Preparation and Characterization of TiB2-(Supra-Nano-Dual-Phase) High-Entropy Alloy Cermet by Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Preparation
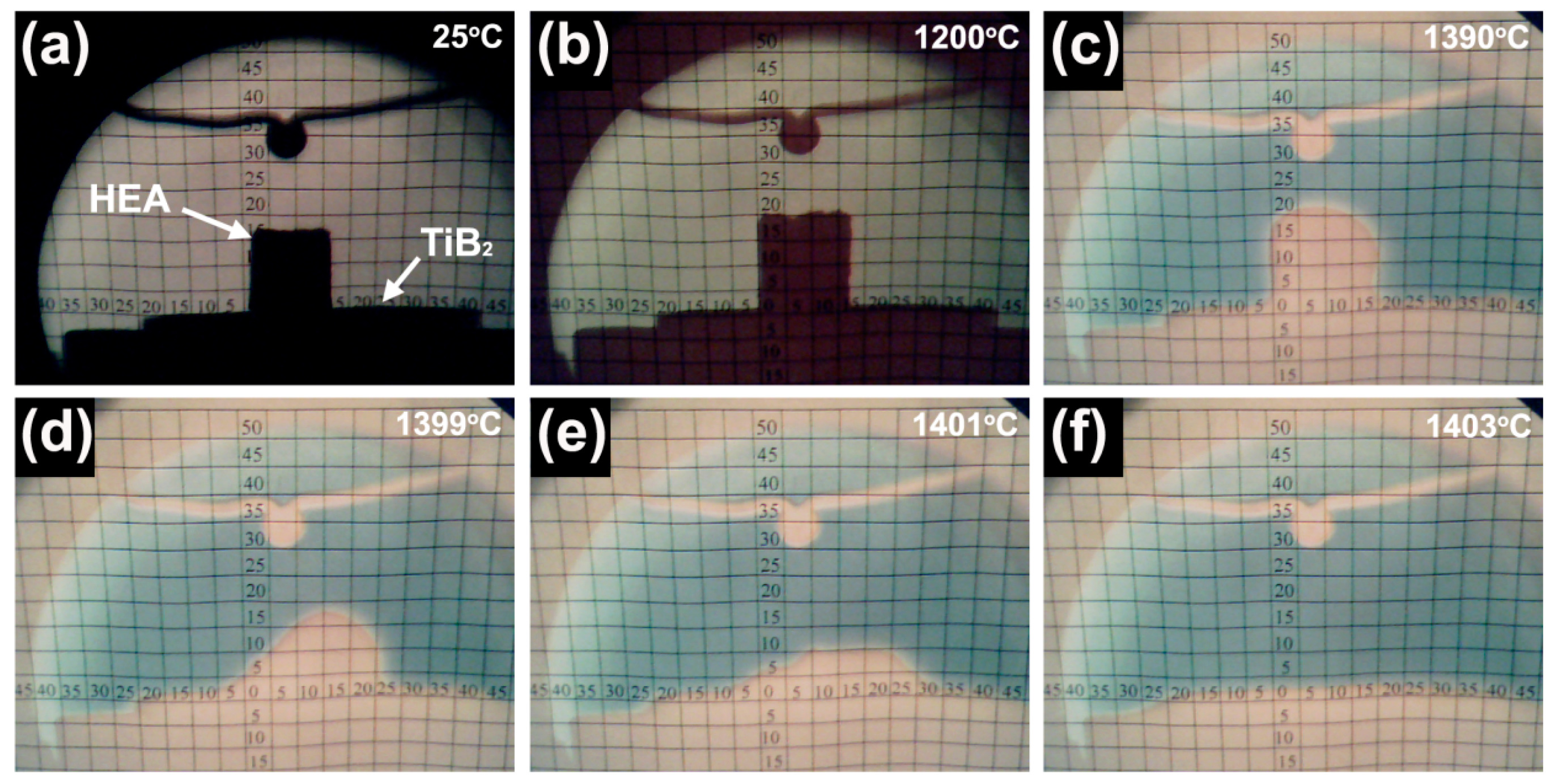
2.2. Wettability Analysis
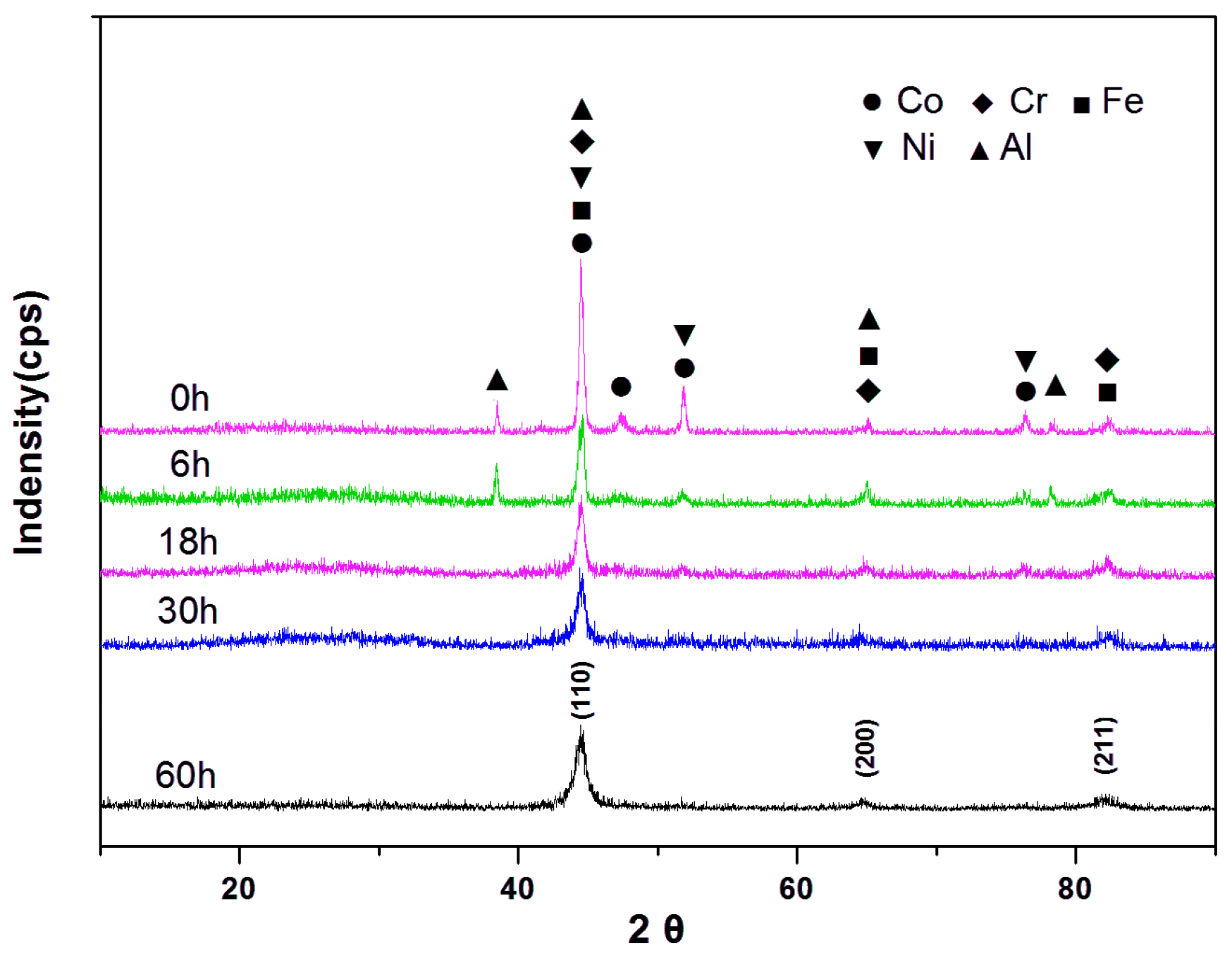
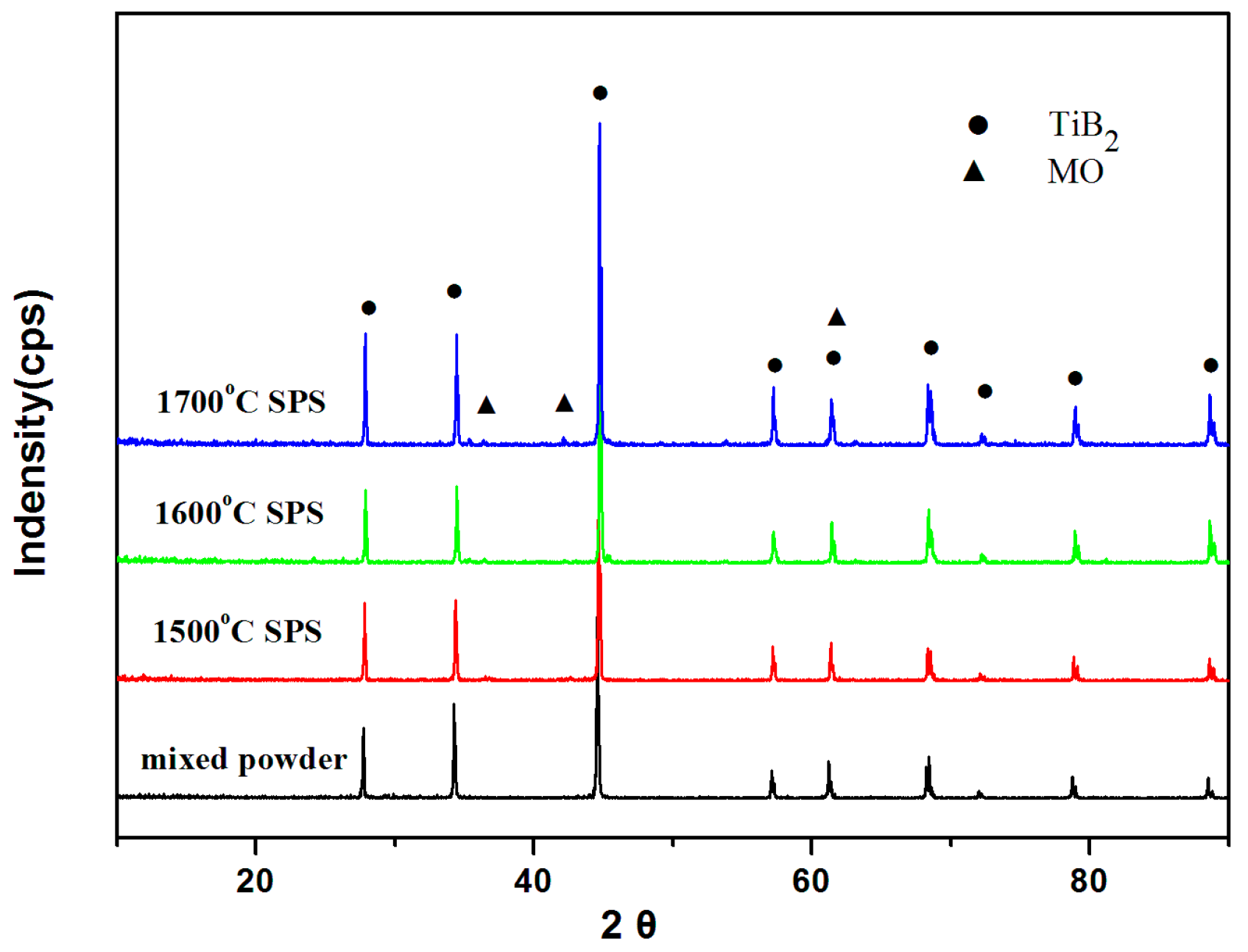
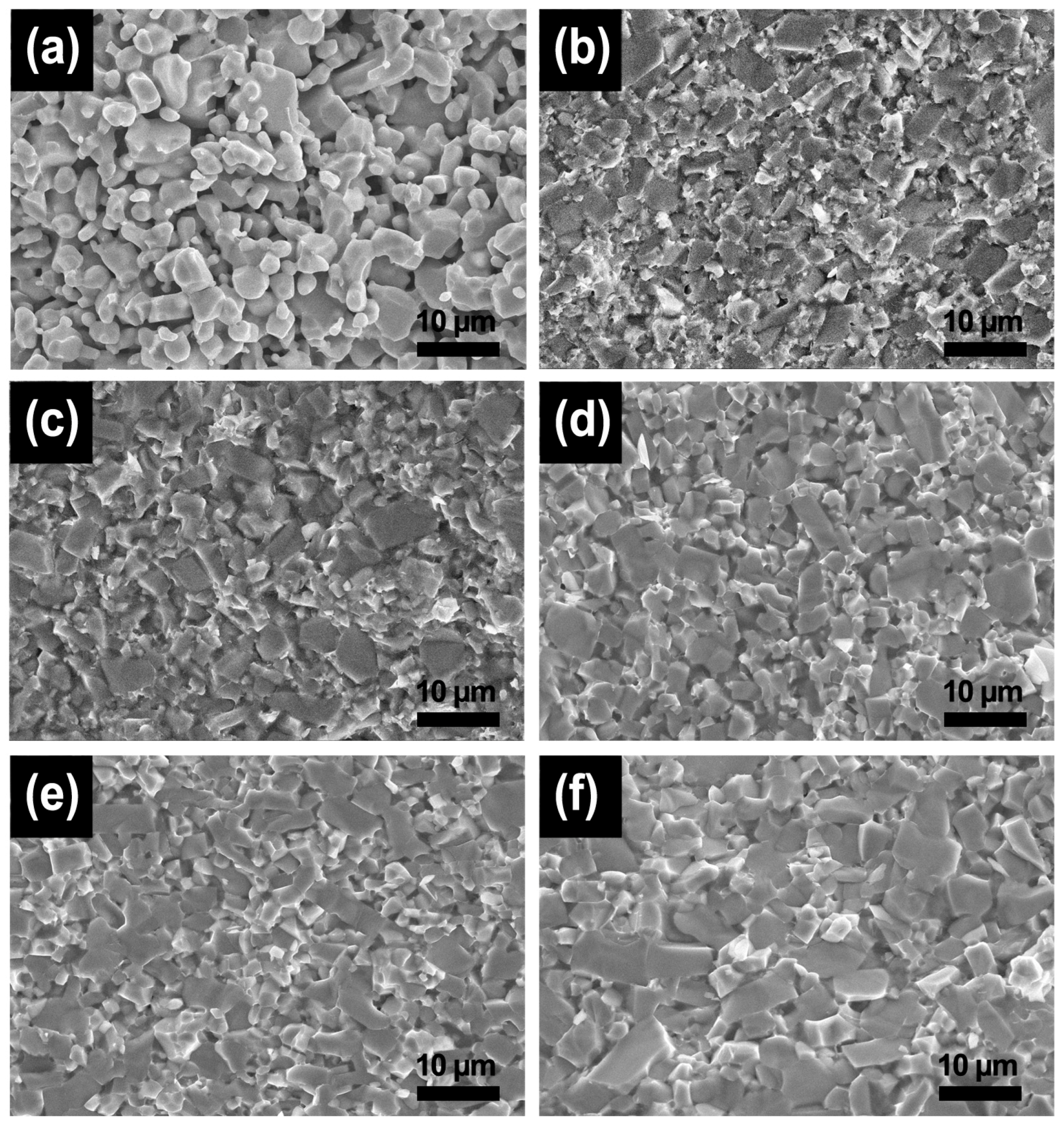
2.3. Sintering and Characterization
2.4. Mechanical Properties
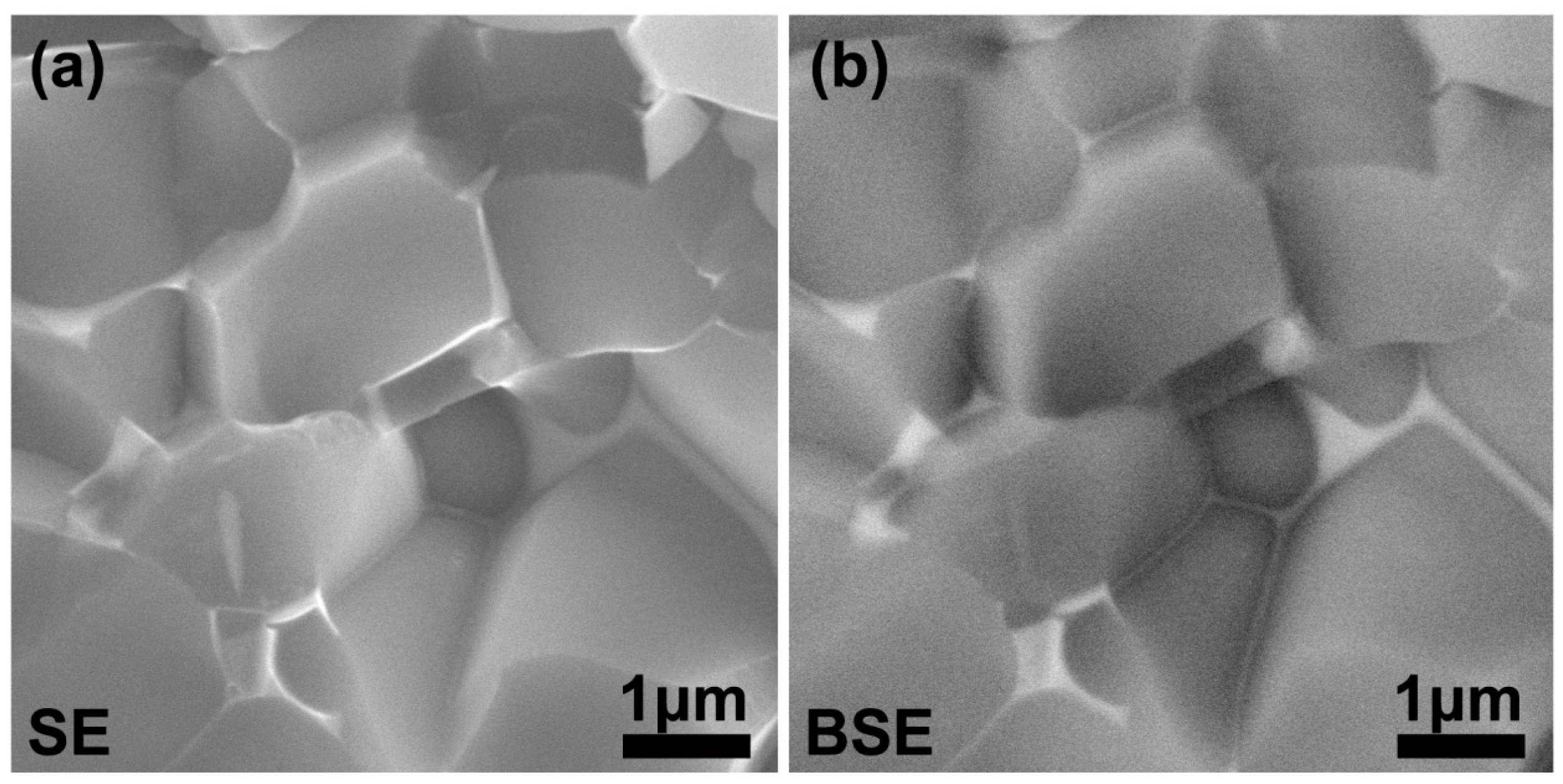
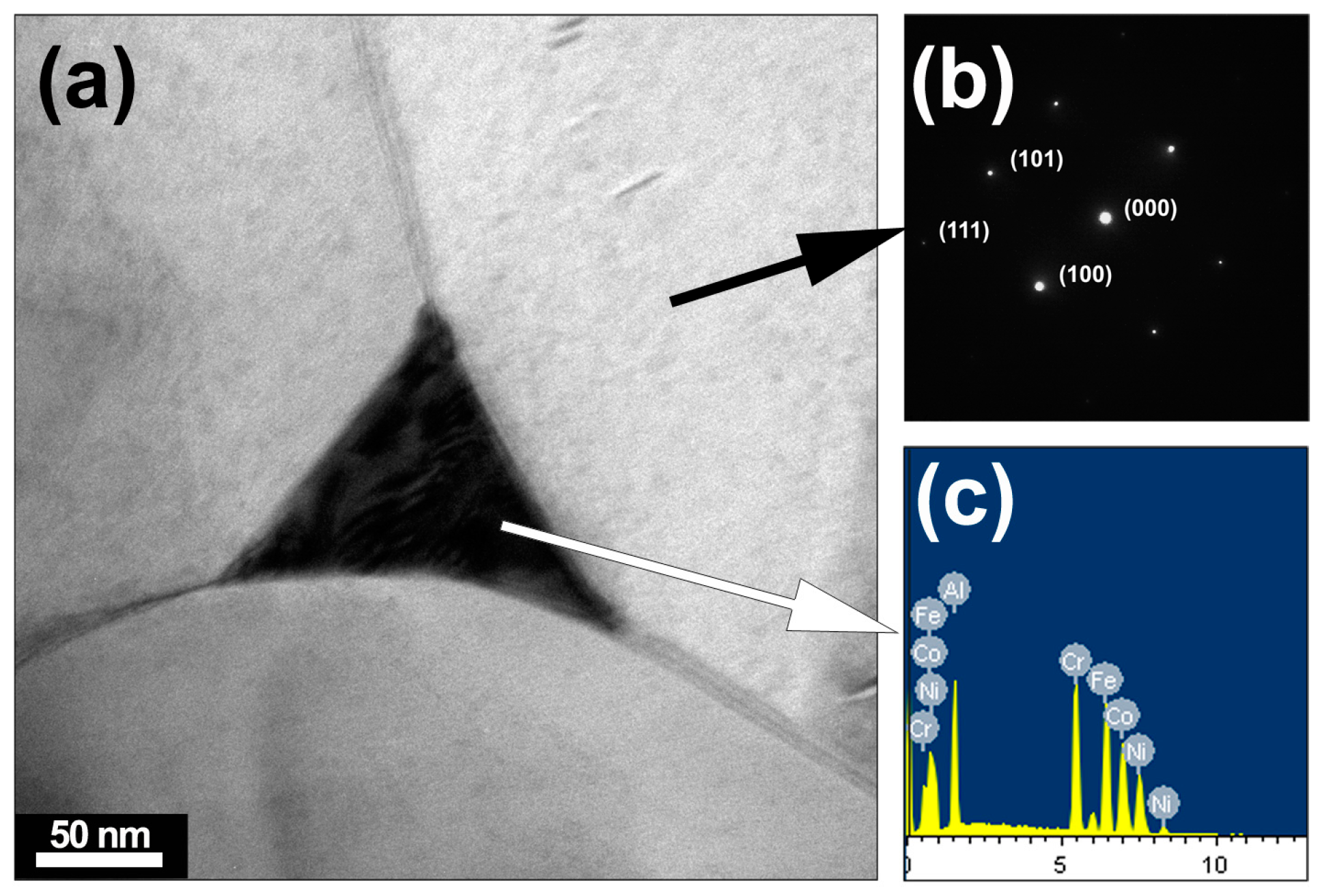
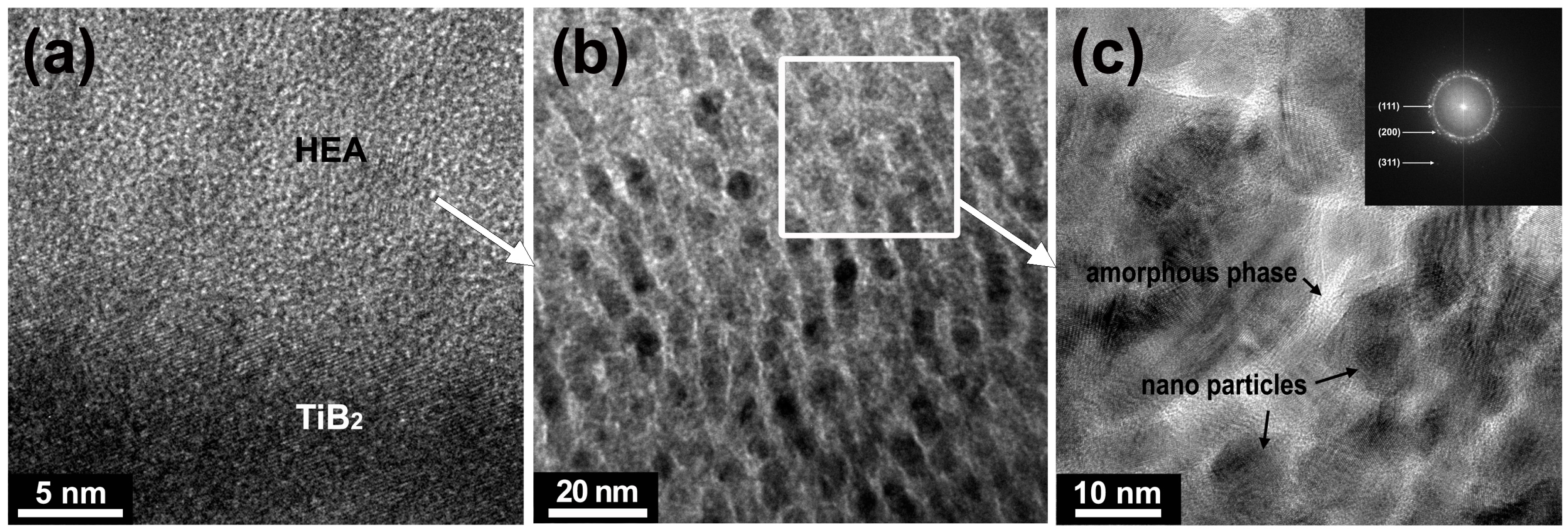
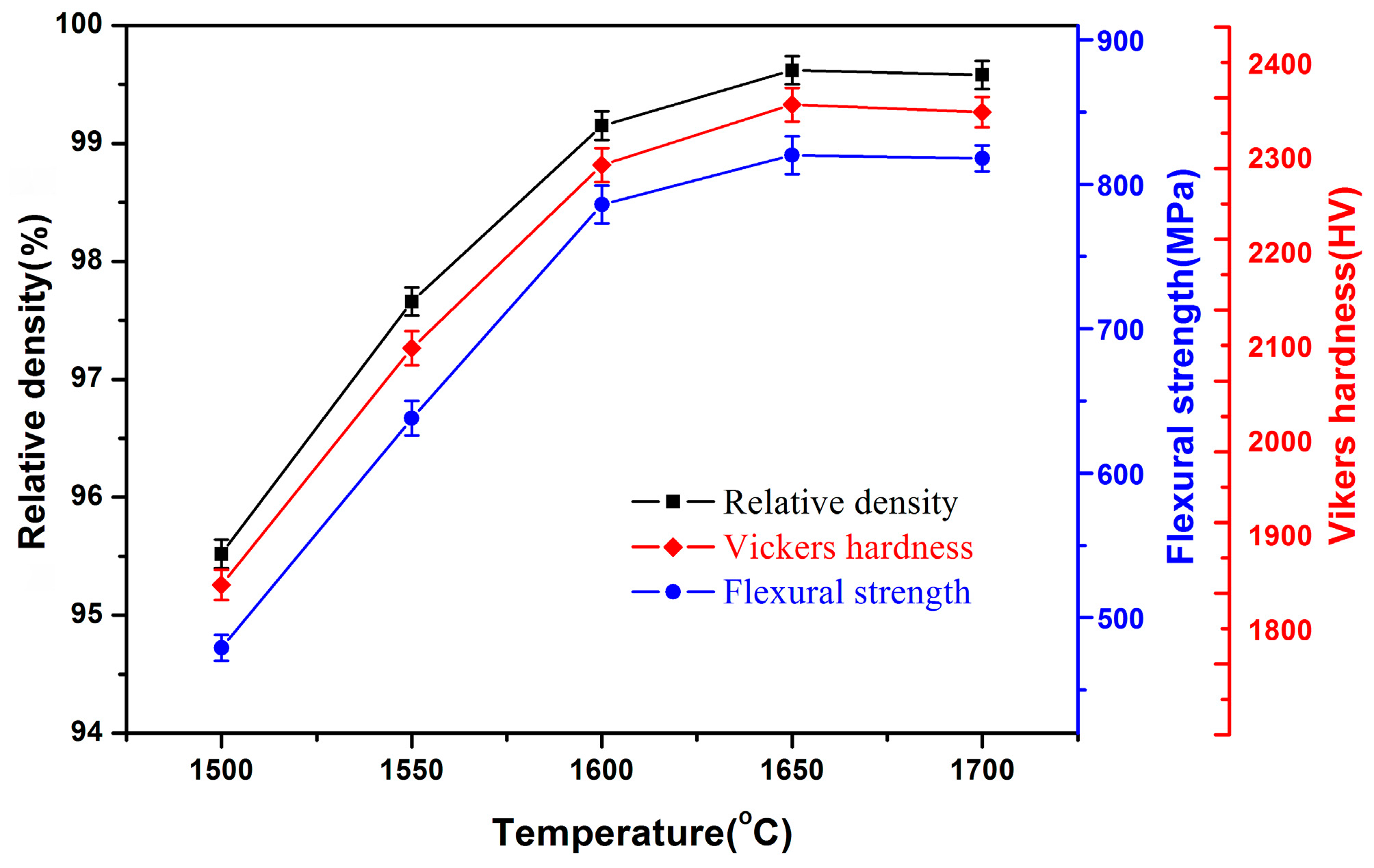
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wen, G.; Li, S.; Zhang, B.; Guo, Z. Reaction synthesis of TiB2–TiC composites with enhanced toughness. Acta Mater. 2001, 49, 1463–1470. [Google Scholar] [CrossRef]
- Gu, M.; Huang, C.; Zou, B.; Liu, B. Effect of (Ni, Mo) and TiN on the microstructure and mechanical properties of TiB2 ceramic tool materials. Mater. Sci. Eng. A 2006, 433, 39–44. [Google Scholar] [CrossRef]
- Basu, B.; Raju, G.B.; Suri, A.K. Processing and properties of monolithic TiB2 based materials. Int. Mater. Rev. 2006, 51, 352–374. [Google Scholar] [CrossRef]
- Vallauri, D.; Adrian, I.C.A.; Chrysanthou, A. TiC-TiB2 composites: A review of phase relationships, processing and properties. J. Eur. Ceram. Soc. 2008, 28, 1697–1713. [Google Scholar] [CrossRef]
- Raju, G.; Mukhopadhyay, A.; Biswas, K.; Basu, B. Densification and high-temperature mechanical properties of hot pressed TiB2–(0–10 wt.%) MoSi2composites. Scr. Mater. 2009, 61, 674–677. [Google Scholar] [CrossRef]
- Wang, W.; Fu, Z.; Wang, H.; Yuan, R. Influence of hot pressing sintering tempera-ture and time on microstructure and mechanical properties of TiB2 ceramics. J. Eur. Ceram. Soc. 2002, 22, 1045–1049. [Google Scholar] [CrossRef]
- Li, L.; Kim, H.; Kang, E. Sintering and mechanical properties of titanium diboride with aluminum nitride as a sintering aid. J. Eur. Ceram. Soc. 2002, 22, 973–977. [Google Scholar] [CrossRef]
- Park, J.; Koh, Y.; Kim, H.; Hwang, C.; Kang, E. Densification and mechanical properties of titanium diboride with silicon nitride as a sintering aid. J. Am. Ceram. Soc. 1999, 82, 3037–3042. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Raju, G.; Basu, B.; Suri, A. Correlation between phase evolution, mechanical properties and instrumented indentation response of TiB2-based ceramics. J. Eur. Ceram. Soc. 2009, 29, 505–516. [Google Scholar] [CrossRef]
- Zheng, L.; Li, F.; Zhou, Y. Preparation, microstructure, and mechanical properties of TiB2 using Ti3AlC2 as a sintering aid. J. Am. Ceram. Soc. 2012, 95, 2028–2034. [Google Scholar] [CrossRef]
- Barandika, M.; Sanchez, J.; Rojo, T.; Cortes, R.; Castro, F. Fe-Ni-Ti binder phases for TiB2-based cermets: A thermodynamic approach. Scr. Mater. 1998, 39, 1395–1400. [Google Scholar] [CrossRef]
- Einarsrud, M.; Hagen, E.; Pettersen, G.; Grande, T. Pressureless sintering of titanium diboride with nickel, nickel boride and iron additives. J. Am. Ceram. Soc. 1997, 8, 3013–3020. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, X.; Wang, F.; Lee, S.; Fan, Q.; Cao, M. Low-temperature densification of TiB2 ceramic by the spark plasma sintering process with Ti as a sintering aid. Scr. Mater. 2012, 66, 167–170. [Google Scholar] [CrossRef]
- Kang, E.; Jang, C.; Lee, C.; Kim, C.; Kim, D. Effect of iron and boron carbide onthe densification and mechanical properties of titanium diboride ceramics. J. Am. Ceram. Soc. 1989, 72, 1868–1872. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Z.; Wang, W.; Wang, H.; Min, X. Wettability between TiB2 ceramic and metals. Acta Metall. Sin. 1999, 12, 395–400. [Google Scholar]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Ye, J. Fabrication and properties of Ti(C, N)-based cermets with multi-component AlCoCrFeNi high-entropy alloys binder. Mater. Lett. 2013, 113, 80–82. [Google Scholar] [CrossRef]
- Fu, Z.; Koc, R. Processing and characterization of TiB2-TiNiFeCrCoAl high-entropy alloy composite. J. Am. Ceram. Soc. 2017, 100, 2803–2813. [Google Scholar] [CrossRef]
- Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F. Mechanical alloying synthesis and spark plasma sintering consolidation of CoCrFeNiAl high-entropy alloy. J. Alloys Compd. 2014, 589, 61–66. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, P.; Han, X.; Lin, Q.; Qiu, F.; Zhang, Y.; Jiang, Q. Wettability and reactivity between B4C and Molten Zr55Cu30Al10Ni5 metallic glass alloy. Mater. Chem. Phys. 2009, 117, 377–383. [Google Scholar] [CrossRef]
- Aizenshtein, M.; Froumin, N.; Frage, N. The nature of TiB2 wetting by Cu and Au. J. Mater. Eng. Perform 2012, 21, 655–659. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B. Synthesis and characterization of nanocrystalline AlFeTiCrZnCu high entropy solid solution by mechanical alloying. J. Alloys Compd. 2008, 460, 253–257. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Qiao, Y.; Chen, G. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Ji, W.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F.; Fu, Z. Alloying behavior and novel properties of CoCrFeNiMn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering. Intermetallics 2015, 56, 2–27. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Kalb, J.A.; Thompson, C.V. Matching glass-forming ability with the density of the amorphous phase. Science 2008, 322, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pang, S.; Men, H.; Ma, C.; Zhang, T. Formation and mechanical propertie sof (Ce-La-Pr-Nd)-Co-Al bulk glassy alloys with superior glass-forming ability. Scr. Mater. 2006, 54, 1123–1126. [Google Scholar] [CrossRef]
- Wu, G.; Chan, K.; Zhu, L.; Sun, L.; Lu, J. Dual-phase nanostructuring as a route to high-strength magnesium alloys. Nature 2017, 545, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Sun, Y.; Ke, B.; Li, Y.; Ji, W.; Wang, W.; Fu, Z. Preparation and Characterization of TiB2-(Supra-Nano-Dual-Phase) High-Entropy Alloy Cermet by Spark Plasma Sintering. Metals 2018, 8, 58. https://doi.org/10.3390/met8010058
Zhang S, Sun Y, Ke B, Li Y, Ji W, Wang W, Fu Z. Preparation and Characterization of TiB2-(Supra-Nano-Dual-Phase) High-Entropy Alloy Cermet by Spark Plasma Sintering. Metals. 2018; 8(1):58. https://doi.org/10.3390/met8010058
Chicago/Turabian StyleZhang, Shulei, Yuchen Sun, Boren Ke, Yulin Li, Wei Ji, Weimin Wang, and Zhengyi Fu. 2018. "Preparation and Characterization of TiB2-(Supra-Nano-Dual-Phase) High-Entropy Alloy Cermet by Spark Plasma Sintering" Metals 8, no. 1: 58. https://doi.org/10.3390/met8010058




