Precipitation Stages and Reaction Kinetics of AlMgSi Alloys during the Artificial Aging Process Monitored by In-Situ Electrical Resistivity Measurement Method
Abstract
:1. Introduction
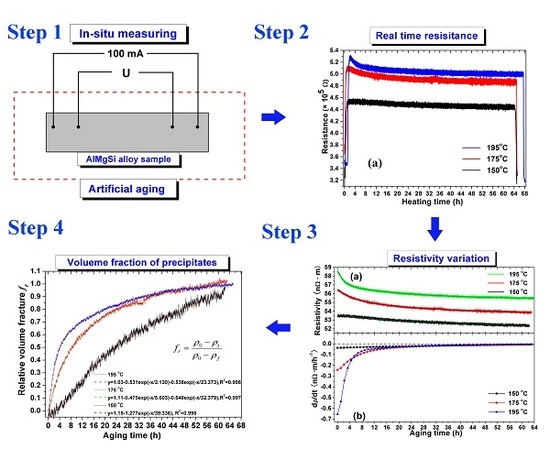
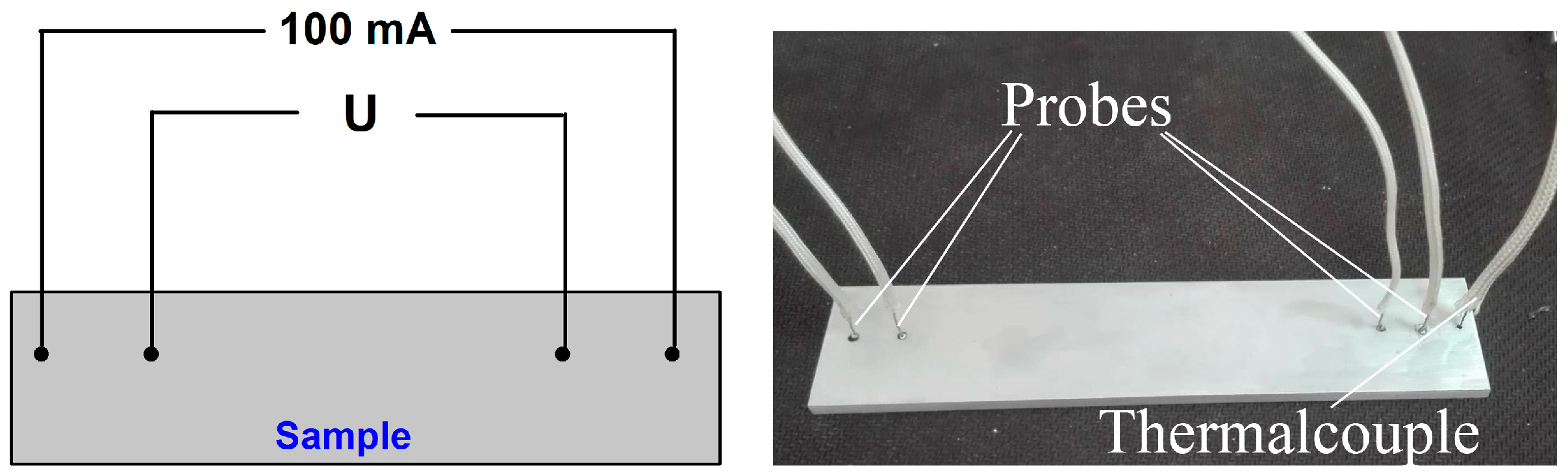
2. Materials and Methods
3. Results
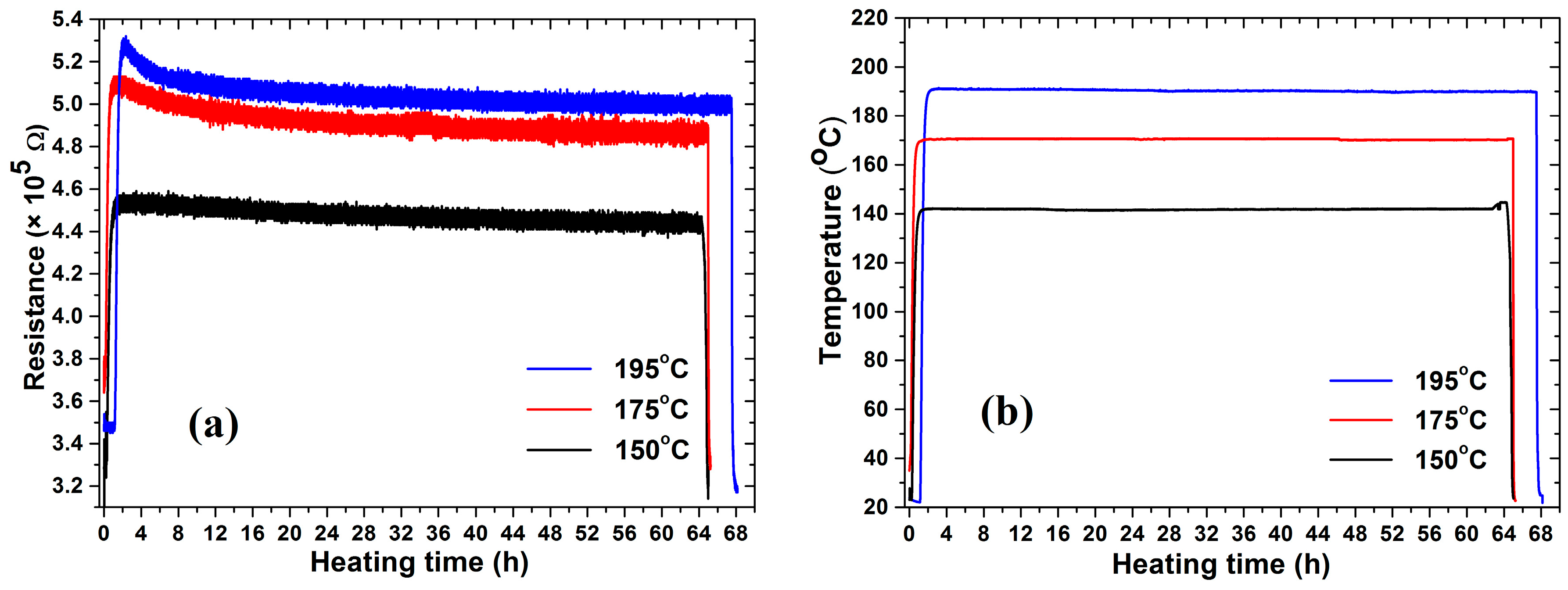
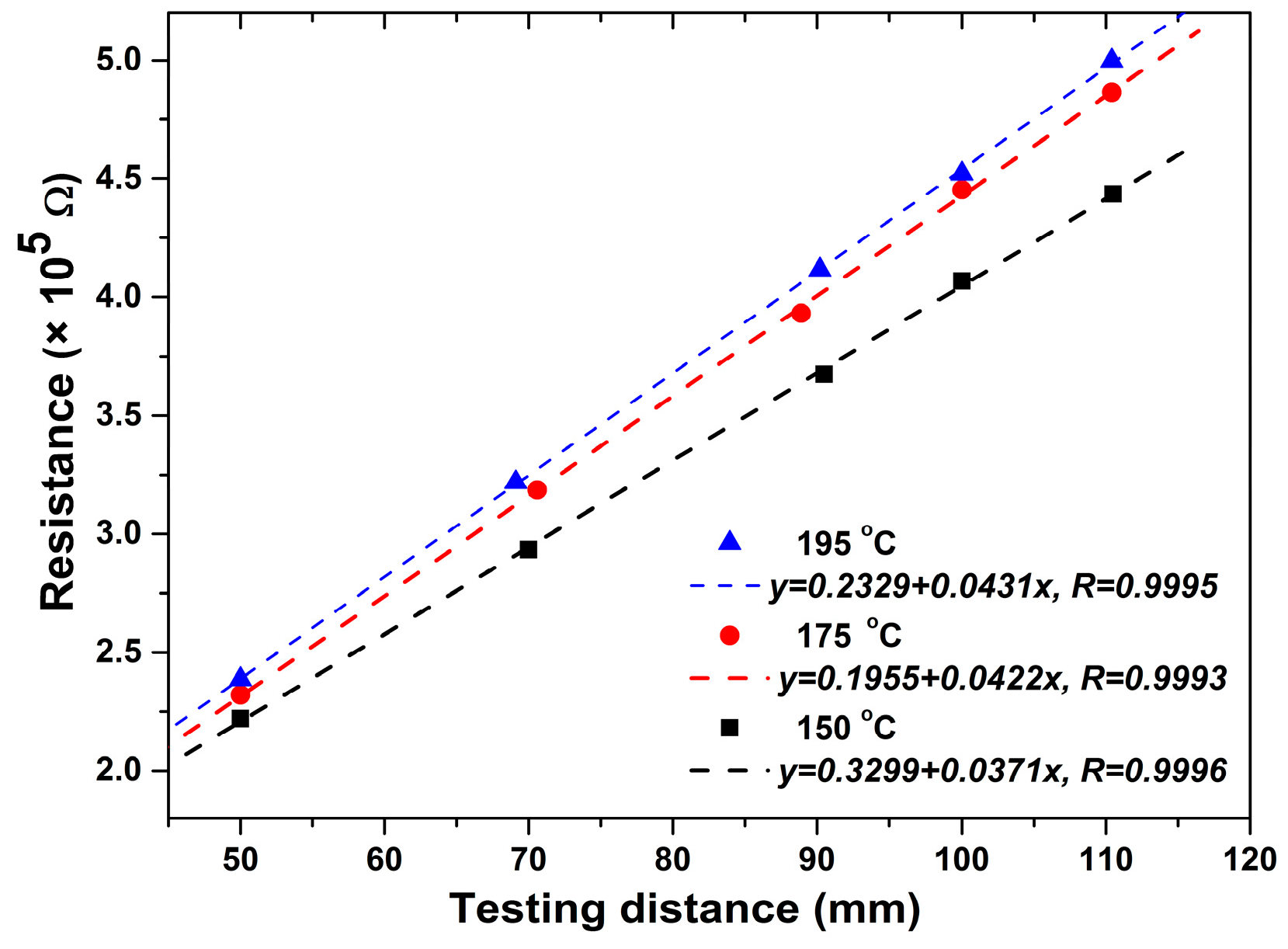
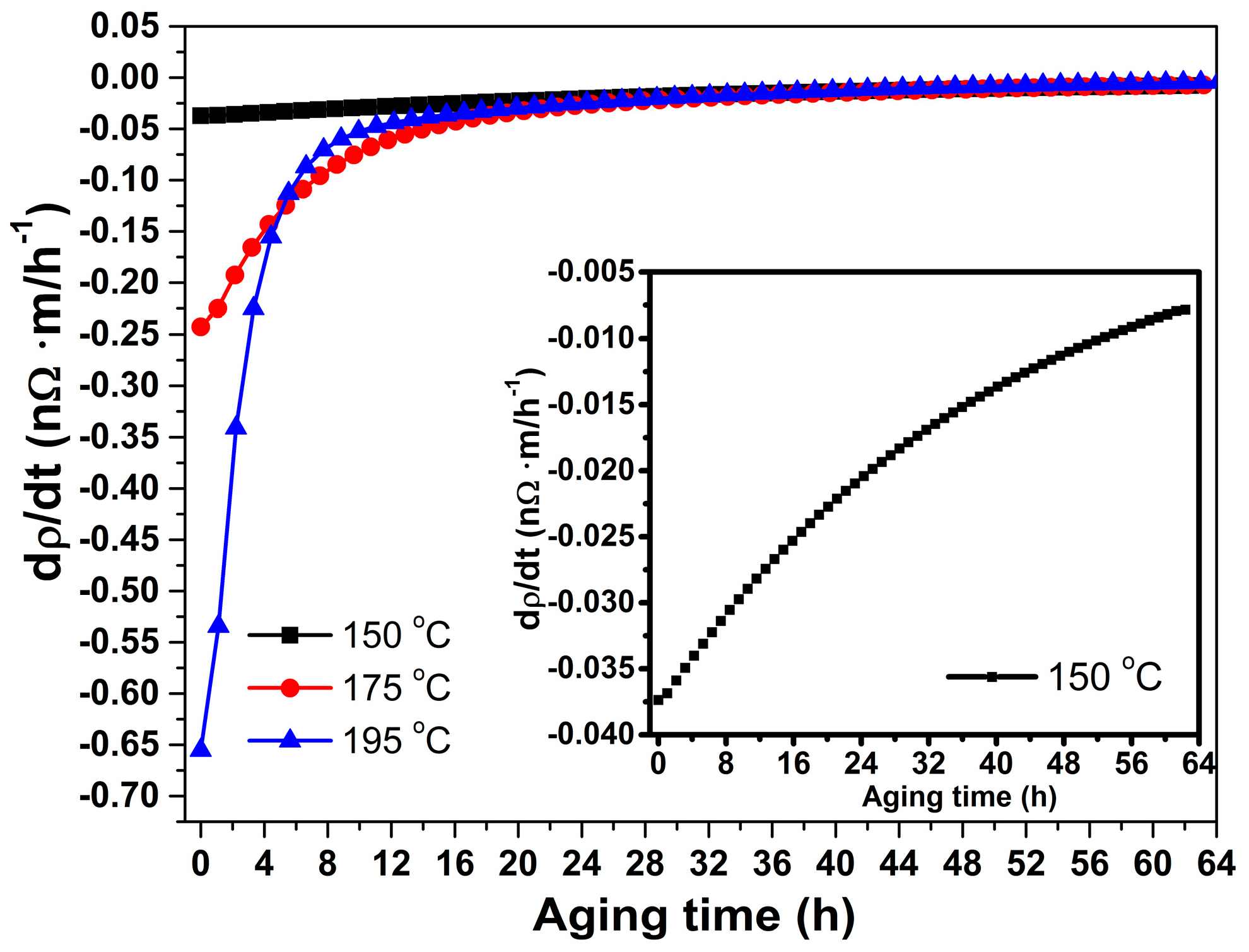
3.1. Resistance and Resistivity
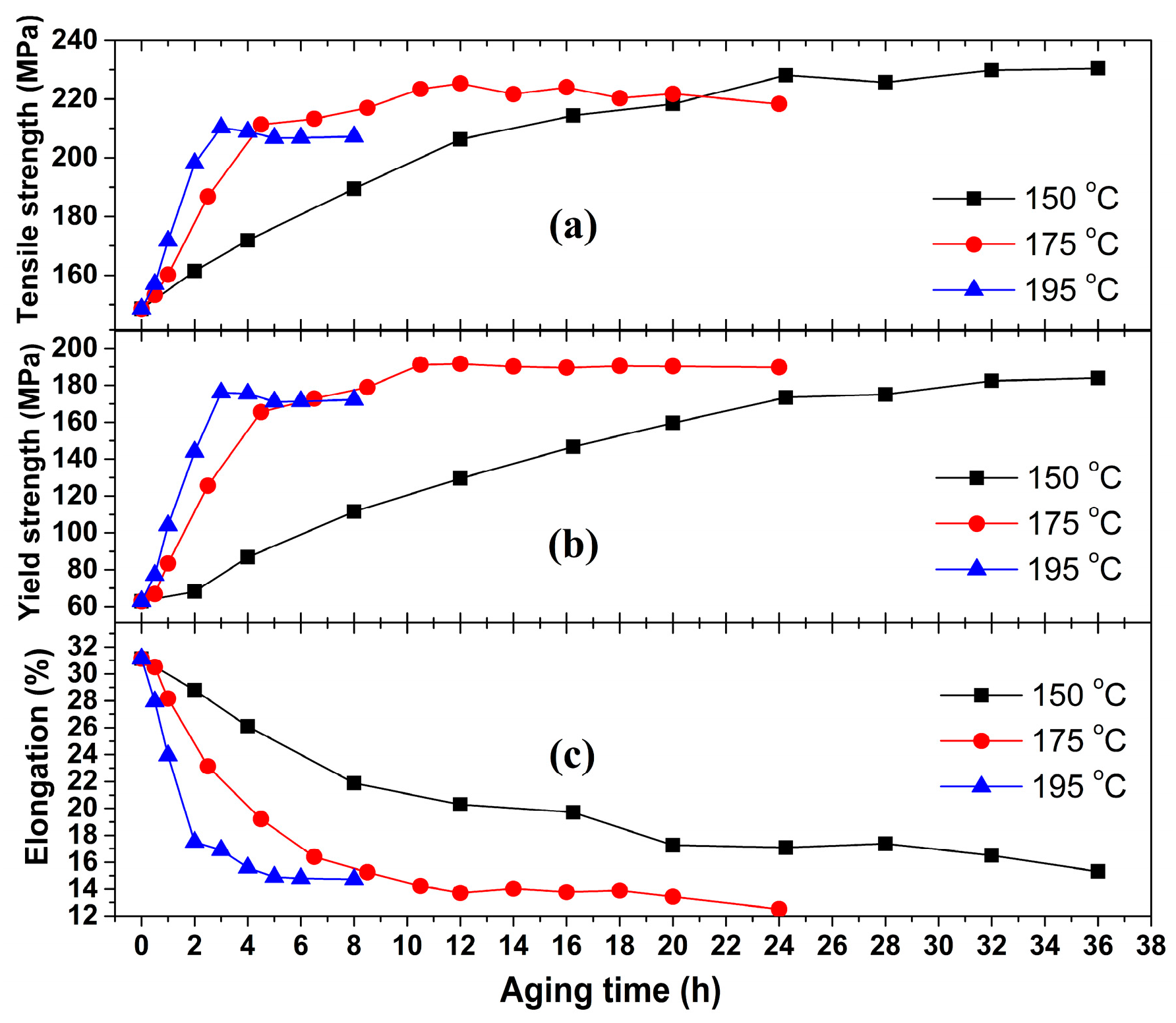
3.2. Mechanical Properties
3.3. Microstructure of Precipitates
4. Discussion
4.1. Precipitation and Phase Transformation Process
4.2. Precipitation Kinetics
4.3. Accuracy and Application of the In-Situ Resistivity Measuring Method
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zandbergen, M.W.; Xu, Q.; Cerezo, A.; Smith, G.D.W. Study of precipitation in Al-Mg-Si alloys by Atom Probe Tomography, I. Microstructural changes as a function of ageing temperature. Acta Mater. 2015, 101, 136–148. [Google Scholar] [CrossRef]
- Chen, J.H.; Costan, E.; Van Huis, M.A.; Xu, Q.; Zandbergen, H.W. Atomic pillar-based nanoprecipitates strengthen AlMgSi alloys. Science 2006, 312, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Pogatscher, S.; Antrekowitsch, H.; Leitner, H.; Ebner, T.; Uggowitzer, P.J. Mechanisms controlling the artificial aging of Al-Mg-Si Alloys. Acta Mater. 2011, 59, 3352–3363. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J. Modeling of precipitation hardening in pre-aged AlMgSi(Cu) alloys. Acta Mater. 2005, 53, 5257–5271. [Google Scholar] [CrossRef]
- Saito, T.; Ehlers, F.J.H.; Lefebvre, W.; Hernandez-Maldonado, D.; Bjørge, R.; Marioara, C.D.; Andersen, S.J.; Mørtsell, E.A.; Holmestad, R. Cu atoms suppress misfit dislocations at the β″ Al interface in Al-Mg-Si alloys. Scr. Mater. 2016, 110, 6–9. [Google Scholar] [CrossRef]
- Werinos, M.; Antrekowitsch, H.; Kozeschnik, E.; Ebner, T.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J.; Pogatscher, S. Ultrafast artificial aging of Al-Mg-Si alloys. Scr. Mater. 2016, 112, 148–151. [Google Scholar] [CrossRef]
- Li, K.; Idrissi, H.; Sha, G.; Song, M.; Lu, J.B.; Shi, H.; Wang, W.L.; Ringer, S.P.; Du, Y.; Schryvers, D. Quantitative measurement for the microstructural parameters of nano-precipitates in Al-Mg-Si-Cu alloys. Mater. Charact. 2016, 118, 352–362. [Google Scholar] [CrossRef]
- Buchanan, K.; Colas, K.; Ribis, J.; Lopez, A.; Garnier, J. Analysis of the metastable precipitates in peak-hardness aged Al-Mg-Si(-Cu) alloys with differing Si contents. Acta Mater. 2017, 132, 209–221. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Jansen, J.; Zandbergen, H.W. The influence of temperature and storage time at RT on nucleation of the β″ phase in a 6082 Al-Mg-Si alloy. Acta Mater. 2003, 51, 789–796. [Google Scholar] [CrossRef]
- Wenner, S.; Marioara, C.D.; Ramasse, Q.M.; Kepaptsoglou, D.M.; Hagec, F.S.; Holmestada, R. Atomic-resolution electron energy loss studies of precipitates in an Al-Mg-Si-Cu-Ag alloy. Scr. Mater. 2014, 74, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Béché, A.; Song, M.; Sha, G.; Lu, X.; Zhang, K.; Du, Y.; Ringer, S.P.; Schryvers, D. Atomistic structure of Cu-containing β″ precipitates in an Al-Mg-Si-Cu alloy. Scr. Mater. 2014, 75, 86–89. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Murashkina, M.Y.; Sabirov, I. A nanostructural design to produce high-strength Al alloys with enhanced electrical conductivity. Scr. Mater. 2014, 76, 13–16. [Google Scholar] [CrossRef]
- Liu, C.H.; Lai, Y.X.; Chen, J.H.; Tao, G.H.; Liu, L.M.; Ma, P.P.; Wu, C.L. Natural-aging-induced reversal of the precipitation pathways in an Al-Mg-Si alloy. Scr. Mater. 2016, 115, 150–154. [Google Scholar] [CrossRef]
- Pogatscher, S.; Kozeschnik, E.; Antrekowitsch, H.; Werinos, M.; Gerstl, S.S.A.; Löffler, J.F.; Uggowitzer, P.J. Process-controlled suppression of natural aging in an Al-Mg-Si alloy. Scr. Mater. 2014, 89, 53–56. [Google Scholar] [CrossRef]
- Aruga, Y.; Kozuka, M.; Takaki, Y.; Sato, T. Effects of natural aging after pre-aging on clustering and bake-hardening behavior in an Al-Mg-Si alloy. Scr. Mater. 2016, 116, 82–86. [Google Scholar] [CrossRef]
- Li, H.; Liu, W. Nanoprecipitates and Their Strengthening Behavior in Al-Mg-Si Alloy during the Aging Process. Metall. Mater. Trans. A 2017, 48, 1990–1998. [Google Scholar] [CrossRef]
- Guo, M.X.; Sha, G.; Cao, L.Y.; Liu, W.Q.; Zhang, J.S.; Zhuang, L.Z. Enhanced bake-hardening response of an Al-Mg-Si-Cu alloy with Zn addition. Mater. Chem. Phys. 2015, 162, 15–19. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J. Characterization of the evolution of the volume fraction of precipitates in aged AlMgSiCu alloys using DSC technique. Mater. Charact. 2005, 55, 307–319. [Google Scholar] [CrossRef]
- Aouabdia, Y.; Boubertakh, A.; Hamamda, S. Precipitation kinetics of the hardening phase in two 6061 aluminium alloys. Mater. Lett. 2010, 64, 353–356. [Google Scholar] [CrossRef]
- Giersberg, L.; Milkereit, B.; Schick, C.; Kessler, O. In Situ Isothermal Calorimetric Measurement of Precipitation Behaviour in Al-Mg-Si Alloys. Mater. Sci. Forum 2014, 794, 939–944. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J.; Poole, W.J. Effect of natural aging on the resistivity evolution during artificial aging of the aluminum alloy AA6111. Mater. Lett. 2005, 59, 575–577. [Google Scholar] [CrossRef]
- Raeisinia, B.; Poole, W.J.; Lloyd, D.J. Examination of precipitation in the aluminum alloy AA6111 using electrical resistivity measurements. Mater. Sci. Eng. A 2006, 420, 245–249. [Google Scholar] [CrossRef]
- Seyedrezai, H.; Grebennikov, D.; Mascher, P.; Zurob, H.S. Study of the early stages of clustering in Al-Mg-Si alloys using the electrical resistivity measurements. Mater. Sci. Eng. A 2009, 525, 186–191. [Google Scholar] [CrossRef]
- Fallah, V.; Langelier, B.; Ofori-Opoku, N.; Raeisinia, B.; Provatas, N.; Esmaeili, S. Cluster evolution mechanisms during aging in Al-Mg-Si alloys. Acta Mater. 2016, 103, 290–300. [Google Scholar] [CrossRef]
- Esmaeili, S.; Poole, W.J.; Lloyd, D.J. Electrical Resistivity Studies on the Precipitation Behaviour of AA6111. Mater. Sci. Forum 2000, 331–337, 995–1000. [Google Scholar] [CrossRef]
- Esmaeili, S.; Vaumousse, D.; Zandbergen, M.W.; Poole, W.J.; Cerezo, A.; Lloyd, D.J. A study on the early-stage decomposition in the Al-Mg-Si-Cu alloy AA6111 by electrical resistivity and three-dimensional atom probe. Philos. Mag. 2007, 87, 3797–3816. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Abdullah, H.A.; Al-Belushi, K.R. Influence of aging parameters on the mechanical properties of 6063 aluminium alloy. J. Mater. Process. Technol. 2000, 102, 234–240. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G.J.; Ding, X.D.; Sun, J.; Chen, K.H. Modeling the strengthening response to aging process of heat-treatable aluminum alloys containing plate/disc- or rod/needle-shaped precipitates. Mater. Sci. Eng. A 2003, 344, 113–124. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø.; Andersen, S.J. Modelling of the age hardening behaviour of Al-Mg-Si alloys. Acta Mater. 2001, 49, 65–75. [Google Scholar] [CrossRef]
- Luo, A.; Lloyd, D.J.; Gupta, A.; Youdelis, W.V. Precipitation and dissolution kinetics in Al-Li-Cu-Mg alloy 8090. Acta Mater. 1993, 41, 769–776. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J.; Poole, W.J. Modeling of precipitation hardening for the naturally aged Al-Mg-Si-Cu alloy AA6111. Acta Mater. 2003, 51, 3467–3481. [Google Scholar] [CrossRef]
- Cluff, D.R.A.; Esmaeili, S. Prediction of the effect of artificial aging heat treatment on the yield strength of an open-cell aluminum foam. J. Mater. Sci. 2008, 43, 1121–1127. [Google Scholar] [CrossRef]
- Raeisinia, B. A Study of Precipitation in the Aluminum Alloy AA6111. Master’s Thesis, Tehran University, Tehran, Iran, 2000. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Zhang, L.; Li, S.; Wu, X.; Zhang, H.; Li, L. Precipitation Stages and Reaction Kinetics of AlMgSi Alloys during the Artificial Aging Process Monitored by In-Situ Electrical Resistivity Measurement Method. Metals 2018, 8, 39. https://doi.org/10.3390/met8010039
He H, Zhang L, Li S, Wu X, Zhang H, Li L. Precipitation Stages and Reaction Kinetics of AlMgSi Alloys during the Artificial Aging Process Monitored by In-Situ Electrical Resistivity Measurement Method. Metals. 2018; 8(1):39. https://doi.org/10.3390/met8010039
Chicago/Turabian StyleHe, Hong, Long Zhang, Shikang Li, Xiaodong Wu, Hui Zhang, and Luoxing Li. 2018. "Precipitation Stages and Reaction Kinetics of AlMgSi Alloys during the Artificial Aging Process Monitored by In-Situ Electrical Resistivity Measurement Method" Metals 8, no. 1: 39. https://doi.org/10.3390/met8010039