1. Introduction
Titanium is a promising material for advanced applications thanks to its high specific strength, excellent corrosion resistance and good biocompatibility. In order to overcome its poor machinability and to reduce material losses, powder metallurgy routes for processing Ti and titanium alloys have been seriously considered since 1980 [
1,
2] and recently comprehensively reviewed in [
3]. Most attention is, naturally, paid to methods of powder production and methods of subsequent compaction. However, powder metallurgy represents an opportunity to affect the properties of the final bulk material via manipulating with the powder. Characteristics of powders such as particle size, particle morphology, material contamination and internal microstructure affect the compaction processing and ultimately the properties of final bulk products.
The properties of bulk materials can be influenced by mechanical milling. The term “milling” itself obviously refers to the reduction of particle sizes by mechanical forces. However, in the case of ductile materials, the particle size reduction may not be easily achievable, which will be discussed below for titanium and its alloys. However, even when the overall particle size is reduced only slightly, internal microstructural changes within each particle might be significant. During mechanical milling, powder particles are repeatedly fragmented and cold-welded together [
4], which determines their final size. However, this process is accompanied by intensive plastic deformation of individual particles and subsequent grain refinement within each of them. Note that the term “particle size” refers to the size of an individual powder particle and shall not be mixed with the term “grain size” that refers to the size of the grain of the polycrystalline structure within a particle. Finally, “crystallite size” refers to the size of a coherently diffracting domain in an X-ray diffraction (XRD) experiment.
High-energy ball milling is one of the most efficient methods for microstructural refinement of powder particles [
4]. It is well known that reducing grain size in the bulk metallic material to submicrometer levels significantly improves its mechanical properties due to the well known Hall-Petch relation. Some scientists therefore aimed on microstructural refinement via reducing the particle size of titanium powder to sub-micrometer levels by ball milling [
5], despite, as described below, particle size reduction is not required for grain size reduction [
6,
7].
The peculiarity of titanium is an immense affinity to oxygen, nitrogen and also hydrogen intake from the environment. Ball milling of Ti in the air atmosphere resulted in formation of titanium oxides, titanium nitrides and to significant intake of O and N in hcp (hexagonal close packed) Ti-matrix [
8]. The formation of uncommon fcc (face centered cubic)-Ti(O,N) was also documented [
8,
9]. Similarly, titanium hydride formed during ball milling of Ti in hydrogen atmosphere [
10]. All these compounds and also hcp Ti-matrix with high O and/or N content are brittle and therefore allow to reduce particle size during milling. For instance, long-term milling led to particle sizes below 50 nm [
5]. Despite the milling was performed in argon atmosphere, nitrogen intake could be demonstrated by observing Ti nitrides by XRD [
11]. On the other hand, when the contamination by H, C, N and O was prevented, the reduction of particle sizes was only minor [
12,
13].
Fortunately, particle size reduction is not required for grain size reduction. High-energy ball milling provides sufficient energy to plastically deform individual powder particles. Each particle is repeatedly severely plastically deformed during the process causing an increase of dislocation density and a grain refinement. Ultra-fine grained (UFG) materials exhibit superior properties [
14] and can be achieved by high-energy mechanical milling followed by appropriate compaction method [
12,
15,
16].
Thermally activated processes of recovery and grain growth may prevent microstructural refinement by severe plastic deformation. Milling under cryogenic temperatures (typically in liquid nitrogen (LN) or liquid argon (LAr) slurry) can be employed to suppress recovery and dynamic recrystallization. Cryogenic milling leads to the reduction of powder crystallite size to the order of tens of nanometers for aluminium [
7] and for titanium and its alloys [
15,
17,
18]. At the same time, the diffusivity of possible contaminating elements such as N or O is significantly reduced [
17]. Despite the reduced diffusivity, liquid nitrogen was found to be unsuitable as a cooling agent, because Ti powder became seriously contaminated by nitrogen (up to 2.99 wt % of N) even at cryogenic temperatures resulting in the brittleness of a bulk material [
19]. This confirmed the earlier results obtained in [
12].
Presented study shows the effect of processing parameters of cryogenic milling on particle size, morphology, microstructure, microhardness and contamination of commercially pure Ti powders. Next to commonly used stainless-steel milling balls, tungsten carbide balls were used. Hard and heavy tungsten carbide balls were successfully applied in producing titanium nitrides, borides or silicides [
9,
20,
21]. However, the use of very hard milling medium can result in the incorporation of material of milling balls into the milled powder [
4,
22]. Tungsten carbide balls for milling titanium were used in a remarkable study by Sekiguchi et al. [
13]. Potential contamination was avoided by covering of tungsten balls by titanium film using preliminary milling of small amount of Ti powder. Contrary to previous investigations, the presented study provides, next to other results, a direct comparison between employing stainless steel and tungsten carbide milling balls.
2. Experimental Methods
Commercially pure Ti powder (Grade 2) manufactured by gas atomization (TLS Technik GmbH & Co. Spezialpulver KG, Bitterfeld, Germany) was used as initial feedstock powder. All handling was performed in air.
Powder milling in liquid nitrogen (LN) or liquid argon (LAr) slurry (wet milling) was performed in Union Process 01-HD attritor (1400
). Stainless steel (SS) and tungsten carbide in Co matrix (WC-Co) balls (6.35 mm in diameter) were used as grinding media. WC-Co balls are significantly harder and twice heavier than SS balls, therefore an increased efficiency of milling is foreseen. Milling conditions are summarized in
Table 1. Note that ball-to-powder ratio (BPR) is calculated as the ratio of mass of balls divided by the mass of powder feed. Therefore, using high-density WC-Co balls led to doubled BPR, despite the volume of balls and the powder remained the same.
Stearic acid (SA, see
Table 1) was used as a process control agent to prevent excessive cold-welding in samples LAr-SS-700/4-SA and LAr-WC-650/3-SA. The used amount (8 wt % of powder) was found to be efficient in suppressing the cold-welding; it was found in a later trial attempt that 0.5 wt % might be sufficient. In order to remove remaining SA adhered to the powder particle surface after milling (and thus to reduce the overall contamination), powders were washed in ethanol and filtered using filtration paper three times.
Milled powder was observed by scanning electron microscope (SEM) FEI Quanta 200F (Hillsboro, OR, USA) equipped with a FEG (field emission gun) cathode and an EDAX energy dispersive X-ray spectroscopy (EDS) detector (Mahwah, NJ, USA). The amount of light elements (N, O, H) was measured by carrier gas hot extraction (CGHE) method. For quantitative analysis of size and morphology, powder particles were hot mounted in an epoxy resin, grinded and polished using standard metallographic methods and examined using SEM. On the cross-section of the specimen, microhardness of powders was measured by Qness Q10A testing machine (Golling, Austria) using Vickers method with very low force of 0.002 kgf (HV0.002) to reduce the size of the indent. 50 selected particles of each sample were manually measured.
In order to observe the powder internal microstructure directly, scanning electron microscopy with the detection of transmitted electrons (STEM) and transmission Kikuchi diffraction (TKD) methods were used. TKD method is also referred to as “transmission EBSD”. The best planar resolution of electron back-scattered diffraction (EBSD) in conventional geometry is typically between 30 and 50 nm, and can be up to 200 nm in the perpendicular longitudinal (primary beam) direction, depending on the material and acceleration voltage [
23]. For ultra-fine grained materials with grain size from few tens to hundreds of nanometers, the resolution of conventional EBSD is not sufficient since the interaction volume is comparable to the grain size. Moreover, in heavily deformed materials, the EBSD analysis is obscured by high dislocation density, residual strains and lattice rotations.
Therefore, to overcome this effect, Keller and Geiss [
24] realized that Kikuchi diffraction pattern observed in common EBSD from back-scattered electrons can be observed also for forward-scattered electrons propagating through a thin sample and suggested appropriate changes in the geometry of observation. It was shown that two points with different crystalline orientation separated by only 2.5 nm [
25] can be successfully measured and indexed. This method can be used in a conventional SEM equipped with an EBSD detector, nevertheless a thin specimen having the thickness of the standard TEM foil is required.
In this work, a lamella was cut from a selected powder particle and carefully thinned using gallium focused ion beam (FIB). Note that it is uneasy to find suitably oriented particle for lamella preparation and that the orientation of the lamella within the particle is unknown. Zeiss Auriga Compact FIB-SEM (Jena, Germany) with EDAX EBSD detector was used for the sample preparation, STEM imaging and TKD measurements with the step size of 5 nm. EDAX OIM software (version 7.3, Mahwah, NJ, USA, 2015) was used for data collection and processing.
Acknowledgments
This work was financially supported by Czech Science Foundation under the project 15-15609S and by ERDF project under the Project No. CZ.02.1.01/0.0/0.0/15_003/0000485.
Author Contributions
Jiří Kozlík, Josef Stráský and Petr Harcuba conceived and designed the experiments; Jiří Kozlík, Petr Harcuba and Ilya Ibragimov performed the experiments; Jiří Kozlík, Josef Stráský and Petr Harcuba analyzed the data; Miloš Janeček and Tomáš Chráska interpreted the achieved results; Josef Stráský and Jiří Kozlík wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Froes, F.H.; Smugeresky, J.E. Powder Metallurgy of Titanium Alloys: Proceedings of a Symposium; Metallurgical Society of AIME: Warrendale, PA, USA, 1980. [Google Scholar]
- Froes, F.H.; Eylon, D.; Eichelman, G.E.; Burte, H.M. Developments in titanium powder metallurgy. J. Microsc. 1980, 32, 47–54. [Google Scholar] [CrossRef]
- Qian, M.; Froes, F.H. Titanium Powder Metallurgy; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Dabhade, V.V.; Rama Mohan, T.R.; Ramakrishnan, P. Nanocrystalline titanium powders by high energy attrition milling. Powder Technol. 2007, 171, 177–183. [Google Scholar] [CrossRef]
- Fecht, H.J. Nanostructure formation by mechanical attrition. Nanostruct. Mater. 1995, 6, 33–42. [Google Scholar] [CrossRef]
- Witkin, D.B.; Lavernia, E.J. Synthesis and mechanical behavior of nanostructured materials via cryomilling. Prog. Mater. Sci. 2006, 51, 1–60. [Google Scholar] [CrossRef]
- Lu, C.J.; Zhang, J.; Li, Z.Q. Structural evolution of titanium powder during ball milling in different atmospheres. J. Alloys Compd. 2004, 381, 278–283. [Google Scholar] [CrossRef]
- Yang, H.; McCormick, P.G. Synthesis of titanium oxynitride by mechanical milling. J. Mater. Sci. 1993, 28, 5663–5667. [Google Scholar] [CrossRef]
- Zhang, H.; Kisi, E.H. Formation of titanium hydride at room temperature by ball milling. J. Phys. 1997, 9, L185. [Google Scholar] [CrossRef]
- Dabhade, V.V.; Rama Mohan, T.R.; Ramakrishnan, P. Synthesis of nanosized titanium powder by high energy milling. Appl. Surf. Sci. 2001, 182, 390–393. [Google Scholar] [CrossRef]
- Ertorer, O.; Zúñiga, A.; Topping, T.; Moss, W.; Lavernia, E.J. Mechanical behavior of cryomilled CP-Ti consoliyeard via quasi-isostatic forging. Metall. Mater. Trans. A 2008, 40, 91–103. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Ono, K.; Fujiwara, H.; Ameyama, K. New microstructure design for commercially pure titanium with outstanding mechanical properties by mechanical milling and hot roll sintering. Mater. Trans. 2010, 51, 39–45. [Google Scholar] [CrossRef]
- Langdon, T.G. Twenty-five years of ultrafine-grained materials: Achieving exceptional properties through grain refinement. Acta Mater. 2013, 61, 7035–7059. [Google Scholar] [CrossRef]
- Ertorer, O.; Topping, T.; Li, Y.; Moss, W.; Lavernia, E.J. Enhanced tensile strength and high ductility in cryomilled commercially pure titanium. Scr. Mater. 2009, 60, 586–589. [Google Scholar] [CrossRef]
- Ertorer, O.; Topping, T.D.; Li, Y.; Moss, W.; Lavernia, E.J. Nanostructured Ti consoliyeard via spark plasma sintering. Metall. Mater. Trans. A 2011, 42, 964–973. [Google Scholar] [CrossRef]
- Sun, F.; Rojas, P.; Zúñiga, A.; Lavernia, E.J. Nanostructure in a Ti alloy processed using a cryomilling technique. Mater. Sci. Eng. A 2006, 430, 90–97. [Google Scholar] [CrossRef]
- Dheda, S.S.; Melnyk, C.; Mohamed, F.A. Effect of titanium nitride nanoparticles on grain size stabilization and consolidation of cryomilled titanium. Mater. Sci. Eng. A 2013, 584, 88–96. [Google Scholar] [CrossRef]
- Kozlik, J.; Becker, H.; Strasky, J.; Harcuba, P.; Janecek, M. Influence of milling parameters on particle size and microhardness of cryomilled and spark plasma sintered CP Ti. In Proceedings of the Metal 2016: 25th Anniversary International Conference on Metallurgy and Materials, Brno, Czech Republic, 25–27 May 2016; pp. 1439–1444. [Google Scholar]
- Kudaka, K.; Iizumi, K.; Sasaku, T. Mechanochemical syntheses of titanium carbide, diboride and nitride. J. Ceram. Soc. Jpn. 1999, 107, 1019–1024. [Google Scholar] [CrossRef]
- Kudaka, K.; Iizumi, K.; Sasaki, T.; Izumi, H. Effect of milling media on the reactionkinetics of the mechanochemical synthesis of pentatitanium trisilicide. J. Am. Ceram. Soc. 2000, 83, 2887–2889. [Google Scholar] [CrossRef]
- Haruyama, O.; Asahi, N. Amorphization of Mixed Ni and Zr Powders by Mechanical Alloying. Mater. Sci. Forum 1992, 88, 333–338. [Google Scholar] [CrossRef]
- Chen, D.; Kuo, J.C.; Wu, W.T. Effect of microscopic parameters on EBSD spatial resolution. Ultramicroscopy 2011, 111, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.; Geiss, R. Transmission EBSD from 10 nm domains in a scanning electron microscope. J. Microsc. 2012, 245, 245–251. [Google Scholar] [CrossRef]
- De Kloe, R. Transmission-EBSD—Taking Transparency to a New Level. 2014. Available online: https://edaxblog.com/2014/10/02/transmisson-ebsd-taking-transparency-to-a-new-level/ (accessed on 27 November 2017).
- Hurlich, A.; Watson, J.F. Selection of Materials for use at cryogenic temperatures. Met. Prog. 1961, 79, 65–72. [Google Scholar]
- Stolyarov, V.V.; Zhu, Y.T.; Alexandrov, I.V.; Lowe, T.C.; Valiev, R.Z. Influence of ECAP routes on the microstructure and properties of pure Ti. Mater. Sci. Eng. A 2001, 299, 59–67. [Google Scholar] [CrossRef]
- Sergueeva, A.V.; Stolyarov, V.V.; Valiev, R.Z.; Mukherjee, A.K. Advanced mechanical properties of pure titanium with ultrafine grained structure. Scr. Mater. 2001, 45, 747–752. [Google Scholar] [CrossRef]
- Wang, Y.N.; Huang, J.C. Texture analysis in hexagonal materials. Mater. Chem. Phys. 2003, 81, 11–26. [Google Scholar] [CrossRef]
- Václavová, K.; Stráský, J.; Polyakova, V.; Stráská, J.; Nejezchlebová, J.; Seiner, H.; Semenova, I.; Janeček, M. Microhardness and microstructure evolution of ultra-fine grained Ti-15Mo and TIMETAL LCB alloys prepared by high pressure torsion. Mater. Sci. Eng. A 2017, 682, 220–228. [Google Scholar] [CrossRef]
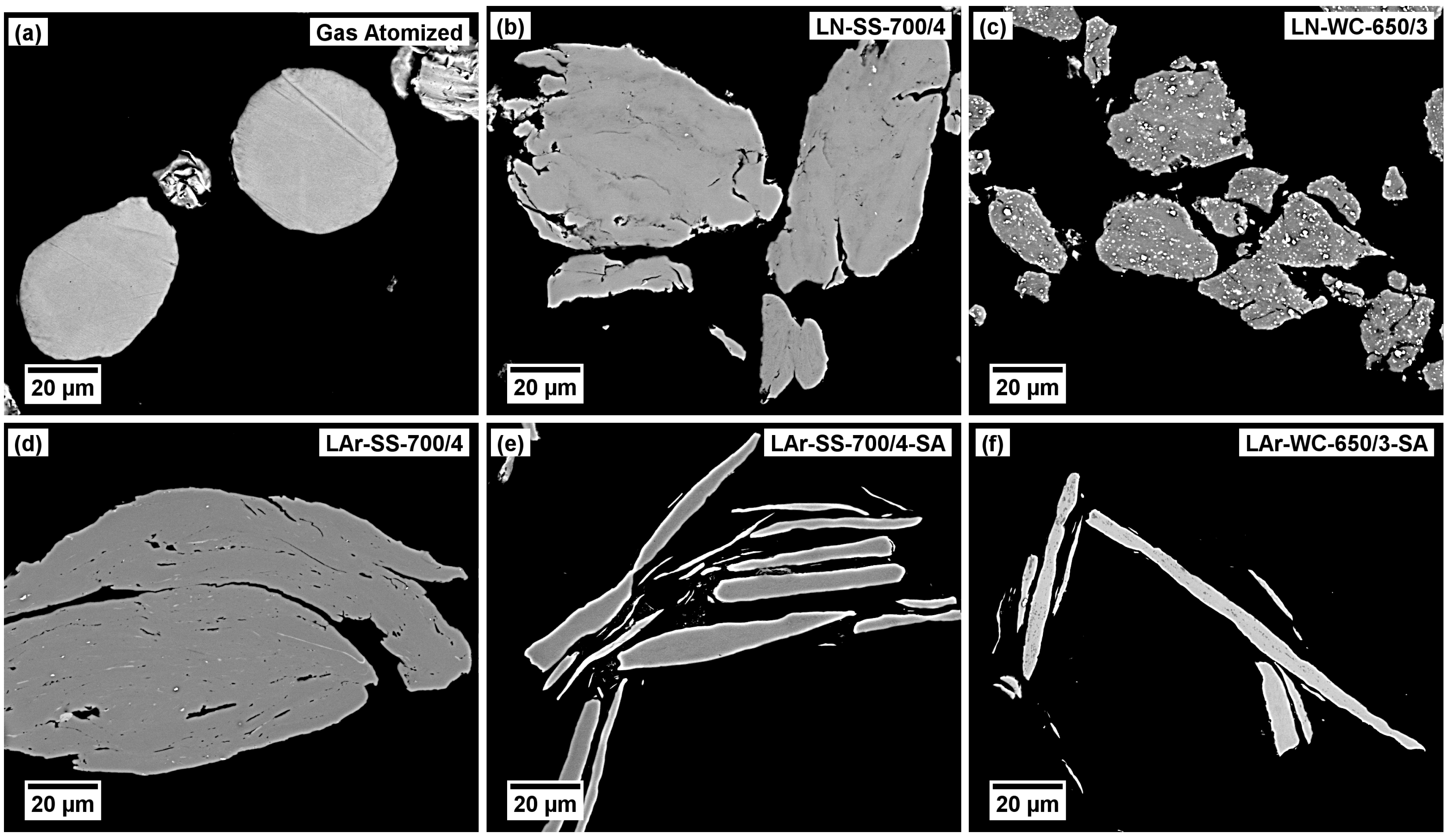
Figure 1.
SEM micrographs of powders: (a) gas-atomized; (b) LN, SS balls, 4 h; (c) LN, WC-Co balls, 3.25 h; (d) LAr, SS balls, 4 h; (e) LAr, SS balls, 4 h, with SA; (f) LAr, WC-Co balls, 3.25 h, with SA.
Figure 2.
SEM micrographs of powder cross-sections: (a) gas-atomized; (b) LN, SS balls, 4 h; (c) LN, WC-Co balls, 3.25 h; (d) LAr, SS balls, 4 h; (e) LAr, SS balls, 4 h, with SA; (f) LAr, WC-Co balls, 3.25 h, with SA.
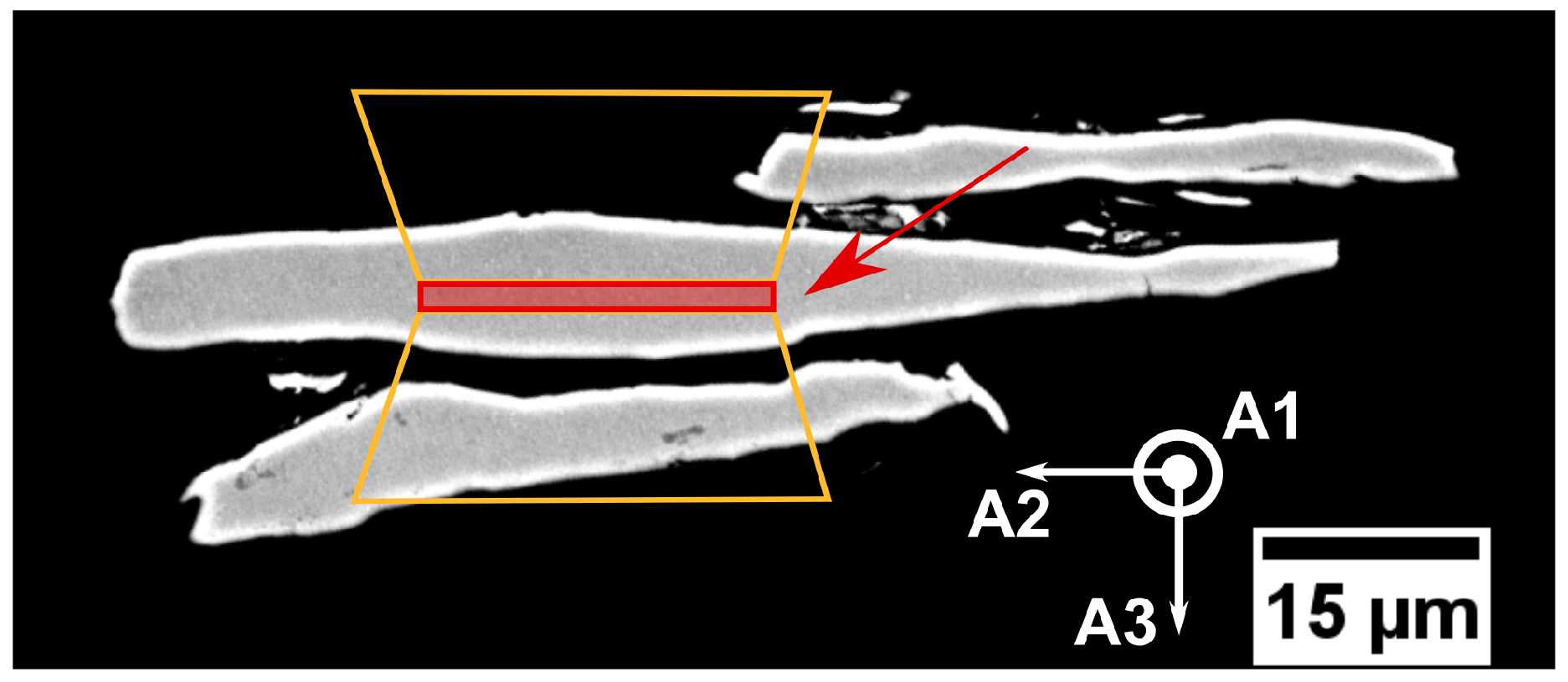
Figure 3.
Cross-section view of powder particles with denoted cuts for the lamella preparation. Red—the lamella, orange—trenches for lamella lift-out. The axes A1–A3 correspond to the axes in pole figures in Figure 5.
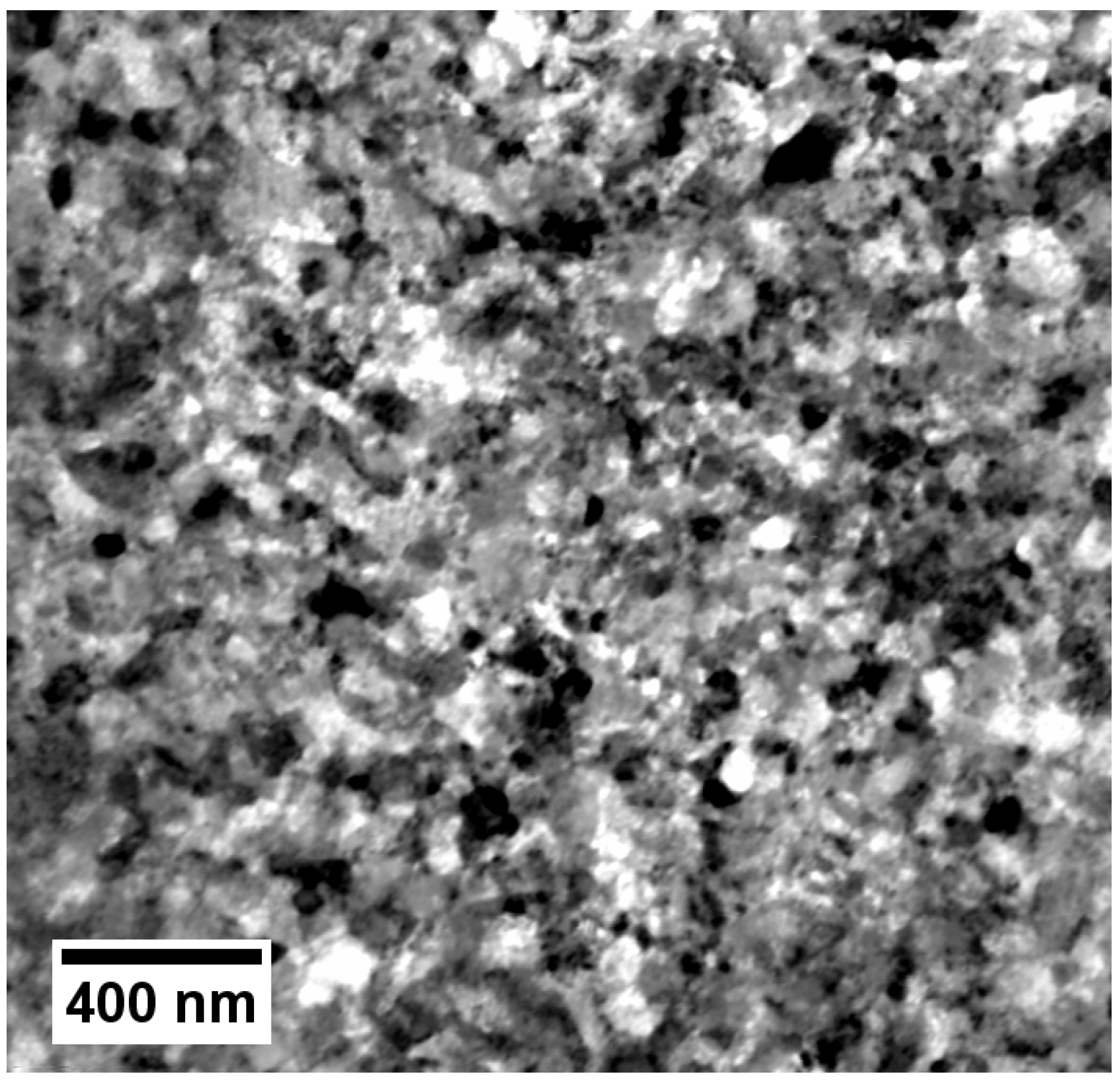
Figure 4.
Orientation dark-field STEM image showing the microstructure of sample LAr-SS-700/4-SA.
Figure 5.
IPF map and corresponding pole figures of a milled powder sample. Scanned area
, step size 5 nm, only well indexed points are shown. High angle grain boundaries (>
°) are marked by black lines. The axes A1 and A2 in the pole figures correspond to the axes shown in
Figure 3.
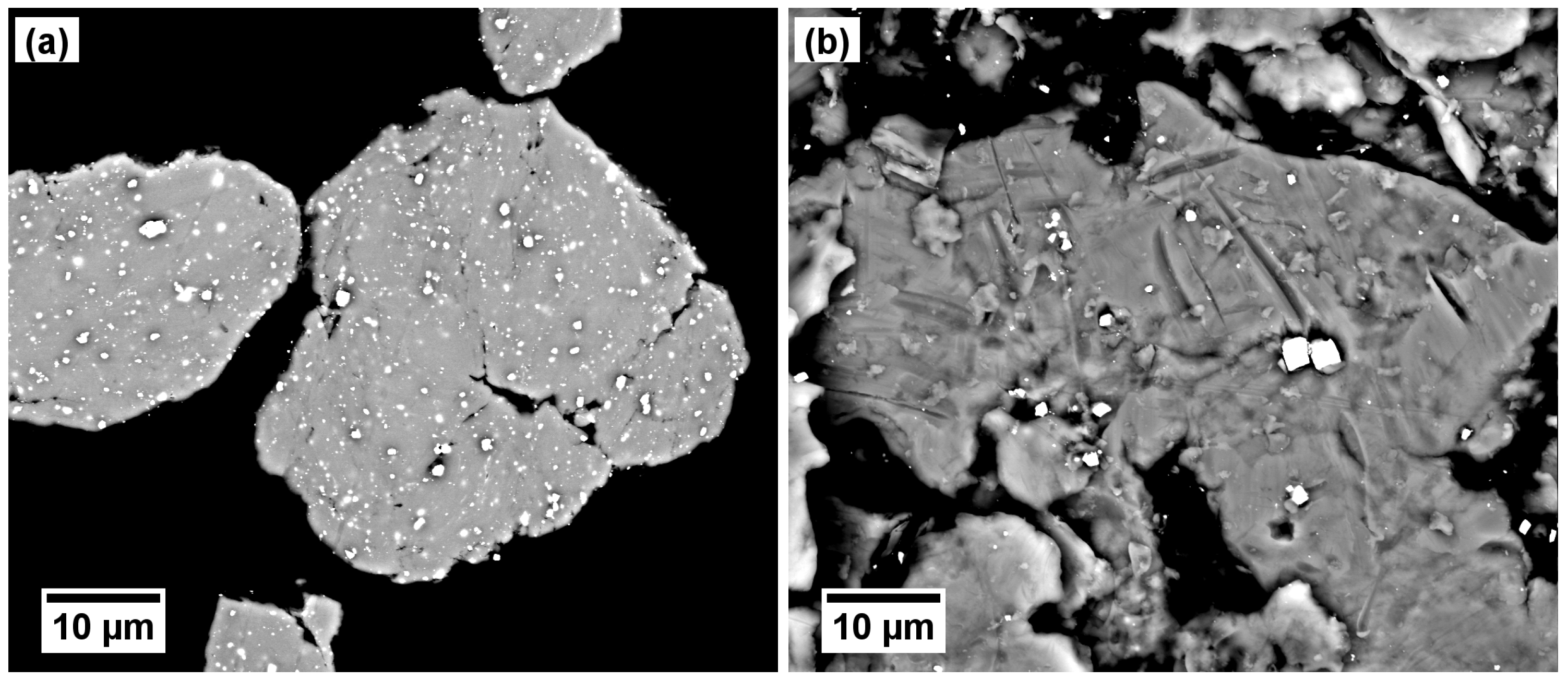
Figure 6.
WC-Co seen as bright spots (back-scattered electron contrast): (a) inside Ti particles; (b) on the particle surface.
Table 1.
Summary of milling process parameters. Milling time and speed for batch with WC-Co balls must have been slightly adjusted due to higher consumption of cryogenic liquid and excessive vibrations.
| Sample | Cooling Liquid | Ball Material | BPR | Milling Speed | Milling Time | Stearic Acid |
|---|
| (rpm) | (h) | (wt %) |
|---|
| LN-SS-700/4 | LN | SS | 16:1 | 700 | 4 | – |
| LN-WC-650/3 | LN | WC-Co | 32:1 | 650 | 3.25 | – |
| LAr-SS-700/4 | LAr | SS | 16:1 | 700 | 4 | – |
| LAr-SS-700/4-SA | LAr | SS | 16:1 | 700 | 4 | 8 |
| LAr-WC-650/3-SA | LAr | WC-Co | 32:1 | 650 | 3.25 | 8 |
Table 2.
Particle size and shape characteristics. The median , 10th and 90th percentile and of Feret diameter and the median of aspect ratio are given for each sample.
| Sample | Feret Diameter () | Aspect Ratio |
|---|
| Max. | Min. |
|---|
| | | | | | |
|---|
| gas atomized | 22 | 37 | 49 | 16 | 30 | 41 | 1.22 |
| LN-SS-700/4 | 30 | 75 | 150 | 15 | 43 | 93 | 1.71 |
| LN-WC-650/3 | 18 | 43 | 87 | 11 | 27 | 54 | 1.55 |
| LAr-SS-700/4 | 110 | 160 | 250 | 54 | 110 | 170 | 1.52 |
| LAr-SS-700/4-SA | 31 | 74 | 150 | 8 | 19 | 74 | 3.47 |
| LAr-WC-650/3-SA | 13 | 38 | 81 | 3 | 7 | 17 | 5.60 |
Table 3.
Contamination of cleaned powders by carrier gas hot extraction (CGHE) analysis (estimated relative error 5%) and Vickers microhardness (HV0.002) of powder particles measured on the cross-section.
| Sample | Hydrogen | Nitrogen | Oxygen | HV0.002 |
|---|
| (wt %) | (wt %) | (wt %) |
|---|
| gas atomized | 0 | 0.02 | 0.15 | |
| LN-SS-700/4 | 0 | 0.80 | 0.43 | |
| LN-WC-650/3 | 0 | 2.99 | 0.36 | |
| LAr-SS-700/4 | 0 | 0.20 | 0.23 | |
| LAr-SS-700/4-SA | 0.05 | 0.06 | 0.36 | |
| LAr-WC-650/3-SA | 0.03 | 0.08 | 0.59 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).