Polyvinyl Phosphate (AAPP). Metals 2017, 7, 278. https://doi.org/10.3390/met7070278
Liu X, Xu B, Min X, Li Q, Yang Y, Jiang T, He Y, Zhang X. Effect of Pyrite on Thiosulfate Leaching of Gold and the Role of Ammonium Alcohol Polyvinyl Phosphate (AAPP). Metals. 2017; 7(7):278. https://doi.org/10.3390/met7070278
Chicago/Turabian StyleLiu, Xiaoliang, Bin Xu, Xin Min, Qian Li, Yongbin Yang, Tao Jiang, Yinghe He, and Xi Zhang. 2017. "Effect of Pyrite on Thiosulfate Leaching of Gold and the Role of Ammonium Alcohol Polyvinyl Phosphate (AAPP)" Metals 7, no. 7: 278. https://doi.org/10.3390/met7070278




