Synthesis of Metastable Au-Fe Alloy Using Ordered Nanoporous Silica as a Hard Template
Abstract
:1. Introduction
2. Materials and Methods
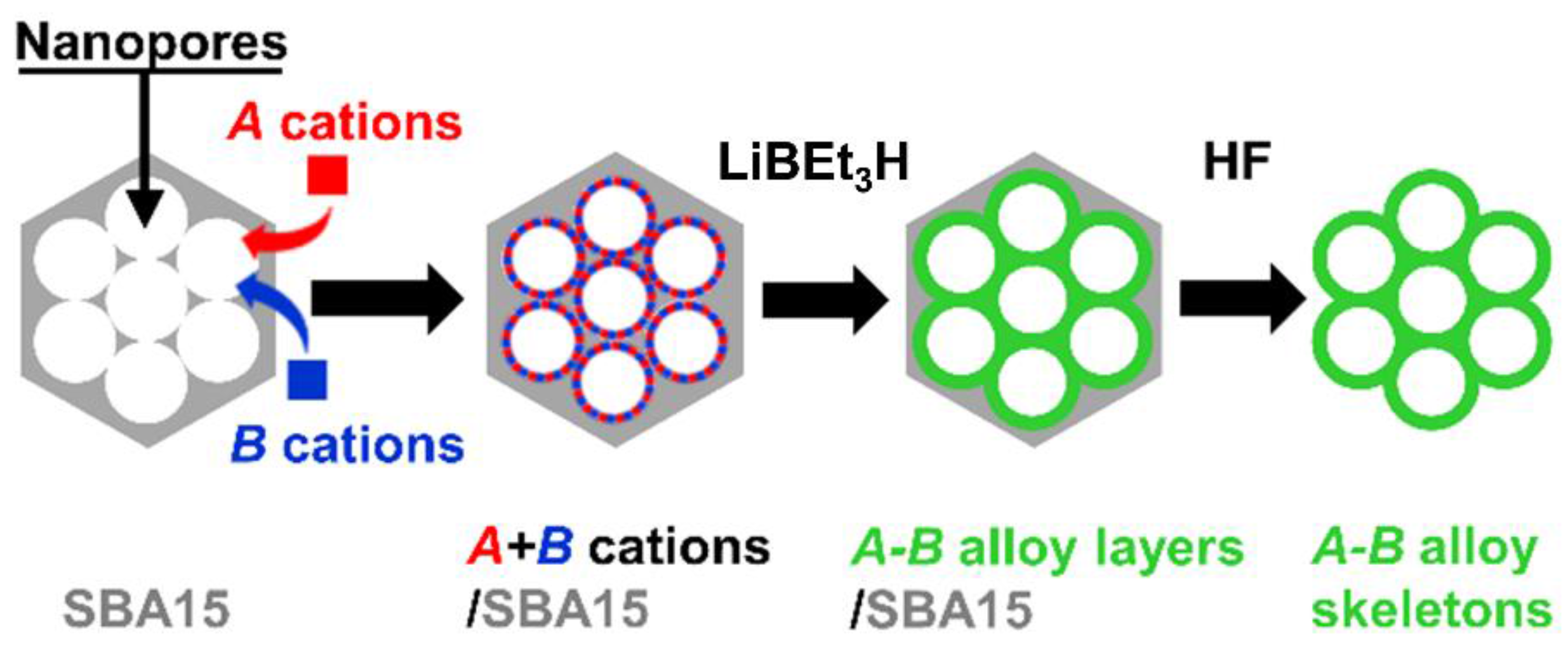
2.1. Sample Preparation
2.2. Characterization
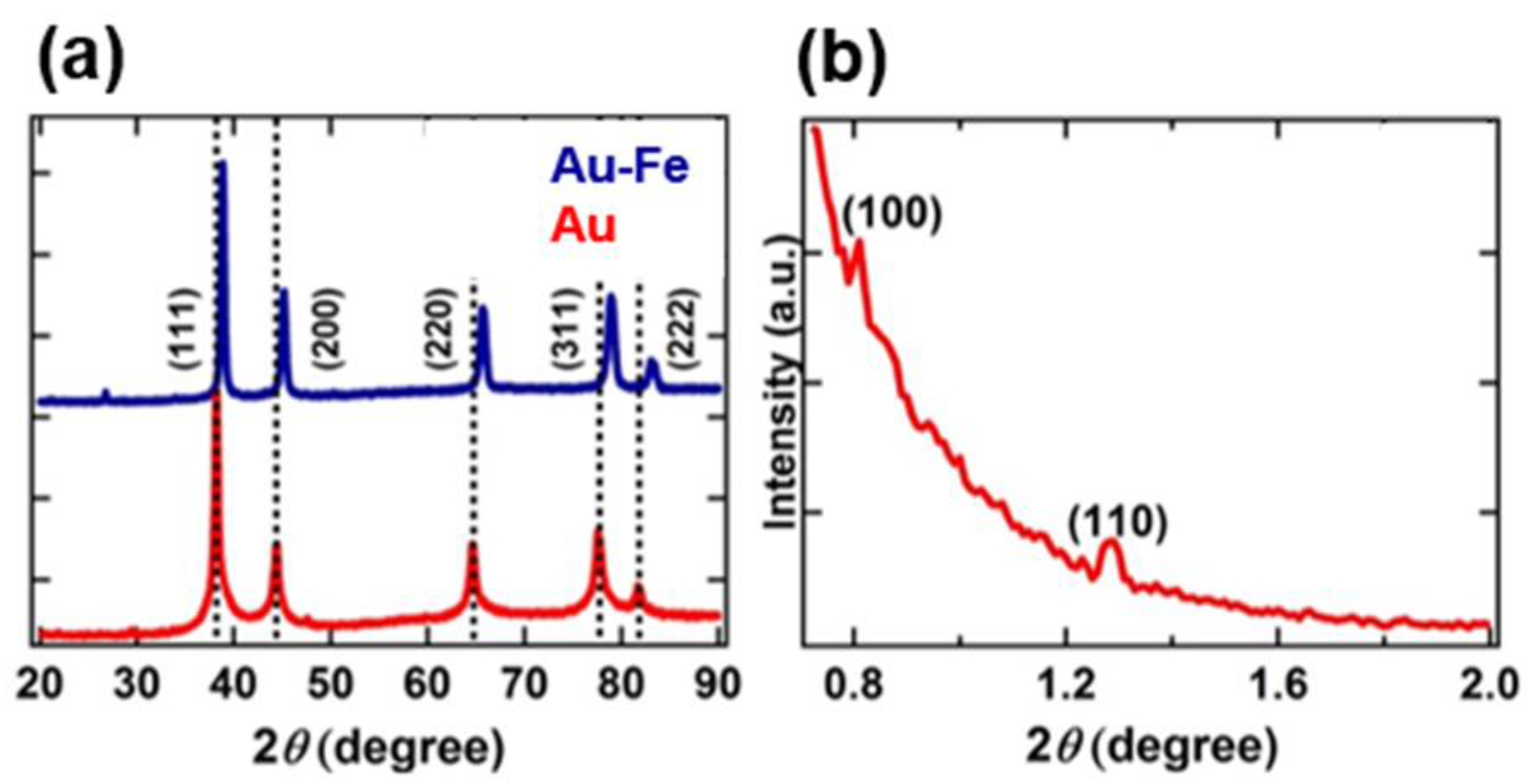
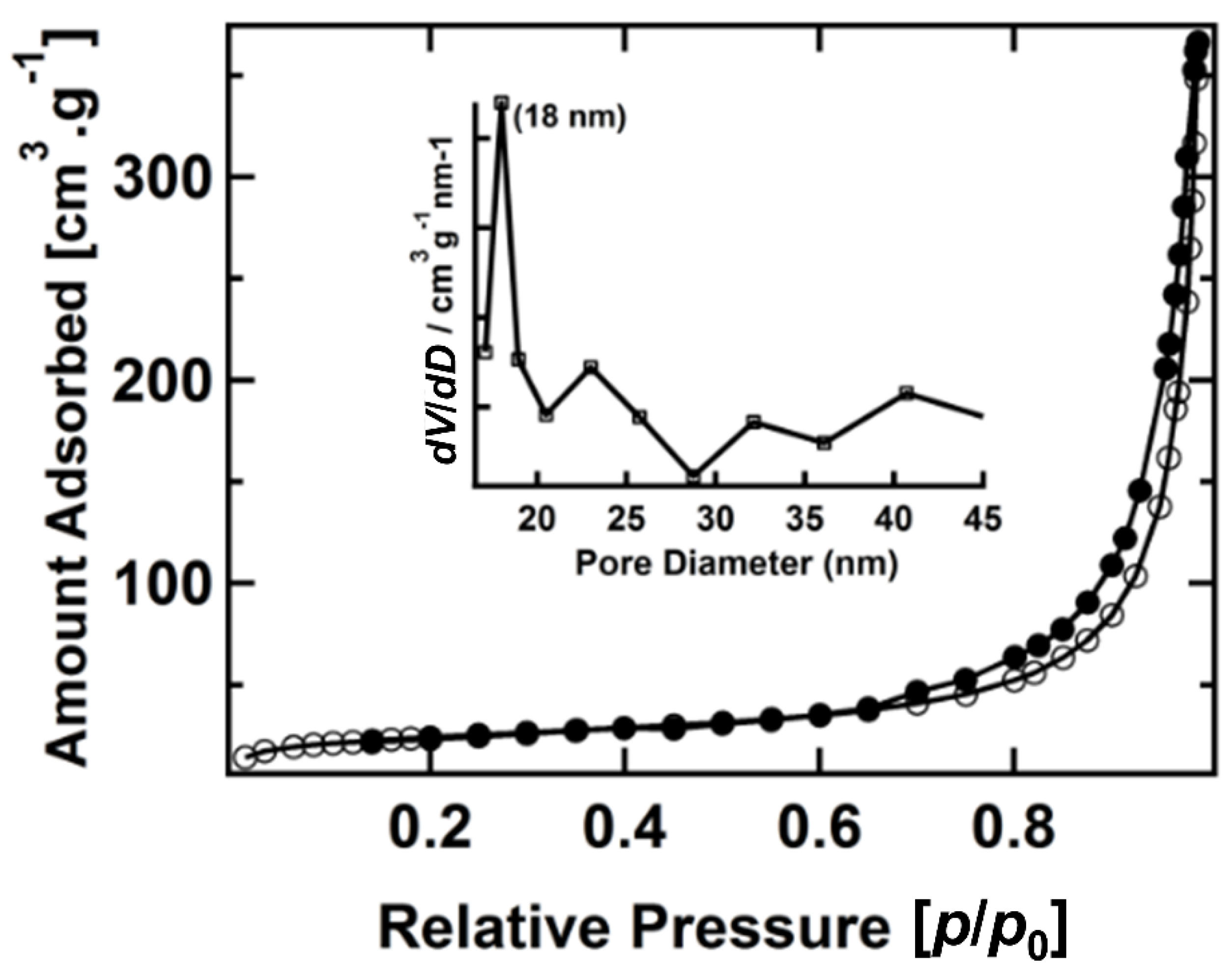
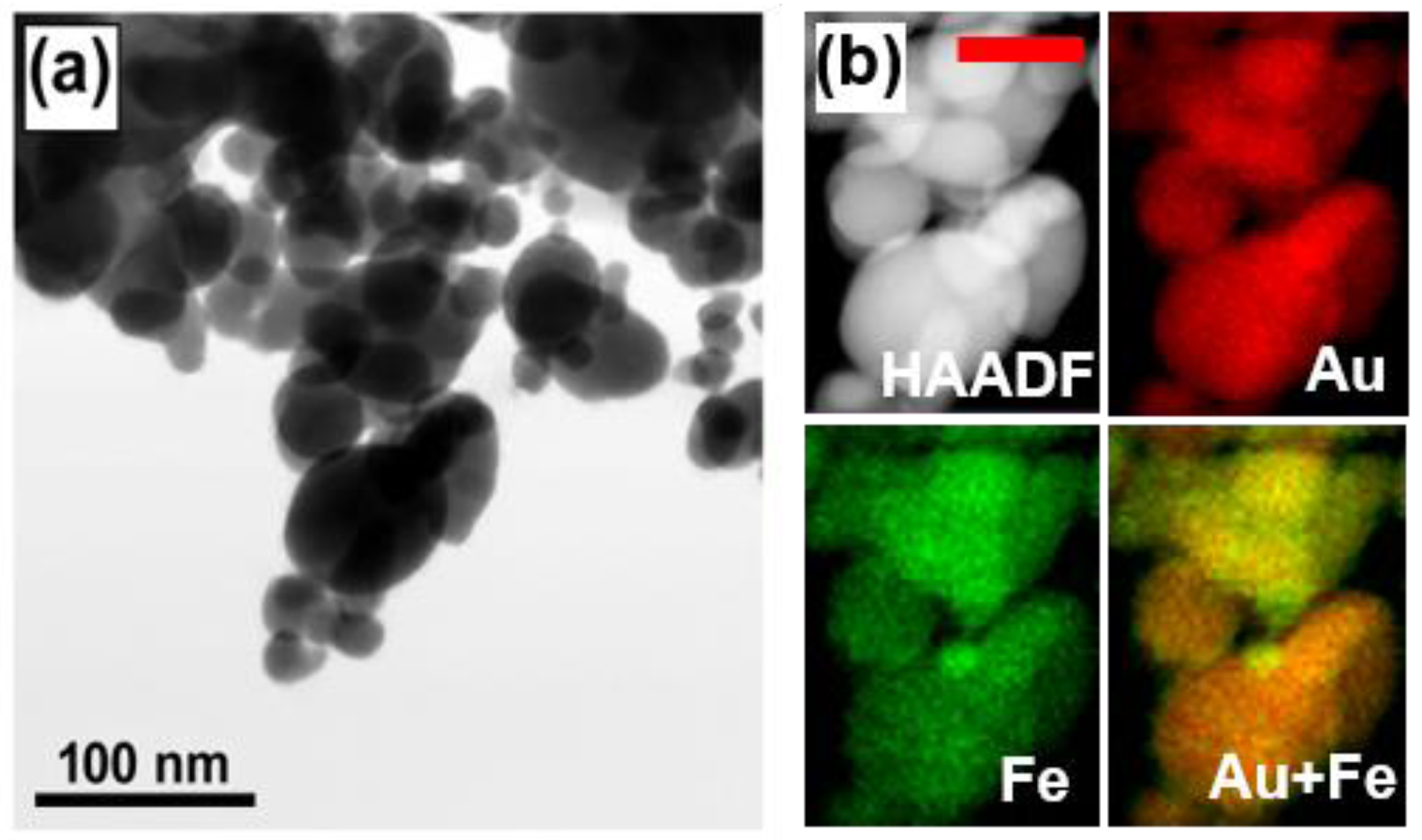
3. Results and Discussions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rick, J.; Tsai, M.C.; Hwang, B.J. Biosensors incorporating bimetallic nanoparticles. Nanomaterials 2016, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Pannu, C.; Bala, M.; Khan, S.A.; Srivastava, S.K.; Kabiraj, D.; Avasthi, D.K. Synthesis and characterization of Au-Fe alloy nanoparticles embedded in a silica matrix by atom beam sputtering. RSC Adv. 2015, 5, 92080–92088. [Google Scholar] [CrossRef]
- Yan, Y.C.; Du, J.S.S.; Gilroy, K.D.; Yang, D.R.; Xia, Y.N.; Zhang, H. Intermetallic nanocrystals: Syntheses and catalytic applications. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, G.; Prasad, P.N.; Swihart, M.T. Synthesis of monodisperse Au, Ag, and Au-Ag alloy nanoparticles with tunable size and surface plasmon resonance frequency. Chem. Mater. 2011, 23, 4098–4101. [Google Scholar] [CrossRef]
- Lehoux, A.; Ramos, L.; Beaunier, P.; Uribe, D.B.; Dieudonne, P.; Audonnet, F.; Etcheberry, A.; Jose-Yacaman, M.; Remita, H. Tuning the porosity of bimetallic nanostructures by a soft templating approach. Adv. Funct. Mater. 2012, 22, 4900–4908. [Google Scholar] [CrossRef]
- Oezaslan, M.; Hasche, F.; Strasser, P. Pt-based core-shell catalyst architectures for oxygen fuel cell electrodes. J. Phys. Chem. Lett. 2013, 4, 3273–3291. [Google Scholar] [CrossRef]
- Chen, H.; Wang, D.L.; Yu, Y.C.; Newton, K.A.; Muller, D.A.; Abruna, H.; DiSalvo, F.J. A surfactant-free strategy for synthesizing, and processing intermetallic platinum-based nanoparticle catalysts. J. Am. Chem. Soc. 2012, 134, 18453–18459. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, Y.C.; Xin, H.L.L.; Newton, K.A.; Holtz, M.E.; Wang, D.L.; Muller, D.A.; Abruna, H.D.; DiSalvo, F.J. Coalescence in the thermal annealing of nanoparticles: An in situ stem study of the growth mechanisms of ordered Pt-Fe nanoparticles in a KCl matrix. Chem. Mater. 2013, 25, 1436–1442. [Google Scholar] [CrossRef]
- Malgras, V.; Ataee-Esfahani, H.; Wang, H.J.; Jiang, B.; Li, C.L.; Wu, K.C.W.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for mesoporous metals. Adv. Mater. 2016, 28, 993–1010. [Google Scholar] [CrossRef] [PubMed]
- Bondi, J.F.; Misra, R.; Ke, X.L.; Sines, I.T.; Schiffer, P.; Schaak, R.E. Optimized synthesis and magnetic properties of intermetallic Au3Fe1−x, Au3Co1−x, and Au3Ni1−x nanoparticles. Chem. Mater. 2010, 22, 3988–3994. [Google Scholar] [CrossRef]
- Naitabdi, A.; Cuenya, B.R. Formation, thermal stability, and surface composition of size-selected aufe nanoparticles. Appl. Phys. Lett. 2007, 91, 113110. [Google Scholar] [CrossRef]
- Zhuravlev, I.A.; Barabash, S.V.; An, J.M.; Belashchenko, K.D. Phase stability, ordering tendencies, and magnetism in single-phase fcc Au-Fe nanoalloys. Phys. Rev. B 2017, 96, 134109. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials 2009, 2, 2188–2215. [Google Scholar] [CrossRef]
- Ding, Y.; Kim, Y.-J.; Erlebacher, J. Nanoporous Gold Leaf: “Ancient Technology”/Advanced Material. Adv. Mater. 2004, 16, 1897–1900. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.S.M.; Sivakumar, T.; Fujita, T.; Jayavel, R.; Abe, H. Synthesis of Metastable Au-Fe Alloy Using Ordered Nanoporous Silica as a Hard Template. Metals 2018, 8, 17. https://doi.org/10.3390/met8010017
Kumar PSM, Sivakumar T, Fujita T, Jayavel R, Abe H. Synthesis of Metastable Au-Fe Alloy Using Ordered Nanoporous Silica as a Hard Template. Metals. 2018; 8(1):17. https://doi.org/10.3390/met8010017
Chicago/Turabian StyleKumar, Paskalis Sahaya Murphin, Thiripuranthagan Sivakumar, Takeshi Fujita, Ramasamy Jayavel, and Hideki Abe. 2018. "Synthesis of Metastable Au-Fe Alloy Using Ordered Nanoporous Silica as a Hard Template" Metals 8, no. 1: 17. https://doi.org/10.3390/met8010017




