In Situ Study of Phase Transformations during Non-Isothermal Tempering of Bainitic and Martensitic Microstructures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
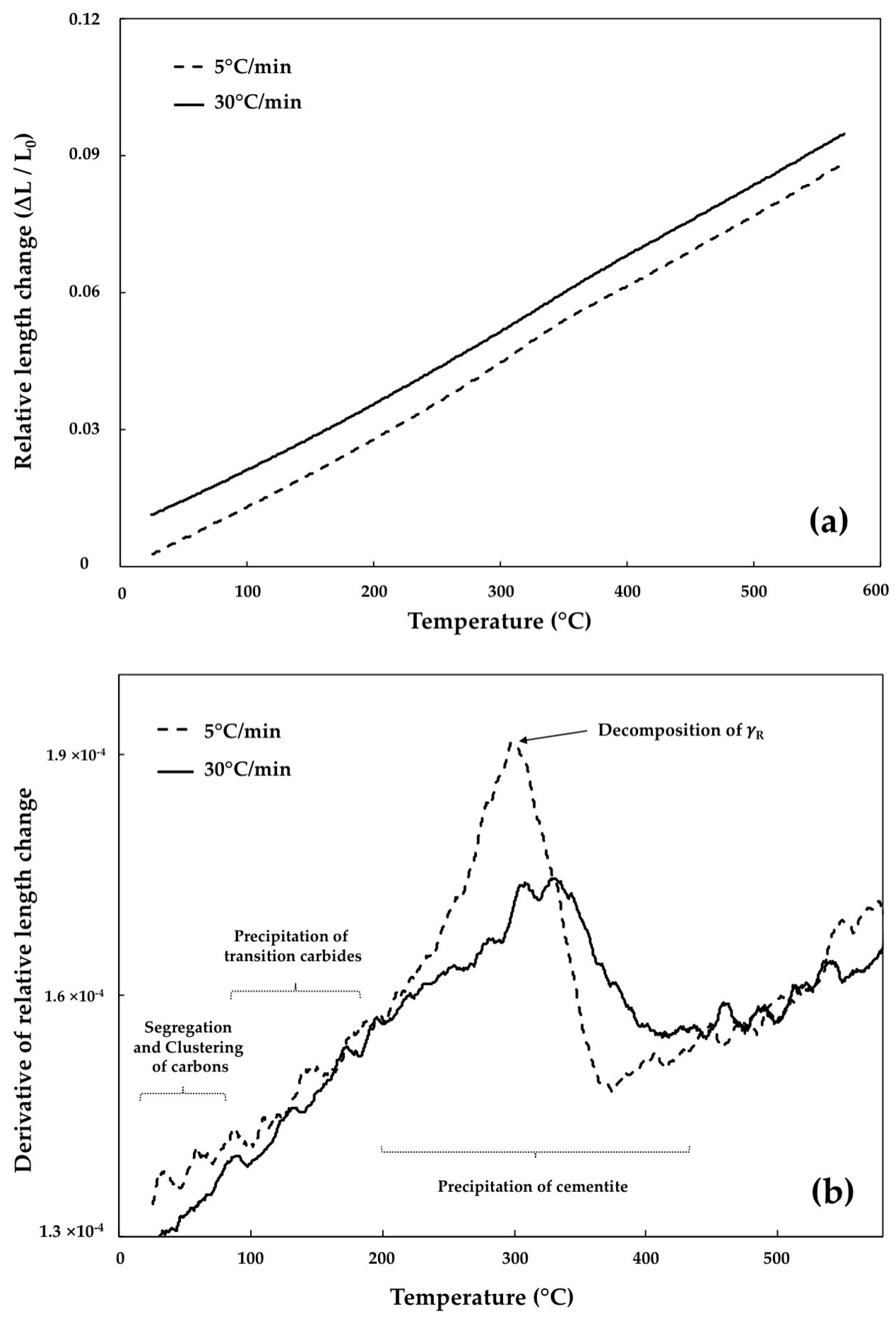
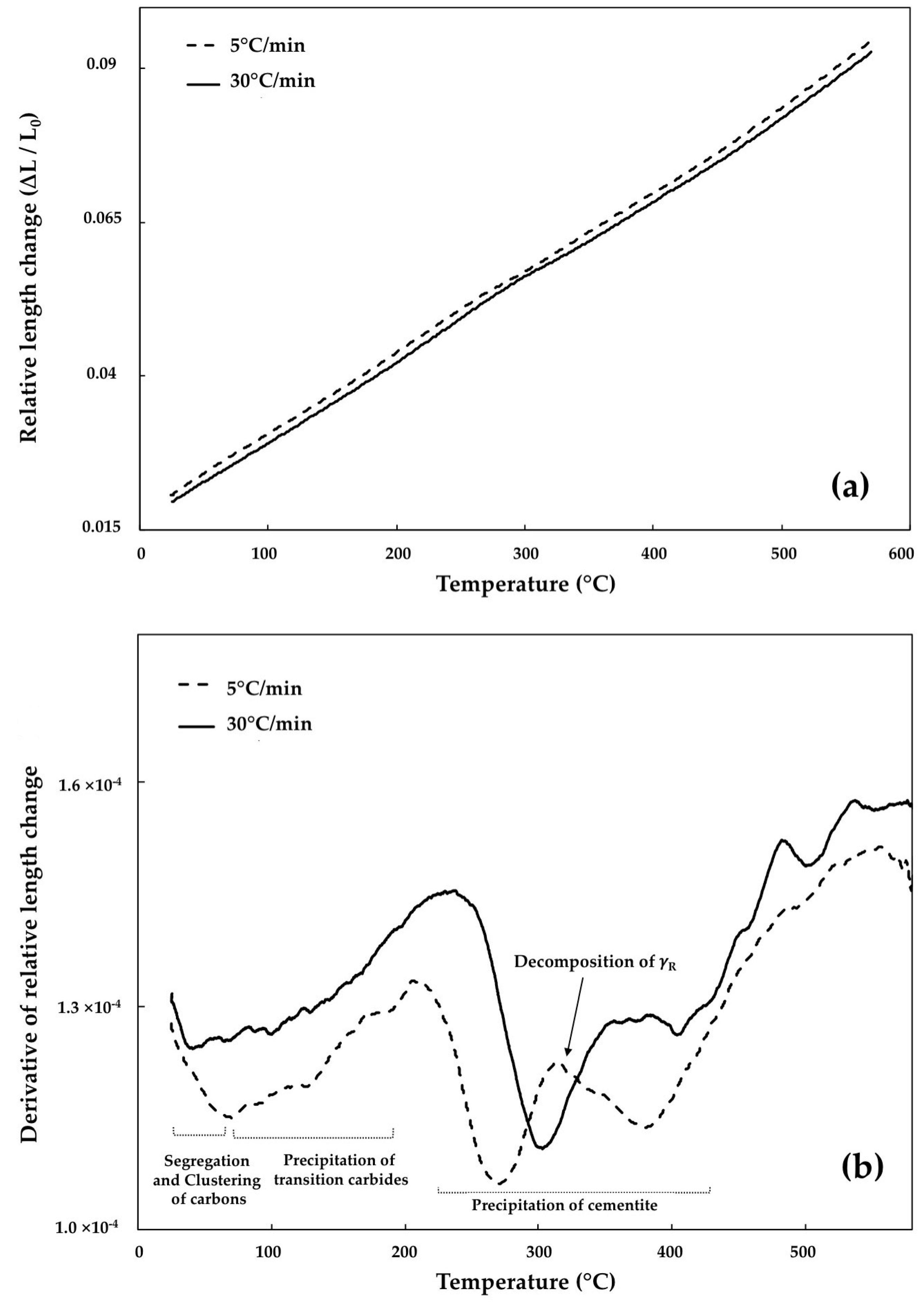
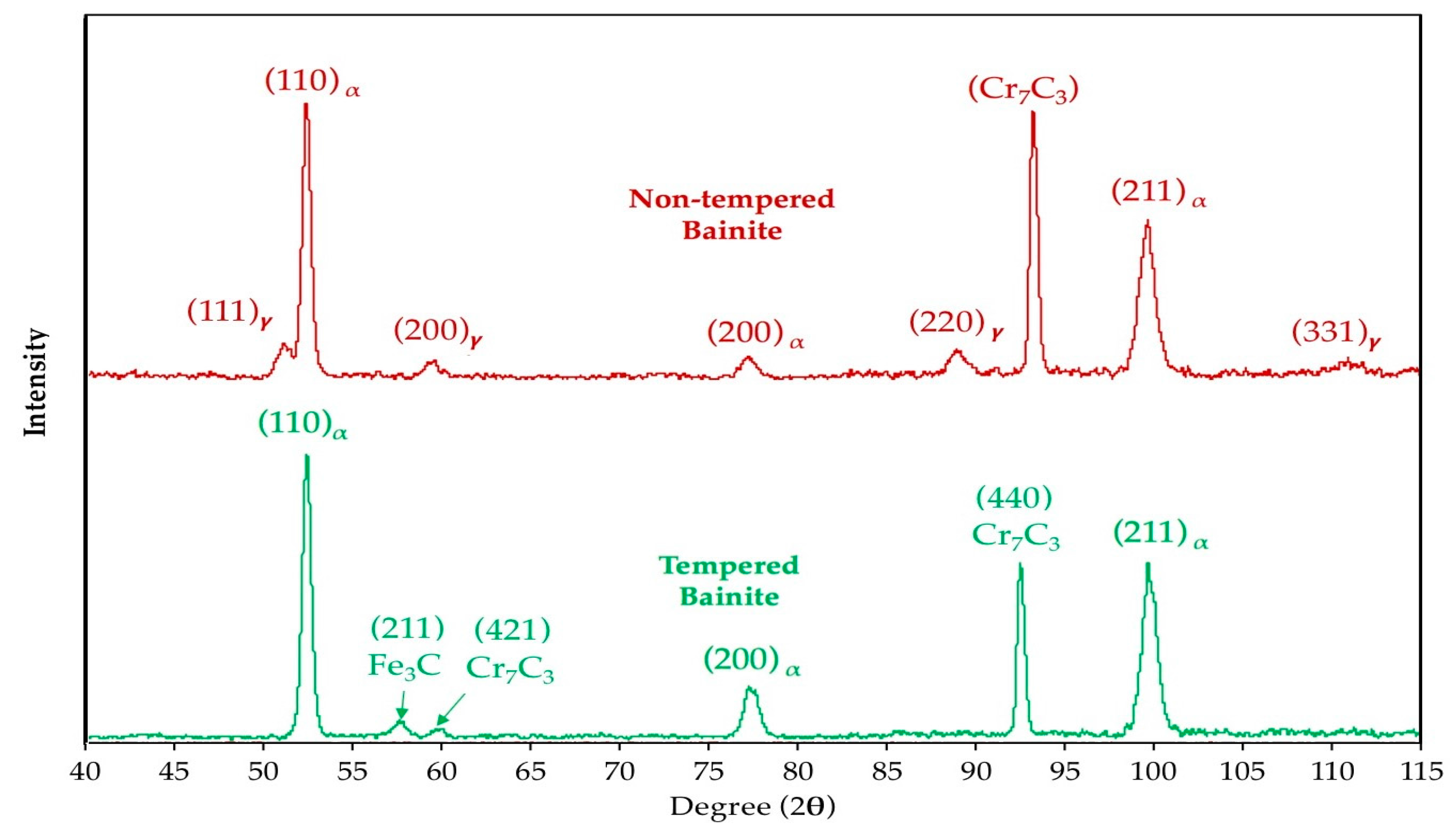
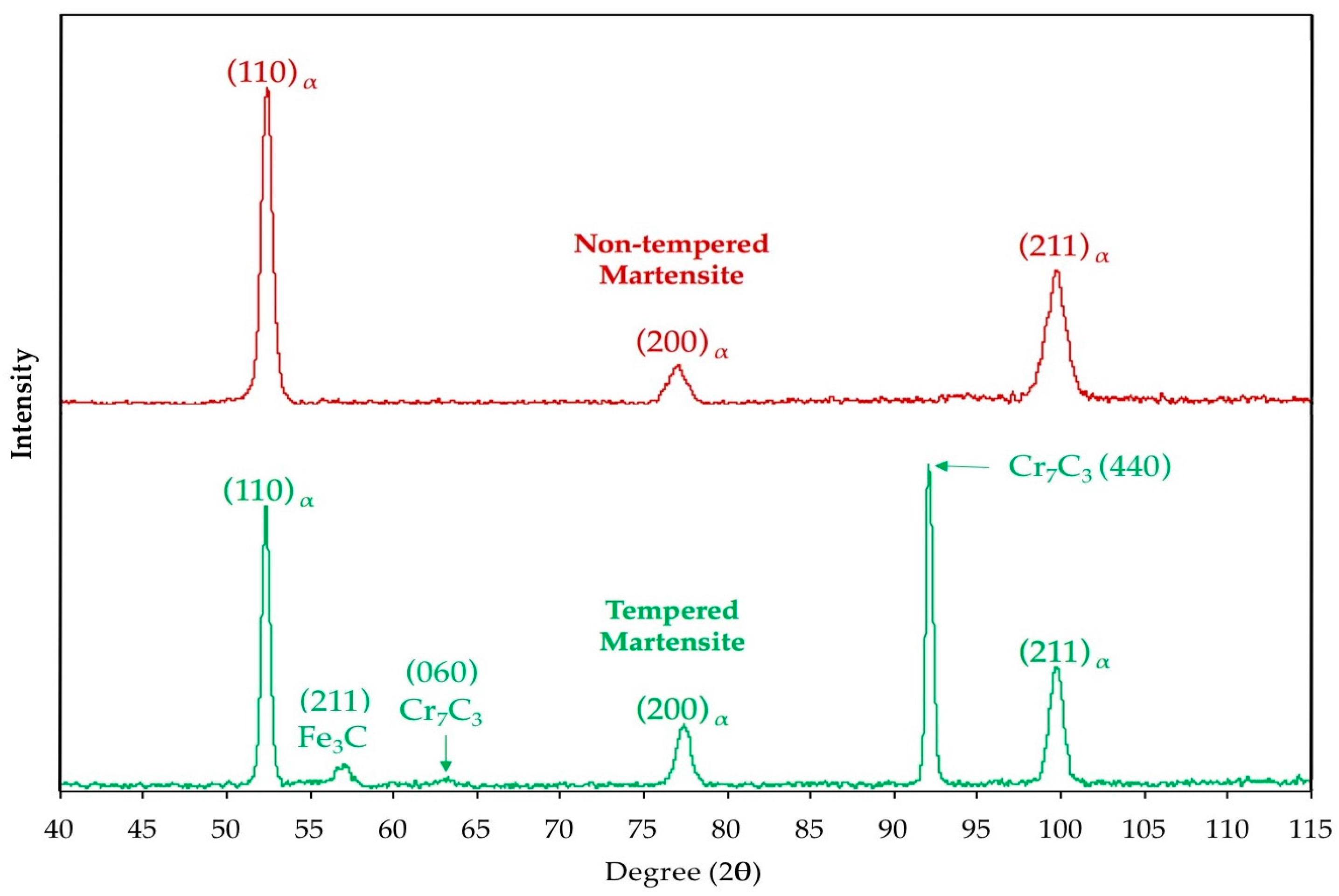
3.1. Segregation and Clustering of Carbon Atoms
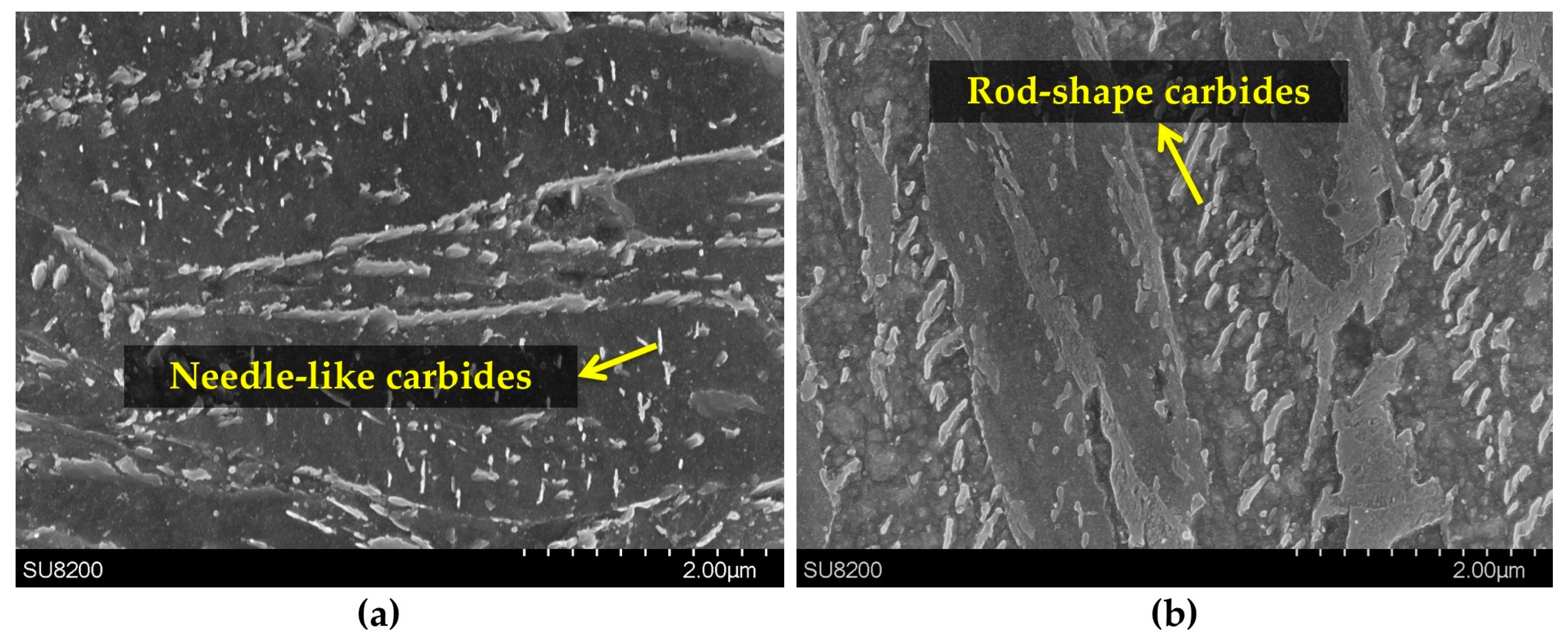
3.2. Precipitation of the ε/η Transition Carbides
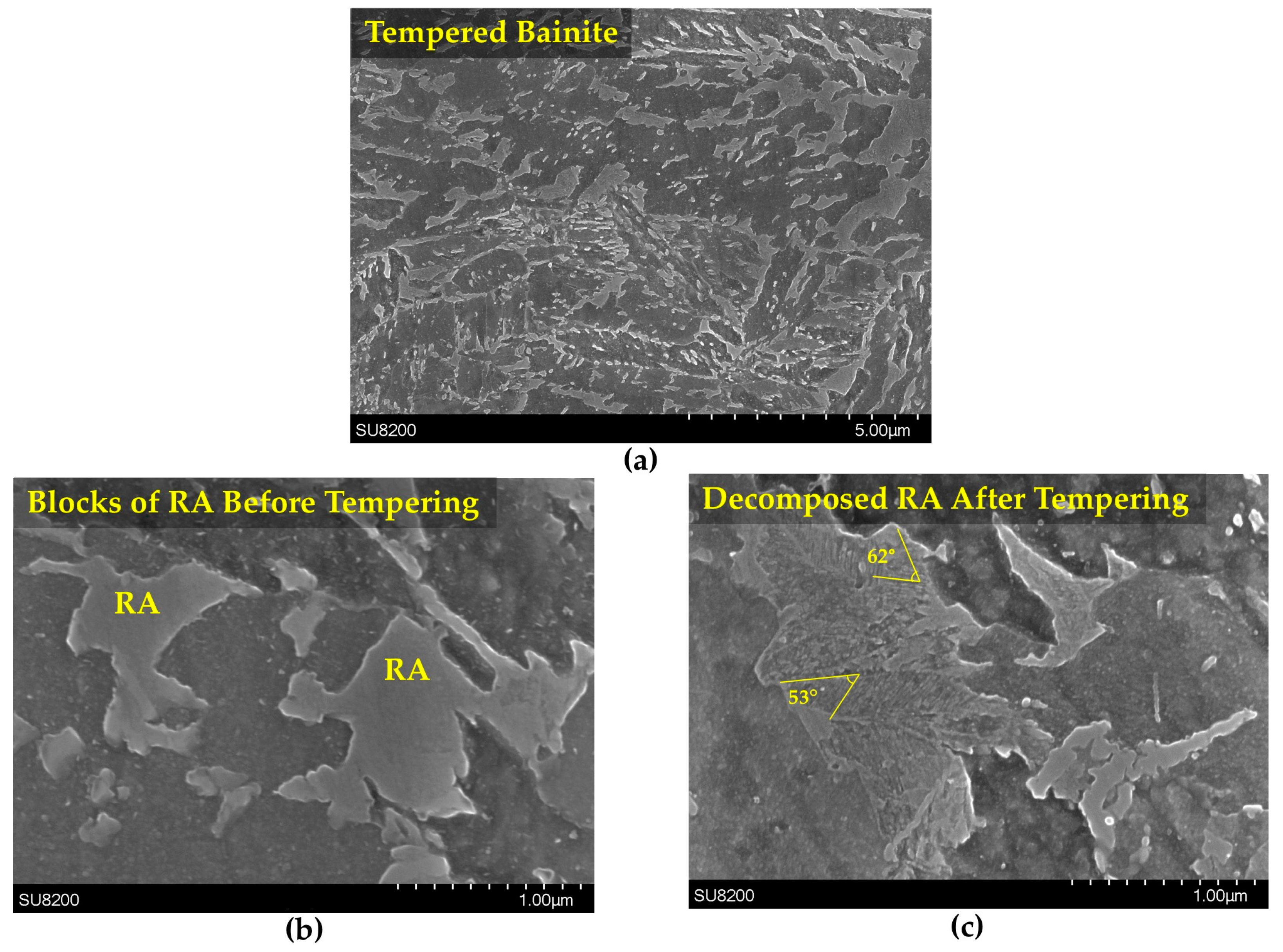
3.3. Retained Austenite Decomposition
3.4. Cementite Precipitation
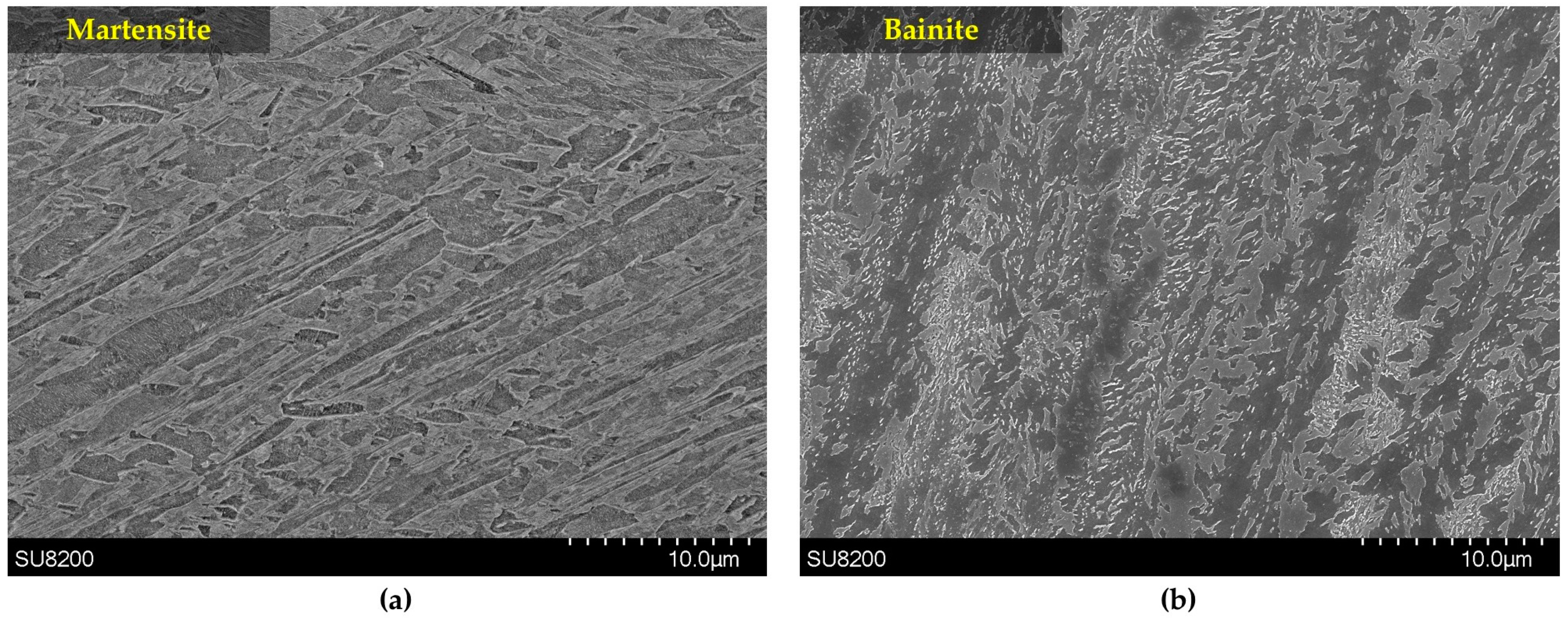
3.5. Microstructural Evolution during Tempering
4. Conclusions
- (1)
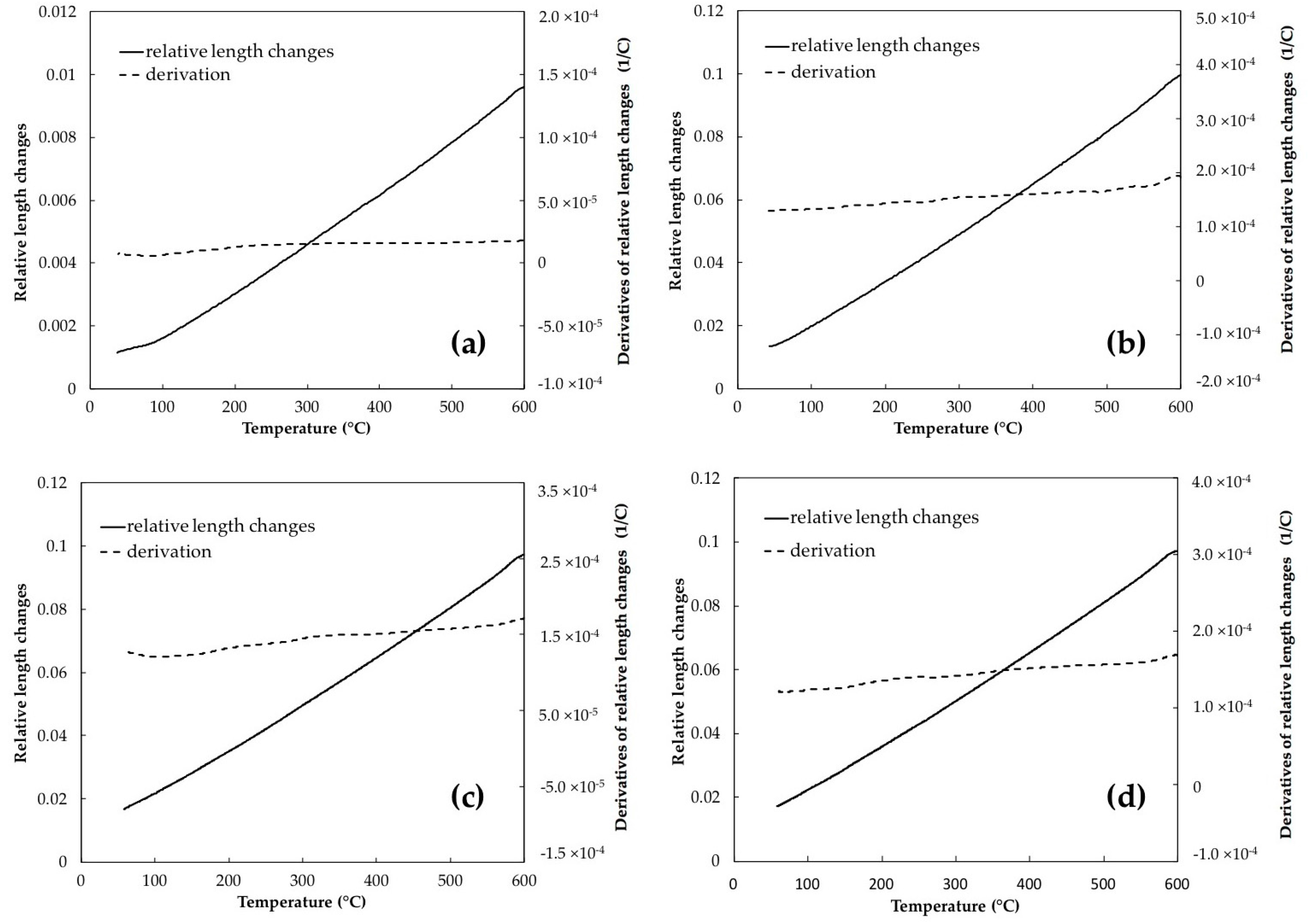
- In the bainitic microstructure, retained austenite decomposed to lower bainite associated with volume expansion during tempering in contrast to martensite tempering where great length decrease occurred due to decomposition of medium-carbon martensite to low carbon martensite plus carbides.
- (2)
- Phase transformations during tempering of martensite occurred at slightly higher temperatures than bainite tempering, owing to the auto-tempering effect through bainite formation.
- (3)
- In tempering of the bainitic specimens which contain 23.2% of retained austenite, its decomposition at the heating rate of 5 °C/min caused 3.74% length increase. This percentage decreased steeply to 2.21% when the heating rate increased to 30 °C/min, demonstrating that some amount of retained austenite remained untransformed after non-isothermal tempering at this heating rate, whilst in martensite, the length increase for both heating rates is almost constant indicating completed transformation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Firrao, D.; Matteis, P.; Russo Spena, P.; Gerosa, R. Influence of the microstructure on fatigue and fracture toughness properties of large heat-treated mold steels. Mater. Sci. Eng. A 2013, 559, 371–383. [Google Scholar] [CrossRef]
- Firrao, D.; Gerosa, R.; Ghidini, A.; Matteis, P.; Mortarino, G.; Pinasco, M.R.; Rivolta, B.; Silva, G.; Stagno, E. Relation between fatigue crack initiation and propagation, toughness and microstructure in large steel blooms for automotive plastic molds. Int. J. Fatigue 2007, 29, 1880–1884. [Google Scholar] [CrossRef]
- Chentouf, S.M.; Jahazi, M.; Lapierre-Boire, L.P.; Godin, S. Characteristics of austenite transformation during post forge cooling of large-size high strength steel ingots. Metallogr. Microstruct. Anal. 2014, 3, 281–297. [Google Scholar] [CrossRef]
- Morra, P.; Böttger, A.; Mittemeijer, E. Decomposition of iron-based martensite. A kinetic analysis by means of differential scanning calorimetry and dilatometry. J. Therm. Anal. Calorim. 2001, 64, 905–914. [Google Scholar] [CrossRef]
- Biss, V.; Cryderman, R.L. Martensite and retained austenite in hot-rolled, low-carbon bainitic steels. Metall. Mater. Trans. B 1971, 2, 2267–2276. [Google Scholar] [CrossRef]
- Gojić, M.; Sućeska, M.; Rajić, M. Thermal analysis of low alloy Cr-Mo steel. J. Therm. Anal. Calorim. 2004, 75, 947–956. [Google Scholar] [CrossRef]
- Rodrigues, P.C.M.; Pereloma, E.V.; Santos, D.B. Mechanical properities of an HSLA bainitic steel subjected to controlled rolling with accelerated cooling. Mater. Sci. Eng. A 2000, 283, 136–143. [Google Scholar] [CrossRef]
- Yan, G.; Han, L.; Li, C.; Luo, X.; Gu, J. Characteristic of retained austenite decomposition during tempering and its effect on impact toughness in SA508 Gr.3 steel. J. Nucl. Mater. 2017, 483, 167–175. [Google Scholar] [CrossRef]
- Cheng, L.; Brakman, C.M.; Korevaar, B.M.; Mittemeijer, E.J. The tempering of iron- carbon martensite; dilatometric and calorimetric analysis. Metall. Trans. A 1988, 19, 2415–2426. [Google Scholar] [CrossRef]
- Nagakura, S.; Hirotsu, Y.; Kusunoki, M.; Suzuki, T.; Nakamura, Y. Crystallographic study of the tempering of martensitic carbon steel by electron microscopy and diffraction. Metall. Trans. A 1983, 14, 1025–1031. [Google Scholar] [CrossRef]
- Speich, G.R.; Leslie, W.C. Tempering of steel. Metall. Trans. 1972, 3, 1043–1054. [Google Scholar] [CrossRef]
- Primig, S.; Leitner, H. Separation of overlapping retained austenite decomposition and cementite precipitation reactions during tempering of martensitic steel by means of thermal analysis. Thermchim. Acta 2011, 526, 111–117. [Google Scholar] [CrossRef]
- Miller, M.; Beaven, P.; Smith, G. A study of the early stages of tempering of iron-carbon martensites by atom probe field ion microscopy. Metall. Trans. A 1981, 12, 1197–1204. [Google Scholar] [CrossRef]
- Taylor, K.; Olson, G.; Cohen, M.; Sande, J.B.V. Carbide precipitation during stage I tempering of Fe-Ni-C martensites. Metall. Mater. Trans. A 1989, 20, 2749–2765. [Google Scholar] [CrossRef]
- Olson, G.B.; Cohen, M. Early stages of aging and tempering of ferrous martensites. Metall. Trans. A 1983, 14, 1057–1065. [Google Scholar] [CrossRef]
- Mittemeher, E.J.; Cheng, L.; van der Schaaf, P.J.; Brakman, C.M.; Korevaar, B.M. Analysis of nonisothermal transformation kinetics; tempering of iron-carbon and iron-nitrogen martensites. Metall. Trans. A 1988, 19, 925–932. [Google Scholar] [CrossRef]
- Saha Podder, A.; Bhadeshia, H.K.D.H. Thermal stability of austenite retained in bainitic steels. Mater. Sci. Eng. A 2010, 527, 2121–2128. [Google Scholar] [CrossRef]
- Ghasemi Nanesa, H.; Jahazi, M. Alternative phase transformation path in cryogenically treated AISI D2 tool steel. Mater. Sci. Eng. A 2015, 634, 32–36. [Google Scholar] [CrossRef]
- Jung, M.; Lee, S.J.; Lee, Y.K. Microstructural and dilatational changes during tempering and tempering kinetics in martensitic medium-carbon steel. Metall. Mater. Trans. A 2009, 40, 551–559. [Google Scholar] [CrossRef]
- Leiva, J.A.V.; Morales, E.V.; Villar-Cociña, E.; Donis, C.A.; de S. Bott, I. Kinetic parameters during the tempering of low-alloy steel through the non-isothermal dilatometry. J. Mater. Sci. 2009, 45, 418. [Google Scholar] [CrossRef]
- Peet, M.J.; Babu, S.S.; Miller, M.K.; Bhadeshia, H.K.D.H. Tempering of low-temperature bainite. Metall. Mater. Trans. A 2017, 48, 3410–3418. [Google Scholar] [CrossRef]
- Krauss, G. Tempering of Lath Martensite in Low and Medium Carbon Steels: Assessment and Challenges. Steel Res. Int. 2017. [Google Scholar] [CrossRef]
- Kang, M.K.; Ai, Y.L.; Zhang, M.X.; Yang, Y.Q.; Zhu, M.; Chen, Y. Carbon content of bainite ferrite in 40CrMnSiMoV steel. Mater. Chem. Phys. 2009, 118, 438–441. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels: Transformations, Microstructure and Properties, 2nd ed.; IOM Communications: London, UK, 2001. [Google Scholar]
- Caballero, F.G.; Miller, M.K.; Babu, S.S.; Garcia-Mateo, C. Atomic scale observations of bainite transformation in a high carbon high silicon steel. Acta Mater. 2007, 55, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C.; Capdevila, C.; Babu, S.S. Redistribution of alloying elements during tempering of a nanocrystalline steel. Acta Mater. 2008, 56, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Jack, K. Structural transformations in the tempering of high-carbon martensitic steels. J. Iron Steel Inst. 1951, 169, 26–36. [Google Scholar]
- Nakamura, Y.; Nagakura, S. Structure of iron-carbon martensite in the transition state from the first to the third stage of tempering studied by electron microscopy and diffraction. Trans. Jpn. Inst. Met. 1986, 27, 842–848. [Google Scholar] [CrossRef]
- Dépinoy, S.; Toffolon-Masclet, C.; Urvoy, S.; Roubaud, J.; Marini, B.; Roch, F.; Kozeschnik, E.; Gourgues-Lorenzon, A.F. Carbide precipitation in 2.25Cr-1Mo bainitic steel: Effect of heating and isothermal tempering conditions. Metall. Mater. Trans. A 2017, 48, 2164–2178. [Google Scholar] [CrossRef]
- Shimizu, K.; Ko, T.; Nishiyama, Z. Transmission electron microscope observation of the bainite of carbon steel. Trans. Jpn. Inst. Met. 1964, 5, 225–230. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M. Phase Transformations in Metals and Alloys (Revised Reprint), 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]

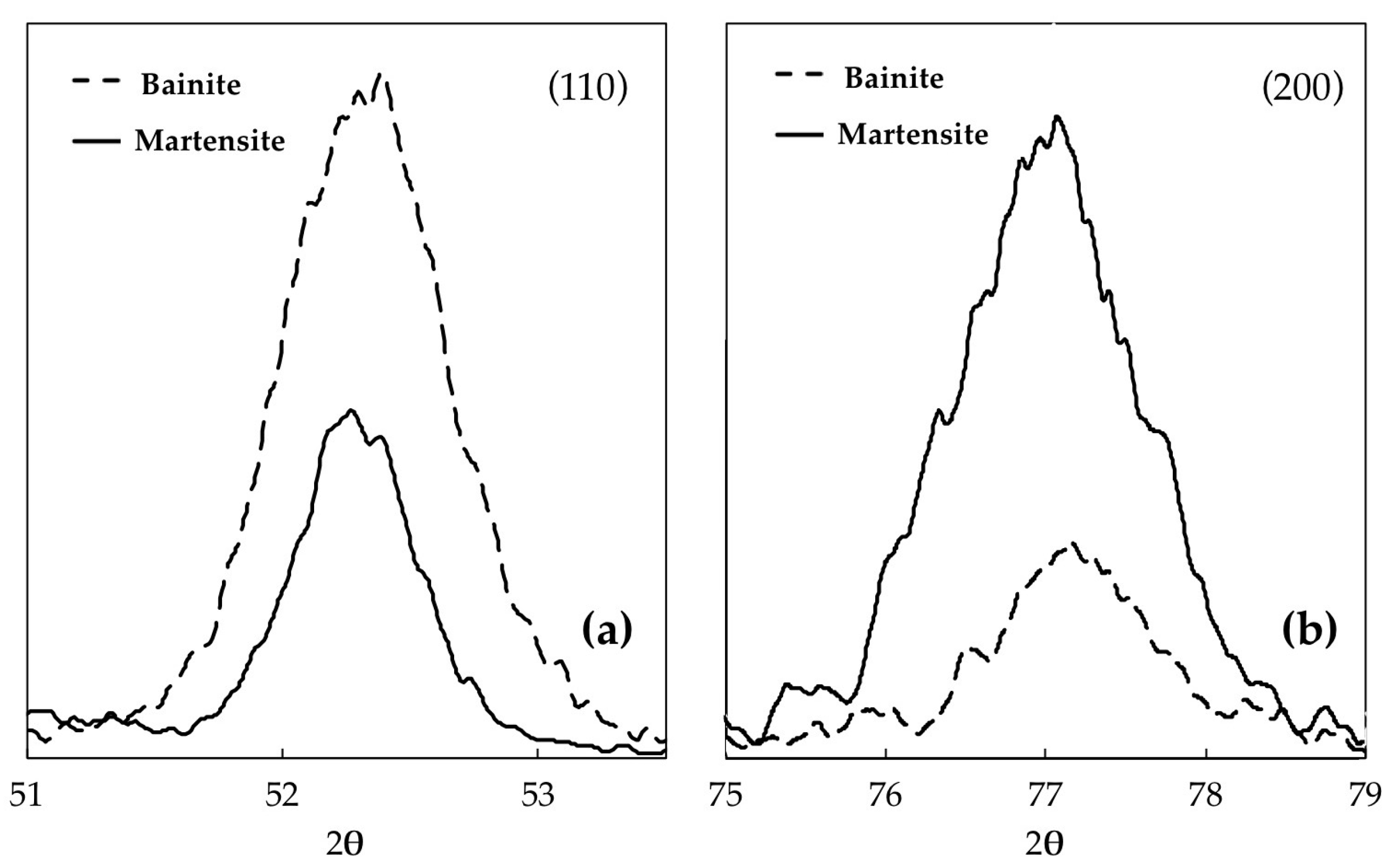

| Microstructure | c(Å) | a(Å) | [C]c (wt %) | [C]a (wt %) | [C] (wt %) |
|---|---|---|---|---|---|
| As-received (Bainite) | 2.8712 | 2.8668 | 0.24 | 0.30 | 0.26 |
| Bainite | 2.8718 | 2.8650 | 0.26 | 0.11 | 0.19 |
| Tempered Bainite (5 °C/min) | 2.8713 | 2.8634 | 0.24 | 0.22 | 0.23 |
| Martensite | 2.8724 | 2.8702 | 0.30 | 0.27 | 0.29 |
| Tempered Martensite (5 °C/min) | 2.8708 | 2.8689 | 0.21 | 0.17 | 0.19 |
| Specimen | Heating Rate (°C/min) | Temperature Range (°C) | Relative Length Change (%) |
|---|---|---|---|
| Bainite | 5 | 255–359 | +3.74 |
| 30 | 276–386 | +2.21 | |
| Martensite | 5 | 269–370 | +0.67 |
| 30 | 300–401 | +0.63 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talebi, S.H.; Ghasemi-Nanesa, H.; Jahazi, M.; Melkonyan, H. In Situ Study of Phase Transformations during Non-Isothermal Tempering of Bainitic and Martensitic Microstructures. Metals 2017, 7, 346. https://doi.org/10.3390/met7090346
Talebi SH, Ghasemi-Nanesa H, Jahazi M, Melkonyan H. In Situ Study of Phase Transformations during Non-Isothermal Tempering of Bainitic and Martensitic Microstructures. Metals. 2017; 7(9):346. https://doi.org/10.3390/met7090346
Chicago/Turabian StyleTalebi, S. Hesamodin, Hadi Ghasemi-Nanesa, Mohammad Jahazi, and Haikouhi Melkonyan. 2017. "In Situ Study of Phase Transformations during Non-Isothermal Tempering of Bainitic and Martensitic Microstructures" Metals 7, no. 9: 346. https://doi.org/10.3390/met7090346





