Precipitate Stability in a Zr–2.5Nb–0.5Cu Alloy under Heavy Ion Irradiation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
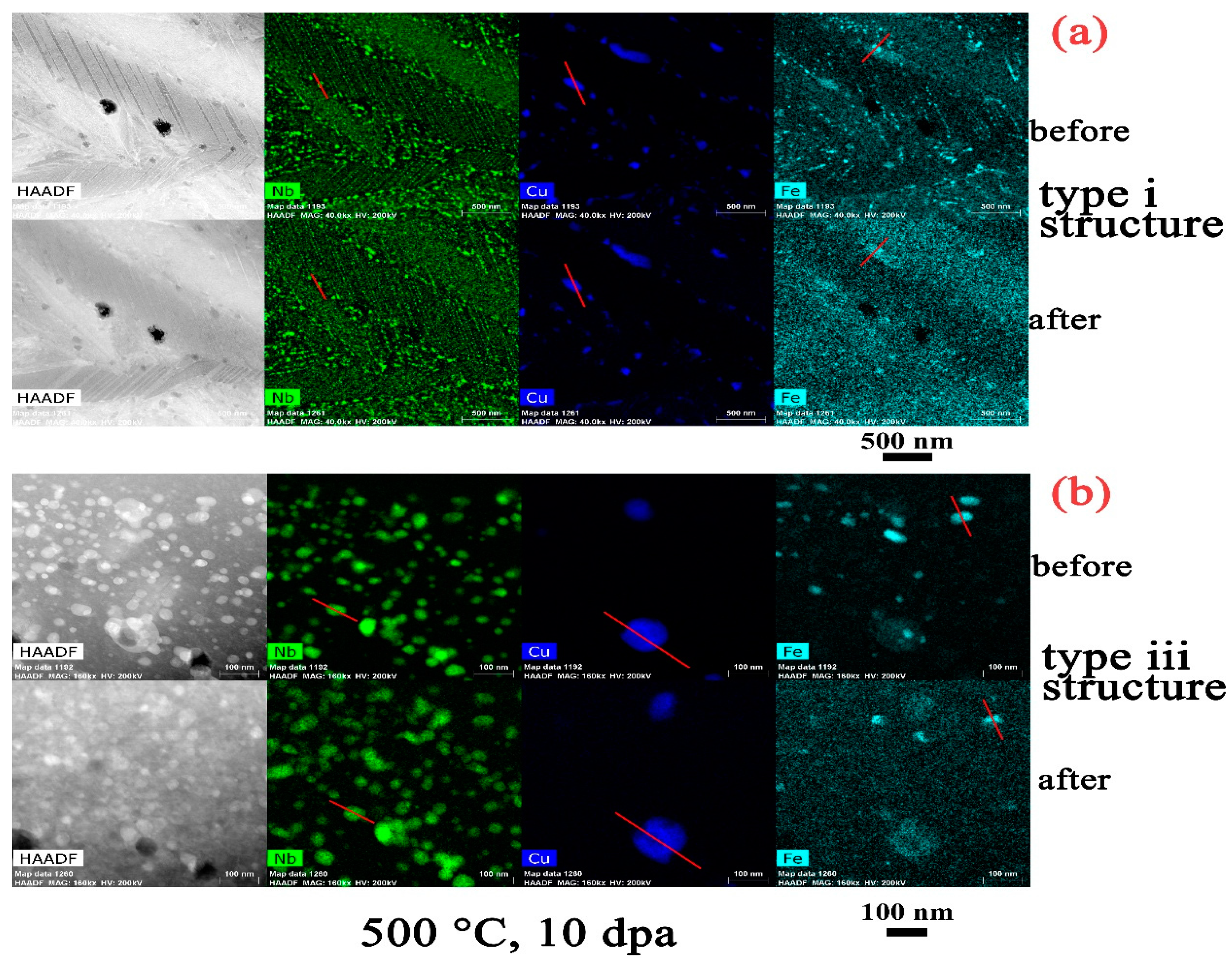
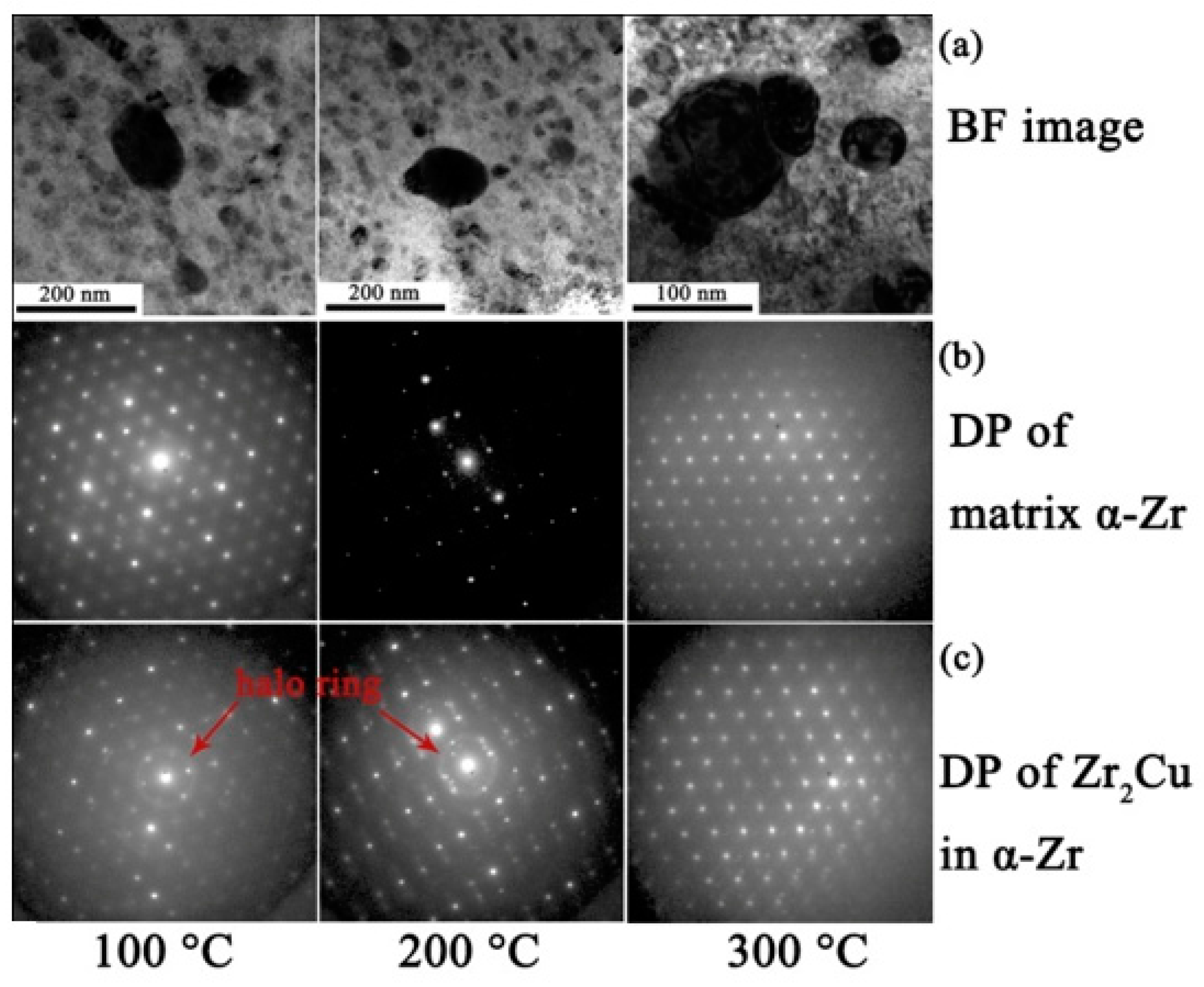
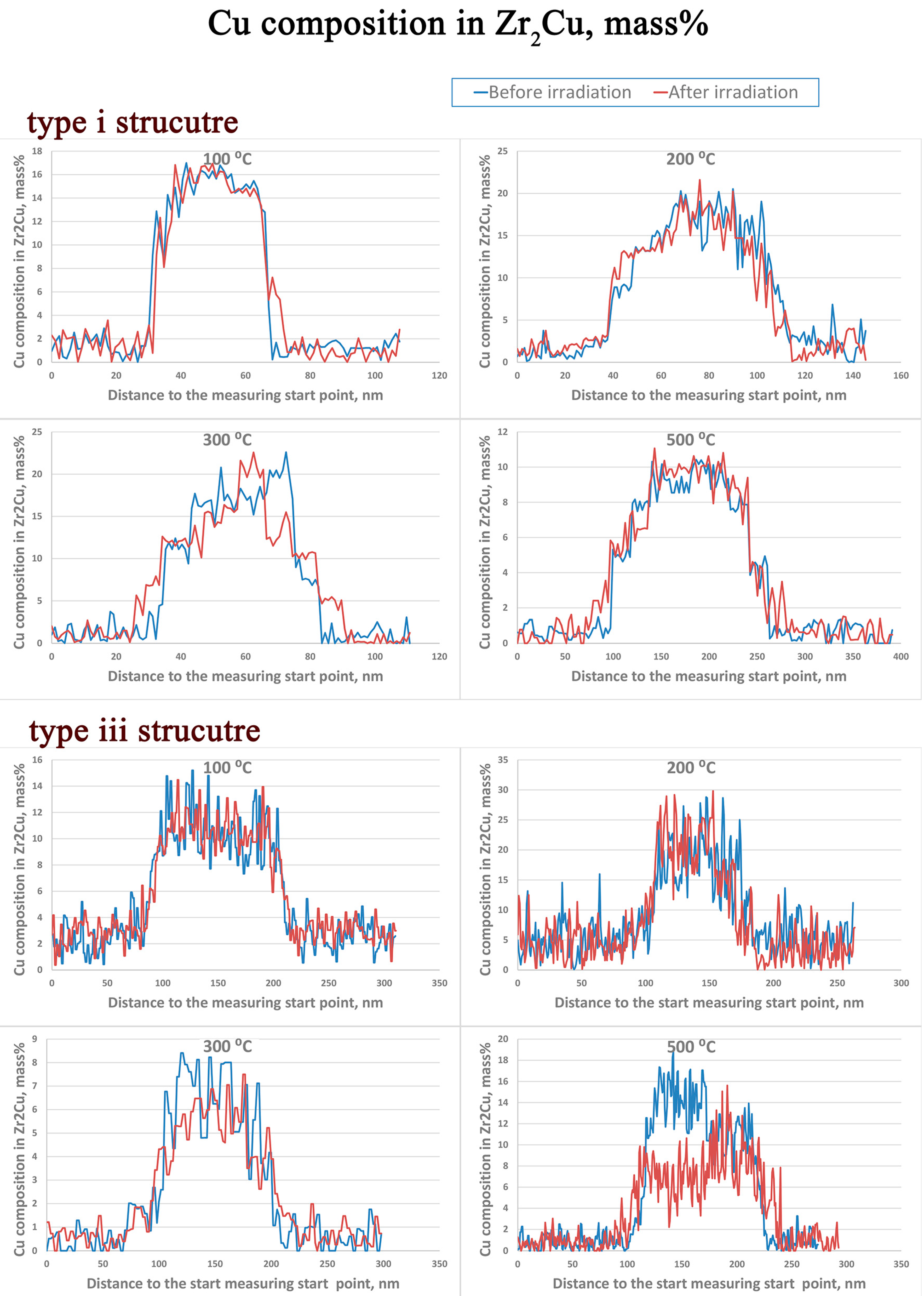
3.1. Zr2Cu
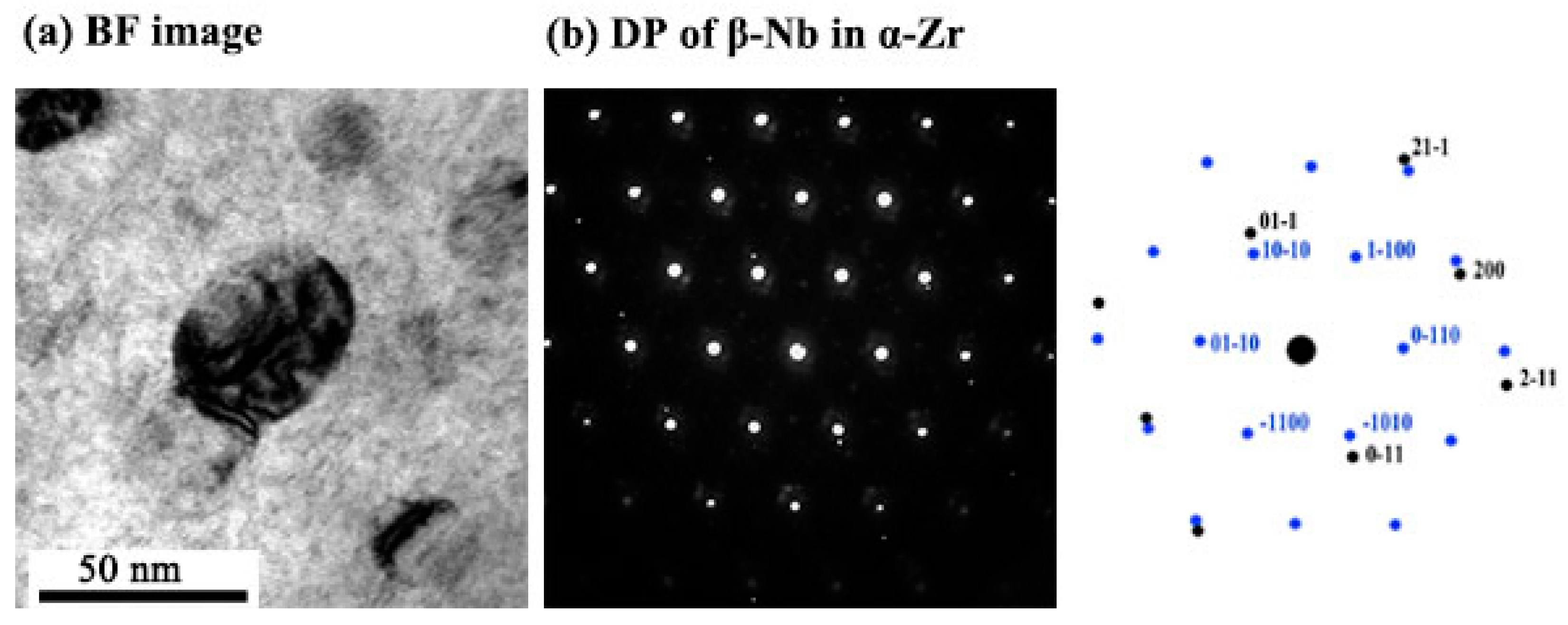
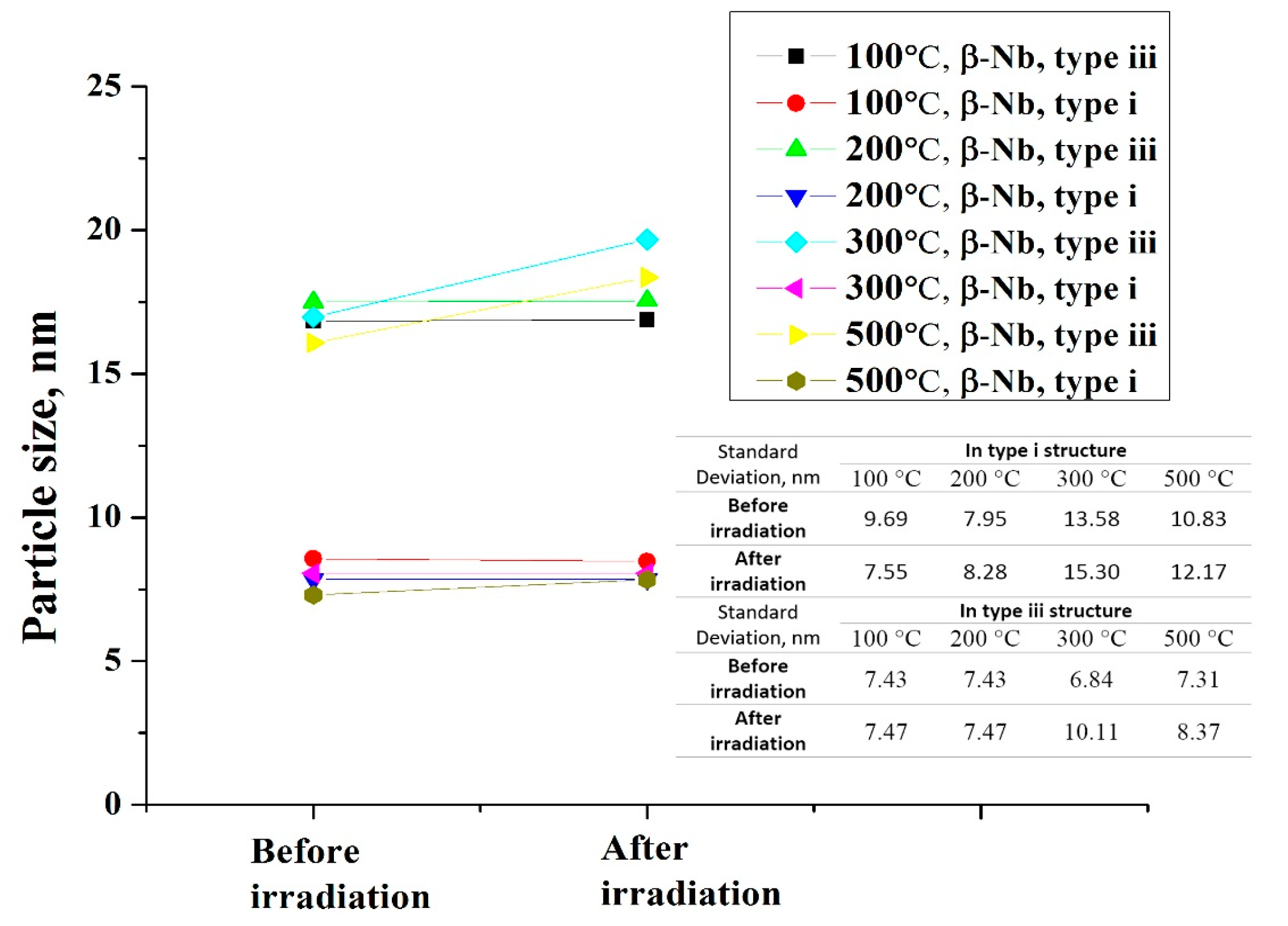
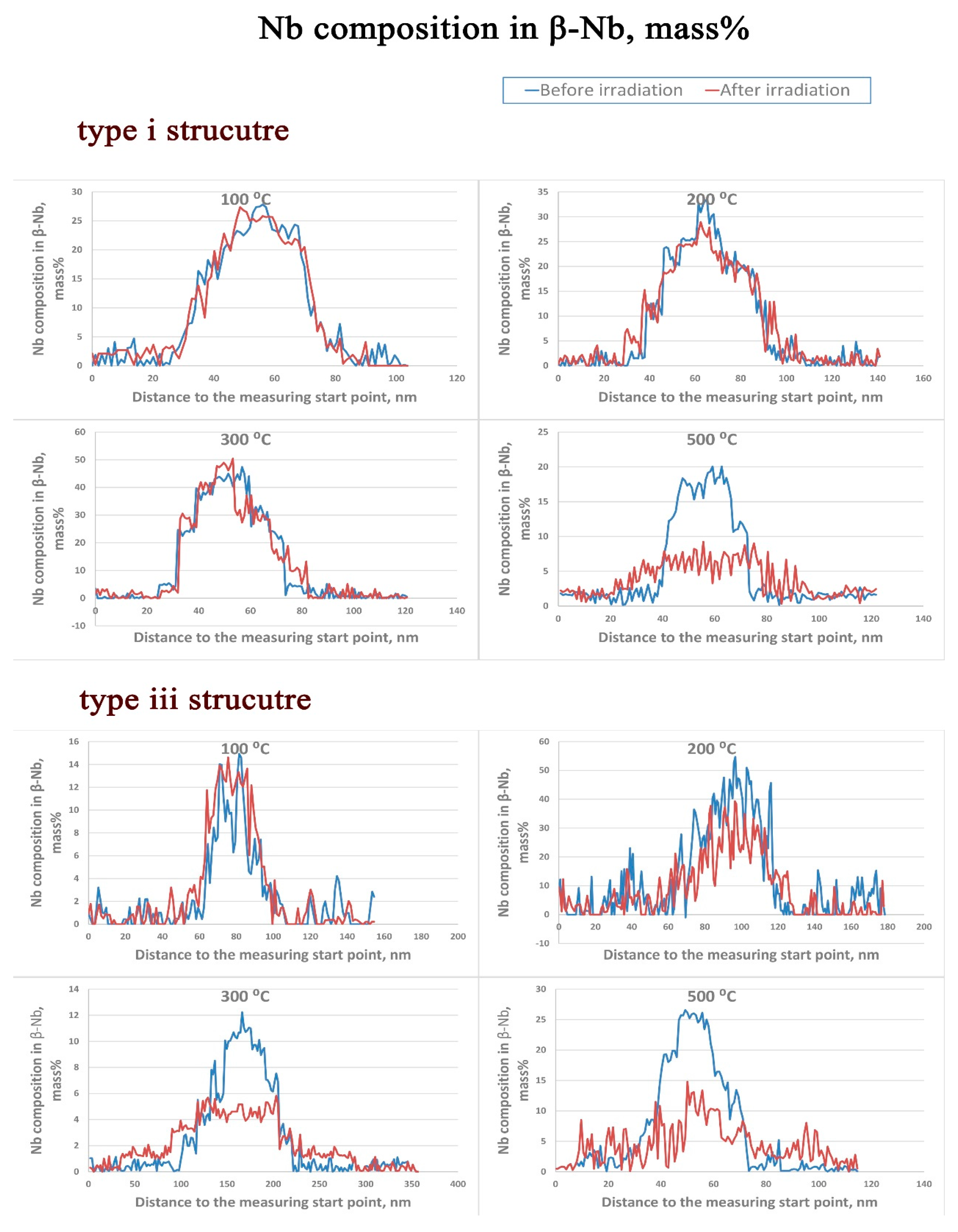
3.2. β–Nb
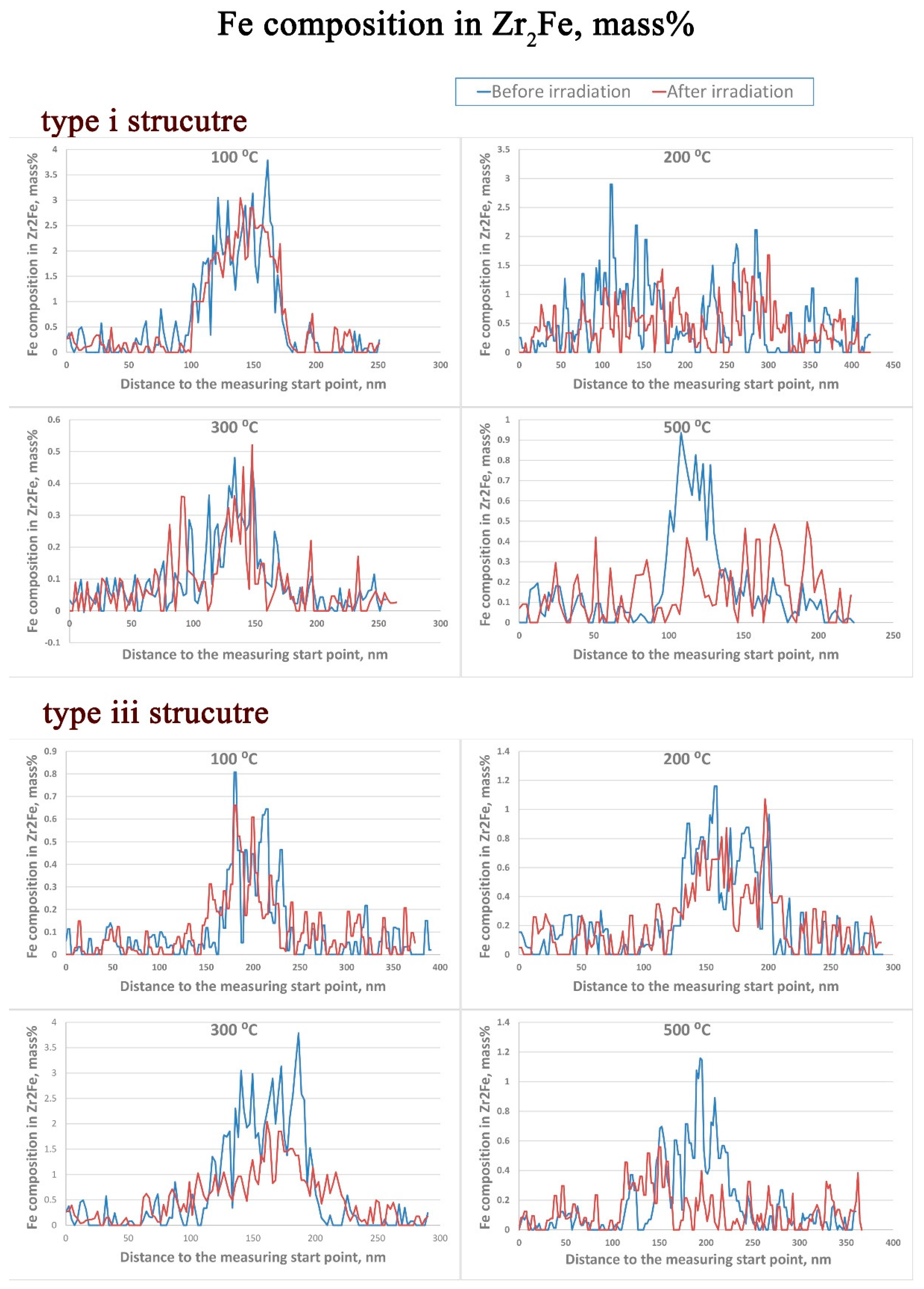
3.3. Zr2Fe
4. Discussion
4.1. Amorphization of Precipitates
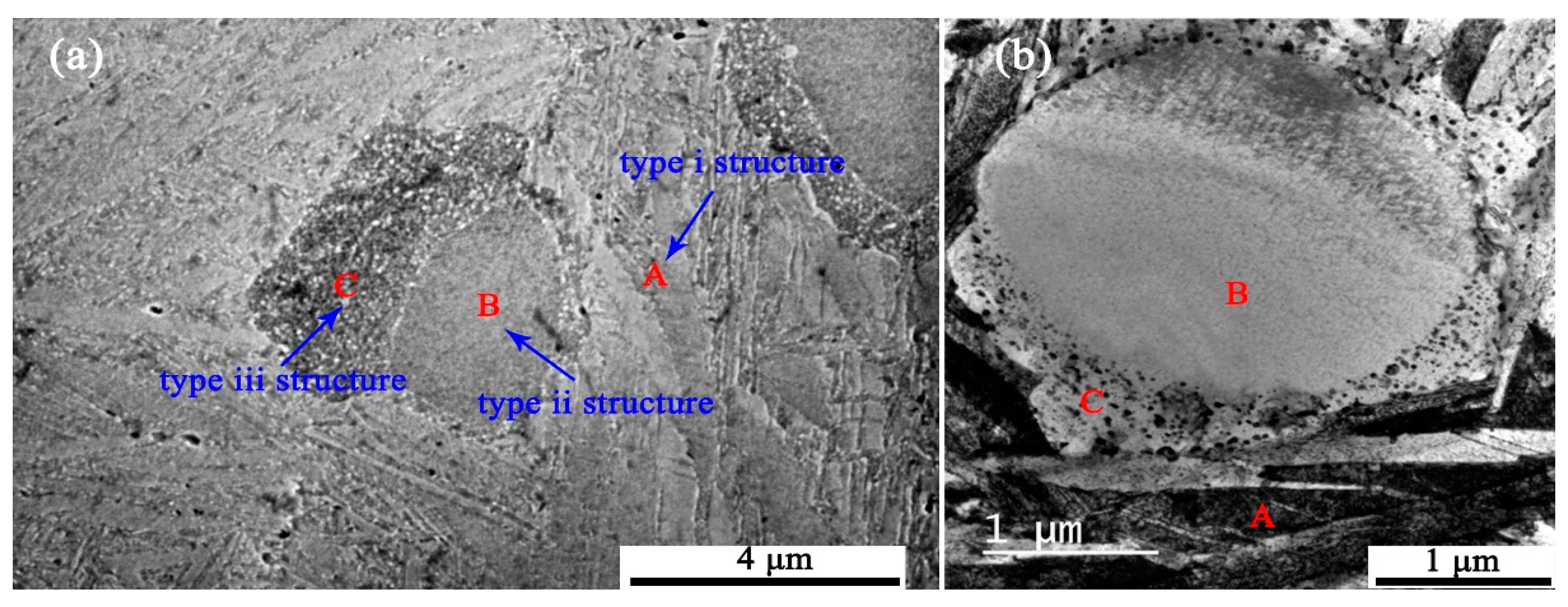
4.2. Effect of Structure on the Precipitate Stability
5. Conclusions
- (1)
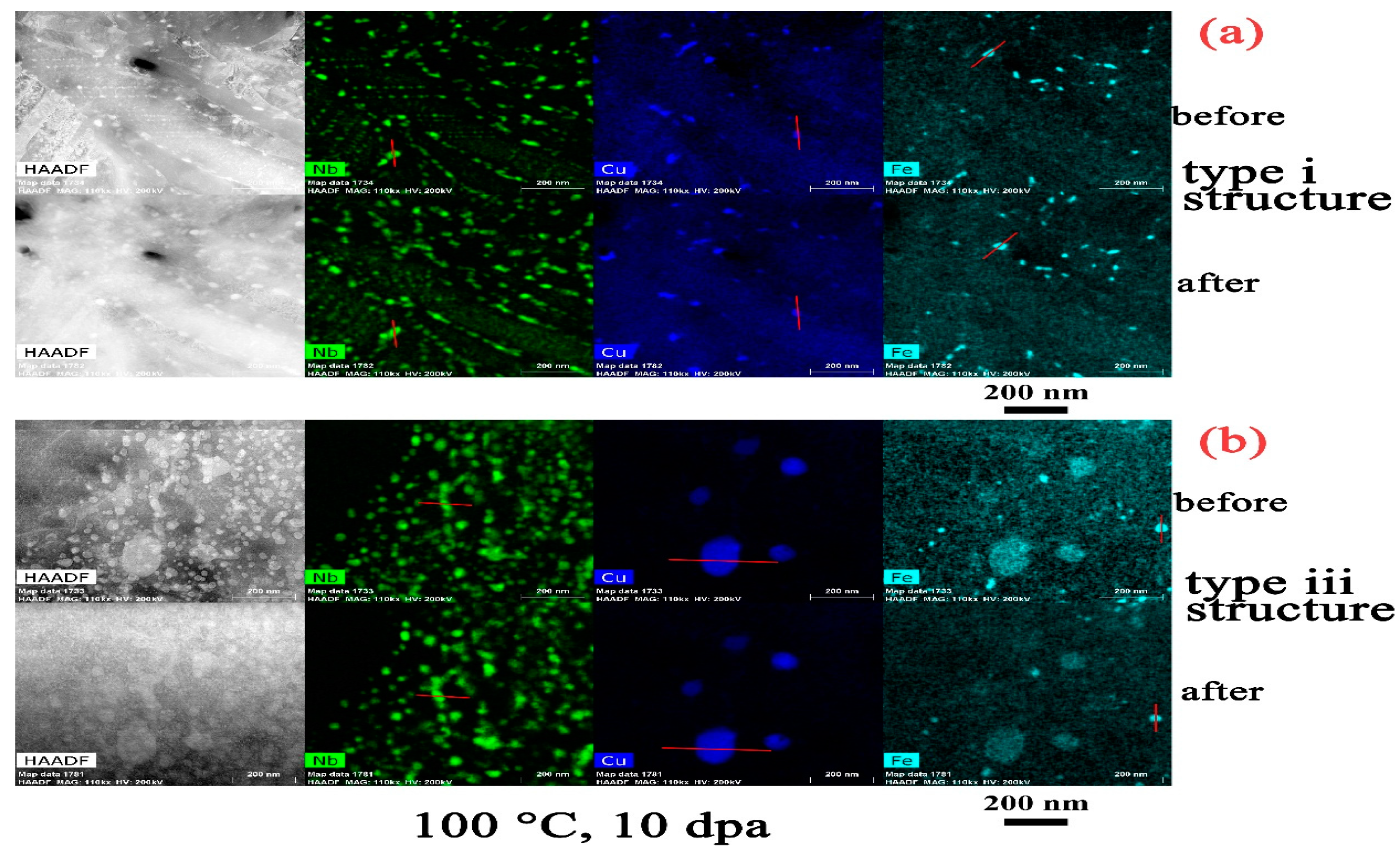
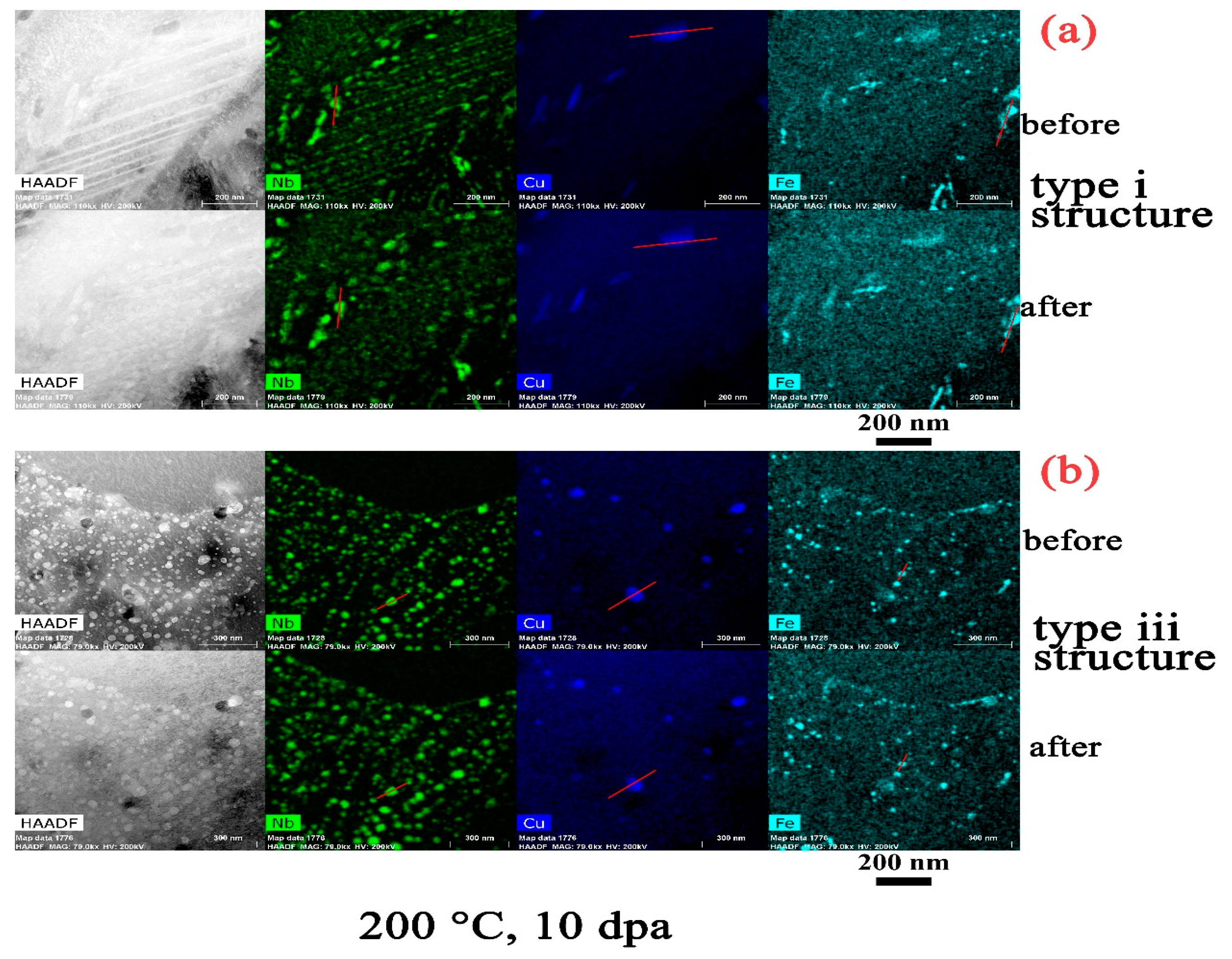
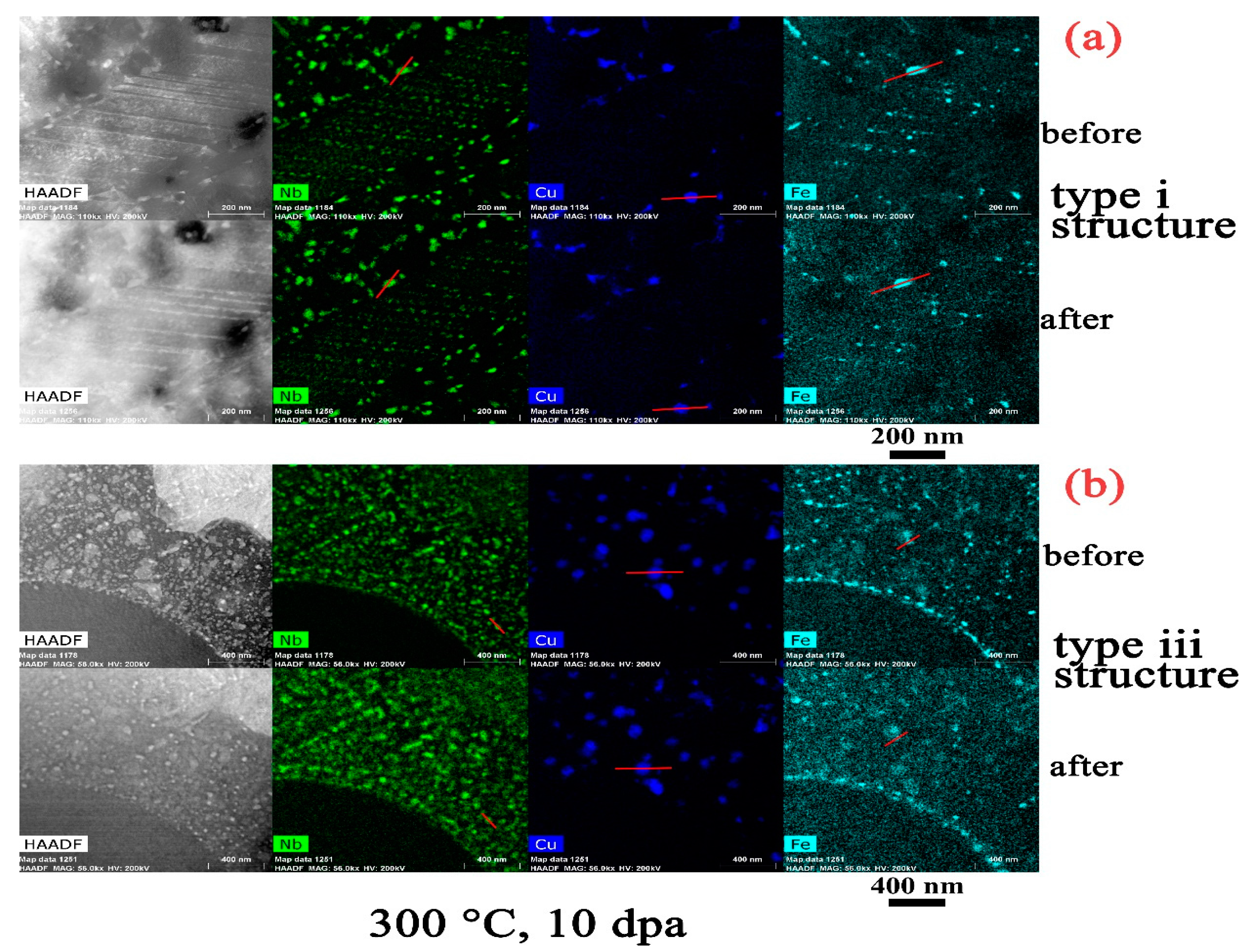
- The amorphization of Zr2Cu is found under irradiation to 10 dpa, and its transformation temperature is found to be between 200 °C to 300 °C, whereas amorphization of β–Nb is not detected at any temperature, including as low as 100 °C.
- (2)
- The surrounding microstructure has significant effects on the precipitate stability. The precipitates are much more stable in microstructures with multiple boundaries than in coarse grain microstructures.
- (3)
- There is an apparent increase of the precipitate size for Zr2Cu in the type iii structure at a temperature of 500 °C, while a size change in the type i structure or at lower irradiation temperatures in the type iii structure is not detected. This size change is in accordance with the measured redistribution of Cu in Zr2Cu.
- (4)
- The redistribution of Nb in β–Nb and the depletion of Fe from Zr2Fe are detected at 300 °C and above in the type iii structure, but such irradiation induced alloying element redistribution only starts at 500 °C in the type i structure.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Griffiths, M.; Gilbert, R.W.; Carpenter, G.J.C. Phase instability, decomposition and redistribution of intermetallic precipitates in Zircaloy–2 and –4 during neutron irradiation. J. Nucl. Mater. 1987, 150, 53–66. [Google Scholar] [CrossRef]
- Etoh, Y.; Shimada, S. Neutron irradiation effects on intermetallic precipitates in Zircaloy as a function of fluence. J. Nucl. Mater. 1993, 200, 59–69. [Google Scholar] [CrossRef]
- Kruger, R.M.; Adamson, R.B. Precipitate behavior in zirconium-based alloys in BWRs. J. Nucl. Mater. 1993, 205, 242–250. [Google Scholar] [CrossRef]
- Pêcheur, D.; Lefebvre, F.; Motta, A.T.; Lemaignan, C.; Charquet, D. Effect of irradiation on the precipitate stability in Zr alloys. J. Nucl. Mater. 1993, 205, 445–451. [Google Scholar] [CrossRef]
- Perovic, V.; Perovic, A.; Weatherly, G.C.; Purdy, G.R. The distribution of Nb and Fe in a Zr–2.5 wt % Nb alloy, before and after irradiation. J. Nucl. Mater. 1995, 224, 93–102. [Google Scholar] [CrossRef]
- Francis, E.M.; Harte, A.; Frankel, P.; Haigh, S.J.; Jädernäs, D.; Romero, J.; Hallstadius, L.; Preuss, M. Iron redistribution in a zirconium alloy after neutron and proton irradiation studied by energy–dispersive X-ray spectroscopy (EDX) using an aberration-corrected (scanning) transmission electron microscope. J. Nucl. Mater. 2014, 454, 387–397. [Google Scholar] [CrossRef]
- Nuttall, K.; Faulkner, D. The effect of irradiation on the stability of precipitates in Zr–2.5 wt % Nb alloys. J. Nucl. Mater. 1977, 67, 131–139. [Google Scholar] [CrossRef]
- Sabol, G.P.; Comstock, R.J.; Weiner, R.A.; Larouere, P.; Stanutz, R.N. In-Reactor Corrosion Performance of ZIRLO and Zinealoy4. In Proceedings of the Zirconium in the Nuclear Industry: Tenth International Symposium, Baltimore, MD, USA, 21–24 June 1993; ASTM International: Philadelphia, PA, USA, 1994. [Google Scholar]
- Anada, H.; Nomoto, K.I.; Shida, Y. Corrosion behavior of Zircaloy–4 sheets produced under various hot-rolling and annealing conditions. In Proceedings of the Zirconium in the Nuclear Industry: Tenth International Symposium, Baltimore, MD, USA, 21–24 June 1993; ASTM International: Philadelphia, PA, USA, 1994. [Google Scholar]
- Jeong, Y.H.; Lee, K.O.; Kim, H.G. Correlation between microstructure and corrosion behavior of Zr–Nb binary alloy. J. Nucl. Mater. 2002, 302, 9–19. [Google Scholar] [CrossRef]
- Kim, H.G.; Jeong, Y.H.; Kim, T.H. Effect of isothermal annealing on the corrosion behavior of Zr–xNb alloys. J. Nucl. Mater. 2004, 326, 125–131. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, B.K.; Jeong, Y.H.; Jung, Y.H. Corrosion behavior of Zr alloys with a high Nb content. J. Nucl. Mater. 2005, 340, 237–246. [Google Scholar] [CrossRef]
- Griffiths, M.; Gilbert, R.W.; Fidleris, V. Accelerated Irradiation Growth of Zirconium Alloys. In Proceedings of the Zirconium in the Nuclear Industry: Eighth International Symposium, San Diego, CA, USA, 19–23 June 1988; ASTM International: Philadelphia, PA, USA, 1989. [Google Scholar]
- Holt, R.A. In-reactor deformation of cold-worked Zr–2.5Nb pressure tubes. J. Nucl. Mater. 2008, 372, 182–214. [Google Scholar] [CrossRef]
- Russell, K.C. Phase stability under irradiation. Prog. Mater. Sci. 1984, 28, 229–434. [Google Scholar] [CrossRef]
- King, A.D.; Hood, G.M.; Holt, R.A. Fe–enhancement of self-diffusion in α–Zr. J. Nucl. Mater. 1991, 185, 174–181. [Google Scholar] [CrossRef]
- Zhang, H.K.; Yao, Z.; Morin, G.; Griffiths, M. TEM characterization of in-reactor neutron irradiated CANDU spacer material Inconel X–750. J. Nucl. Mater. 2014, 451, 88–96. [Google Scholar] [CrossRef]
- Field, G.J.; Dunn, J.T.; Cheadle, B.A. Analysis of the Pressure Tube Failure at Pickering NGS “A” Unit 2 Nuclear Systems Department. Can. Metall. Q. 1985, 24, 181–188. [Google Scholar] [CrossRef]
- Coleman, C.E.; Gilbert, R.W.; Carpenter, G.J.C.; Wetherly, G.C. Precipitation in Zr–2.5 wt % Nb during neutron irradiation. In Proceedings of the Phase Stability during Irradiation Symposium, Pittsburgh, PA, USA, 5–9 October 1980; The Metallurgical Society of AIME: New York, NY, USA, 1981; pp. 587–599. [Google Scholar]
- Dong, Q.; Yu, H.; Yao, Z.; Long, F.; Balogh, L.; Daymond, M.R. Study of microstructure and precipitates of a Zr–2.5Nb–0.5Cu CANDU spacer material. J. Nucl. Mater. 2016, 481, 153–163. [Google Scholar] [CrossRef]
- Was, G.S. Fundamentals of Radiation Materials Science: Metals and Alloys; Springer: New York, NY, USA, 2007; p. 52. [Google Scholar]
- Gilbon, D.; Soniak, A.; Doriot, S.; Mardon, J.P. Irradiation Creep and Growth Behavior, and Microstructural Evolution of Advanced Zr–Base Alloys. In Proceedings of the Zirconium in the Nuclear Industry: Twelfth International Symposium, Toronto, ON, Canada, 15–18 June 1988; ASTM International: Philadelphia, PA, USA, 2000. [Google Scholar]
- Griffiths, M.; Müllejans, H. A TEM study of α–phase stability in Zr–2.5 Nb pressure tubes following neutron irradiation (A TEM study of α–phase stability). Micron 1995, 26, 555–557. [Google Scholar] [CrossRef]
- Cann, C.D.; So, C.B.; Styles, R.C.; Coleman, C.E. Precipitation in Zr–2.5Nb enhanced by proton irradiation. J. Nucl. Mater. 1993, 205, 267–272. [Google Scholar] [CrossRef]
- Griffiths, M.; Gilbert, R.W.; Coleman, C.E. Grain boundary sinks in neutron-irradiated Zr and Zr-alloys. J. Nucl. Mater. 1988, 159, 405–416. [Google Scholar] [CrossRef]
- Yang, W.J.S.; Tucker, R.P.; Cheng, B.; Adamson, R.B. Precipitates in zircaloy: Identification and the effects of irradiation and thermal treatment. J. Nucl. Mater. 1986, 138, 185–195. [Google Scholar] [CrossRef]
- Yang, W.J.S. Precipitate stability in neutron-irradiated Zircaloy–4. J. Nucl. Mater. 1988, 158, 71–80. [Google Scholar] [CrossRef]
- Motta, A.T.; Howe, L.M.; Okamoto, P.R. Amorphization kinetics of Zr3Fe under electron irradiation. J. Nucl. Mater. 1993, 205, 258–266. [Google Scholar] [CrossRef]
- Nagase, T.; Umakoshi, Y. Phase stability of amorphous and crystalline phases in melt-spun Zr66.7Cu33.3 alloy under electron irradiation. Scr. Mater. 2003, 48, 1237–1242. [Google Scholar] [CrossRef]
- Brimhall, J.L.; Kissinger, H.E.; Charlot, L.A. Amorphous phase formation in irradiated intermetallic compounds. Radiat. Eff. 1983, 77, 273–293. [Google Scholar] [CrossRef]
- Abriata, J.P.; Bolcich, J.C. The Nb–Zr (Niobium-Zirconium) system. Bull. Alloy Phase Diagr. 1982, 3, 34–44. [Google Scholar] [CrossRef]
- Arias, D.; Abriata, J.P. Cu–Zr (Copper–Zirconium). Bull. Alloy Phase Diagr. 1990, 11, 452–459. [Google Scholar] [CrossRef]
- Dey, G.K.; Singh, R.N.; Tewari, R.; Srivastava, D.; Banerjee, S. Metastability of the β–phase in Zr-rich Zr–Nb alloys. J. Nucl. Mater. 1995, 224, 146–157. [Google Scholar] [CrossRef]
- Jung, Y.I.; Lee, M.H.; Kim, H.G.; Park, J.Y.; Jeong, Y.H. Behavior of a recrystallization in HANA–4 and HANA-6 zirconium-based alloys. J. Alloys Compd. 2009, 479, 423–426. [Google Scholar] [CrossRef]
- Bai, X.M.; Voter, A.F.; Hoagland, R.G.; Nastasi, M.; Uberuaga, B.P. Efficient Annealing of Radiation Damage Near Grain Boundaries via Interstitial Emission. Science 2010, 327, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Han, W.Z.; Demkowicz, M.J.; Fu, E.G.; Wang, Y.Q.; Misra, A. Effect of grain boundary character on sink efficiency. Acta Mater. 2012, 60, 6341–6351. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Yu, K.Y.; Wang, H.; Kirk, M.A.; Li, M.; Zhang, X. In situ studies on radiation tolerance of nanotwinned Cu. Acta Mater. 2016, 111, 148–156. [Google Scholar] [CrossRef]
- Shu, S.; Bellon, P.; Averback, R.S. Role of point-defect sinks on irradiation-induced compositional patterning in model binary alloys. Phys. Rev. B 2015, 91, 214107. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, S.; Bellon, P.; Averback, R.S. Precipitate stability in Cu–Ag–W system under high-temperature irradiation. Acta Mater. 2015, 97, 348–356. [Google Scholar] [CrossRef]
- Di, C.; Wang, J.; Chen, T.; Shao, L. Defect annihilation at grain boundaries in alpha–Fe. Sci. Rep. 2013, 3, 1450. [Google Scholar]
- Song, M.; Wu, Y.D.; Chen, D.; Wang, X.M.; Sun, C.; Yu, K.Y.; Chen, Y.; Shao, L.; Yang, Y.; Hartwig, K.T.; et al. Response of equal channel angular extrusion processed ultrafine-grained T91 steel subjected to high temperature heavy ion irradiation. Acta Mater. 2014, 74, 285–295. [Google Scholar] [CrossRef]
- Sun, C.; Song, M.; Yu, K.Y.; Chen, Y.; Kirk, M.; Li, M.; Wang, H.; Zhang, X. In situ Evidence of Defect Cluster Absorption by Grain Boundaries in Kr Ion Irradiated Nanocrystalline Ni. Metall. Mater. Trans. A 2013, 44, 1966–1974. [Google Scholar] [CrossRef]
- Idrees, Y.; Yao, Z.; Kirk, M.A.; Daymond, M.R. In situ study of defect accumulation in zirconium under heavy ion irradiation. J. Nucl. Mater. 2013, 433, 95–107. [Google Scholar] [CrossRef]
- Singh, B.N.; Foreman, A.J.E. Calculated grain size-dependent vacancy supersaturation and its effect on void formation. Philos. Mag. 1974, 29, 847–858. [Google Scholar] [CrossRef]
- Motta, A.T.; Faldowski, J.A.; Howe, L.M.; Okamoto, P.R. In situ studies of phase transformations in zirconium alloys and compounds under irradiation. In Proceedings of the Zirconium in the Nuclear Industry: Eleventh International Symposium, Garmisch-Partenkirchen, Germany, 11–14 September 1995; ASTM International: Philadelphia, PA, USA, 1996. [Google Scholar]
- Baštecká, J. Interaction of dislocation loop with free surface. Cechoslovackij Fiziceskij Zurnal B 1964, 14, 430–442. [Google Scholar] [CrossRef]
- Narayan, J.; Washburn, J. Stability of dislocation loops near a free surface. J. Appl. Phys. 1972, 43, 4862–4865. [Google Scholar] [CrossRef]
- Yi, X.; Jenkins, M.L.; Hattar, K.; Edmondson, P.D.; Roberts, S.G. Characterisation of radiation damage in W and W-based alloys from 2 MeV self-ion near-bulk implantations. Acta Mater. 2015, 92, 163–177. [Google Scholar] [CrossRef]
- Dong, Q.; Yu, H.; Qin, H.; Yao, Z.; Daymond, M.R. A direct comparison of annealing in TEM thin foils and bulk material in a zirconium alloy. To be submitted.

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Yao, Z.; Wang, Q.; Yu, H.; Kirk, M.A.; Daymond, M.R. Precipitate Stability in a Zr–2.5Nb–0.5Cu Alloy under Heavy Ion Irradiation. Metals 2017, 7, 287. https://doi.org/10.3390/met7080287
Dong Q, Yao Z, Wang Q, Yu H, Kirk MA, Daymond MR. Precipitate Stability in a Zr–2.5Nb–0.5Cu Alloy under Heavy Ion Irradiation. Metals. 2017; 7(8):287. https://doi.org/10.3390/met7080287
Chicago/Turabian StyleDong, Qingshan, Zhongwen Yao, Qiang Wang, Hongbing Yu, Mark A. Kirk, and Mark R. Daymond. 2017. "Precipitate Stability in a Zr–2.5Nb–0.5Cu Alloy under Heavy Ion Irradiation" Metals 7, no. 8: 287. https://doi.org/10.3390/met7080287




