Ion Implantation of Calcium and Zinc in Magnesium for Biodegradable Implant Applications
Abstract
:1. Introduction
2. Materials and Methods
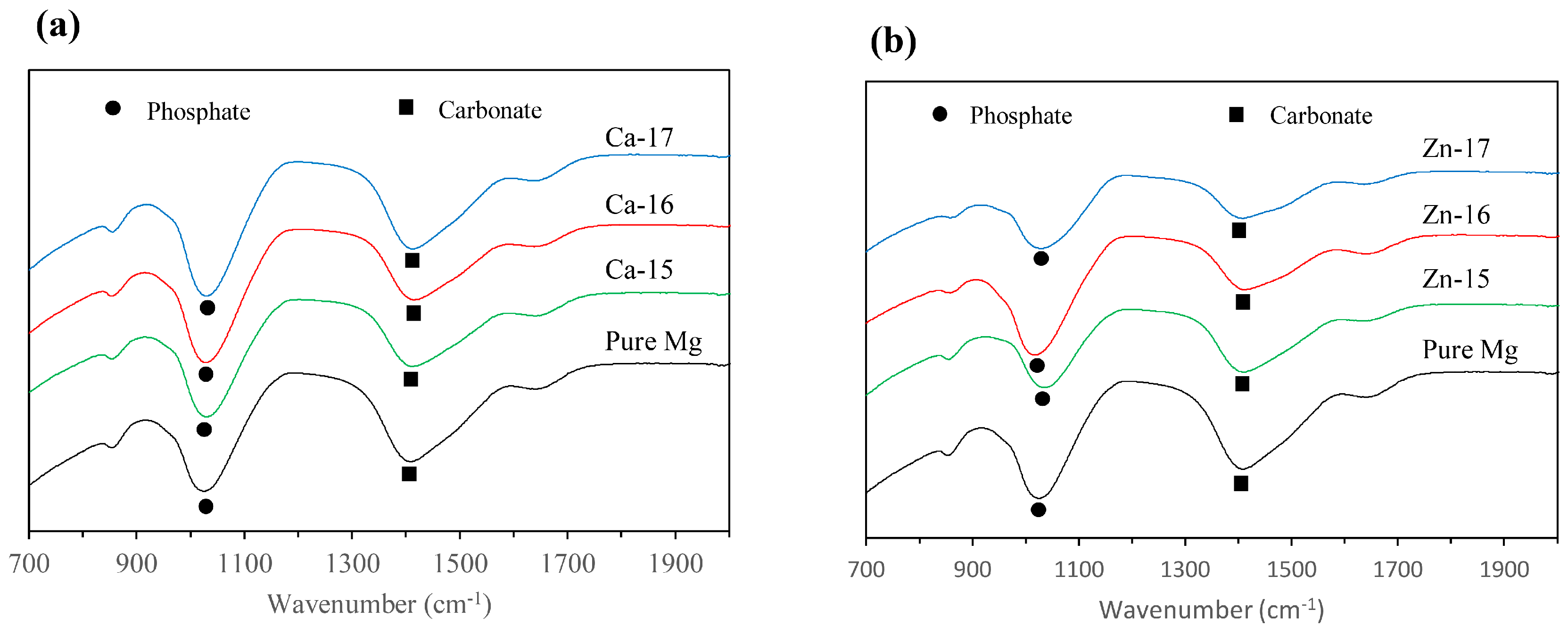
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartwig, A.; Decker, M.; Klein, O.; Karl, H. Stoichiometric titanium dioxide ion implantation in aisi 304 stainless steel for corrosion protection. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2015, 365, 94–99. [Google Scholar] [CrossRef]
- Guo, Z.; Pang, X.; Yan, Y.; Gao, K.; Volinsky, A.A.; Zhang, T.-Y. Cocrmo alloy for orthopedic implant application enhanced corrosion and tribocorrosion properties by nitrogen ion implantation. Appl. Surf. Sci. 2015, 347, 23–34. [Google Scholar] [CrossRef]
- Huang, H.-H.; Huang, H.-M.; Lin, M.-C.; Zhang, W.; Sun, Y.-S.; Kai, W.; Liaw, P.K. Enhancing the bio-corrosion resistance of ni-free zrcufeal bulk metallic glass through nitrogen plasma immersion ion implantation. J. Alloys Compd. 2014, 615 (Suppl. 1), S660–S665. [Google Scholar] [CrossRef]
- Escalada, L.; Lutz, J.; Brühl, S.P.; Fazio, M.; Márquez, A.; Mändl, S.; Manova, D.; Simison, S.N. Microstructure and corrosion behavior of aisi 316l duplex treated by means of ion nitriding and plasma based ion implantation and deposition. Surf. Coat. Technol. 2013, 223, 41–46. [Google Scholar] [CrossRef]
- Feng, K.; Wang, Y.; Li, Z.; Chu, P.K. Characterization of carbon ion implantation induced graded microstructure and phase transformation in stainless steel. Mater. Charact. 2015, 106, 11–19. [Google Scholar] [CrossRef]
- Hongxi, L.; Qian, X.; Xiaowei, Z.; Chuanqi, W.; Baoyin, T. Wear and corrosion behaviors of ti6al4v alloy biomedical materials by silver plasma immersion ion implantation process. Thin Solid Films 2012, 521, 89–93. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, S.; Xu, K.; Chu, P.K. Corrosion resistance of praseodymium-ion-implanted tin coatings in blood and cytocompatibility with vascular endothelial cells. Vacuum 2015, 117, 73–80. [Google Scholar] [CrossRef]
- Grayeli-Korpi, A.-R.; Savaloni, H. Effect of nitrogen ion implantation on corrosion inhibition of nickel coated 316 stainless steel and correlation with nano-structure. Appl. Surf. Sci. 2012, 258, 9982–9988. [Google Scholar] [CrossRef]
- Zhang, E.; Chen, Y.; Tang, Y. Effect of copper ion implantation on corrosion morphology and corrosion behavior of LaFe11.6Si1.4 alloy. J. Rare Earths 2012, 30, 269–273. [Google Scholar] [CrossRef]
- Feng, K.; Cai, X.; Li, Z.; Chu, P.K. Improved corrosion resistance of stainless steel 316l by ti ion implantation. Mater. Lett. 2012, 68, 450–452. [Google Scholar] [CrossRef]
- Williams, J.M.; Gonzales, A.; Quintana, J.; Lee, I.S.; Buchanan, R.A.; Burns, F.C.; Culbertson, R.J.; Levy, M.; Treglio, J.R. Ion implantation for corrosion inhibition of aluminum alloys in saline media. Nucl. Instrum. Methods Phys. Res. Sect. B 1991, 59–60, 845–850. [Google Scholar] [CrossRef]
- Abreu, C.M.; Cristóbal, M.J.; Figueroa, R.; Pena, G. Influence of molybdenum ion implantation on the localized corrosion resistance of a high strength aluminium alloy. Corros. Sci. 2012, 54, 143–152. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zu, X.T.; Wang, L.; Qiu, S.Y. Role of aluminum ion implantation on microstructure, microhardness and corrosion properties of titanium alloy. Vacuum 2008, 83, 444–447. [Google Scholar] [CrossRef]
- Ali, N.; Fulazzaky, M.A.; Mustapa, M.S.; Ghazali, M.I.; Ridha, M.; Sujitno, T. Assessment of fatigue and corrosion fatigue behaviours of the nitrogen ion implanted cpti. Int. J. Fatigue 2014, 61, 184–190. [Google Scholar] [CrossRef]
- Zhao, Y.; Jamesh, M.I.; Li, W.K.; Wu, G.; Wang, C.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K. Enhanced antimicrobial properties, cytocompatibility, and corrosion resistance of plasma-modified biodegradable magnesium alloys. Acta Biomater. 2014, 10, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Effects of zirconium and oxygen plasma ion implantation on the corrosion behavior of ZK60 Mg alloy in simulated body fluids. Corros. Sci. 2014, 82, 7–26. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; Jin, W.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Effects of zirconium and nitrogen plasma immersion ion implantation on the electrochemical corrosion behavior of Mg–Y–Re alloy in simulated body fluid and cell culture medium. Corros. Sci. 2014, 86, 239–251. [Google Scholar] [CrossRef]
- Wu, G.; Xu, R.; Feng, K.; Wu, S.; Wu, Z.; Sun, G.; Zheng, G.; Li, G.; Chu, P.K. Retardation of surface corrosion of biodegradable magnesium-based materials by aluminum ion implantation. Appl. Surf. Sci. 2012, 258, 7651–7657. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Chen, J.; Zou, Z. Surface characteristics and corrosion resistance of biodegradable magnesium alloy ZK60 modified by Fe ion implantation and deposition. Prog. Nat. Sci. Mater. Int. 2014, 24, 547–553. [Google Scholar] [CrossRef]
- Koç, E.; Kannan, M.B.; Ünal, M.; Candan, E. Influence of zinc on the microstructure, mechanical properties and in vitro corrosion behavior of magnesium–zinc binary alloys. J. Alloys Compd. 2015, 648, 291–296. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D. Structural characteristics and corrosion behavior of biodegradable Mg-Zn, Mg-Zn-Gd alloys. J. Mater. Sci. Mater. Med. 2013, 24, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gong, L.; Feng, K.; Wu, S.; Zhao, Y.; Chu, P.K. Rapid degradation of biomedical magnesium induced by zinc ion implantation. Mater. Lett. 2011, 65, 661–663. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Xiong, G.Y.; Luo, H.L.; He, F.; Huang, Y.; Wang, Y.L. Influence of zinc ion implantation on surface nanomechanical performance and corrosion resistance of biomedical magnesium-calcium alloys. Appl. Surf. Sci. 2008, 254, 5514–5516. [Google Scholar] [CrossRef]
- Xu, R.; Yang, X.; Suen, K.W.; Wu, G.; Li, P.; Chu, P.K. Improved corrosion resistance on biodegradable magnesium by zinc and aluminum ion implantation. Appl. Surf. Sci. 2012, 263, 608–612. [Google Scholar] [CrossRef]
- Myasnikov, A.M.; Gerasimenko, N.N. Chapter 8 ion implantation and thermal annealing of III-V compound semiconducting systems: Some problems of III-V narrow gap semiconductors. In Semiconductors and Semimetals; Constantinos, C., Gérard, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 46, pp. 257–293. [Google Scholar]
- Yang, H.; Zhang, S.; Yu, D.; Li, K.; Hu, Q.; Yang, Y.; Zhang, K.; Li, H. Corrosion resistance and magnetostrictive properties of (Tb0.3Dy0.7)Fe2 alloy modified by nitrogen ion implantation. J. Rare Earths 2015, 33, 629–632. [Google Scholar] [CrossRef]
- Song, Y.; Han, E.-H.; Dong, K.; Shan, D.; Yim, C.D.; You, B.S. Effect of hydrogen on the corrosion behavior of the Mg–xZn alloys. J. Magnes. Alloys 2014, 2, 208–213. [Google Scholar] [CrossRef]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Rosalbino, F.; De Negri, S.; Saccone, A.; Angelini, E.; Delfino, S. Bio-corrosion characterization of Mg-Zn-x (x = Ca, Mn, Si) alloys for biomedical applications. J. Mater. Sci. Mater. Med. 2010, 21, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Harandi, S.E.; Mirshahi, M.; Koleini, S.; Idris, M.H.; Jafari, H.; Kadir, M.R.A. Effect of calcium content on the microstructure, hardness and in-vitro corrosion behavior of biodegradable Mg-Ca binary alloy. Mater. Res. 2013, 16, 11–18. [Google Scholar] [CrossRef]
- Kim, K.H.; Nam, N.D.; Kim, J.G.; Shin, K.S.; Jung, H.C. Effect of calcium addition on the corrosion behavior of Mg–5Al alloy. Intermetallics 2011, 19, 1831–1838. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Biliński, A.; Lewandowska-Szumieł, M.; Rajchel, B. Effect of calcium-ion implantation on the corrosion resistance and biocompatibility of titanium. Biomaterials 2001, 22, 2139–2151. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Galván, J.; Pardo, A.; Merino, M.; Arrabal, R. Native air-formed oxide film and its effect on magnesium alloys corrosion. Open Corros. J. 2010, 3, 80–91. [Google Scholar]
- Zucchi, F.; Grassi, V.; Frignani, A.; Monticelli, C.; Trabanelli, G. Electrochemical behaviour of a magnesium alloy containing rare earth elements. J. Appl. Electrochem. 2006, 36, 195–204. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D. Electrochim. Acta 2007, 52, 3785–3793. [Google Scholar] [CrossRef]
- Alabbasi, A.; Bobby Kannan, M.; Walter, R.; Störmer, M.; Blawert, C. Performance of pulsed constant current silicate-based peo coating on pure magnesium in simulated body fluid. Mater. Lett. 2013, 106, 18–21. [Google Scholar] [CrossRef]
- Walter, R.; Bobby Kannan, M. In-vitro degradation behaviour of we54 magnesium alloy in simulated body fluid. Mater. Lett. 2011, 65, 748–750. [Google Scholar] [CrossRef]
- Jin, S.; Amira, S.; Ghali, E. Electrochemical impedance spectroscopy evaluation of the corrosion behavior of die cast and thixocast AXJ530 magnesium alloy in chloride solution. Adv. Eng. Mater. 2007, 9, 75–83. [Google Scholar] [CrossRef]
- Virtanen, S.; Schmuki, P.; Frankel, G.S.; Division, E.S.C.; Meeting, E.S. Critical Factors in Localized Corrosion IV: A Symposium in Honor of the 65th Birthday of Hans Böhni: Proceedings of the International Symposium; Electrochemical Society: Pennington, NJ, USA, 2003. [Google Scholar]
- Bobby Kannan, M.; Singh, R.K.R. A mechanistic study of in vitro degradation of magnesium alloy using electrochemical techniques. J. Biomed. Mater. Res. Part A 2010, 93A, 1050–1055. [Google Scholar]
- Pramatarova, L.; Pecheva, E.; Presker, R.; Pham, M.T.; Maitz, M.F.; Stutzmann, M. Hydroxyapatite growth induced by native extracellular matrix deposition on solid surfaces. Eur. Cells Mater. 2005, 9, 9–12. [Google Scholar] [CrossRef]
- Agha, N.A.; Feyerabend, F.; Mihailova, B.; Heidrich, S.; Bismayer, U.; Willumeit-Römer, R. Magnesium degradation influenced by buffering salts in concentrations typical of in vitro and in vivo models. Mater. Sci. Eng. C 2016, 58, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.; Baby, S.; Menon, K.V. Spinal fixation device: A 6-year postimplantation study. J. Biomater. Appl. 2003, 18, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Shi, Z.; Song, G.; Atrens, A. Corrosion resistance of anodised single-phase Mg alloys. Surf. Coat. Technol. 2006, 201, 492–503. [Google Scholar] [CrossRef]
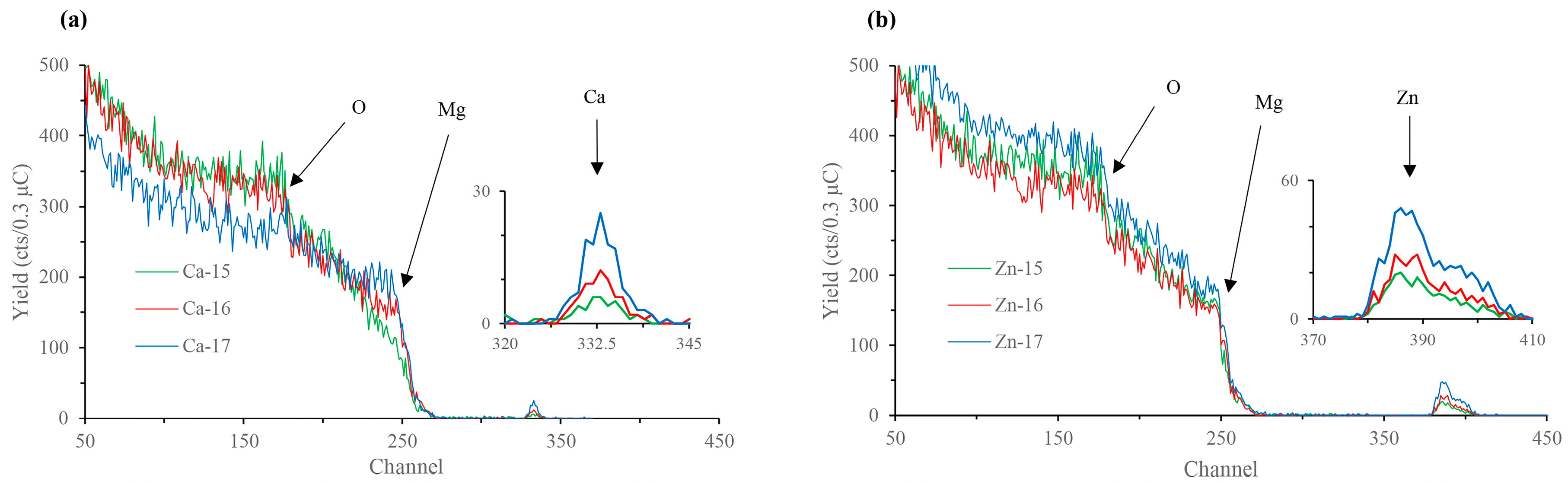
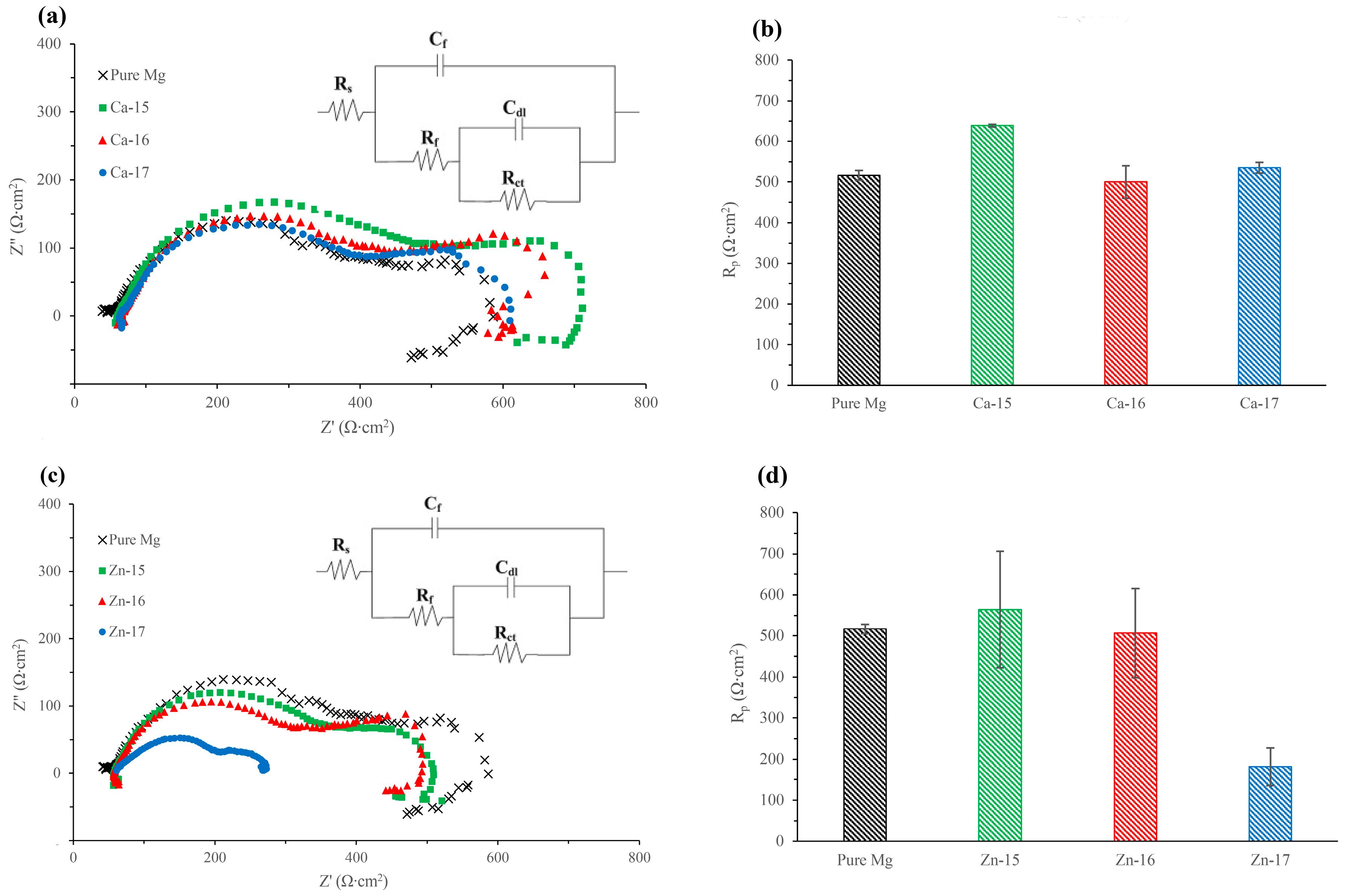
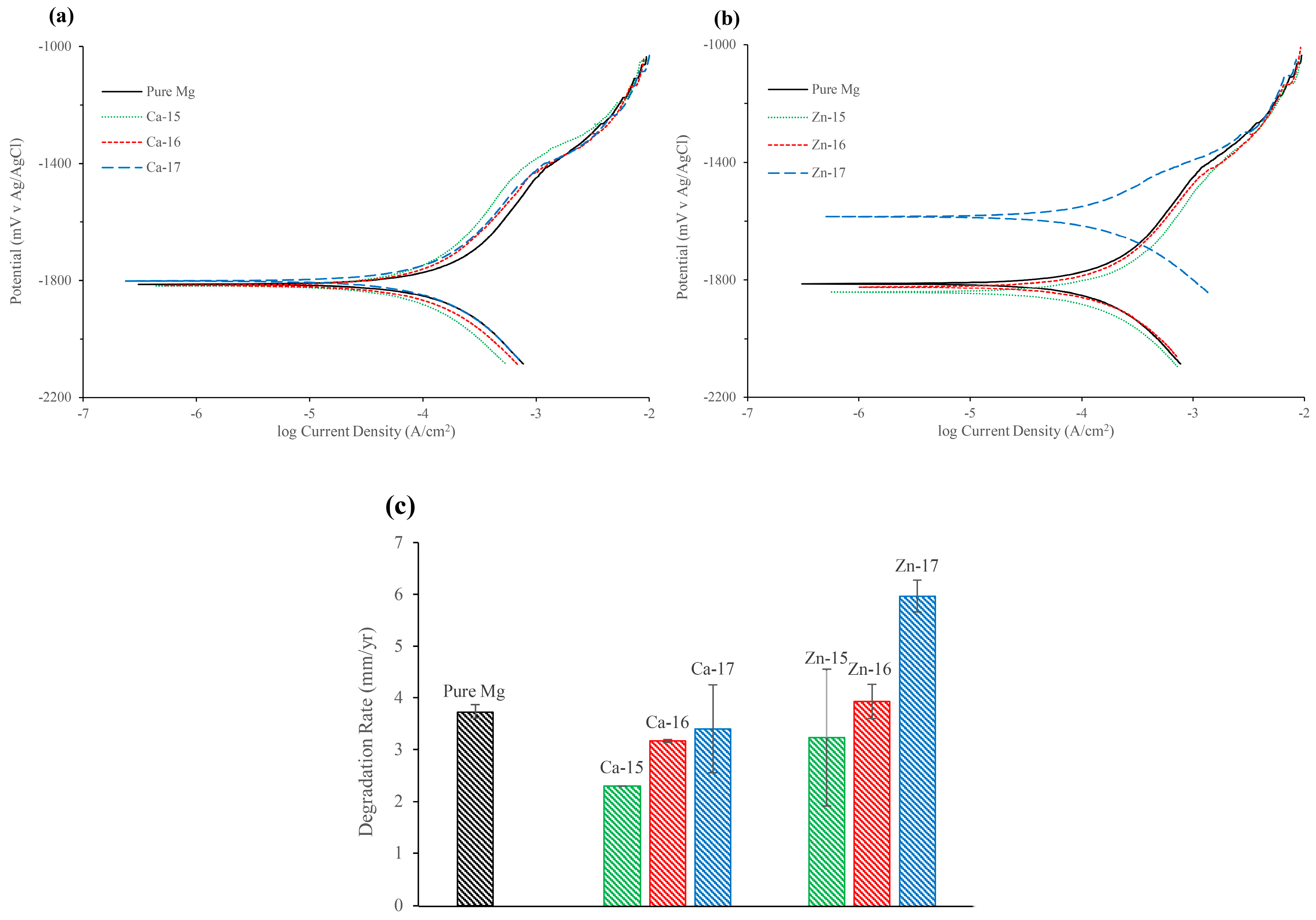
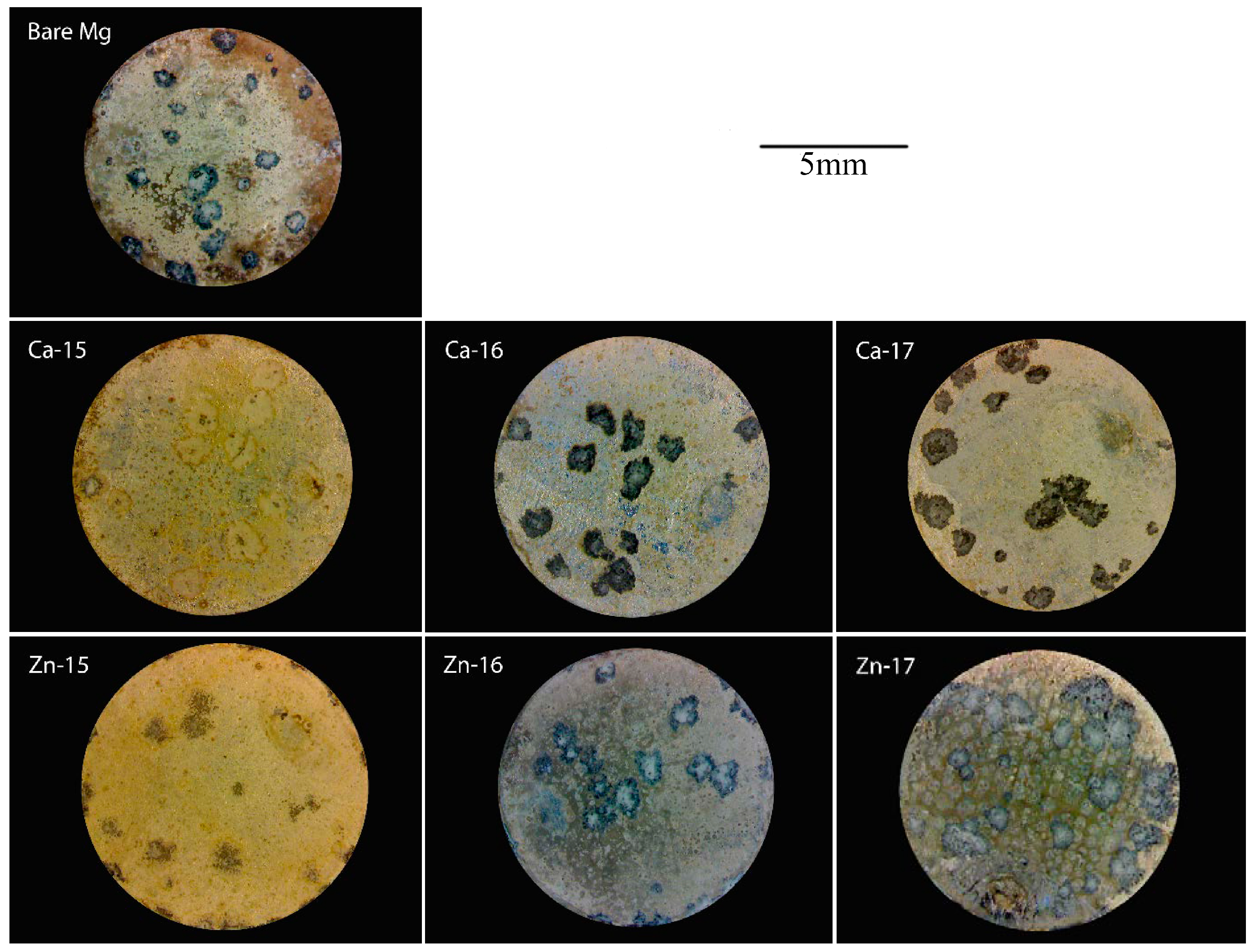
| Element | Zn | Ca | Fe | Cu | Al | Mn | Si | Mg |
|---|---|---|---|---|---|---|---|---|
| Weight % | 0.008 | 0.003 | 0.004 | 0.001 | 0.007 | 0.002 | 0.01 | Bal. |
| Reagent | Amount |
|---|---|
| NaCl (g/L) | 8.036 |
| NaHCO3 (g/L) | 0.352 |
| KCl (g/L) | 0.225 |
| K2HPO4∙3H2O (g/L) | 0.230 |
| MgCl2∙6H2O (g/L) | 0.311 |
| 1.0 M HCl (mL/L) | 40.0 |
| CaCl2 (g/L) | 0.293 |
| Na2SO4 (g/L) | 0.072 |
| TRIS Buffer (g/L) | 6.063 |
| Sample | Cdl (Ω−1∙cm−2∙s−n) (× 10−3) | n-Cdl | Rt (Ω∙cm2) | Cf (Ω−1∙cm−2∙s−n) (×10−5) | n-Cf | Rf (Ω∙cm2) |
|---|---|---|---|---|---|---|
| Pure Mg | 1.05 ± 1.72 | 0.74 | 94.93 ± 59.68 | 11.25 ± 15.62 | 0.69 | 429.80 ± 89.75 |
| Ca-15 | 2.61 ± 0.16 | 0.99 | 164.65 ± 33.59 | 5.28 ± 0.19 | 0.80 | 466.80 ± 34.51 |
| Ca-16 | 2.95 ± 0.05 | 0.92 | 173.65 ± 60.25 | 5.32 ± 0.20 | 0.79 | 357.71 ± 47.80 |
| Ca-17 | 3.07 ± 0.30 | 0.99 | 147.30 ± 24.46 | 5.64 ± 0.23 | 0.78 | 366.62 ± 4.38 |
| Zn-15 | 3.10 ± 0.16 | 0.98 | 130.80 ± 10.61 | 5.53 ± 0.61 | 0.79 | 404.95 ± 133.14 |
| Zn-16 | 2.53 ± 0.45 | 0.95 | 177.05 ± 31.18 | 5.22 ± 0.42 | 0.80 | 315.90 ± 61.37 |
| Zn-17 | 2.73 ± 3.72 | 0.84 | 80.46 ± 50.83 | 9.02 ± 8.23 | 0.66 | 98.62 ± 97.34 |
| Sample | Ecorr (mV v Ag/AgCl) | icorr (µA/cm2) | Ebd (mV v Ag/AgCl) | Epass (mV) |
|---|---|---|---|---|
| Pure Mg | −1820 ± 24.27 | 161.66 ± 5.50 | −1418 ± 18.50 | 401 ± 4.72 |
| Ca-15 | −1825 ± 16.26 | 100 ± 0.00 | −1384 ± 16.97 | 441 ± 33.23 |
| Ca-16 | −1817 ± 4.24 | 138 ± 1.41 | −1443 ± 19.79 | 374 ± 24.04 |
| Ca-17 | −1811 ± 12.72 | 148 ± 36.77 | −1437 ± 15.55 | 374 ± 2.82 |
| Zn-15 | −1833 ± 9.89 | 140.5 ± 57.27 | −1399 ± 20.50 | 433 ± 10.61 |
| Zn-16 | −1809 ± 15.55 | 171 ± 14.14 | −1411 ± 16.97 | 398 ± 32.53 |
| Zn-17 | −1583 ± 0.00 | 259.5 ± 13.43 | −1440 ± 6.36 | 142.5 ± 6.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somasundaram, S.; Ionescu, M.; Mathan, B.K. Ion Implantation of Calcium and Zinc in Magnesium for Biodegradable Implant Applications. Metals 2018, 8, 30. https://doi.org/10.3390/met8010030
Somasundaram S, Ionescu M, Mathan BK. Ion Implantation of Calcium and Zinc in Magnesium for Biodegradable Implant Applications. Metals. 2018; 8(1):30. https://doi.org/10.3390/met8010030
Chicago/Turabian StyleSomasundaram, Sahadev, Mihail Ionescu, and Bobby Kannan Mathan. 2018. "Ion Implantation of Calcium and Zinc in Magnesium for Biodegradable Implant Applications" Metals 8, no. 1: 30. https://doi.org/10.3390/met8010030




