Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media
Abstract
:1. Introduction
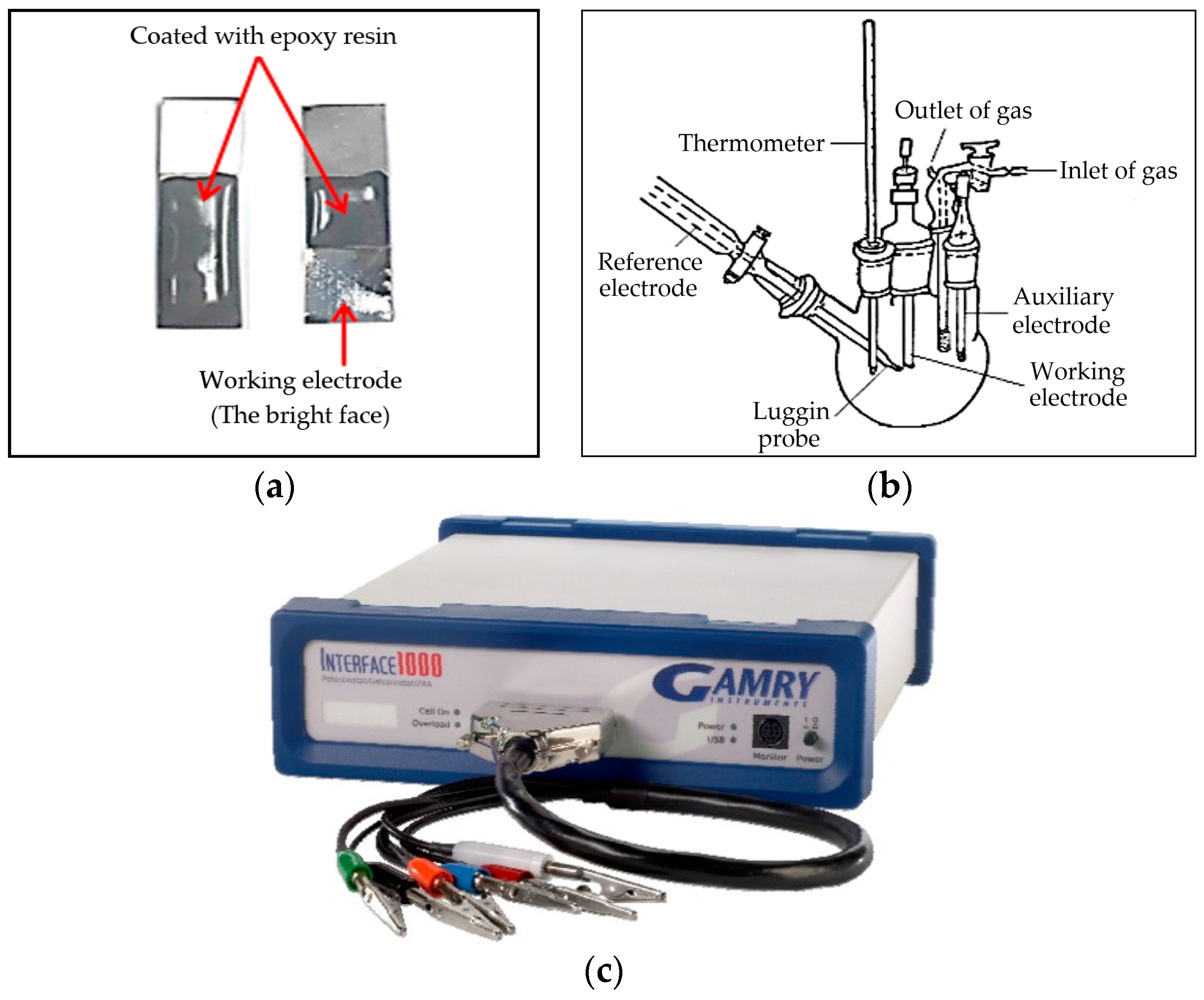
2. Experiments
3. Results and Discussion
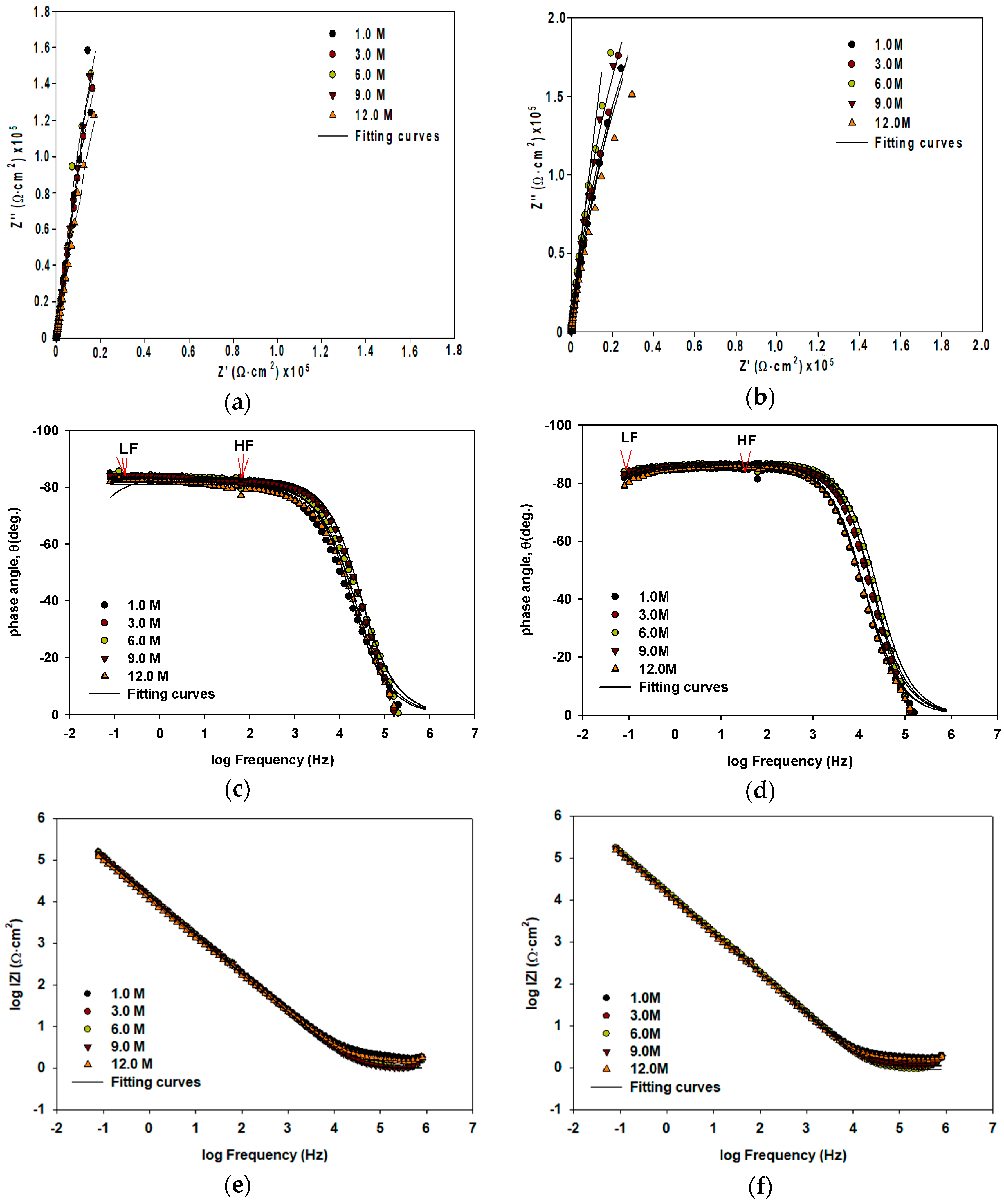
3.1. Passivation and Resistance Characterization
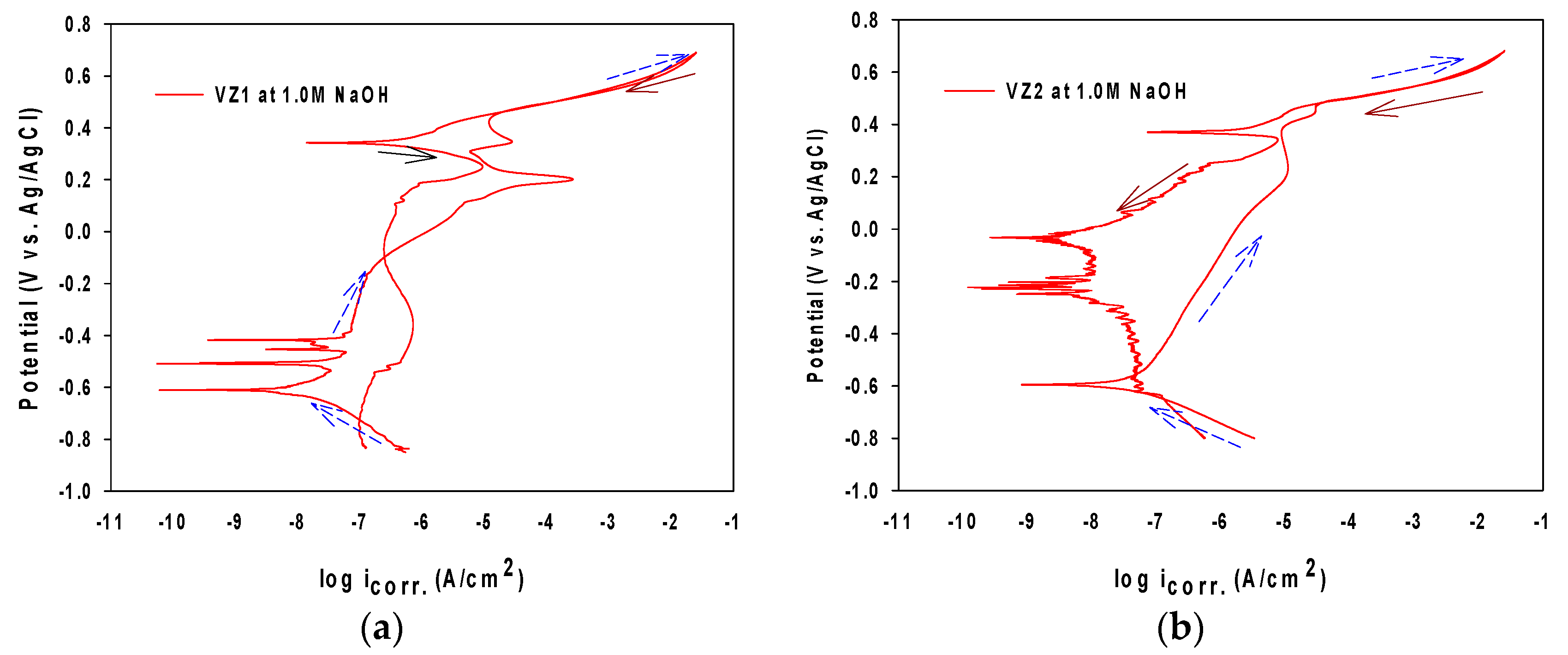
3.2. The Formation of Corrosion Film in Immersion Solution
3.3. Morphological and Structural Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Axinte, E. Metallic glasses from “alchemy” to pure science: Present and future of design, processing and applications of glassy metals. Mater. Des. 2012, 35, 518–556. [Google Scholar] [CrossRef]
- Gebert, A.; Buchholz, K.; Leonhard, A.; Mummert, K.; Eckert, J.; Schultz, L. Investigations on the electrochemical behaviour of Zr-based bulk metallic glasses. Mater. Sci. Eng. A 1999, 267, 294–300. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Zhang, H.; Wang, A.; Hu, Z. The influence of Zr substitution for Nb on the corrosion behaviors of the Ni-Nb-Zr bulk metallic glasses. Sci. China Phys. Mech. Astron. 2012, 55, 2362–2366. [Google Scholar] [CrossRef]
- Zeng, Y.; Qin, C.; Nishiyama, N.; Inoue, A. New nickel-based bulk metallic glasses with extremely high nickel content. J. Alloys Compd. 2010, 489, 80–83. [Google Scholar] [CrossRef]
- Qin, C.; Asami, K.; Kimura, H.; Zhang, W.; Inoue, A. Electrochemical and XPS studies of Ni-based metallic glasses in boiling nitric acid solutions. Electrochim. Acta 2009, 54, 1612–1617. [Google Scholar] [CrossRef]
- Arab, S.; Emran, K. Influence of Cr addition on the electrochemical behavior of Ni-base metallic glasses in HCl. Phys. Chem. News 2009, 50, 130–138. [Google Scholar]
- Arab, S.T.; Emran, K.M.; Al-Turaif, H.A. Passivity characteristics on Ni(Cr)(Fe)SiB glassy alloys in phosphate solution. J. Saudi Chem. Soc. 2014, 18, 169–182. [Google Scholar] [CrossRef]
- Al-Mobarak, N.; Khaled, K.; Hamed, M.N.; Abdel-Azim, K. Employing electrochemical frequency modulation for studying corrosion and corrosion inhibition of copper in sodium chloride solutions. Arab. J. Chem. 2011, 4, 185–193. [Google Scholar] [CrossRef]
- Tomashov, N.D. Passivity and Protection of Metals against Corrosion; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Lavigne, O.; Shoji, T.; Takeda, Y. EIS pitting temperature determination of A182 nickel based alloy in simulated BWR environment containing dilute seawater. Nucl. Eng. Des. 2014, 273, 435–439. [Google Scholar] [CrossRef]
- BenSalah, M.; Sabot, R.; Triki, E.; Dhouibi, L.; Refait, P.; Jeannin, M. Passivity of Sanicro28 (UNS N-08028) stainless steel in polluted phosphoric acid at different temperatures studied by electrochemical impedance spectroscopy and Mott-Schottky analysis. Corros. Sci. 2014, 86, 61–70. [Google Scholar] [CrossRef]
- Arab, S.T.; Emran, K.M.; Al-Turaif, H.A. The electrochemical behavior of Ni-base metallic glasses containing Cr in H2SO4 solutions. J. Korean Chem. Soc. 2012, 56, 448–458. [Google Scholar] [CrossRef]
- Ter-Ovanessian, B.; Alemany-Dumont, C.; Normand, B. Electronic and transport properties of passive films grown on different Ni-Cr binary alloys in relation to the pitting susceptibility. Electrochim. Acta 2014, 133, 373–381. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Lavigne, O.; Alemany-Dumont, C.; Normand, B.; Berger, M.-H.; Duhamel, C.; Delichére, P. The effect of nitrogen on the passivation mechanisms and electronic properties of chromium oxide layers. Corros. Sci. 2011, 53, 2087–2096. [Google Scholar] [CrossRef]
- Belo, M.D.C.; Hakiki, N.; Ferreira, M. Semiconducting properties of passive films formed on nickel-base alloys type Alloy 600: Influence of the alloying elements. Electrochim. Acta 1999, 44, 2473–2481. [Google Scholar] [CrossRef]
- Jakupi, P.; Zagidulin, D.; Noël, J.; Shoesmith, D. The impedance properties of the oxide film on the Ni–Cr–Mo Alloy-22 in neutral concentrated sodium chloride solution. Electrochim. Acta 2011, 56, 6251–6259. [Google Scholar] [CrossRef]
- Karimi, S.; Nickchi, T.; Alfantazi, A. Effects of bovine serum albumin on the corrosion behaviour of AISI 316L, Co–28Cr–6Mo, and Ti–6Al–4V alloys in phosphate buffered saline solutions. Corros. Sci. 2011, 53, 3262–3272. [Google Scholar] [CrossRef]
- Macdonald, D.D. Passivity—The key to our metals-based civilization. Pure Appl. Chem. 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Rosalbino, F.; Delsante, S.; Borzone, G.; Scavino, G. Influence of noble metals alloying additions on the corrosion behaviour of titanium in a fluoride-containing environment. J. Mater. Sci. Mater. Med. 2012, 23, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Mahdi, E. Evaluating corrosion inhibitors with the help of electrochemical measurements including electrochemical frequency modulation. Int. J. Electrochem. Sci. 2012, 7, 4673–4685. [Google Scholar]
- Fontana, M.G. Corrosion Engineering; Mcgraw-Hill Book Company: New York, NY, USA, 1986. [Google Scholar]
- Zhang, B.-P.; Habazaki, H.; Kawashima, A.; Asami, K.; Hashimoto, K. The corrosion behavior of amorphous Ni-Cr-19P alloys in concentrated hydrofluoric acid. Corros. Sci. 1992, 33, 1519–1528. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, V.; Quraishi, M. Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: Electrochemical, SEM, AFM, and XPS studies. J. Mol. Liq. 2016, 216, 164–173. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhou, T.; Yin, B.; Yin, S.; Zhang, Y. Effect of tin on the corrosion behavior of sea-water corrosion-resisting steel. Mater. Des. 2015, 84, 1–9. [Google Scholar] [CrossRef]
- De Oliveira, R.; Albuquerque, D.; Leite, F.; Yamaji, F.; Cruz, T. Measurement of the Nanoscale Roughness by Atomic Force Microscopy: Basic Principles and Applications; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
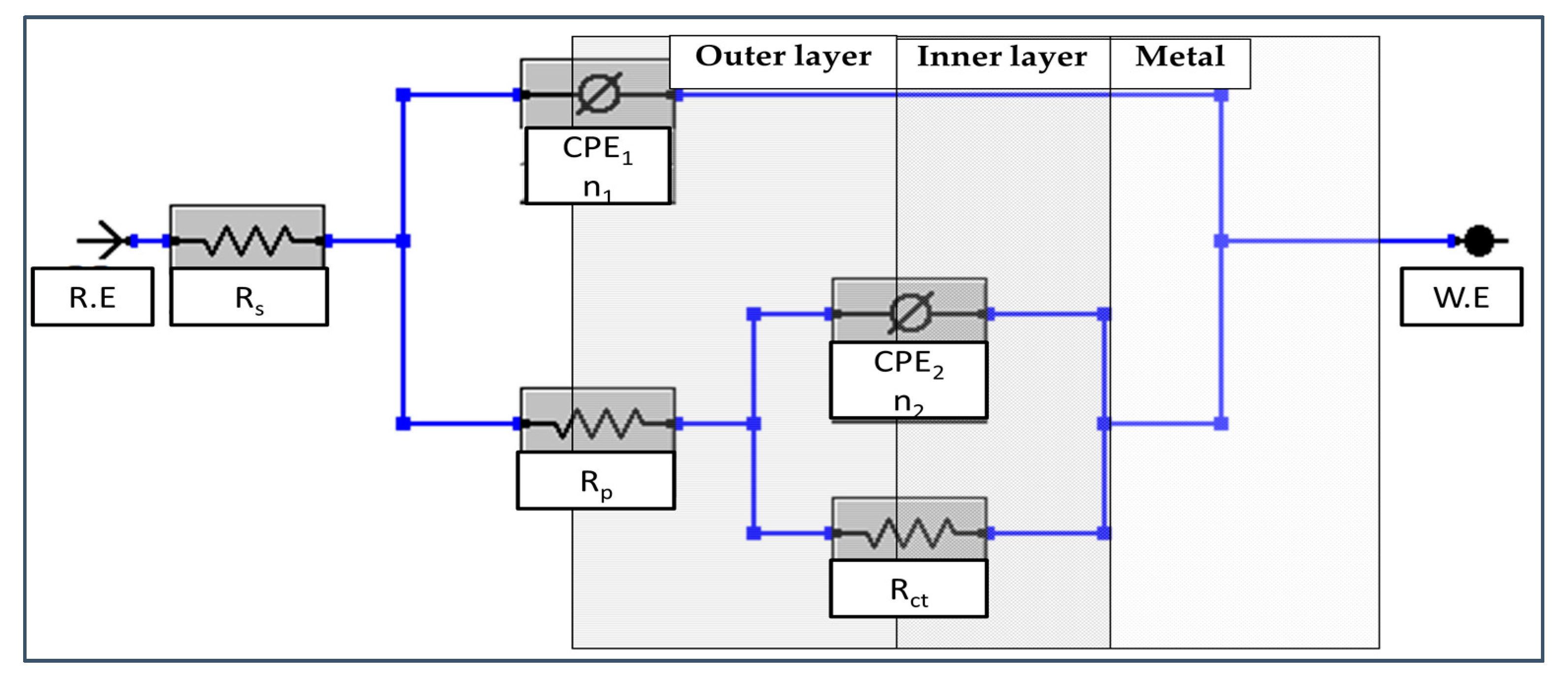
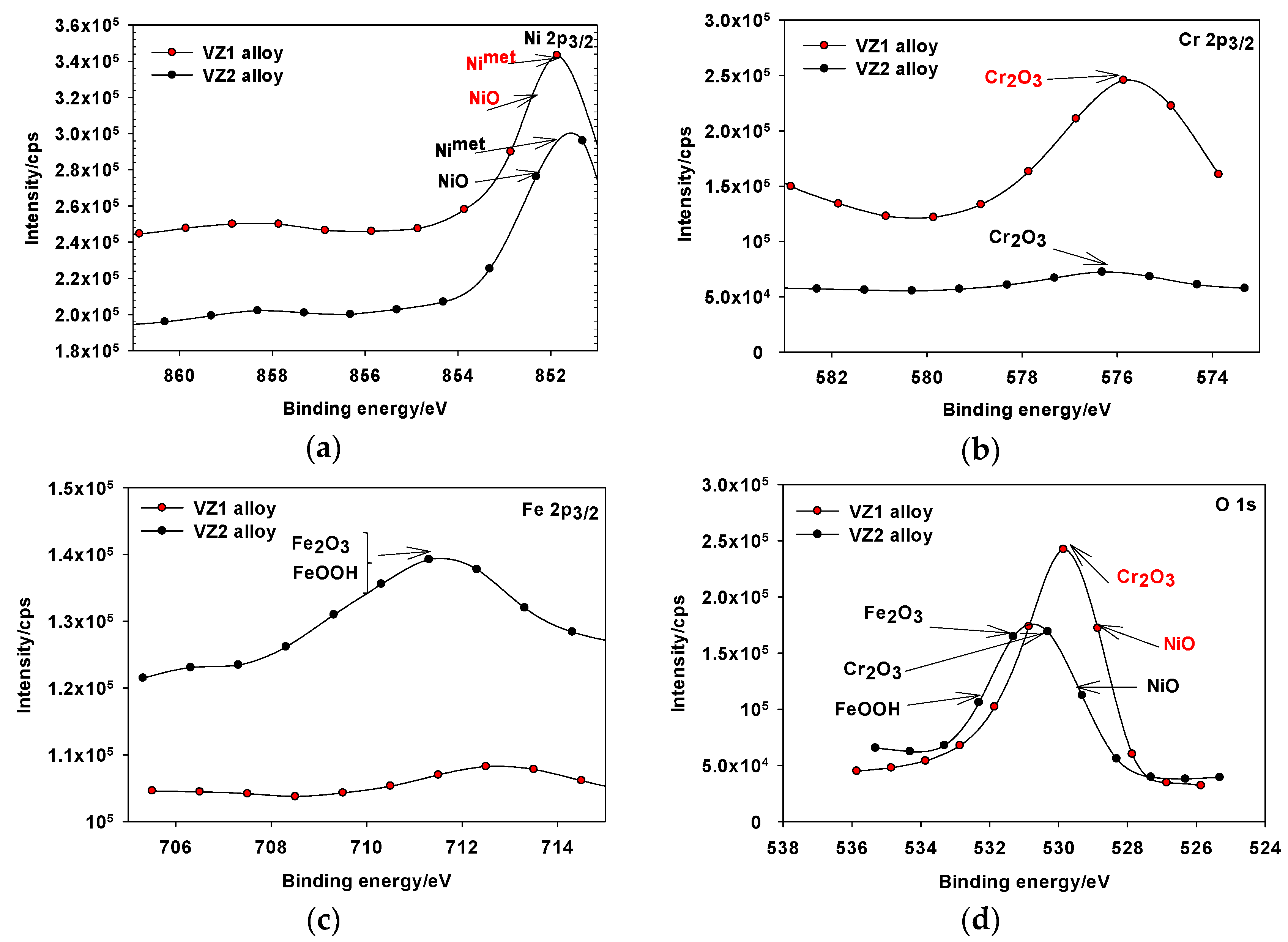
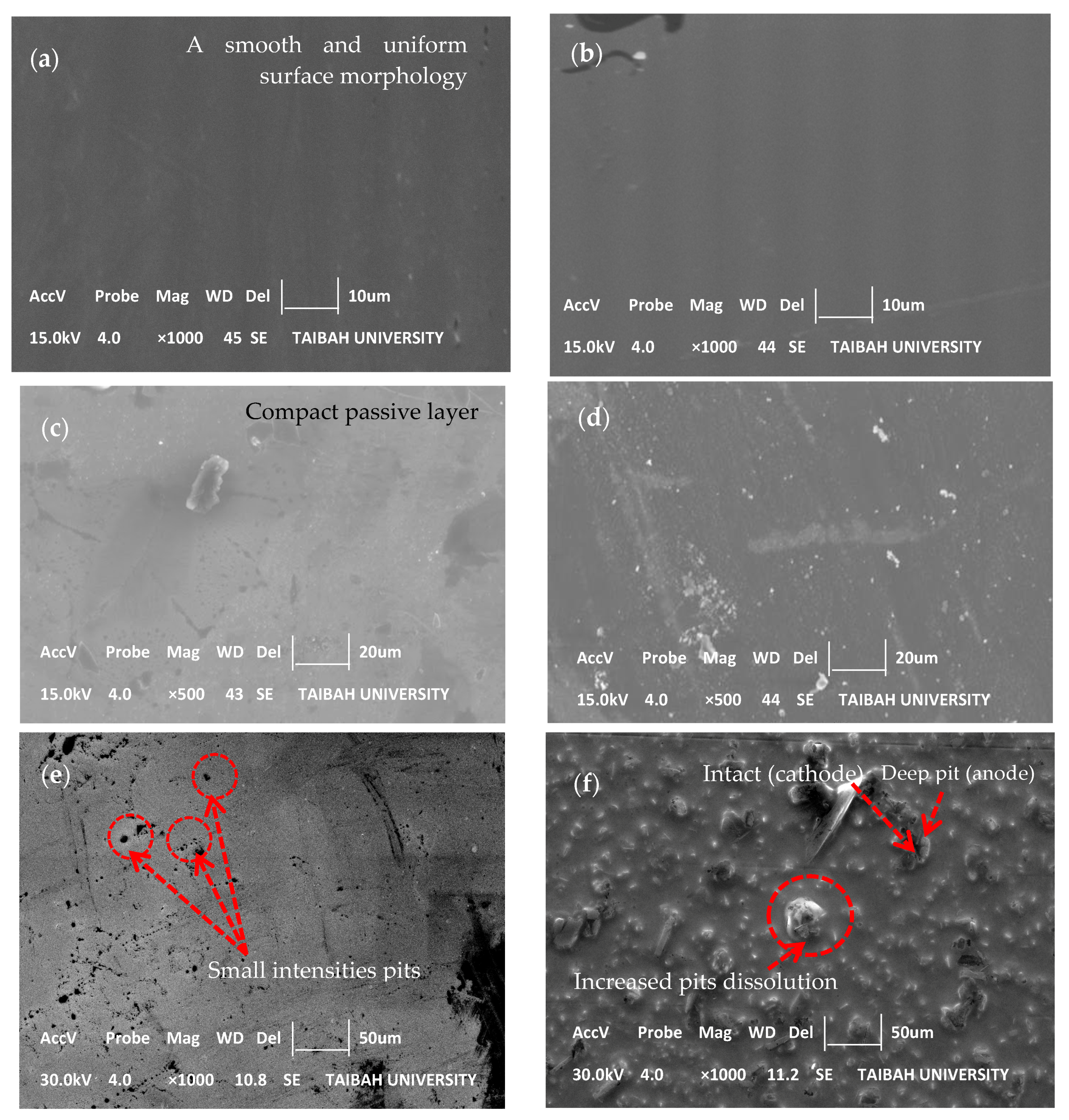
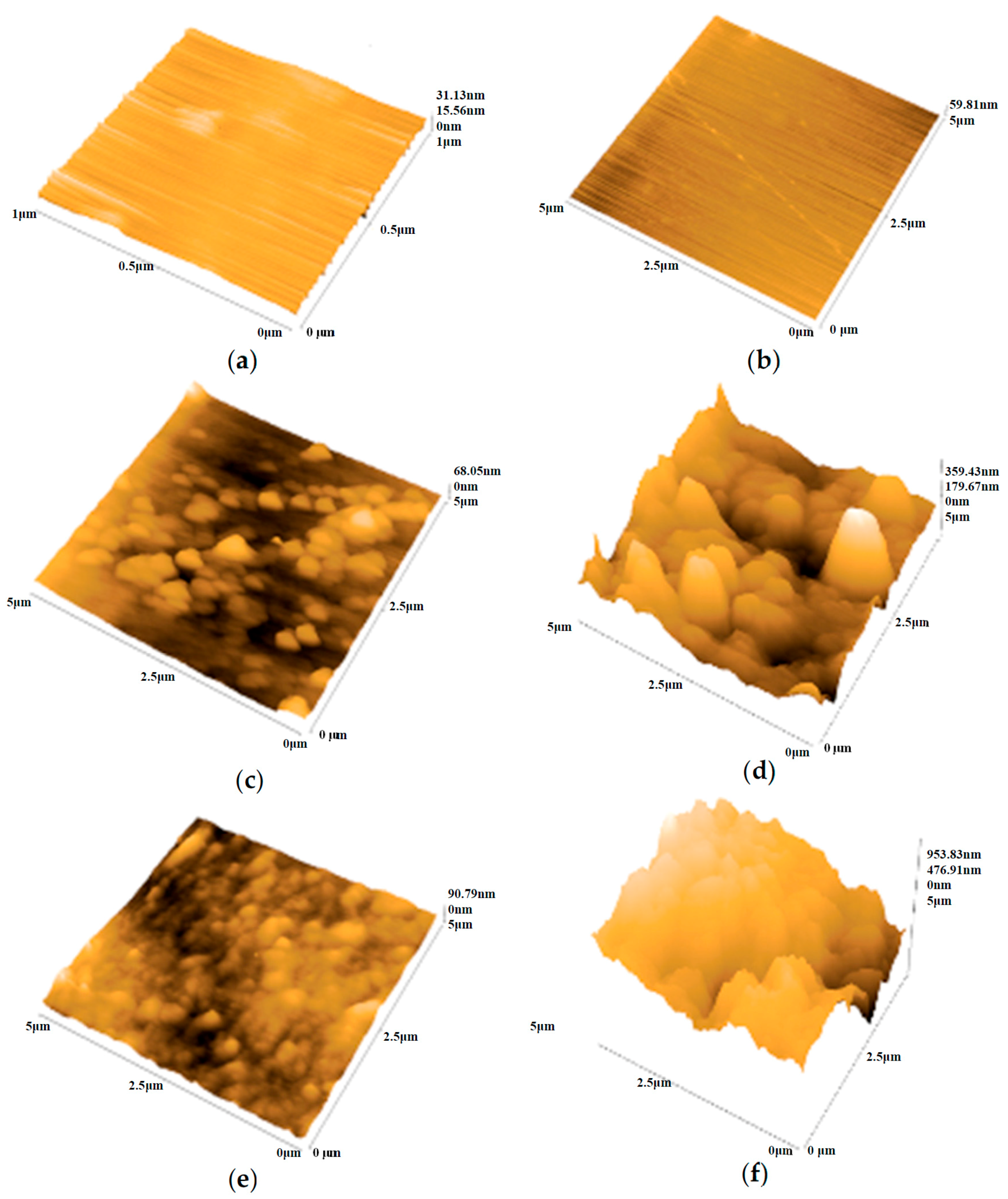
| Alloy | NaOH (M) | Rp (Ω·cm2) | CPE1, Q1 × 10−5 (Ω−1·cm−2·sn1) | n1 | Ceff1, ×10−5 (F·cm−2) | d1 (nm) | Rct, ×106 (Ω·cm2) | CPE2, Q2 × 10−5 (Ω−1·cm−2·sn2) | n2 | Ceff2, ×10−5 (F·cm−2) | d2 (nm) | Total Thickness (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VZ1 | 1.0 | 5.361 × 103 | 5.220 | 0.775 | 3.61 | 0.294 | 29.35 | 1.287 | 0.999 | 1.291 | 2.051 | 2.351 |
| 3.0 | 2.474 × 103 | 8.322 | 0.863 | 6.47 | 0.164 | 29.31 | 1.418 | 0.955 | 1.881 | 1.411 | 1.581 | |
| 6.0 | 2.226 × 103 | 8.602 | 0.849 | 6.42 | 0.165 | 19.21 | 1.662 | 0.933 | 2.511 | 1.061 | 1.221 | |
| 9.0 | 2.126 × 103 | 10.60 | 0.773 | 6.85 | 0.155 | 17.71 | 1.766 | 0.925 | 2.812 | 0.947 | 1.101 | |
| 12.0 | 1.751 × 103 | 35.78 | 0.743 | 30.4 | 0.035 | 17.48 | 1.787 | 0.912 | 3.111 | 0.855 | 0.890 | |
| VZ2 | 1.0 | 41.73 | 1.161 | 0.951 | 0.784 | 1.351 | 2.608 | 2.461 | 0.892 | 4.082 | 6.511 | 2.00 |
| 3.0 | 50.28 | 3.081 | 0.960 | 2.36 | 0.451 | 2.246 | 3.441 | 0.872 | 6.501 | 4.091 | 0.860 | |
| 6.0 | 19.73 | 3.461 | 0.968 | 2.72 | 0.391 | 2.090 | 4.651 | 0.892 | 8.071 | 3.292 | 0. 720 | |
| 9.0 | 10.12 | 4.511 | 0.933 | 2.59 | 0.410 | 0.278 | 7.481 | 0.868 | 11.91 | 2.241 | 0.634 | |
| 12.0 | 6.363 | 4.461 | 0.957 | 3.09 | 0.344 | 0.123 | 7.852 | 0.874 | 10.91 | 2.441 | 0.588 |
| Alloys | NaOH (M) | −Eocp (mV) | −Ecorr (mV) | icorr (μA·cm−2) | −βc (V/decade) | Epit (mV) | Erep (mV) | −Epass (mV) | ipass (μA·cm−2) | Erep − Epit (mV) | Ecorr − Epass (mV) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VZ1 | 1.0 | 623.8 | 613.1 | 0.011 | 96.81 | −66.07 | 601.9 | 474.7 | 0.082 | - | −138.4 |
| 3.0 | 700.8 | 696.4 | 0.015 | 99.08 | −149.5 | 534.2 | 560.9 | 0.086 | - | −135.5 | |
| 6.0 | 790.6 | 775.7 | 0.026 | 92.08 | −230.7 | 463.2 | 642.8 | 0.098 | - | −132.9 | |
| 9.0 | 820.6 | 809.3 | 0.069 | 92.08 | −247.8 | 456.4 | 681.8 | 0.241 | 704.2 | −127.5 | |
| 12.0 | 790.9 | 782.2 | 0.763 | 92.89 | −271.8 | 590.7 | 708.7 | 0.979 | 862.5 | −73.5 | |
| VZ2 | 1.0 | 600.5 | 599.1 | 0.026 | 93.16 | 101.2 | 577.1 | 362.3 | 0.475 | - | −236.8 |
| 3.0 | 613.8 | 600.6 | 0.028 | 98.24 | 51.16 | 551.5 | 410.7 | 0.300 | - | −189.9 | |
| 6.0 | 678.9 | 668.6 | 0.030 | 93.48 | −129.2 | 530.3 | 498.6 | 0.113 | - | −170 | |
| 9.0 | 770.8 | 763.3 | 0.034 | 94.02 | −229.3 | 448.9 | 597.3 | 0.120 | 678.2 | −166 | |
| 12.0 | 840.5 | 830.8 | 0.042 | 95.13 | −240.4 | 443.1 | 658.1 | 0.638 | 683.5 | −172.7 |
| Alloys | NaOH (M) | icorr (μA·cm−2) | −βc (mV/Decade) | Corrosion Rate × 10−3 (mm/year) | CF-2 | CF-3 |
|---|---|---|---|---|---|---|
| VZ1 | 1.0 | 0.034 | 59.31 | 1.214 | 2.180 | 1.239 |
| 3.0 | 0.057 | 59.22 | 1.442 | 1.395 | 1.429 | |
| 6.0 | 0.168 | 60.81 | 2.093 | 2.005 | 1.469 | |
| 9.0 | 0.171 | 60.49 | 2.136 | 2.124 | 1.277 | |
| 12.0 | 0.191 | 59.65 | 2.378 | 1.877 | 1.196 | |
| VZ2 | 1.0 | 0.530 | 59.45 | 2.132 | 1.781 | 2.891 |
| 3.0 | 0.793 | 63.12 | 2.175 | 2.395 | 2.479 | |
| 6.0 | 0.805 | 58.56 | 6.596 | 1.561 | 1.671 | |
| 9.0 | 0.877 | 59.56 | 7.363 | 1.761 | 1.231 | |
| 12.0 | 0.898 | 59.31 | 8.802 | 2.180 | 1.239 |
| Alloys | NaOH (M) | Ra (nm) | Rp (nm) | Rpm (nm) | RT (nm) | RTm (nm) |
|---|---|---|---|---|---|---|
| VZ1 | as-received | 0.501 | 1.861 | 0.882 | 3.051 | 1.531 |
| 1.0 | 2.713 | 19.58 | 7.253 | 27.16 | 11.41 | |
| 3.0 | 17.78 | 85.22 | 37.86 | 115.8 | 61.25 | |
| 6.0 | 23.52 | 73.33 | 34.72 | 120.4 | 71.65 | |
| 9.0 | 25.27 | 89.94 | 24.76 | 129.5 | 53.01 | |
| 12.0 | 74.02 | 209.3 | 66.07 | 365.4 | 137.1 | |
| VZ2 | as-received | 0.621 | 1.981 | 0.899 | 3.071 | 1.781 |
| 1.0 | 5.953 | 19.32 | 7.783 | 32.35 | 15.41 | |
| 3.0 | 12.723 | 31.28 | 14.40 | 52.95 | 22.92 | |
| 6.0 | 14.23 | 49.81 | 31.16 | 75.23 | 52.00 | |
| 9.0 | 19.04 | 51.71 | 35.99 | 97.20 | 66.01 | |
| 12.0 | 178.0 | 319.3 | 86.54 | 677.5 | 170.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emran, K.M.; Al-Refai, H. Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media. Metals 2018, 8, 64. https://doi.org/10.3390/met8010064
Emran KM, Al-Refai H. Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media. Metals. 2018; 8(1):64. https://doi.org/10.3390/met8010064
Chicago/Turabian StyleEmran, Khadijah M., and Hanaa Al-Refai. 2018. "Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media" Metals 8, no. 1: 64. https://doi.org/10.3390/met8010064





