1. Introduction
Accident tolerant fuel (ATF) systems have attracted significant attention regarding the safety margins of commercial light water reactors (LWRs) since the Fukushima Daiichi nuclear accident in 2011. The desired ATF needs to not only buffer against a loss of cooling for a considerably long period, but also improve fuel performance while enhancing fuel safety at normal operation. One way to meet these demands is to develop a UO
2 composite fuel. The low thermal conductivity of UO
2 fuel has become one of the major concerns limiting nuclear reactor performance and safety. Incorporation of UO
2 with high thermal conductivity particles such as BeO or SiC could improve fuel thermal conductivity, one of the key criteria in balancing thermal energy and reactor safety demands [
1,
2]. Another way to mitigate against severe accidents is to develop enhanced strength and ductility ATF cladding, as this would alleviate the severity of reactivity-initiated accidents, and oxidation-resistant ATF cladding in the case of a loss-of-coolant accident (LOCA) [
3]. Zr alloys, which have low neutron absorption cross-sections and a good neutron irradiation resistance, are currently used in nuclear fuel cladding for commercial LWRs [
4]. However, in accident scenarios, Zr alloys react with hot steam, generating massive oxidation heat and hydrogen in a very short period. Additionally, the mechanical strength of Zr alloys undergoes significant recession and degradation during accidents. Therefore, it is important to develop new accident-tolerant fuel cladding materials [
4]. SiC possesses an extremely low hydrogen liberation rate, excellent high temperature mechanical properties, a high melting point, and little irradiation creep compared with Zr alloys [
5]. FeCrAl alloys present slower oxidation rates in high-temperature steam, which may help to preempt further damage resulting from an accident [
6]. Surface-coated Zr alloy cladding is also a candidate for ATF owing to its improved corrosion performance compared with Zr alloy cladding and its high neutron economy compared with FeCrAl alloys [
7].
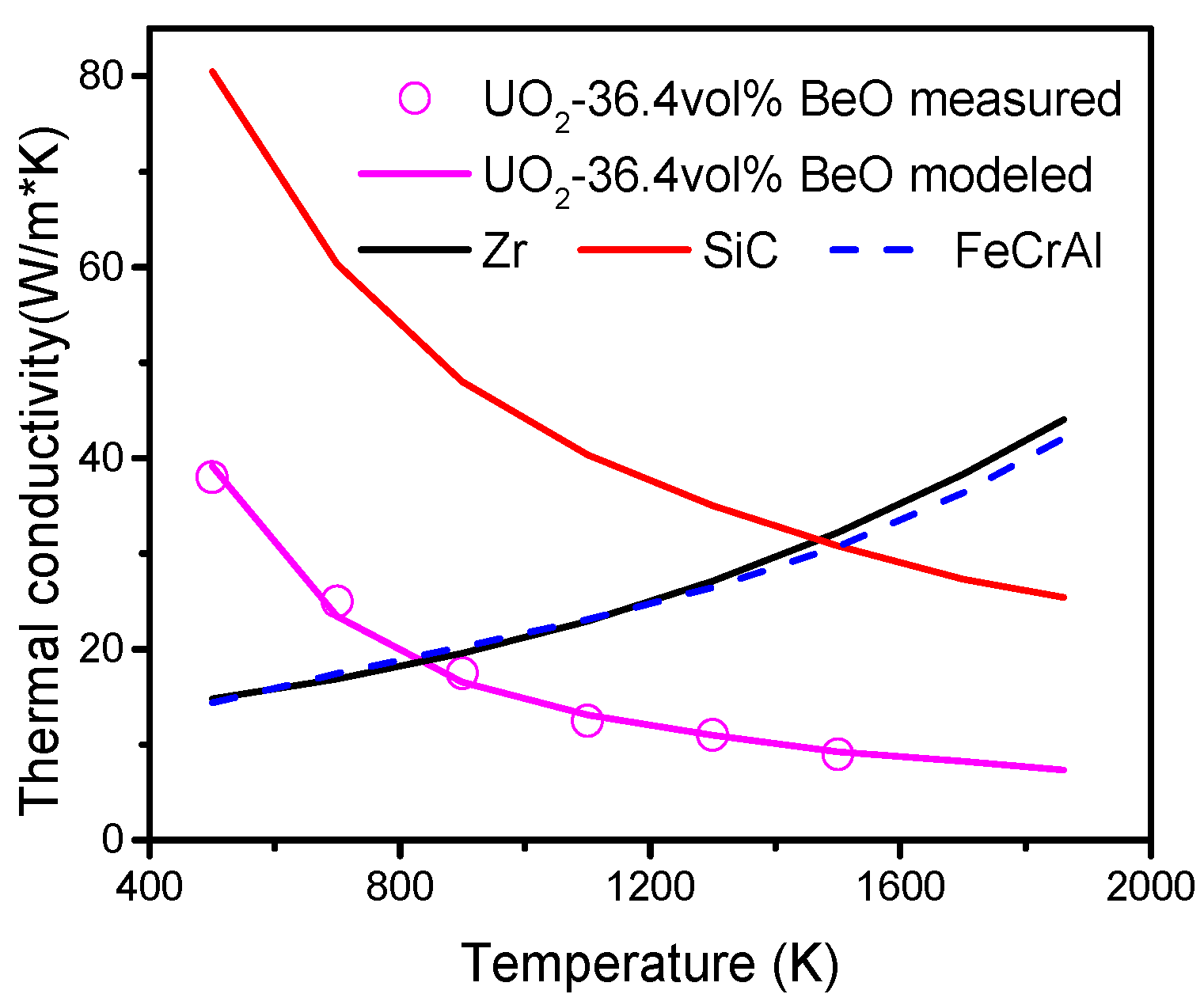
The mechanical interactions between fuel and cladding are essential factors for ATF development. In this study, a fully coupled multiphysics fuel performance and solid mechanics analysis is performed with different cladding materials, namely, Zr, SiC, FeCrAl, SiC-coated Zr, and FeCrAl-coated Zr, to compare the mechanical interactions of fuel and cladding materials, using the CityU Advanced Multiphysics Nuclear Fuels Performance with User-defined Simulations (CAMPUS) code. The effects of coating thickness (0.5, 1 and 1.5 mm) on fuel performance and mechanical interaction are also discussed in this study. A fuel material of UO2-36.4vol % BeO is chosen as optimal due to its promising application in commercial LWRs to achieve better fuel performance. Temperature-dependent material properties, such as thermal conductivity, density, specific heat capacity, and thermal expansion, are incorporated into the CAMPUS code, and the fuel centerline temperature, gap size evolution, fission gas release, plenum pressure, and fuel and cladding axial and radial displacements are calculated and discussed in detail.
3. Results and Discussions
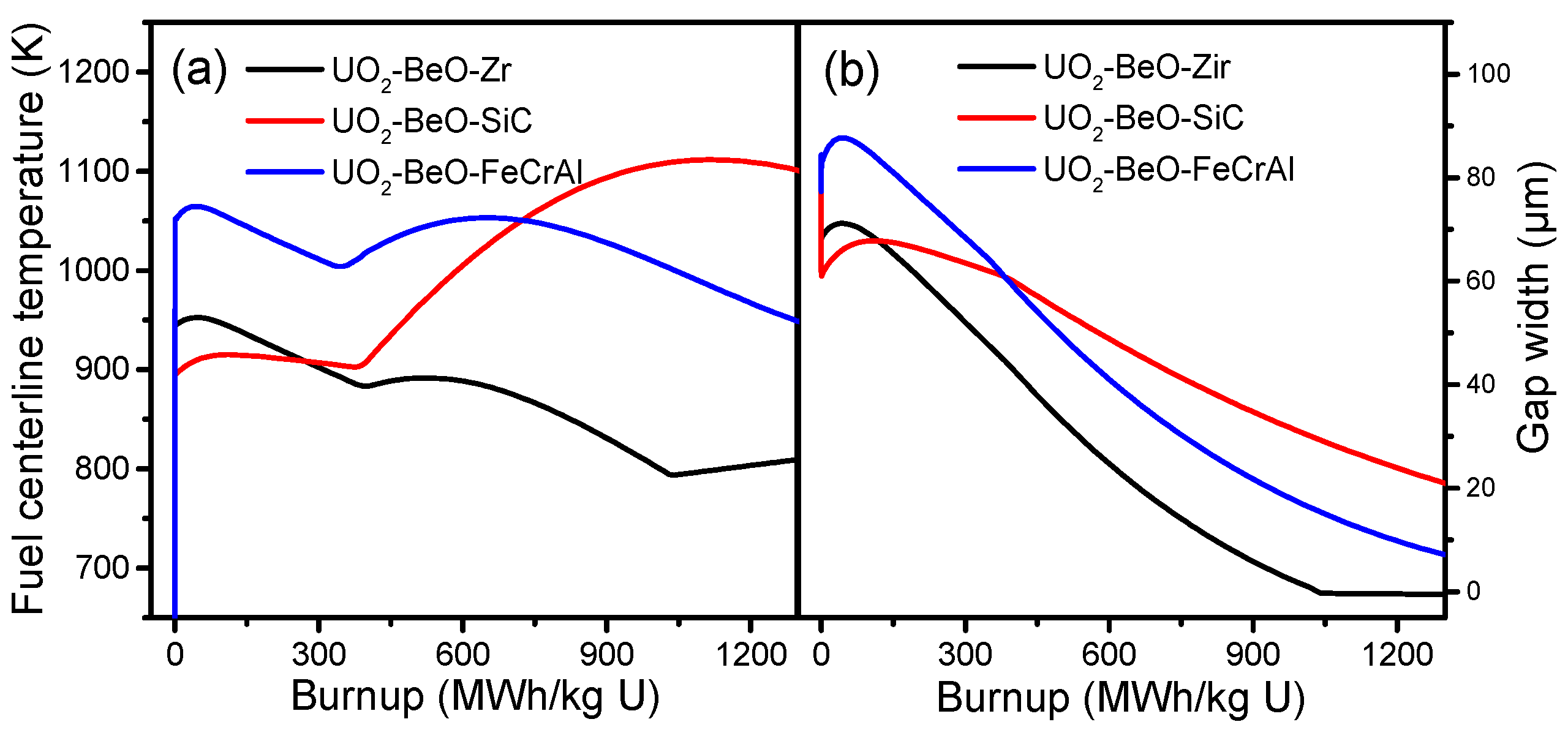
Figure 3a shows fuel centerline temperature of Zircaloy, SiC, and FeCrAl claddings. From
Figure 3a, we can see that the fuel temperature tendencies of the Zircaloy and FeCrAl claddings are almost equivalent, while the SiC cladding is quite different. At the beginning of burnup, the SiC cladding has a lower fuel centerline temperature than the Zircaloy and FeCrAl claddings. After the initial power-up, the fuel centerline temperatures of the Zircaloy, SiC, and FeCrAl claddings all increase slightly and then decrease. At a burnup of around 400 MWh/kg U, the fuel centerline temperature of the SiC cladding increases dramatically, while the fuel centerline temperatures of the Zircaloy and FeCrAl claddings increase only slightly. The fuel centerline temperatures of the Zircaloy and FeCrAl claddings begin to decrease at a burnup of around 700 MWh/kg U, while the fuel centerline temperature of the SiC cladding continues to increase. At burnup of 750 MWh/kg U, the fuel centerline temperature of the SiC cladding begins to exceed the fuel centerline temperature of the FeCrAl cladding and finally reaches 1100 K. These differences in fuel center temperature are due mainly to the lack of creep in the SiC cladding during irradiation. This leads to a delayed gap closure, resulting in a poor performance of pellet cladding mechanical contact. As shown in
Figure 3a, neither the SiC nor FeCrAl cladding can effectively decrease the fuel centerline temperature compared with the Zircaloy cladding at a high burnup.
The gap width is compared across the different cladding materials, and the tendencies of the Zircaloy, SiC, and FeCrAl claddings are shown in
Figure 3b. The gap size evolution tendencies of the Zircaloy and FeCrAl claddings are almost equivalent, while the SiC cladding shows a gentle incline. From
Figure 3b, the initial values of the Zircaloy, SiC, and FeCrAl claddings are different. The SiC cladding has the smallest gap size, around 60 µm, while the FeCrAl cladding has the largest gap size, around 85 µm, due to the different thermal expansions of Zircaloy, SiC, and FeCrAl. Then, due to fuel densification, the gap sizes of the Zircaloy, SiC, and FeCrAl claddings all show a slight increase during the short burnup range, after which the gap size decreases almost linearly with burnup increase. Finally, fuel and cladding contact is achieved, which leads to gap size becoming zero for the Zircaloy cladding at a burnup of 1050 MWh/kg U. At a burnup of 1350 MWh/kg U, the gaps of the SiC and FeCrAl claddings are not yet closed.
The fuel displacements against the axial positions of the Zircaloy, SiC, and FeCrAl claddings, at a burnup of 1200 MWh/kg U, are presented in
Figure 4.
Figure 4a shows that the fuel radial displacement of the Zircaloy cladding is the smallest, due to it having the lowest fuel centerline temperature, while the fuel radial displacement of the SiC cladding is the largest, due it having the highest fuel centerline temperature. From
Figure 4b, we can see that the fuel axial displacements of different cladding materials show the same trend as fuel radial displacements. This demonstrates that both fuel radial and axial displacements depend significantly on fuel temperature. At a burnup of 1200 MWh/kg U, UO
2-BeO fuel with SiC cladding possesses the highest fuel centerline temperature, leading to the largest fuel radial and axial displacements, while UO
2-BeO fuel with Zircaloy cladding possesses the lowest fuel centerline temperature, which leads to the smallest radial and axial displacements.
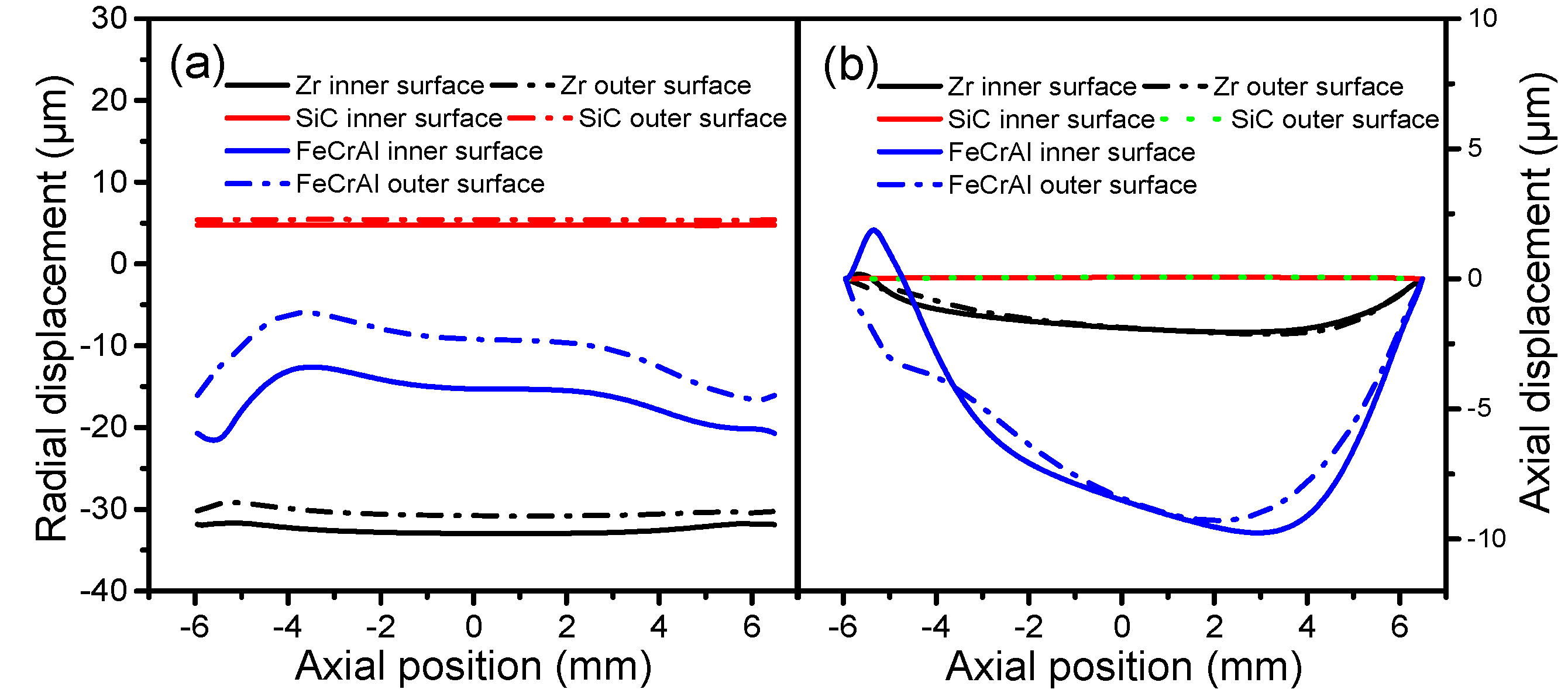
The cladding displacements on both the inner and outer surfaces of Zircaloy, SiC, and FeCrAl claddings at a burnup of 1200 MWh/kg U are depicted in
Figure 5 as a function of axial position. From
Figure 5a we can see that for all cladding types, the radial displacements of the inner and outer surfaces change simultaneously at the same axial position. The radial displacements of the Zircaloy and FeCrAl claddings arch toward the fuel, while the radial displacement of the SiC cladding shifts toward the coolant. The different displacement directions illustrate that plenum pressure, thermal expansion, and creep all contribute to the radial displacement of cladding. For the Zircaloy and FeCrAl claddings, the inner surface experiences larger radial displacements than the outer surface, which may be due to larger temperature-driven thermal expansions of the inner surface. For the SiC cladding, the outer and inner surfaces experience almost the same radial displacement. The inner and outer surfaces of the FeCrAl cladding show the largest differences at the same axial position, while the inner and outer surfaces of the SiC cladding show the least differences at the same axial position. This means that the FeCrAl cladding endures more deformation inside the cladding material. There are no significant changes in the axial displacement of the SiC cladding on either the inner or outer surfaces in any of the axial positions shown in
Figure 5b, perhaps due to the effects of plenum pressure and the thermal expansion of the SiC cladding. For the Zircaloy cladding, the axial displacements of both inner and outer surfaces slightly increase as axial position changes from −6 to 4.5 mm, and then decrease again. The axial displacements of the inner and outer surfaces of the FeCrAl cladding show dramatic changes as the axial position changes; these become increasingly disparate at the same axial position, which may indicate that the effect of plenum pressure suppresses the effect of thermal expansion of the FeCrAl cladding.
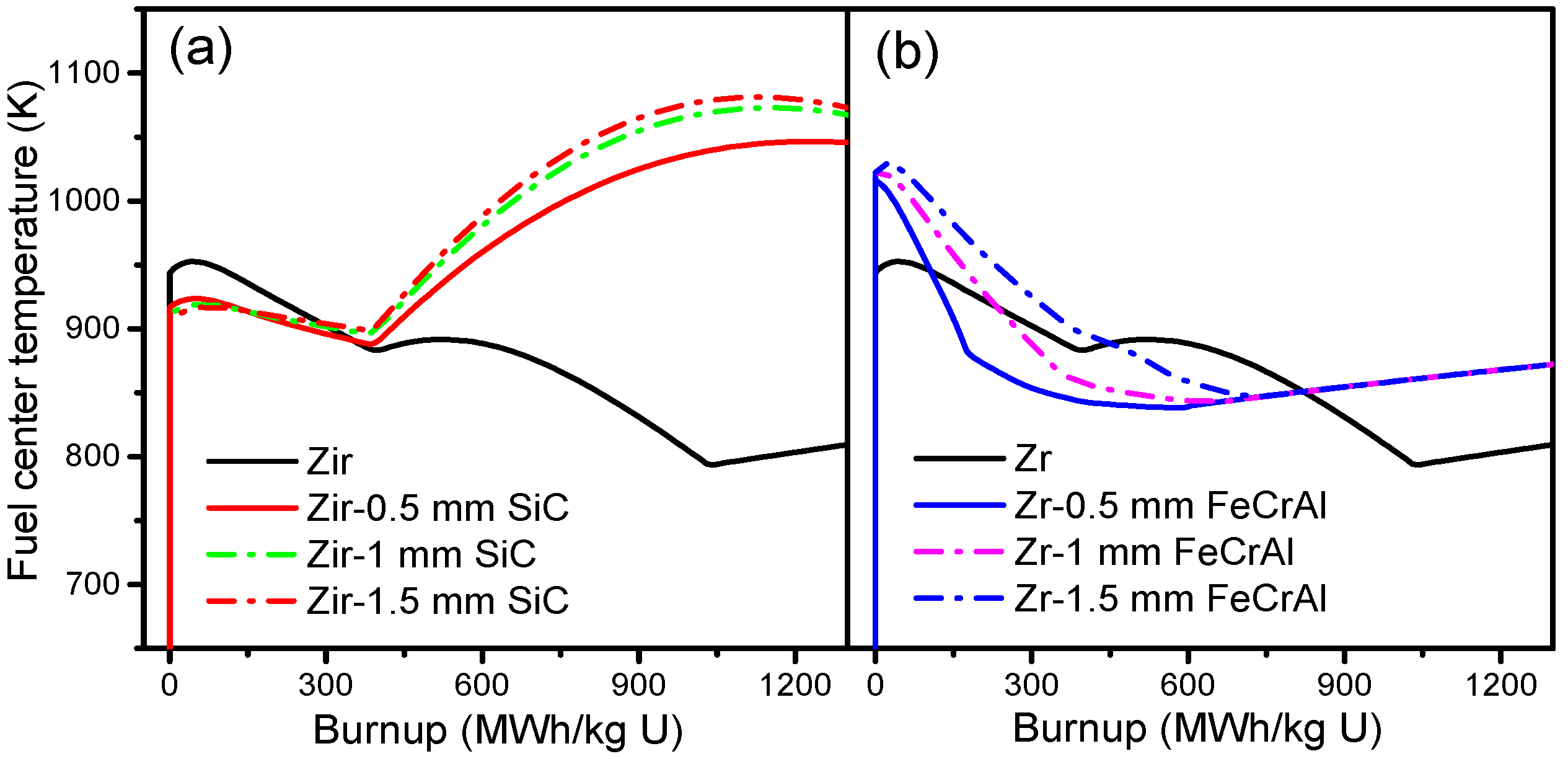
Figure 6 shows the burnup-dependent fuel centerline temperatures of Zircaloy, Zircaloy coated with 0.5, 1 and 1.5 mm SiC claddings, and Zircaloy coated with 0.5, 1 and 1.5 mm FeCrAl claddings, respectively. From
Figure 6a, we can see that the fuel centerline temperature tendencies of the SiC-coated claddings are quite different from that of the Zircaloy cladding. For the SiC-coated claddings, the fuel centerline temperatures are lower than those of the Zircaloy cladding at the beginning of a burnup and then show a slight decrease as the burnup increases. However, as the burnup reaches 400 MWh/kg U, the fuel centerline temperature tendencies of the SiC-coated claddings increase rapidly, while the fuel centerline temperature of the Zircaloy cladding continues to decrease. The lower fuel centerline temperature of the SiC-coated claddings at the early burnup stage is due mainly to the smaller gap size (shown in
Figure 7a), which causes better gap conductance. There is no big difference in the fuel centerline temperature of Zircaloy coated with 1 and 1.5 mm SiC claddings. Zircaloy coated with 0.5 mm SiC cladding shows lower fuel centerline temperatures than the 1 and 1.5 mm coated claddings; this is due to better gap conductance resulting from a smaller gap width. From
Figure 6b, we can see that the initial fuel centerline temperature of coated claddings is higher than that of the Zircaloy cladding at power-up, due to its relatively poor gap conductance. The fuel centerline temperature of Zircaloy coated with FeCrAl claddings then decreases rapidly as gap size decreases, and finally shows a slight increase as the gap closes. The fuel centerline temperature of Zircaloy coated with FeCrAl claddings increases as coating thickness increases at the same burnup, a result of the gap size increasing as the thickness increases at the same burnup rates, as shown in
Figure 7b.
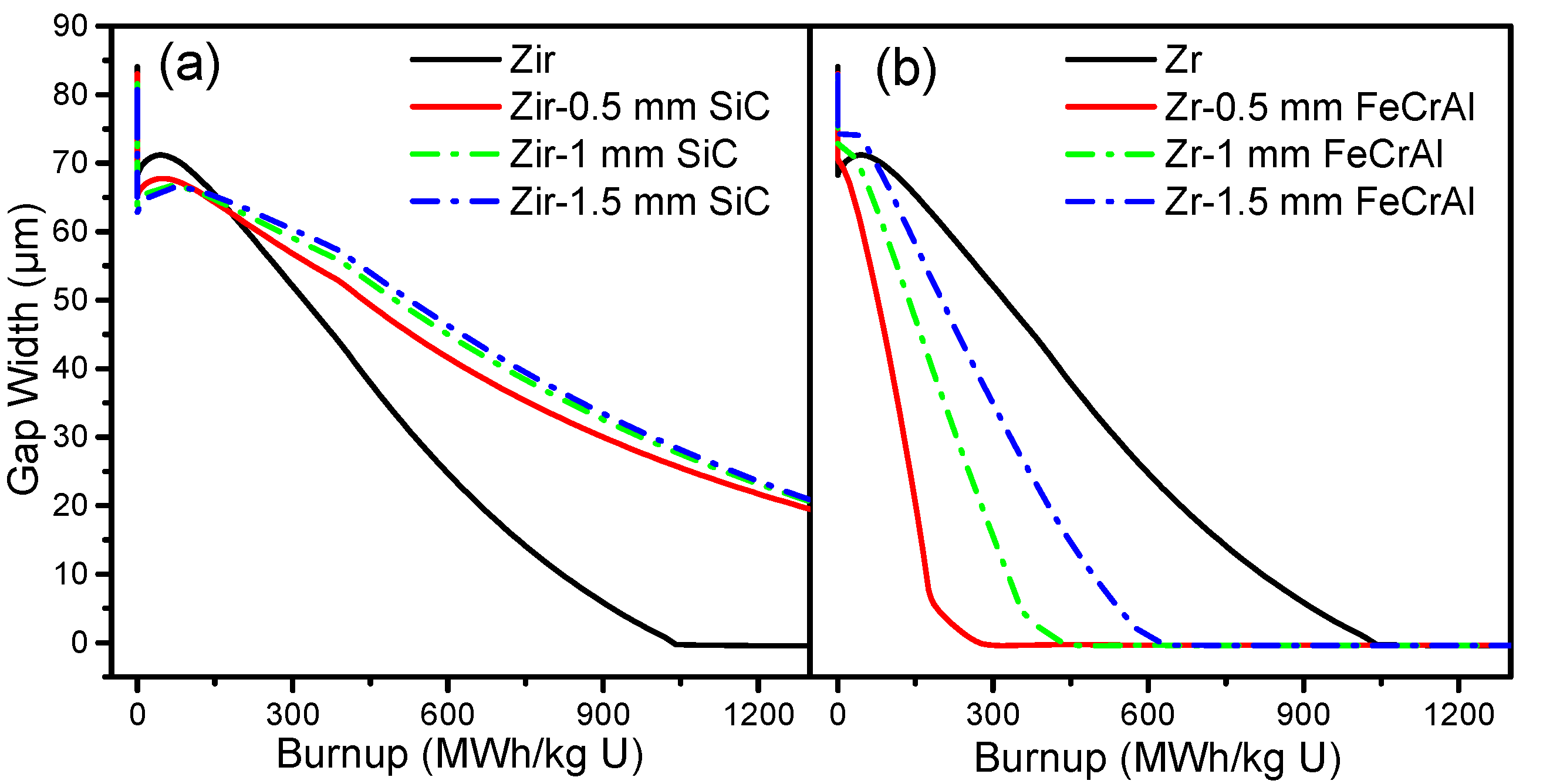
Figure 7 shows the gap size evolutions of Zircaloy, Zircaloy coated with 0.5, 1 and 1.5 mm SiC claddings, and Zircaloy coated with 0.5, 1 and 1.5 mm FeCrAl claddings, respectively. For the SiC-coated Zircaloy claddings, the gap size is smaller than pure Zircaloy claddings before the burnup reaches 250 MWh/kg U, but then tends to be larger than Zircaloy claddings as the burnup rates increase. The gap sizes of Zircaloy coated with 1 and 1.5 mm SiC claddings have almost the same gap size evolution, while Zircaloy coated with 0.5 mm SiC cladding shows a smaller gap size at the same burnup rates. For the FeCrAl-coated Zircaloy claddings, all of the gap sizes are smaller than for the pure Zircaloy cladding and are closed before the burnup reaches 650 MWh/kg U. As the coating thickness of FeCrAl increases, the gap size increases at the same burnup rate. The gap size is affected by plenum pressure, fission gas release, and other factors, as demonstrated in our previous work [
19].
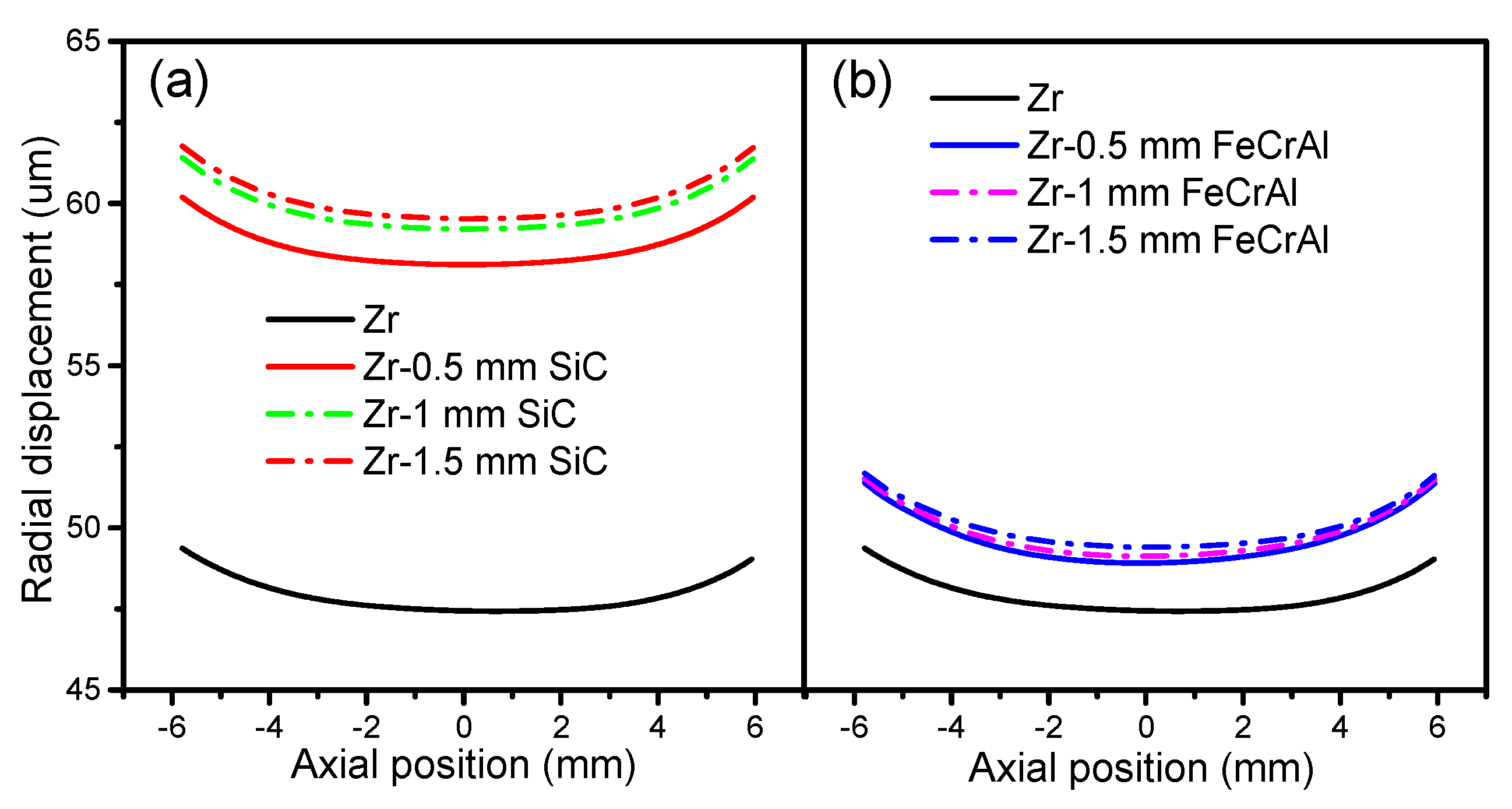
Figure 8 presents the fuel radial displacement evolutions of Zircaloy, Zircaloy coated with 0.5, 1 and 1.5 mm SiC claddings, and Zircaloy coated with 0.5, 1 and 1.5 mm FeCrAl claddings at a burnup of 1200 MWh/kg U. For both the SiC- and FeCrAl-coated claddings, the fuel radial displacement increases as the thickness increases. However, the SiC-coated claddings show more obvious differences in fuel radial displacement at a burnup of 1200 MWh/kg U. The coating causes larger fuel radial displacements than pure Zircaloy when examined at the same axial position as SiC- and FeCrAl-coated claddings, which have higher fuel centerline temperatures than pure Zircaloy. The SiC-coated claddings have more influence on fuel radial displacement than the FeCrAl-coated claddings, as the SiC-coated claddings have higher fuel centerline temperatures.
Figure 9 plots the fuel axial displacement evolutions of Zircaloy, Zircaloy coated with 0.5, 1 and 1.5 mm SiC claddings, and Zircaloy coated with 0.5, 1 and 1.5 mm FeCrAl claddings, at a burnup of 1200 MWh/kg U. This shows that the coatings result in more fuel axial displacement overall than the pure Zircaloy cladding when considered at the same burnup rate, and that the SiC-coated Zircaloy claddings experience more axial displacement than the FeCrAl-coated Zircaloy due to its higher fuel centerline temperature. However, no significant difference in fuel axial displacement can be observed with different coating thicknesses.
Figure 10 depicts the cladding radial displacement evolutions of the inner and outer surfaces of Zircaloy, Zircaloy coated with 0.5, 1 and 1.5 mm SiC claddings, and Zircaloy coated with 0.5, 1 and 1.5 mm FeCrAl claddings, at a burnup of 1200 MWh/kg U. The SiC-coated claddings show little radial displacement, while the FeCrAl-coated claddings show large displacements toward the fuel direction. The difference between the displacements of the FeCrAl-coated cladding on the inner and outer surfaces is more obvious than those of the pure Zircaloy cladding. More radial displacements occur at the extremities for the FeCrAl-coated claddings.
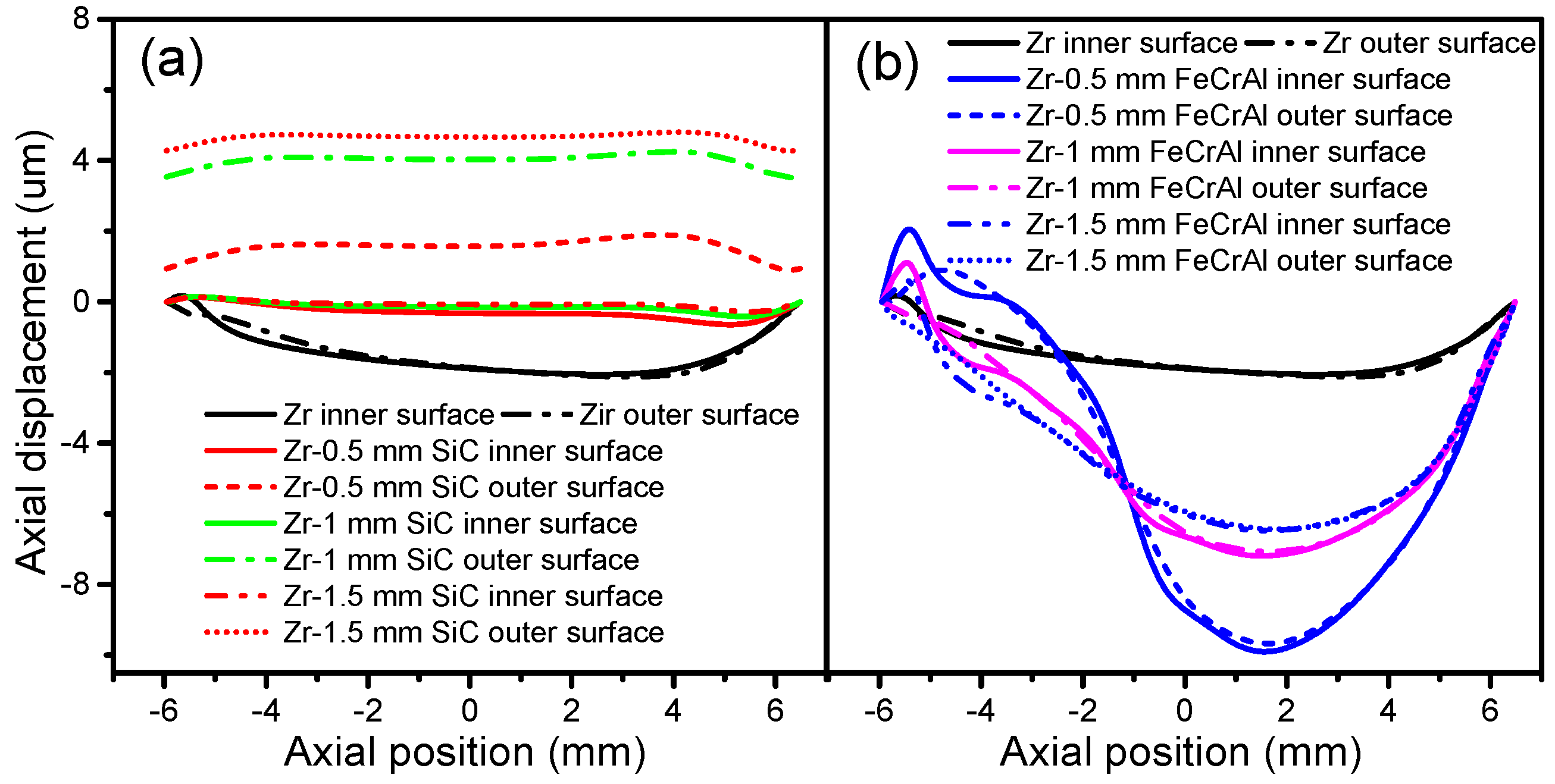
Figure 11 shows the cladding axial displacement evolutions of Zircaloy, Zircaloy coated with 0.5, 1 and 1.5 mm SiC claddings and Zircaloy coated with 0.5, 1 and 1.5 mm FeCrAl claddings, at a burnup of 1200 MWh/kg U. The SiC-coated claddings swell in the axial direction, while the Zircaloy and FeCrAl-coated claddings shrink axially. The axial expansions of the SiC-coated claddings increase as the coating thickness increases. For the FeCrAl-coated claddings, the axial displacement changes dramatically as the axial position changes. At axial point 0.015 m, the largest displacement occurs for 0.5, 1 and 1.5 mm FeCrAl-coated claddings. From 0 to −6 mm, Zircaloy coated with 0.5 mm FeCrAl cladding has more displacement than the thicker 1 and 1.5 mm FeCrAl claddings.