Crystallization and Beneficiation of Magnetite for Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Equipmental and Analytical Method
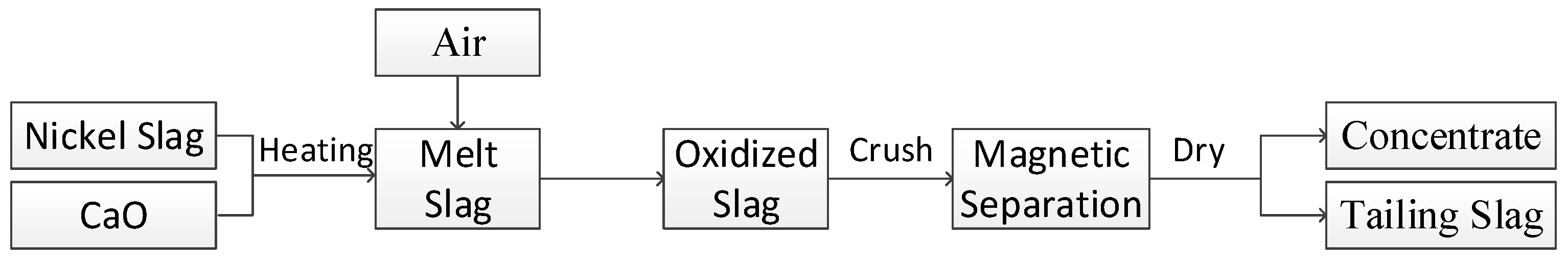
2.3. Experimental Procedure
3. Results and Discussion
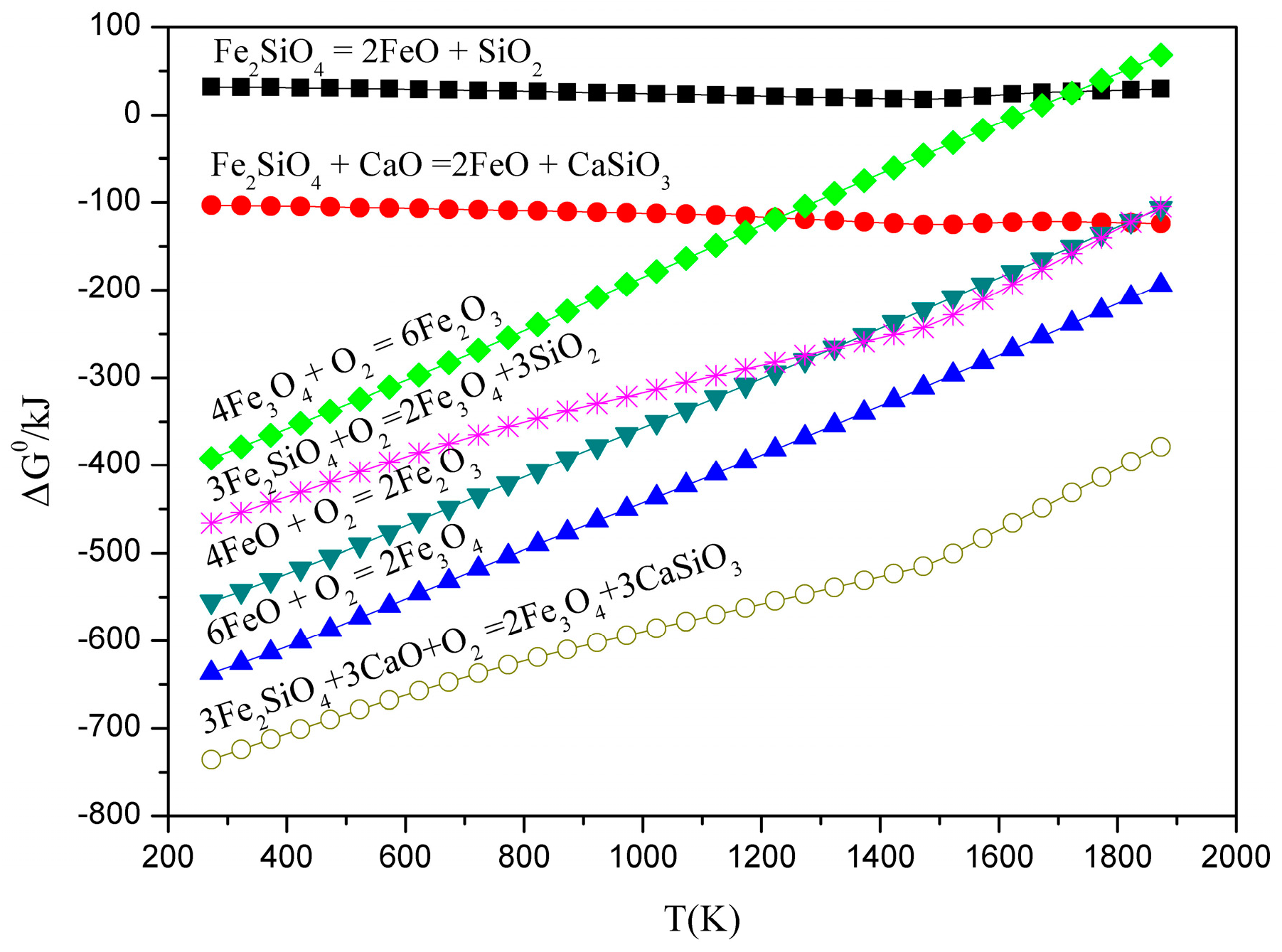
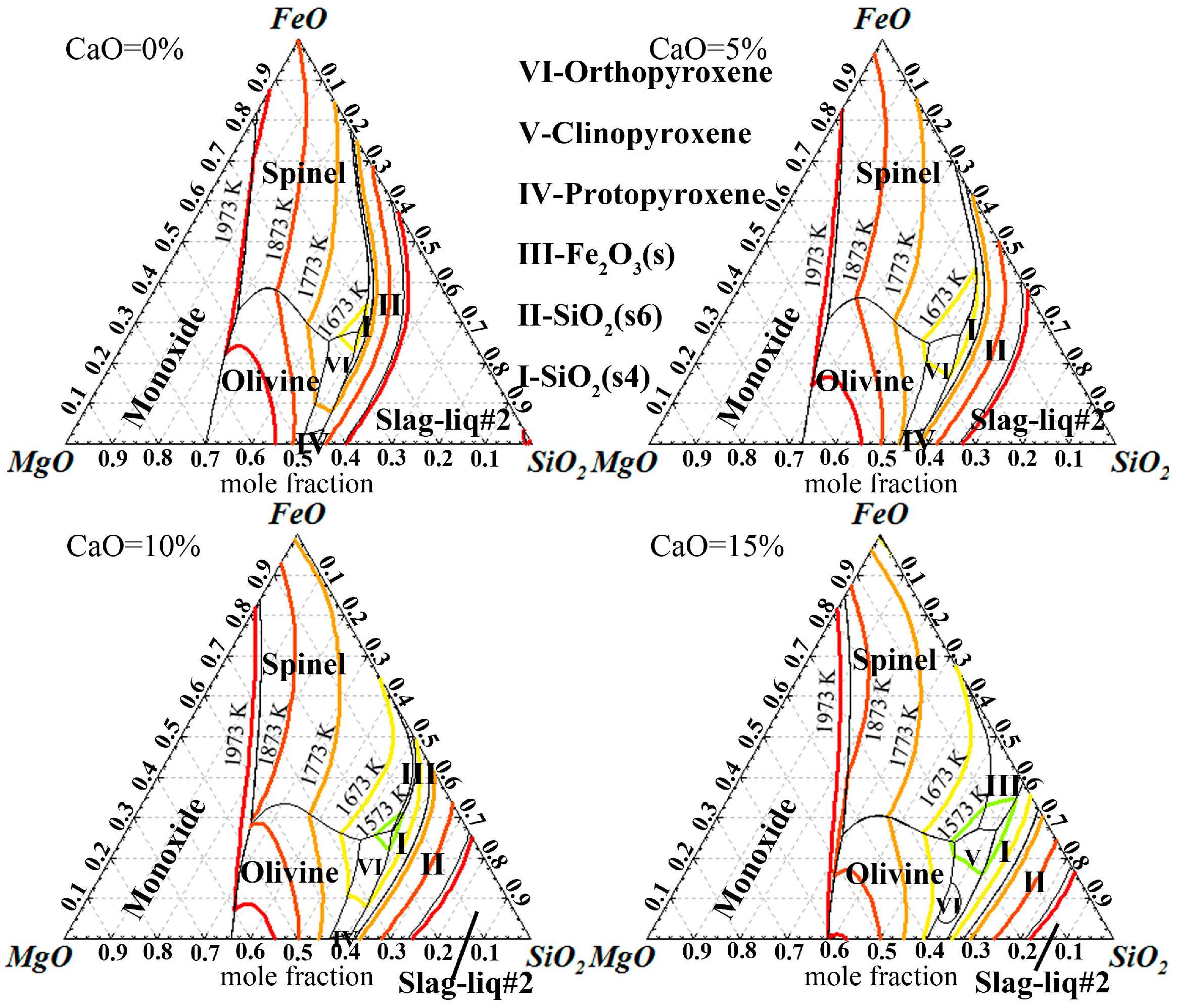
3.1. Thermodynamic Feasibility Analysis
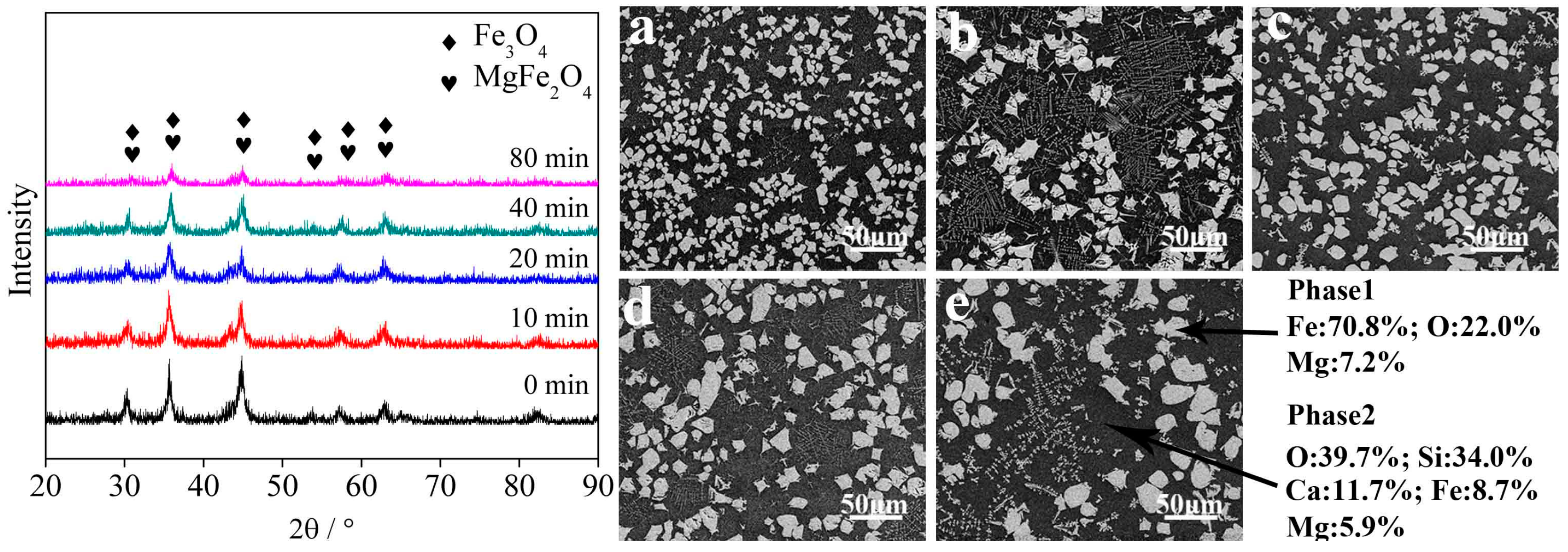
3.2. Influence Factors on the Crystallization of Magnetite
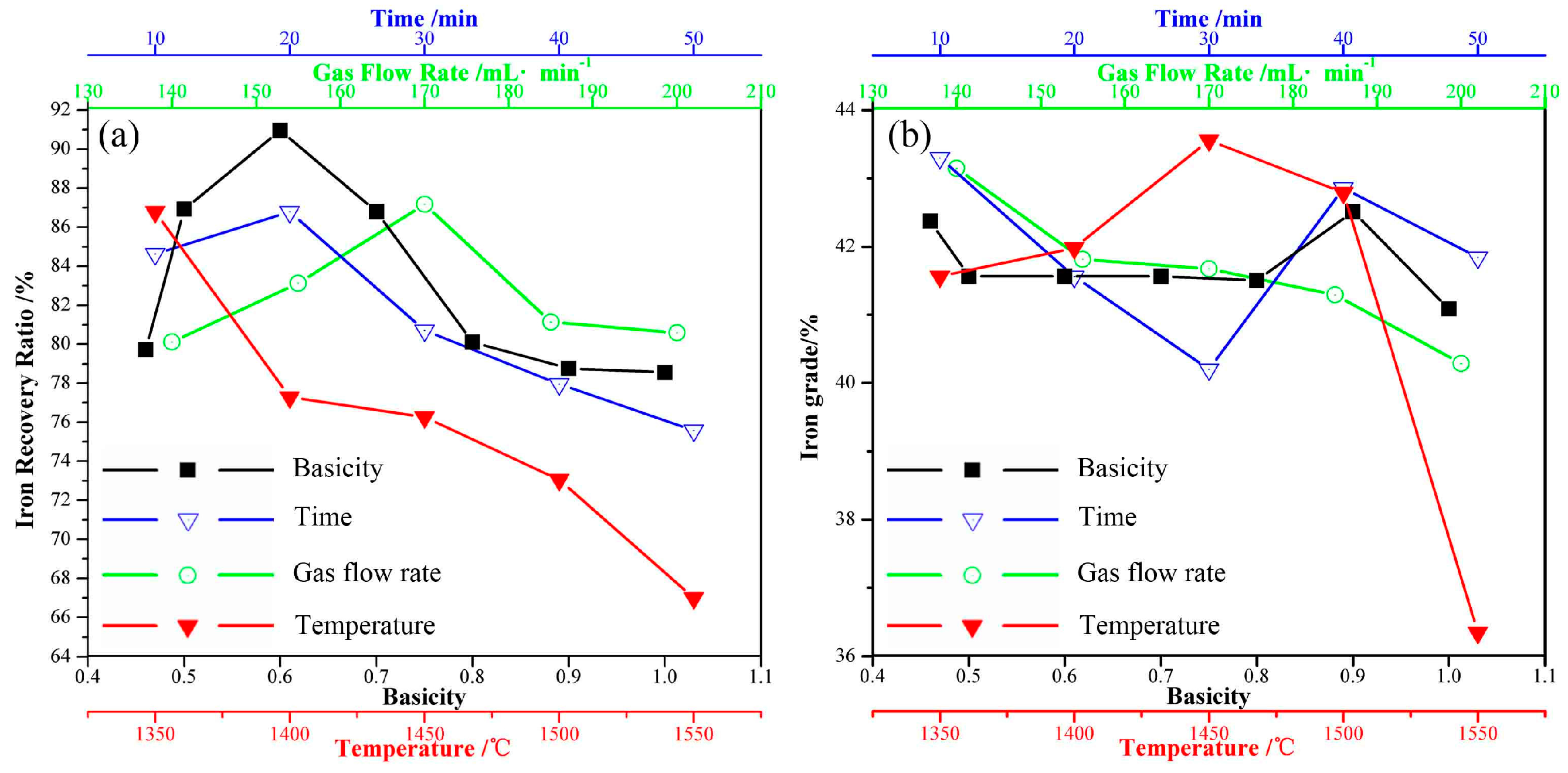
3.2.1. Holding Time
3.2.2. Temperature
3.2.3. Air Flow Rate
3.2.4. Basicity
3.3. Magnetic Separation
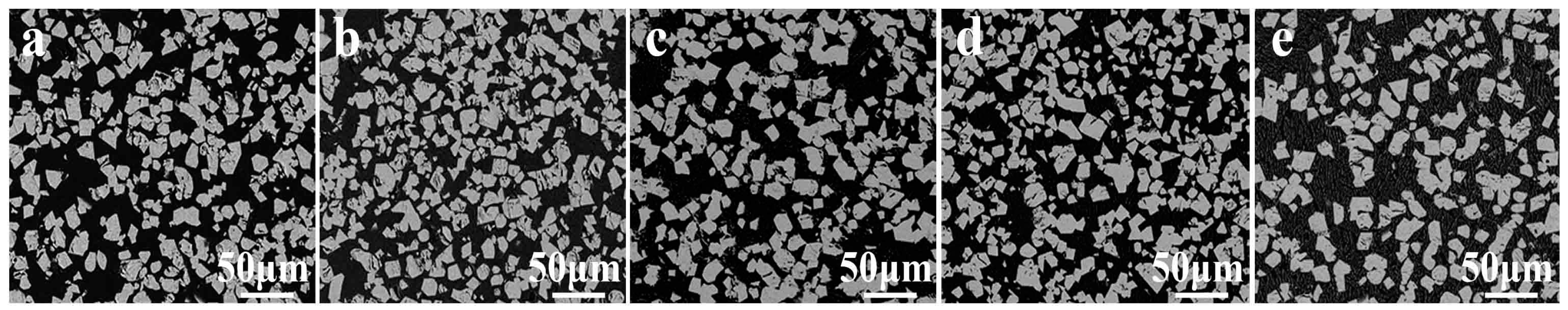
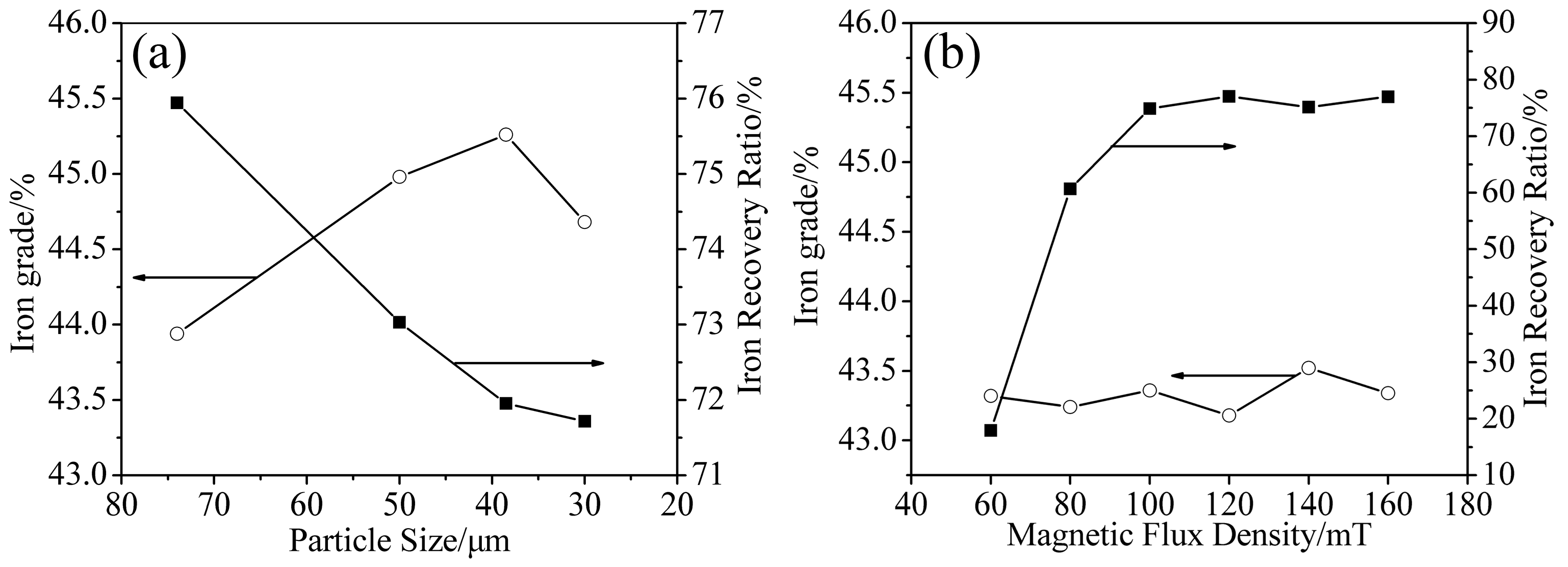
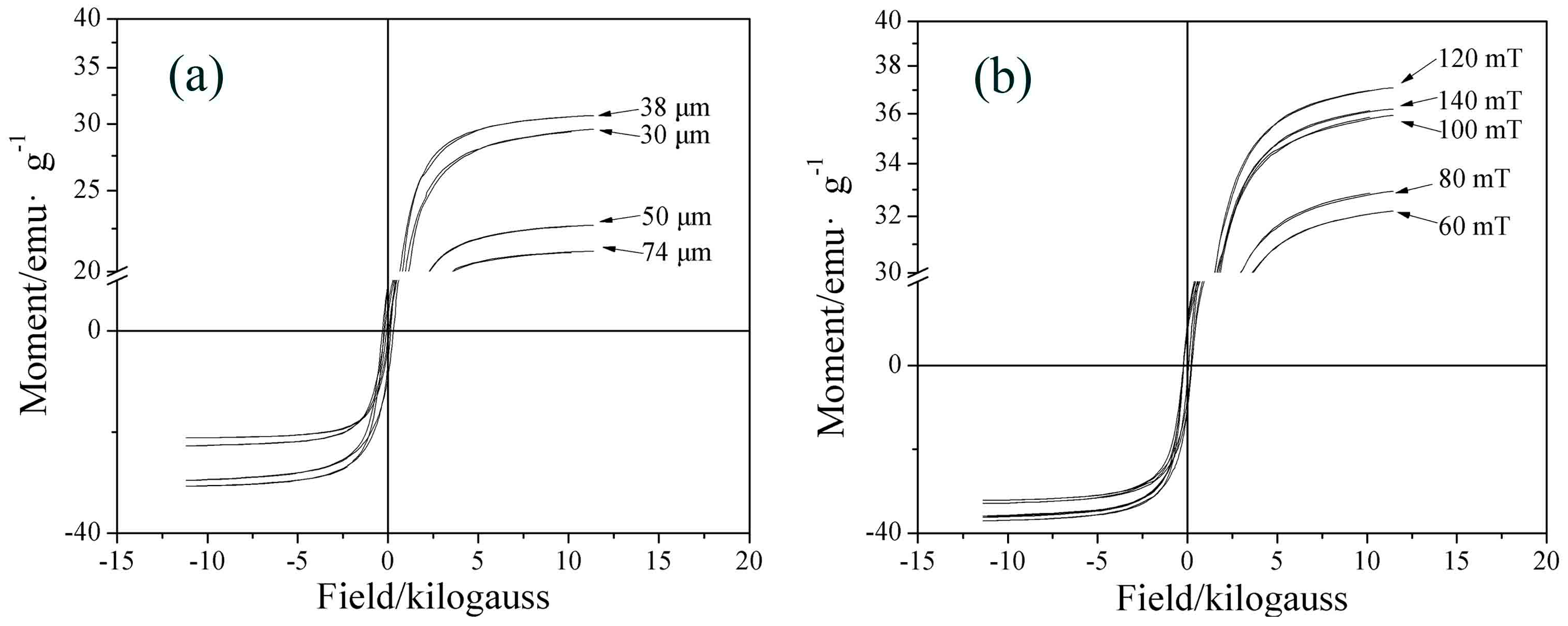
3.3.1. Particle Size
3.3.2. Magnetic Flux Density
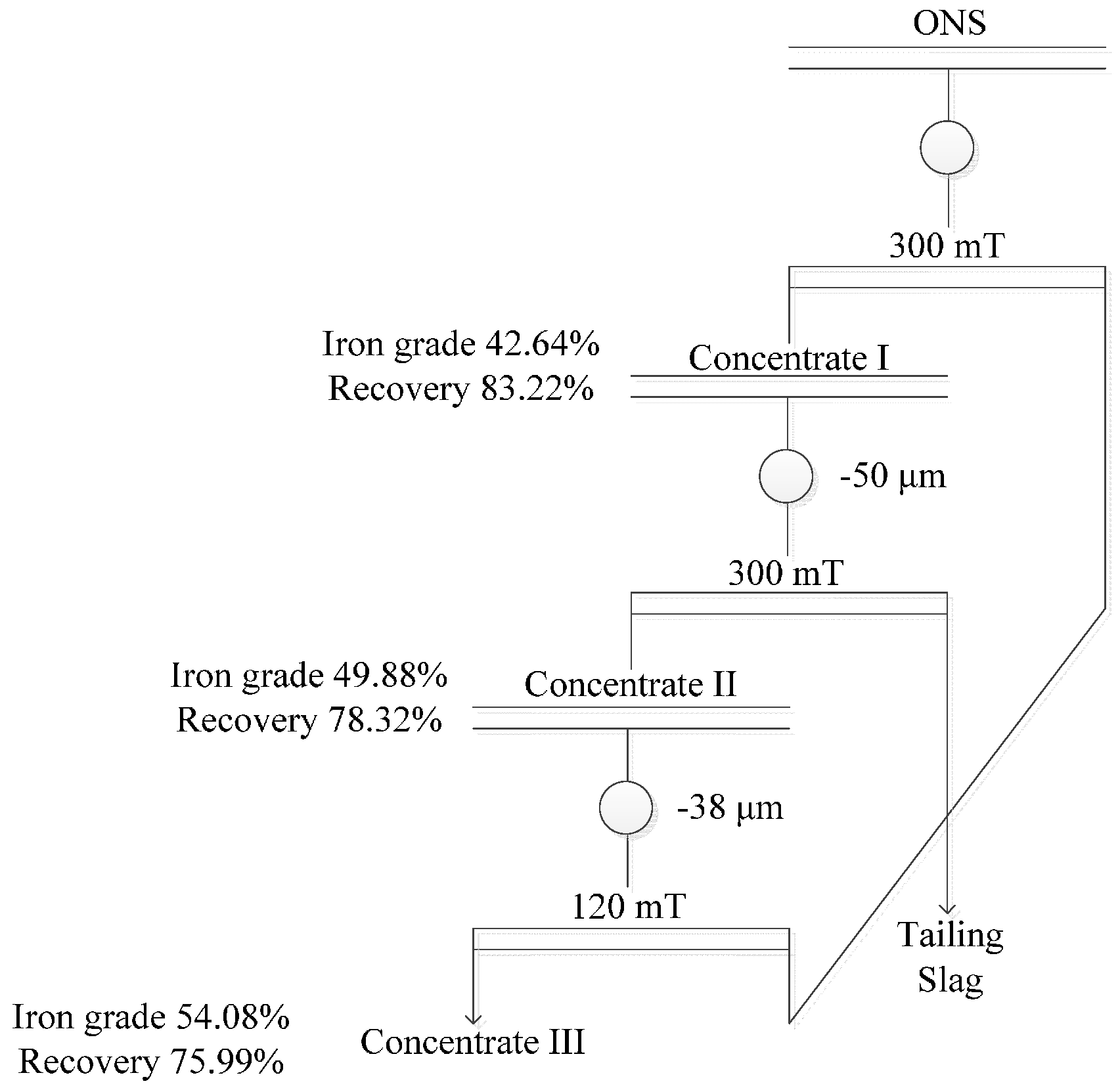
3.3.3. Optimal Conditions
4. Conclusions
- (1)
- It can be seen from the thermodynamic analysis that fayalite in nickel slag cannot be decomposed spontaneously, even with temperatures of up to 1873 K, but can be oxidized into magnetite with the help of CaO and O2. Liquidus projection of FeO-SiO2-MgO-CaO-O2 shows that the addition of CaO can not only expand the spinel phase zone, but also obviously decrease the liquidus temperature.
- (2)
- Fayalite in nickel slags can be oxidized to form magnetite phase in air at high temperature. The holding time, temperature, and air flow rate significantly affect the crystallization behavior of magnetite, while the addition of CaO affects it slightly. The average diameter size of the magnetite reaches 20 μm after undergoing a holding process at 1623 K for 20 min. Temperature and air flow rate obviously affect the shapes of magnetite, which exhibits spherical, polyhedral and skeletal shapes.
- (3)
- The iron recovery and grade are obviously affected by particle size and magnetic flux density. The iron recovery decreases with the decrease of particle size, while the iron grade reaches a maximal value at 38 μm. As the magnetic flux density increases, the iron recovery initially increases rapidly, reaching a maximal value at 120 mT, while the iron grade remains almost constant.
- (4)
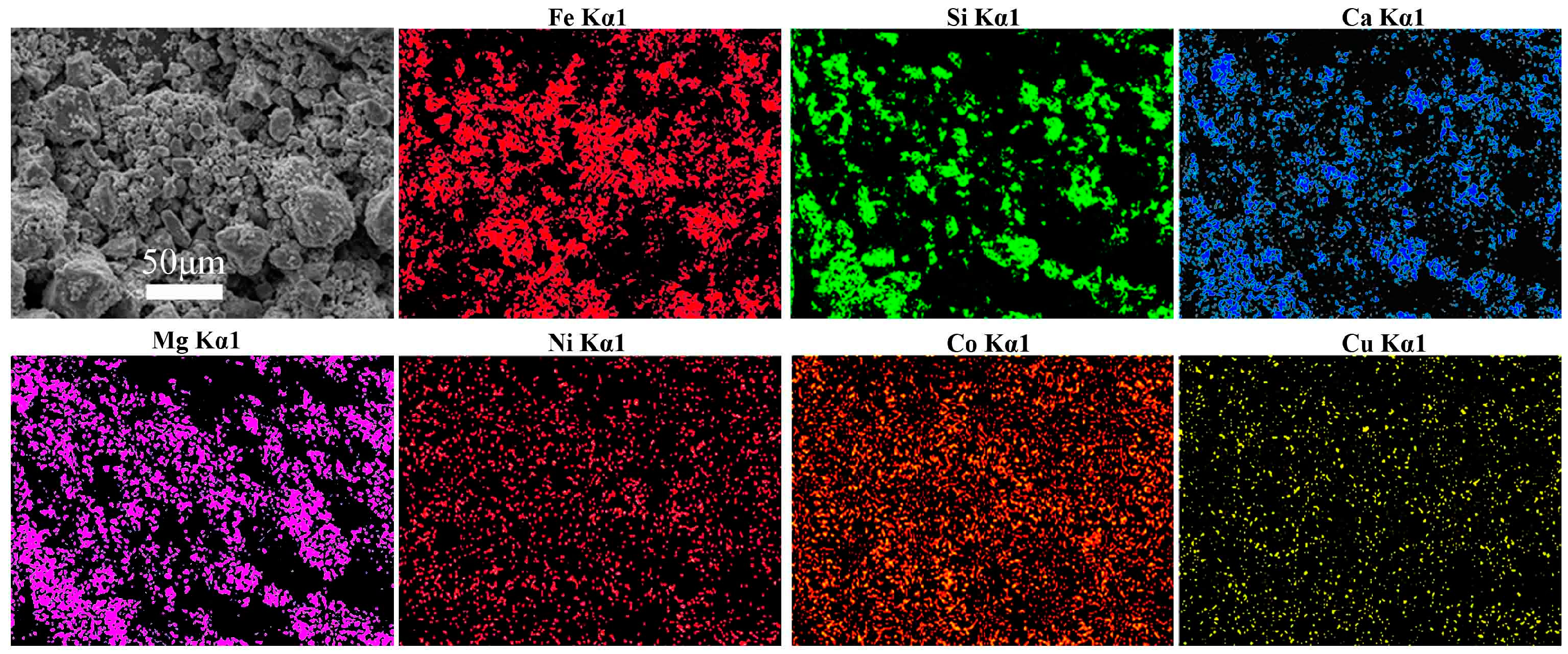
- The final iron recovery and grade reaches 75.99% and 54.08%, respectively, via multi-step magnetic separation, instead of single magnetic separation. Iron in the concentrate mainly exists in the form of magnetite and magnesium ferrite; and the contents of siderophile elements (Ni, Co) in the final concentrate were also higher than that of the raw slags.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, R.T. Economic and environmentally beneficial treatment of slags in DC arc furnaces. In Proceedings of the VII International Conference on Molten Slags, Fluxes and Salts, Cape Town, South Africa, 25–28 January 2004. [Google Scholar]
- Pan, J.; Zheng, G.L.; Zhu, D.Q.; Zhou, X.L. Utilization of nickel slag using selective reduction followed by magnetic separation. Trans. Nonferrous Met. Soc. China 2013, 11, 3421–3427. [Google Scholar] [CrossRef]
- Ni, W.; Ma, M.S.; Wang, Y.L.; Wang, Z.J.; Liu, F.M. Thermodynamic and kinetic in recovery of iron from nickel residue. J. Univ. Sci. Technol. B 2009, 2, 163–168. [Google Scholar]
- Jin, Y.; Wang, W.Z.; Zhang, C.M. Research the kinetic of abstracting iron from Jin Chuan waste slag. In Proceedings of the VII National Conference on Metallurgical Reaction Engineering, Shenyang, China, 15–19 May 1998. [Google Scholar]
- Semykina, A.; Shatokha, V.; Iwase, M.; Seetharaman, S. Kinetics of oxidation of divalent iron to trivalent state in liquid FeO-CaO-SiO2 slags. Metall. Mater. Trans. B 2010, 12, 1230–1239. [Google Scholar] [CrossRef]
- Zhang, L.N.; Zhang, L.; Wang, M.Y.; Sui, Z.T. Oxidization mechanism in CaO-FeOx-SiO2 slag with high iron content. Trans. Nonferrous Met. Soc. China 2005, 4, 938–943. [Google Scholar]
- Semykina, A.; Nakano, J.; Sridhar, S.; Shatokha, V.; Seetharaman, S. Confocal scanning laser microscopy studies of crystal growth during oxidation of a liquid FeO-CaO-SiO2 slag. Metall. Mater. Trans. B 2011, 6, 471–476. [Google Scholar] [CrossRef]
- Fan, Y.; Shibata, E.; Iizuka, A.; Nakamura, T. Crystallization behavior of copper smelter slag during molten oxidation. Metall. Mater. Trans. B 2015, 5, 2158–2164. [Google Scholar] [CrossRef]
- Liu, H.L.; Hu, J.H.; Wang, H.; Wang, C.; Li, J.Q. Multiphase transformation during process of copper slag calcination. J. Cent. South Univ. 2013, 8, 3159–3165. [Google Scholar]
- Zhang, G.F.; Yang, Q.R.; Yang, Y.D.; Wu, P.; McLean, A. Recovery of iron from waste slag of pyrite processing using reduction roasting magnetic separation method. Can. Metall. Q. 2013, 2, 153–159. [Google Scholar] [CrossRef]
- Peng, N.; Peng, B.; Chai, L.Y.; Li, M.; Wang, J.M.; Yan, H.; Yuan, Y. Recovery of iron from zinc calcines by reduction roasting and magnetic separation. Miner. Eng. 2012, 35, 57–60. [Google Scholar] [CrossRef]
- Li, G.H.; Gu, F.Q.; Jiang, T.; Luo, J.; Deng, B.N.; Peng, Z.W. Beneficiation of Aluminum-, iron-, and titanium-bearing constituents from diasporic bauxite ores. JOM 2017, 2, 315–322. [Google Scholar] [CrossRef]
- Semykina, A.; Shatokha, V.; Sridhar, S. Innovative approach to recovery of iron from steelmaking slags. Ironmak. Steelmak. 2010, 7, 536–540. [Google Scholar] [CrossRef]
- Wang, P.; Dong, F.Z. Research on Preparation of Iron Concentrate from Iron Tailings. In Proceedings of the 2010 International Symposium on Low-Carbon Economy and Technology Science, Zibo, China, 29–31 October 2010. [Google Scholar]
- Ku, J.G.; Chen, H.H.; He, K.; Yan, Q.X. Force analysis and dynamic simulation of ferromagnetic mineral particles in magnetic separation process. J. Cent. South Univ. 2015, 5, 1577–1582. [Google Scholar]
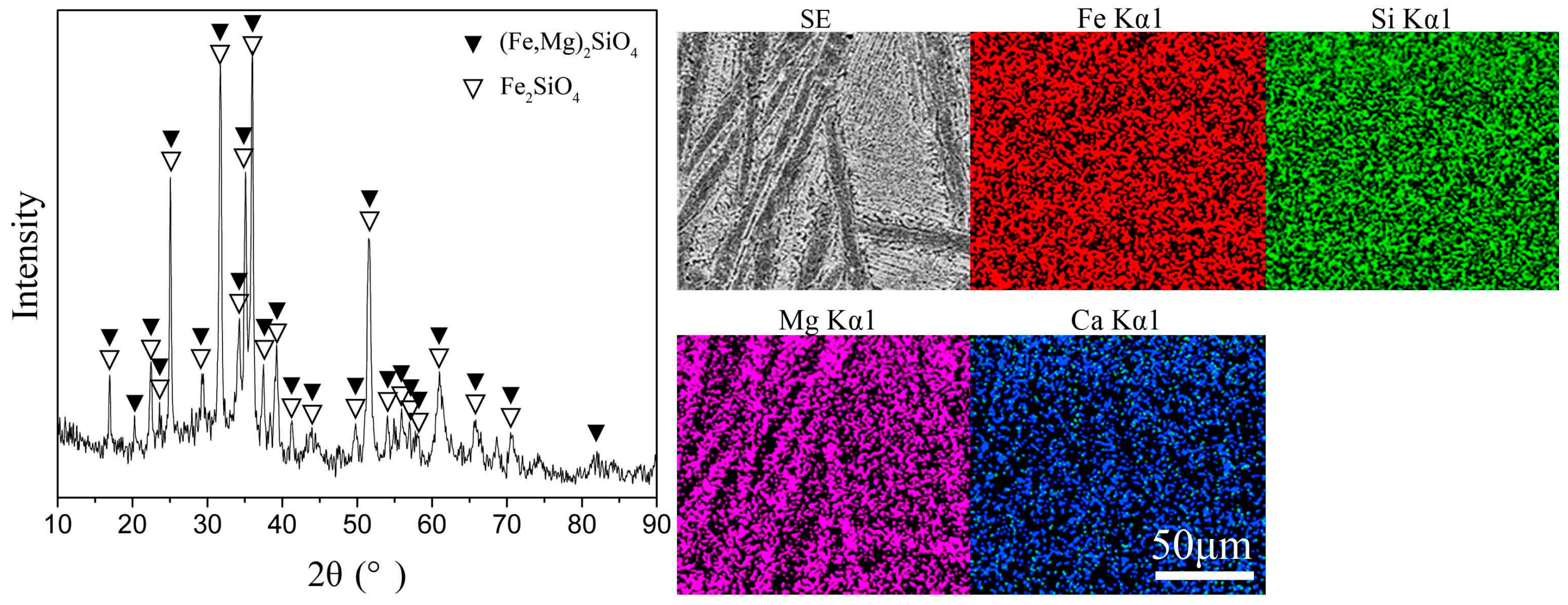


| Fe | Si | Mg | Ca | S | Ni | Co | Cu | Pb | Zn | As |
|---|---|---|---|---|---|---|---|---|---|---|
| 39.19 | 20.14 | 5.07 | 2.56 | 1.20 | 0.16 | 0.08 | 0.22 | 0.01 | 0.05 | 0.00048 |
| Products | Iron Grade | Yield | Recovery | ||
|---|---|---|---|---|---|
| Individual | Cumulative | Individual | Cumulative | ||
| Concentrate I | 42.64 | 70.39 | 70.39 | 83.22 | 83.22 |
| Concentrate II | 49.88 | 80.45 | 56.63 | 94.11 | 78.32 |
| Concentrate III | 54.08 | 89.48 | 50.67 | 97.02 | 75.99 |
| Products | TFe | Si | Mg | Ca | Ni | Co | Cu | Pb | Zn | As |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentrate I | 42.64 | 8.83 | 4.81 | 3.20 | 0.18 | 0.10 | 0.21 | 0.002 | 0.040 | <0.001 |
| Concentrate II | 49.88 | 7.42 | 4.66 | 2.48 | 0.20 | 0.12 | 0.23 | 0.001 | 0.049 | <0.001 |
| Concentrate III | 54.08 | 5.04 | 3.15 | 0.82 | 0.21 | 0.12 | 0.24 | 0.005 | 0.049 | <0.001 |
| Tailing slag | 18.71 | 20.26 | 5.64 | 7.03 | 0.06 | 0.04 | 0.14 | 0.015 | 0.05 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Du, X.; Shen, Y.; Li, G.; Li, M. Crystallization and Beneficiation of Magnetite for Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation. Metals 2017, 7, 321. https://doi.org/10.3390/met7080321
Ma Y, Du X, Shen Y, Li G, Li M. Crystallization and Beneficiation of Magnetite for Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation. Metals. 2017; 7(8):321. https://doi.org/10.3390/met7080321
Chicago/Turabian StyleMa, Yongbo, Xueyan Du, Yingying Shen, Guozhou Li, and Ming Li. 2017. "Crystallization and Beneficiation of Magnetite for Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation" Metals 7, no. 8: 321. https://doi.org/10.3390/met7080321




