Microstructure Evolution and Microstructural Characteristics of Al–Mg–Si Aluminum Alloys Fabricated by a Modified Strain-Induced Melting Activation Process
Abstract
:1. Introduction
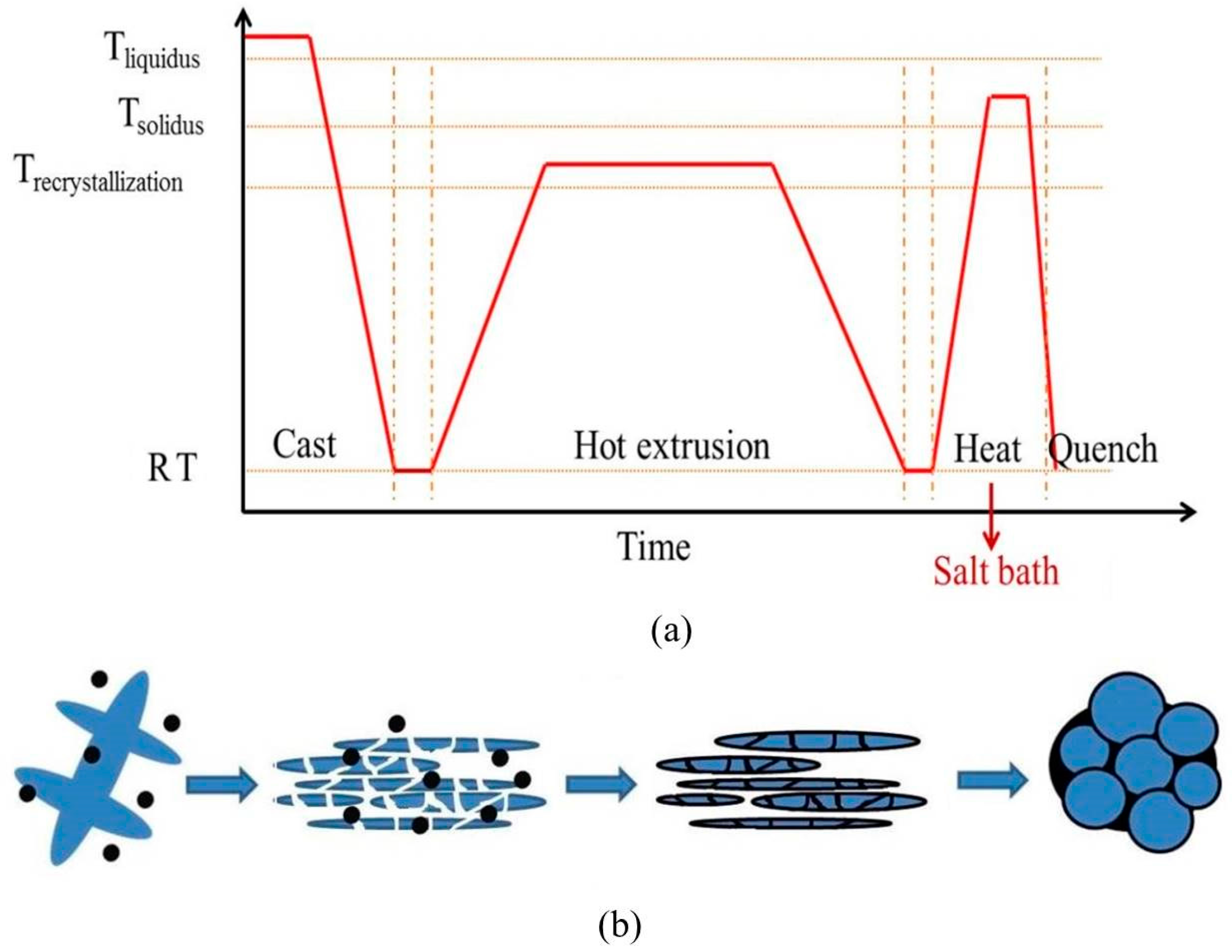
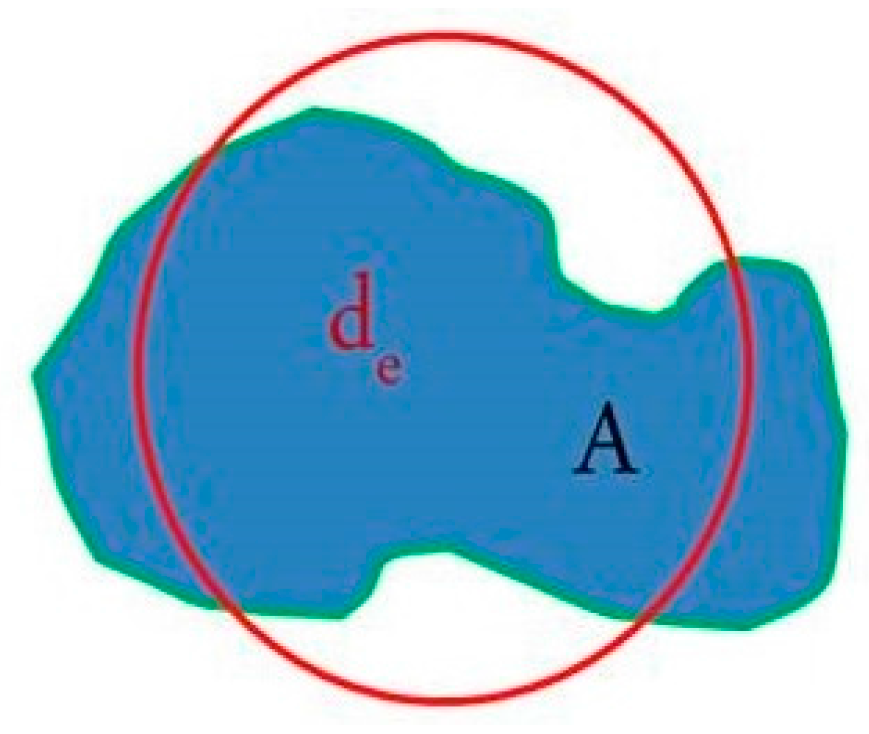
2. Materials and Methods
3. Results and Discussion
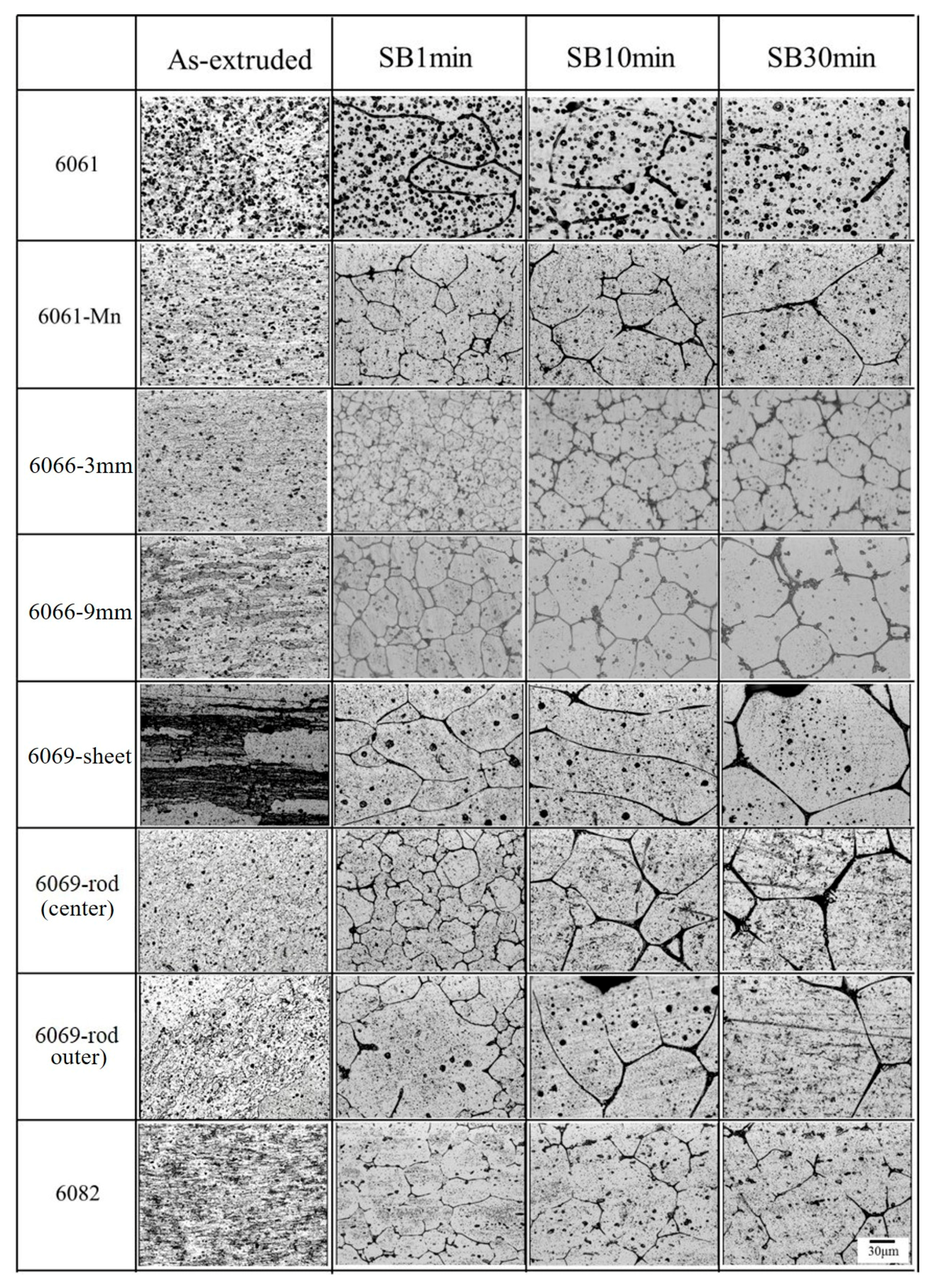
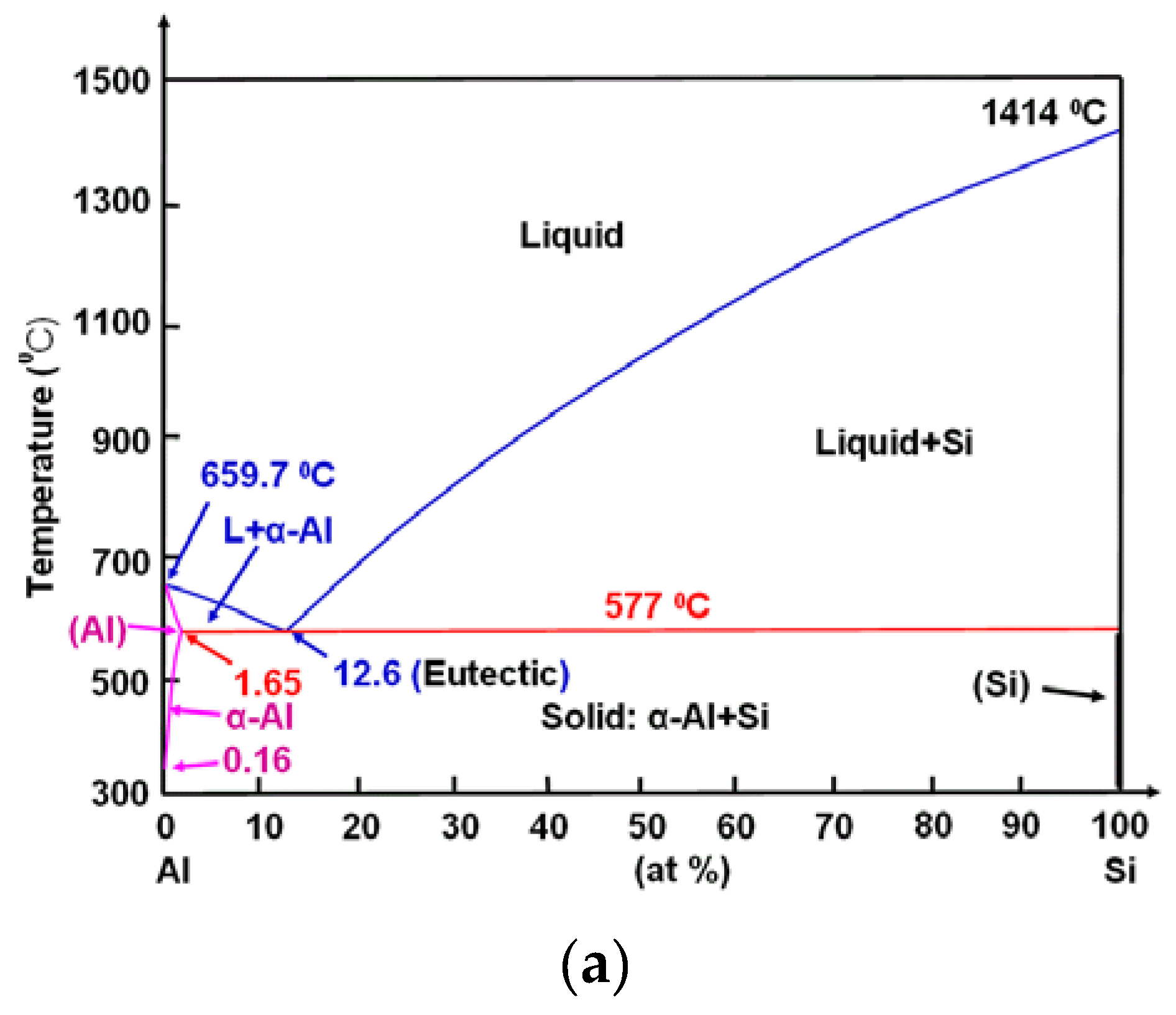
3.1. Effects of Extrusion Conditions and Chemical Composition on Microstructure
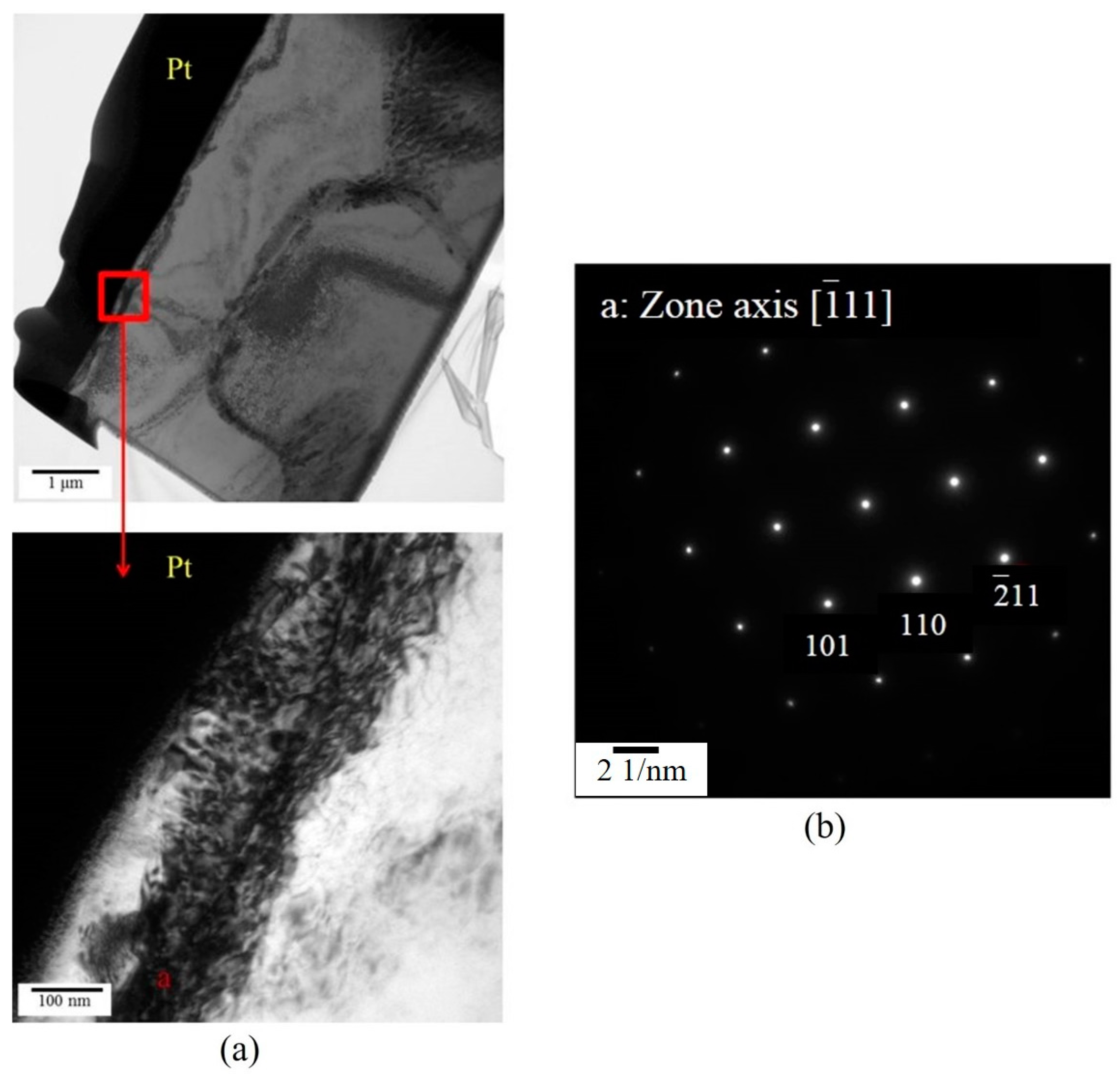
3.2. Microstructural Evolution of SIMA-Processed Alloys
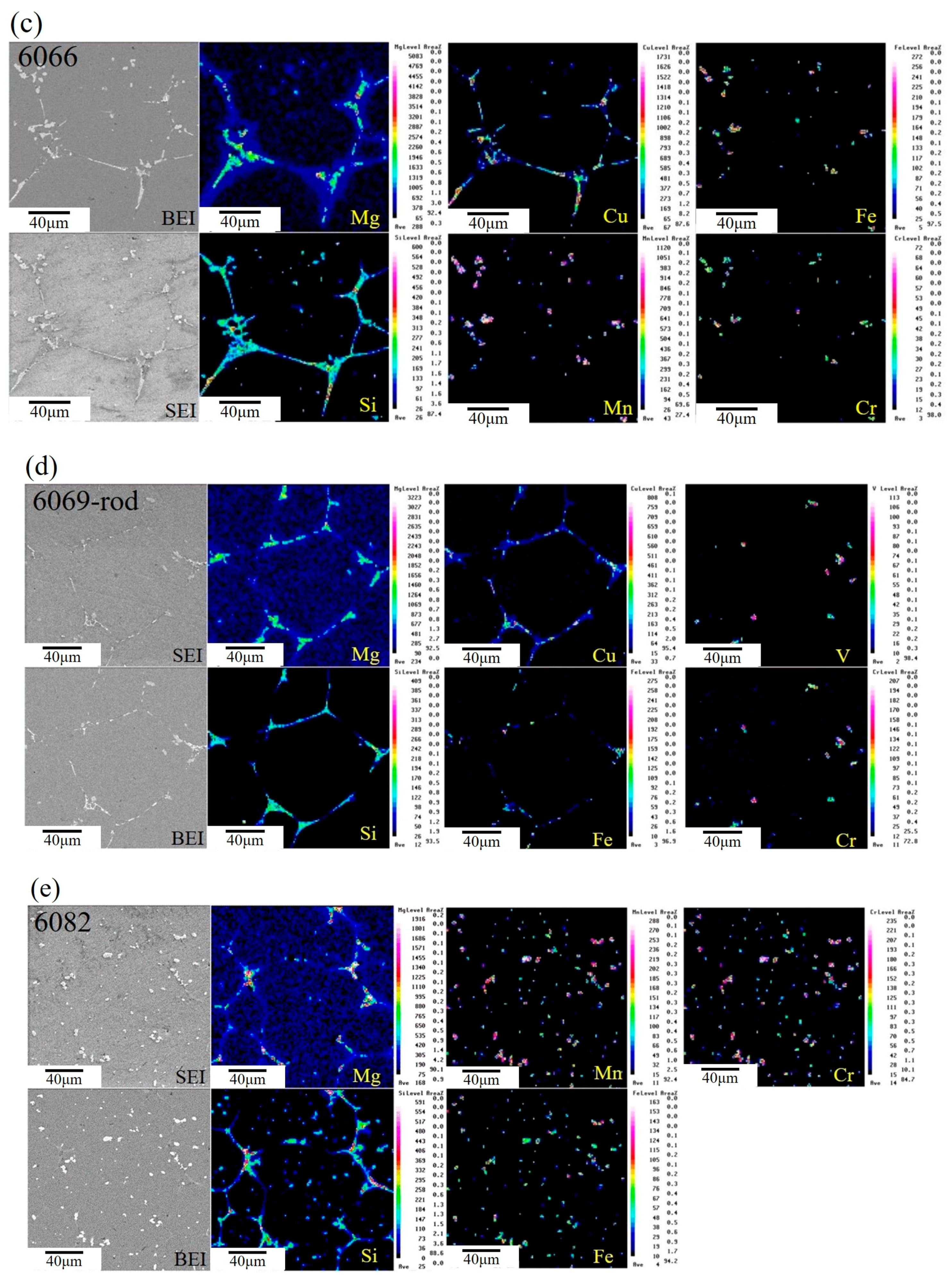
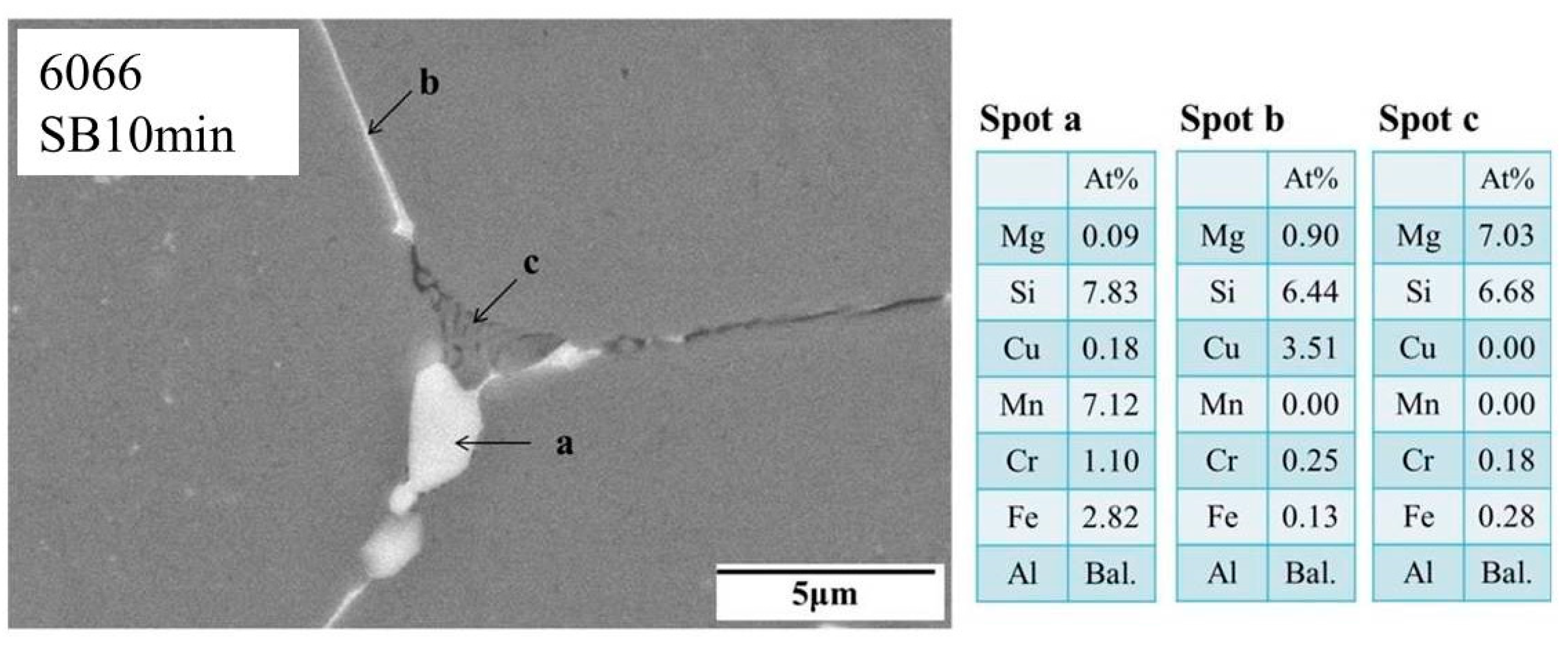
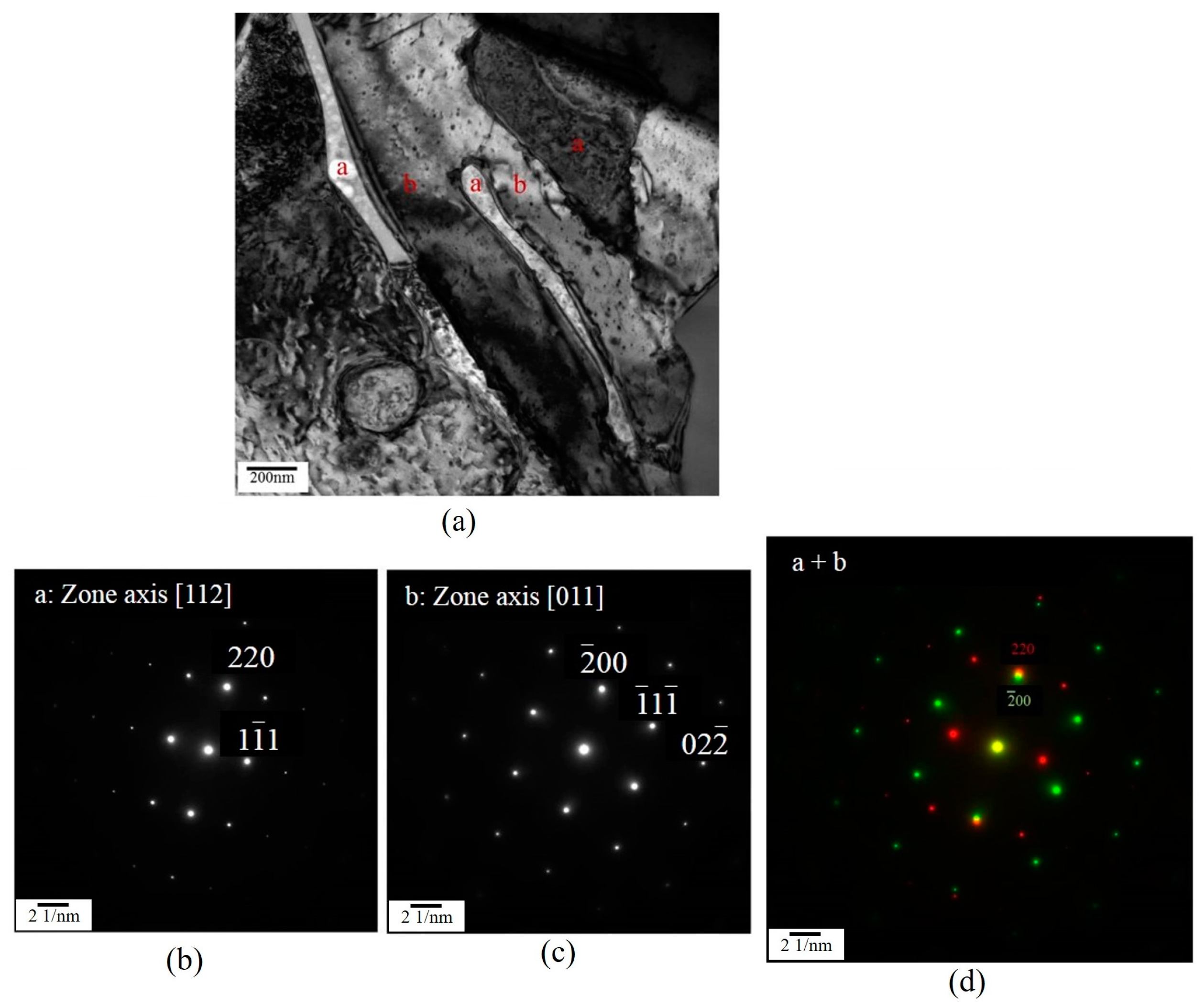
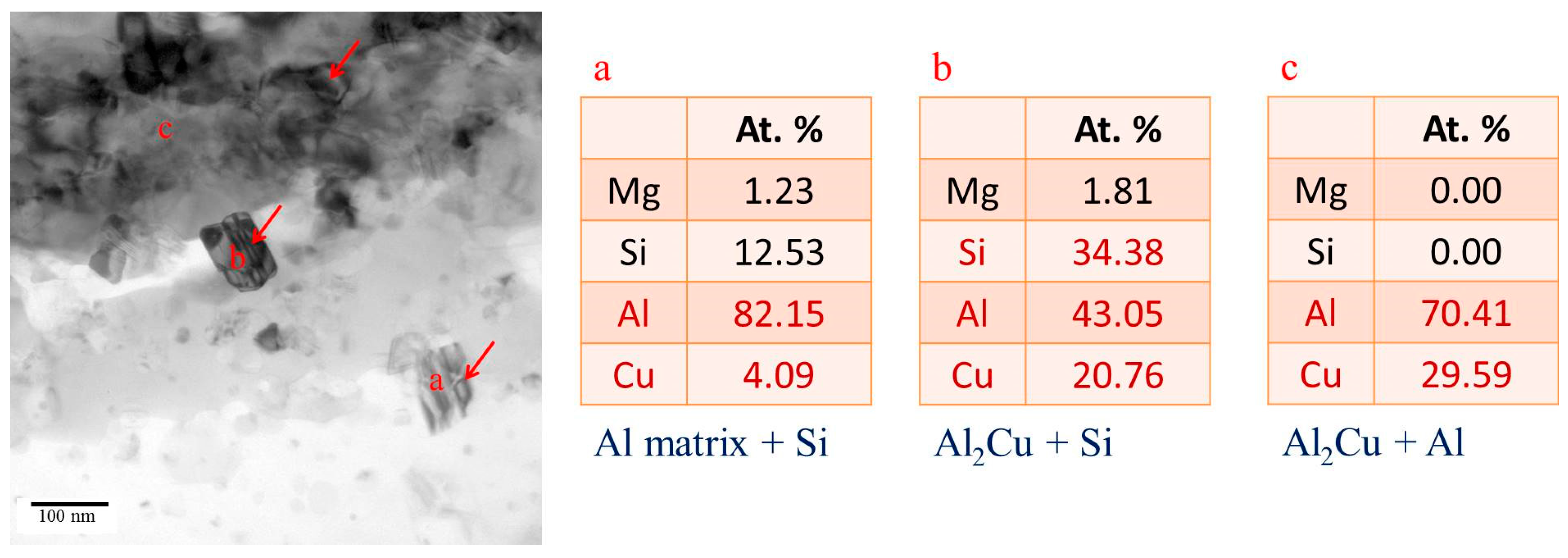
3.3. Microstructural Characteristics of Phases Located at Globule Boundaries in SIMA-Processed 6066 Alloy
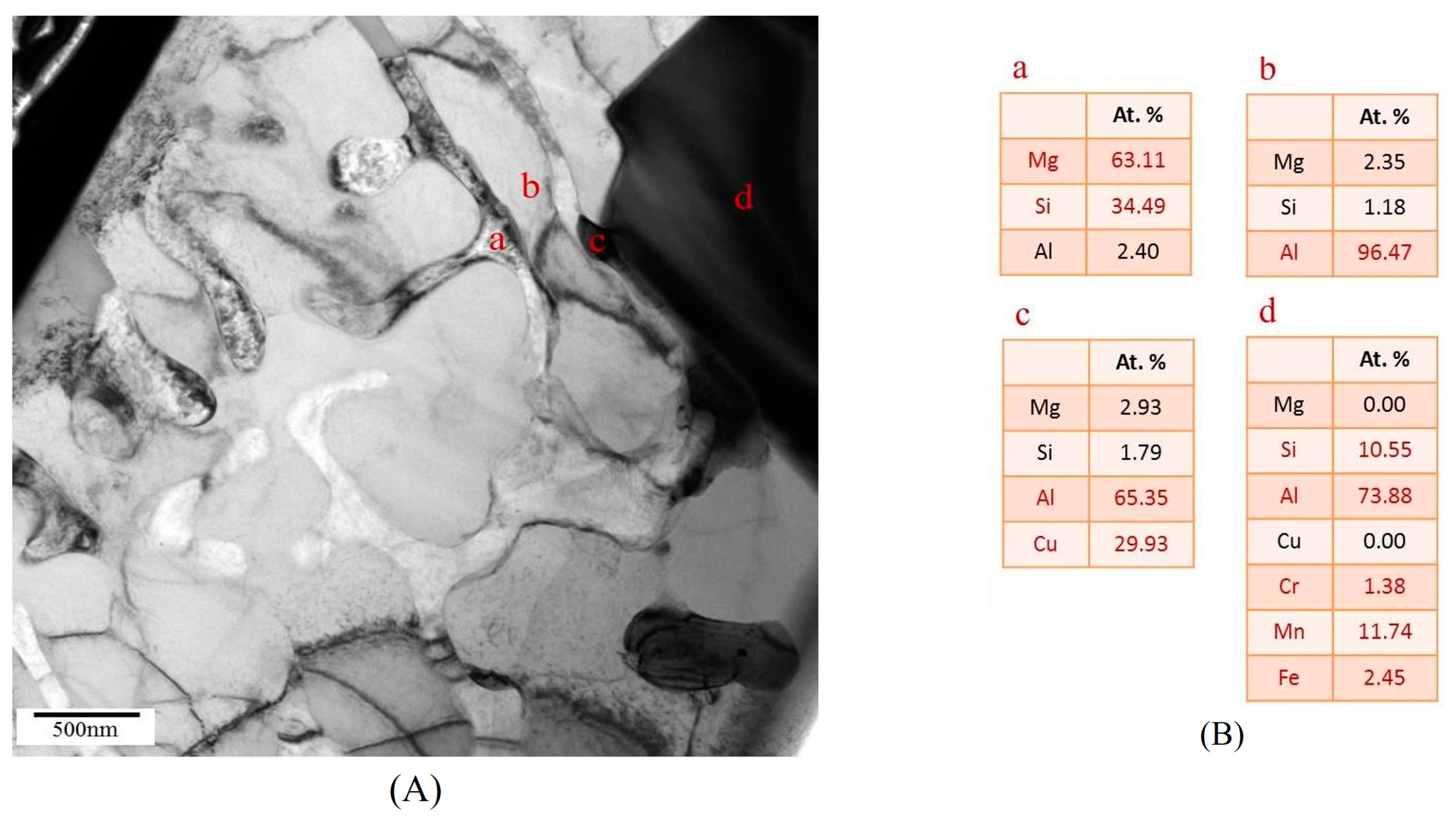
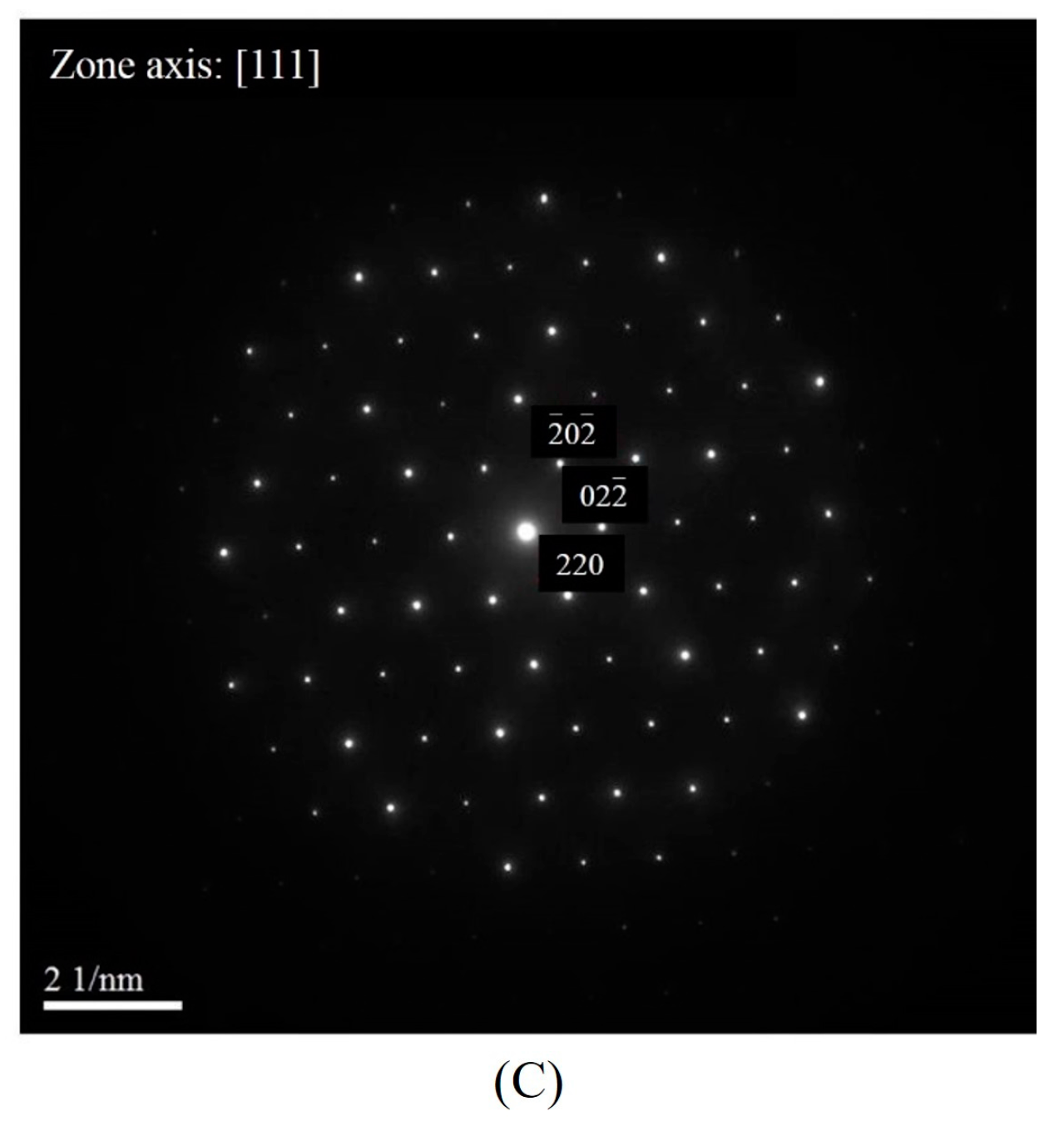
3.3.1. TEM Analysis of Liquid Pool Mostly Composed of Si and Mg
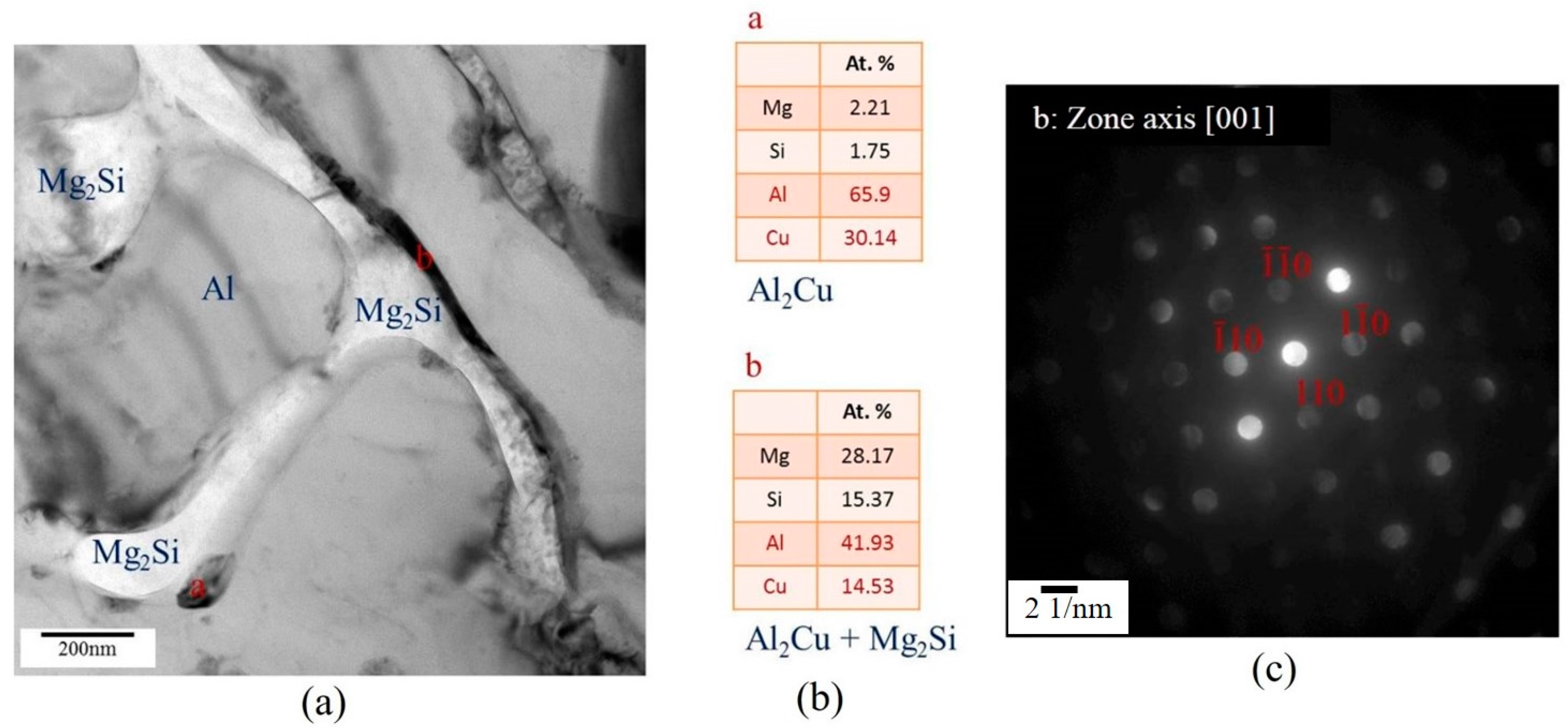
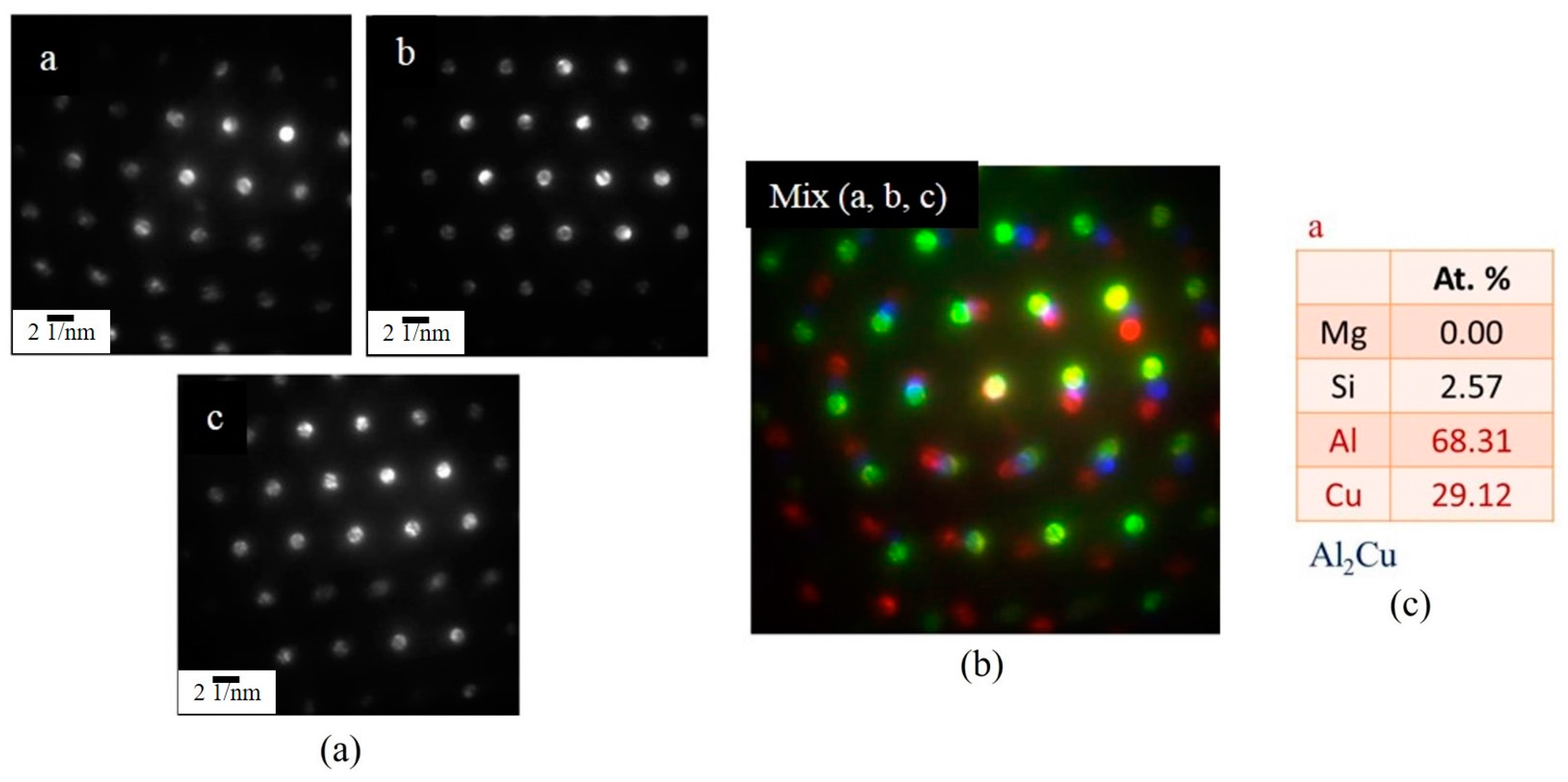
3.3.2. TEM Analysis of Globule Boundaries Mostly Composed of Si and Cu
4. Conclusions
- Three major factors affect the generation of fine, uniform, and highly spheroidized globular grains with high liquid fractions in this modified SIMA process: (a) sufficient elements to form low melting point phases; (b) the addition of particular elements for inhibiting grain growth; and (c) proper extrusion parameters to get a fine and uniform initial extrusion microstructure.
- Of the 6xxx series alloys, 6066 aluminum alloy is the most suitable for the modified SIMA process. Mg, Si, and Cu can generate low melting point phases, and Mn can inhibit grain growth.
- According to TEM results, the eutectic phases of Al and Mg2Si, Al and Al2Cu, and Al and Si are the major phases at the globule boundaries. EPMA data confirm this result. The high melting-point phase Al15(Fe,Mn,Cr)3Si2 was also identified by TEM.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fan, Z. Semisolid metal processing. Int. Mater. Rev. 2002, 47, 49–85. [Google Scholar] [CrossRef]
- Tzimas, E.; Zavaliangos, A. A comparative characterization of near-equiaxed microstructures as produced by spray casting, magnetohydronamic casting and the stress induced, melt activated process. Mater. Sci. Eng. A 2000, 289, 217–227. [Google Scholar] [CrossRef]
- Tzimas, E.; Zavaliangos, A. Evolution of near-equizxed microstructure in the semisolid state. Mater. Sci. Eng. A 2000, 289, 228–240. [Google Scholar] [CrossRef]
- Song, Y.B.; Park, K.T.; Hong, C.P. Grain refinement of 6061 Al alloy by the modified strain-induced melt-activated (SIMA) process for semi-solid processing. Mater. Trans. 2006, 47, 1250–1256. [Google Scholar] [CrossRef]
- Parshizfard, E.; Shabestari, S.G. An investigation on the microstructural evolution and mechanical properties of A380 aluminum alloy during SIMA process. J. Alloys Compd. 2011, 509, 9654–9658. [Google Scholar] [CrossRef]
- Paes, M.; Zoqui, E.J. Semi-solid behavior of new Al–Si–Mg alloy for thixoforming. Mater. Sci. Eng. A 2005, 406, 63–73. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. High-temperature deformation resistance and forming behavior of two-step SIMA-processed 6066 alloy. Mater. Sci. Eng. A 2016, 659, 143–157. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. Microstructure evolution and high-temperature compressibility of modified two-step strain-induced melt activation-processed Al-Mg-Si aluminum alloy. Metals 2015, 6, 113. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. High-temperature compressive resistance and mechanical properties improvement of strain-induced melt activation-processed Al–Mg–Si aluminum alloy. Metals 2016, 6, 183. [Google Scholar] [CrossRef]
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy; American Society for Metals: Materials Park, OH, USA, 1984; pp. 224–240. [Google Scholar]
- Totten, G.E.; Mackenzie, D.S. Handbook of Aluminum-Physical Metallurgy and Process; Metal Dekker Inc.: Valley Forge, PA, USA, 2003; pp. 168–185. [Google Scholar]
- Zhen, L.; Fei, W.D.; Kang, S.B.; Kim, H.W. Precipitation behavior of Al–Si–Mg alloys with high silicon content. J. Mater. Sci. 1997, 32, 1895–1902. [Google Scholar] [CrossRef]
- Jeniski, R.A., Jr. Effects of Cr addition on the microstructure and mechanical behavior of 6061-T6 continuously cast and rolled redraw rod. Mater. Sci. Eng. A 1997, 237, 52–64. [Google Scholar] [CrossRef]
- Claves, S.R.; Elias, D.L.; Misiolek, W.Z. Analysis of the intermetallic phase transformation occurring during homogenization of 6xxx aluminum alloys. Mater. Sci. Forum 2002, 396, 667–674. [Google Scholar] [CrossRef]
- Miao, W.F.; Laughlin, D.E. Effects of Cu content and presaging on precipitation characteristics in Aluminum alloy 6022. Metall. Mater. Trans. A 2000, 31, 361–371. [Google Scholar] [CrossRef]
- Man, J.; Jing, L.; Jie, S.G. The effects of Cu addition on the microstructure and thermal stability of Al–Si–Mg alloy. J. Alloys Compd. 2007, 437, 146–150. [Google Scholar] [CrossRef]
- Sepehrband, P.; Mahmudi, R.; Khomamizadeh, F. Effect of Zr addition on the aging behavior of A319 aluminum cast alloy. Scr. Mater. 2005, 52, 253–257. [Google Scholar] [CrossRef]
- Cabibbo, M.; Evangelista, E.; Scalabroni, C.; Bonetti, E. A transmission electron microscopy study of the role of Sc + Zr addition to a 6082-T8 alloy subjected to equal channel angular pressing. Mater. Sci. Forum 2006, 503–504, 841–846. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, Z.; Hu, M.; Xu, H. Evolution of the semi-solid microstructure of ADC12 alloy in a modified SIMA process. Mater. Charact. 2011, 62, 925–930. [Google Scholar] [CrossRef]
- Yan, G.; Zhao, S.; Ma, S.; Shou, H. Microstructural evolution of A356.2 alloy prepared by the SIMA process. Mater. Charact. 2012, 69, 45–51. [Google Scholar] [CrossRef]
- Hardy, S.C.; Voorhees, P.W. Ostwald ripening in a system with a high volume fraction of coarsening phase. Metall. Trans. A 1988, 19, 2713–2721. [Google Scholar] [CrossRef]
- Huang, H.J.; Cai, Y.H.; Cui, H.; Huang, J.F.; He, J.P.; Zhang, J.S. Influence of Mn addition on microstructure and phase formation of spray-deposited Al-25Si-xFe-yMn alloy. Mater. Sci. Eng. A 2009, 502, 118–125. [Google Scholar] [CrossRef]



| Code | Mg | Si | Cu | Mn | Fe | Cr | V | Zr | Al |
|---|---|---|---|---|---|---|---|---|---|
| 6061 | 0.88 | 0.67 | 0.16 | 0.03 | 0.19 | 0.04 | 0 | 0 | Bal. |
| 6061-Mn | 0.83 | 0.75 | 0.20 | 0.31 | 0.19 | 0.07 | 0 | 0 | Bal. |
| 6066-3mm | 1.02 | 1.29 | 0.95 | 1.02 | 0.19 | 0.18 | 0 | 0 | Bal. |
| 6066-9mm | 1.02 | 1.29 | 0.95 | 1.02 | 0.19 | 0.18 | 0 | 0 | Bal. |
| 6069-sheet | 1.25 | 0.75 | 0.73 | 0.05 | 0.13 | 0.16 | 0 | 0.11 | Bal. |
| 6069-rod | 1.30 | 0.85 | 0.72 | 0.09 | 0.13 | 0.14 | 0.12 | 0 | Bal. |
| 6082 | 0.71 | 1.10 | 0.06 | 0.71 | 0.19 | 0.23 | 0 | 0 | Bal. |
| Code | Average Initial Grain Size (μm) | Liquid Fraction (%) in 10-min Salt Bath | K (μm3·min−1) |
|---|---|---|---|
| 6061 | 40 | 3.2 | 42,329 |
| 6061-Mn | 7 | 7.2 | 30,054 |
| 6066-3mm | 3–5 | 16 | 16,806 |
| 6066-9mm | 5–7 | 15.5 | 17,024 |
| 6069-sheet | 7 & 300 | 10.4 | 31,579 |
| 6069-rod (center) | 15 | 11.2 | 32,678 |
| 6082 | 8 | 7.5 | 24,357 |
| Material | Mg2Si | Al2Cu | Excess Si | Sum |
|---|---|---|---|---|
| 6061 | 1.11 | 0.33 | 0.28 | 1.72 |
| 6066 | 1.61 | 1.76 | 0.7 | 3.97 |
| 6069 | 2.01 | 1.35 | 0.07 | 3.43 |
| 6082 | 1.12 | 0.11 | 0.7 | 1.93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Hung, F.-Y.; Lui, T.-S. Microstructure Evolution and Microstructural Characteristics of Al–Mg–Si Aluminum Alloys Fabricated by a Modified Strain-Induced Melting Activation Process. Metals 2018, 8, 3. https://doi.org/10.3390/met8010003
Lin C-W, Hung F-Y, Lui T-S. Microstructure Evolution and Microstructural Characteristics of Al–Mg–Si Aluminum Alloys Fabricated by a Modified Strain-Induced Melting Activation Process. Metals. 2018; 8(1):3. https://doi.org/10.3390/met8010003
Chicago/Turabian StyleLin, Chia-Wei, Fei-Yi Hung, and Truan-Sheng Lui. 2018. "Microstructure Evolution and Microstructural Characteristics of Al–Mg–Si Aluminum Alloys Fabricated by a Modified Strain-Induced Melting Activation Process" Metals 8, no. 1: 3. https://doi.org/10.3390/met8010003





