Determination of Three-Dimensional Morphology and Inner Structure of Second-Phase Inclusions in Metals by Non-Aqueous Solution Electrolytic and Room Temperature Organic Methods
Abstract
:1. Introduction
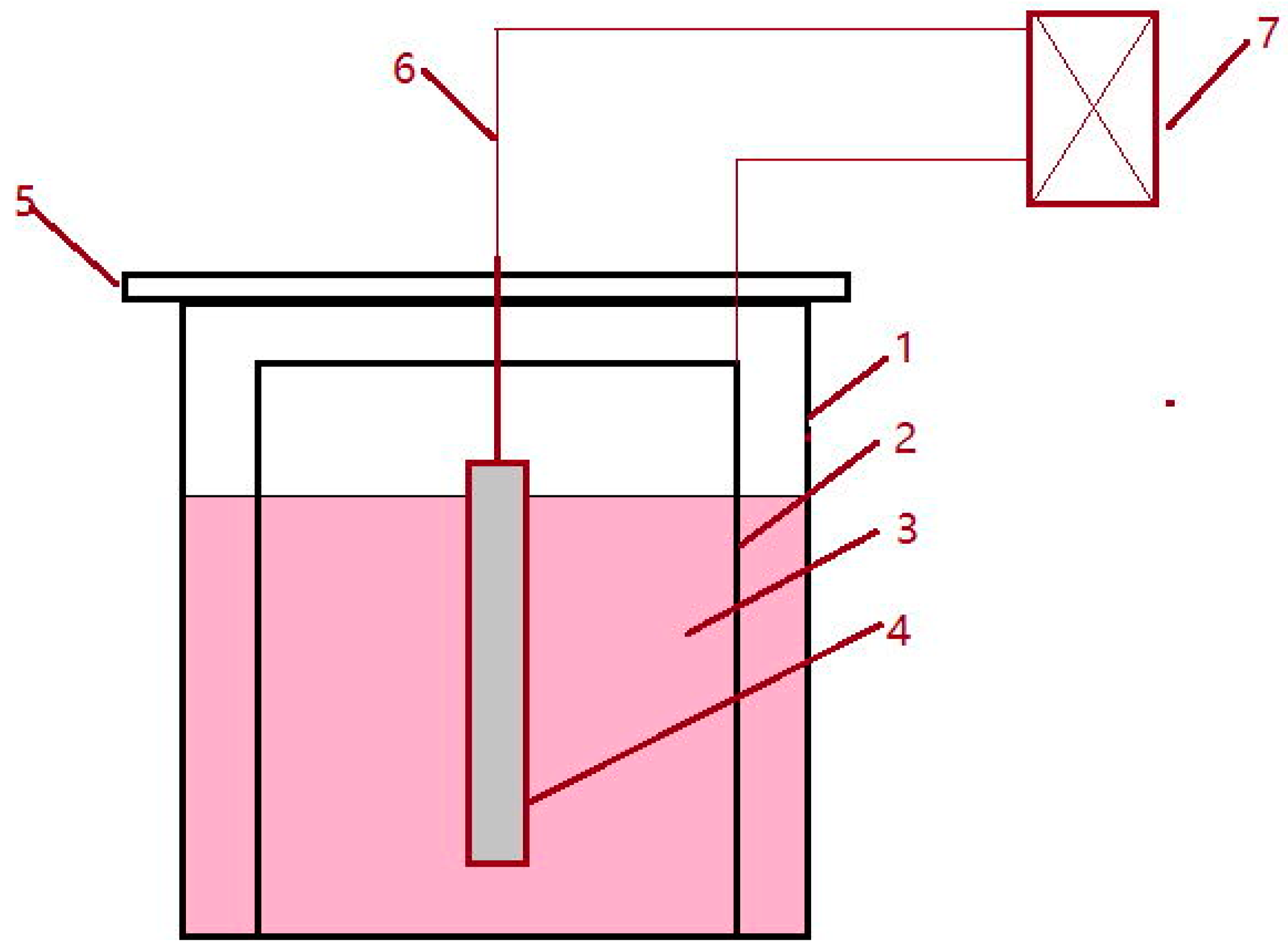
2. Experimental Methods
3. Results and Discussions
4. Conclusions
- (1)
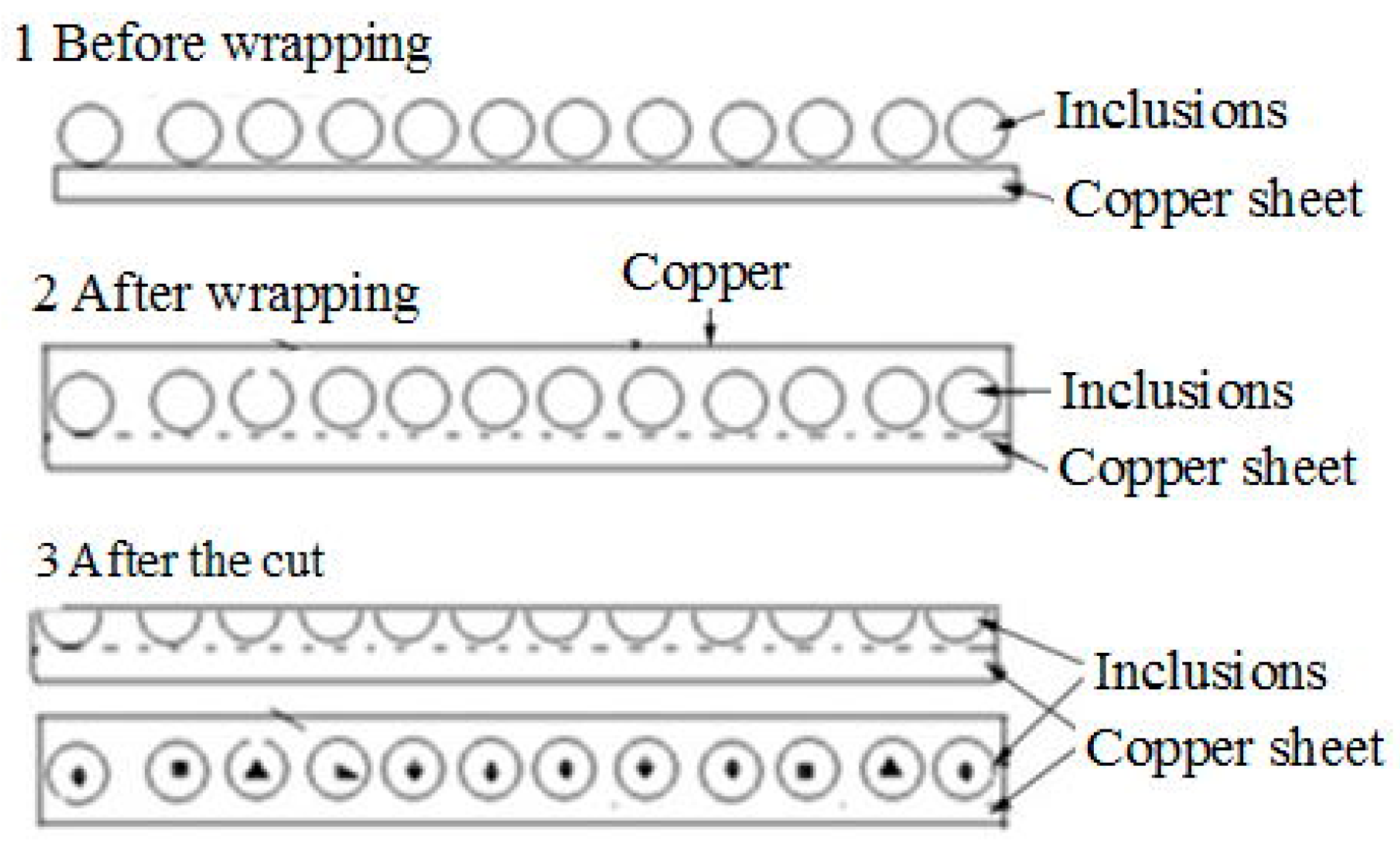
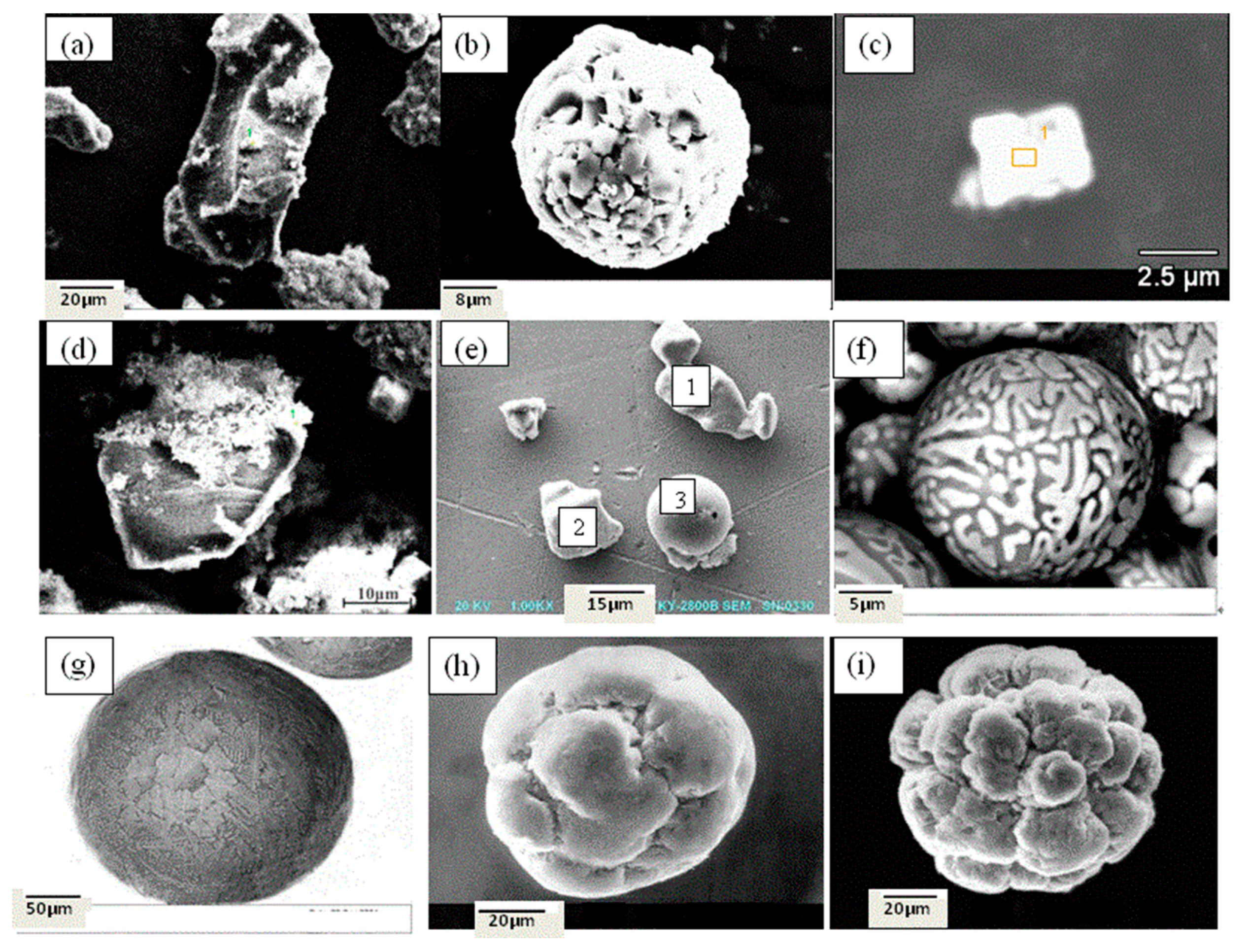
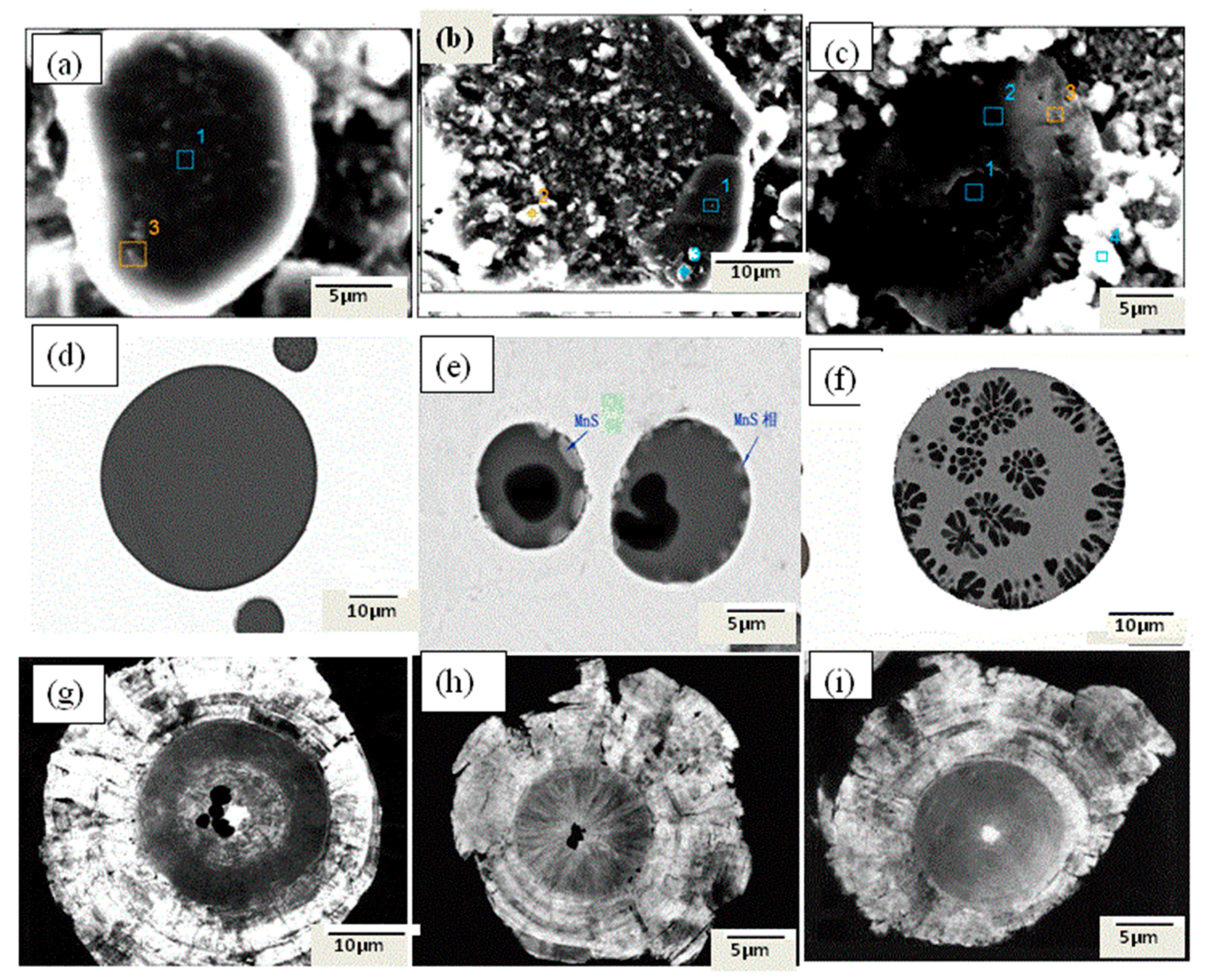
- The inclusions in Al-killed steel, Si-killed steel, and ductile cast iron can be extracted without destruction of their morphology and chemical composition, and their three-dimensional morphology and surface structure can be detected by SEM or FESEM. A RTO technique can be applied to cut the extracted inclusions and obtain more information regarding their inner structures.
- (2)
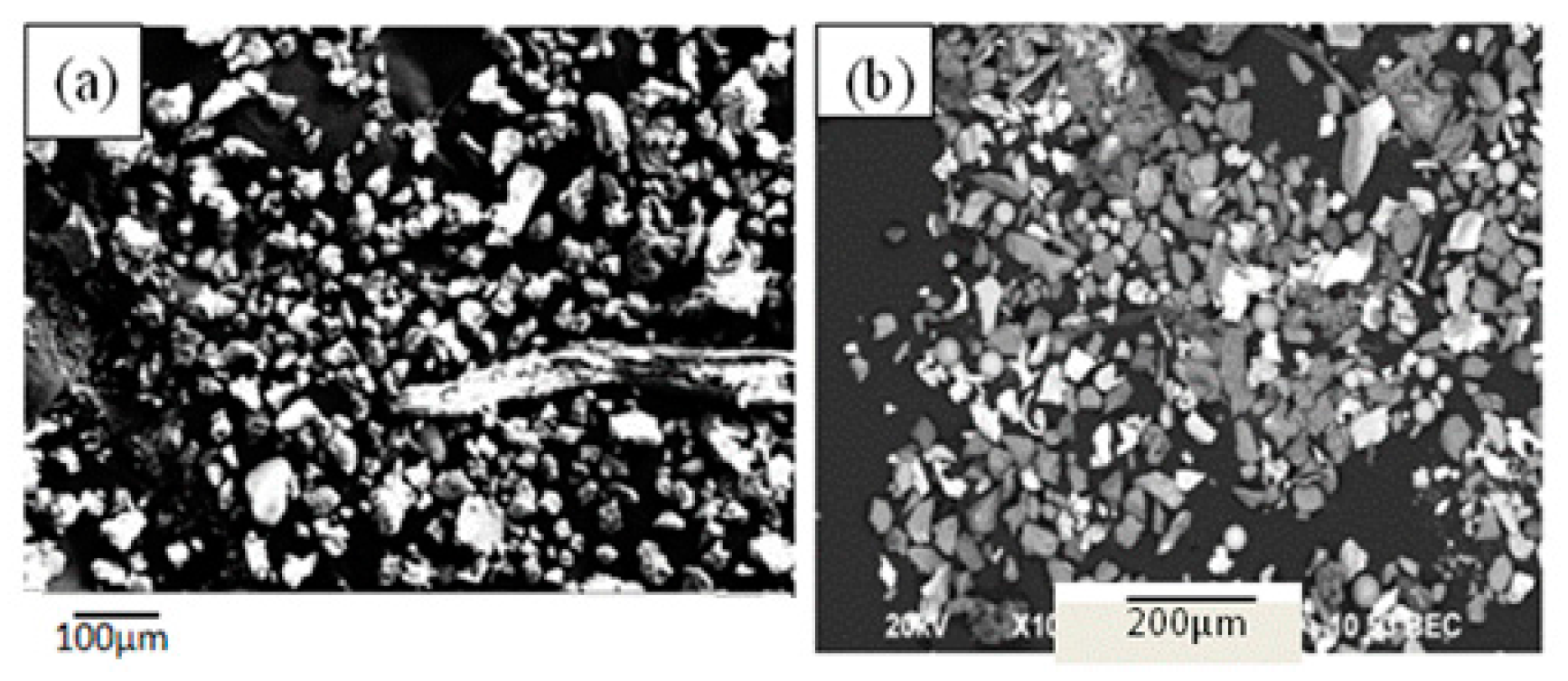
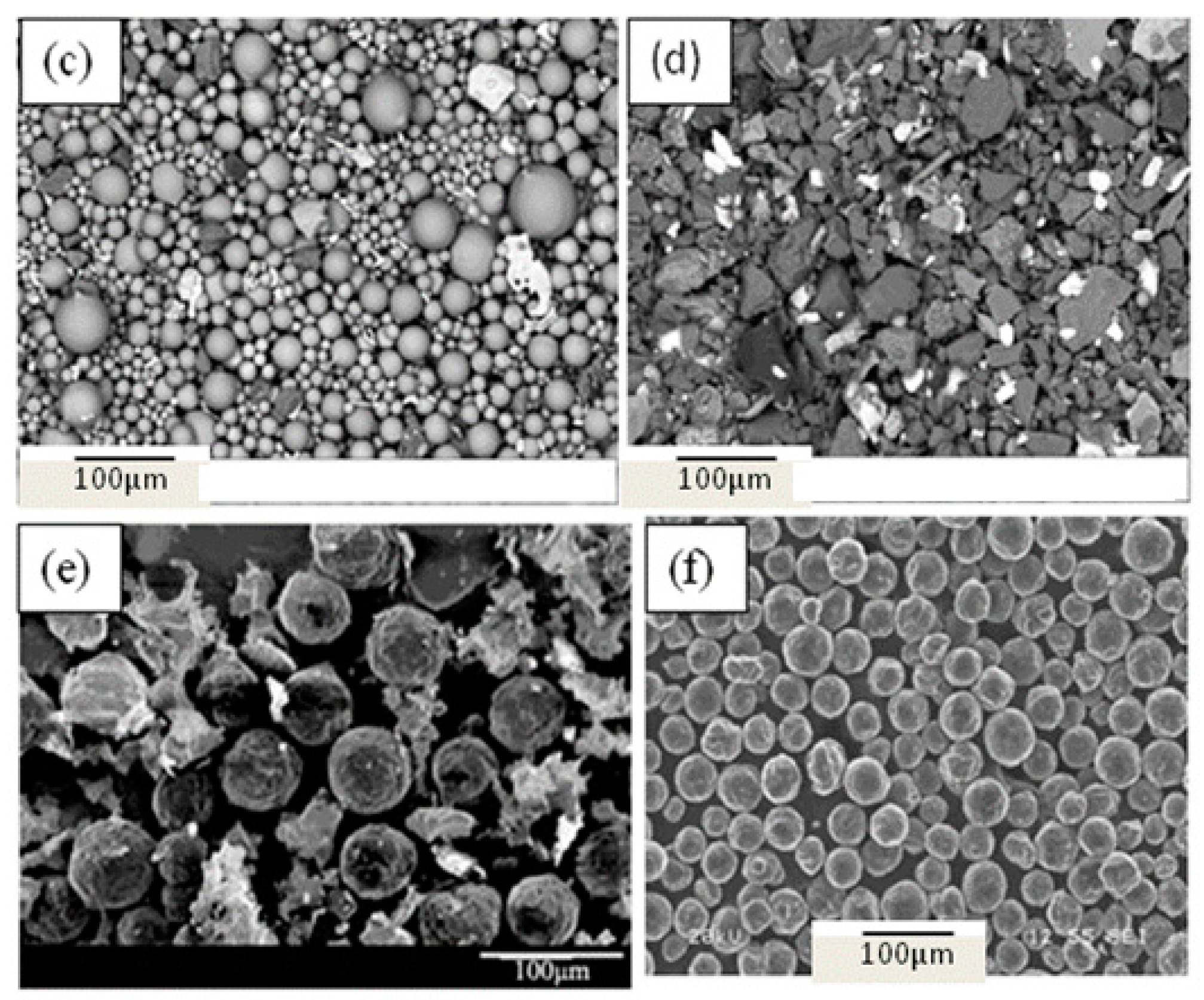
- The majority of the inclusions in Al-killed steel slab had an irregular morphology, those in the Si-killed steel were mainly spherical, and almost all the spheroidal graphite in the ductile cast iron featured a uniform globular morphology.
- (3)
- When the inclusions were cut by the RTO technique, nucleation was not observed in the inner structures of Al-killed steel. For silicate inclusions, some dendritic or rod-like MnS phase precipitates appeared on their surfaces, and some silicate-rich phases were detected in their inner matrix. For spheroidal graphite, rare-earth oxides (one particle or more) were observed in the center of almost every graphite particle as nuclei.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Ende, M.-A.; Guo, M.X.; Proost, J.; Blanpain, B.; Wollants, P. Formation and morphology of Al2O3 inclusions at the onset of liquid Fe deoxidation by Al addition. ISIJ Int. 2011, 51, 27–34. [Google Scholar] [CrossRef]
- Van Ende, M.-A.; Guo, M.X.; Zinngrebe, E.; Dekkers, R.; Proost, J.; Blanpain, B.; Wollants, P. Morphology and growth of alumina inclusions in Fe–Al alloys at low oxygen partial pressure. Ironmak. Steelmak. 2009, 36, 201–208. [Google Scholar] [CrossRef]
- Fang, K.M.; Nan, R.M. Research on determination of the rare-earth content in metal phases of steel. Metall. Mater. Trans. A 1986, 17, 315–323. [Google Scholar]
- Chen, X.C.; Shi, C.B.; Guo, H.J.; Wan, F.; Ren, H. Investigation of oxide inclusions and primary carbonitrides in Inconel 718 superalloy refined through electroslag remelting process. Metall. Mater. Trans. B 2012, 43, 1596–1607. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, L.F.; Yang, W.; Wang, Y.; Liu, Y.; Dong, Y.C. Characterization of the Three-Dimensional Morphology and Formation Mechanism of Inclusions in Linepipe Steels. Metall. Mater. Trans. B 2017, 48, 701–712. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, H.G.; Oh, K.-S. MnS precipitation in association with manganese silicate inclusions in Si/Mn deoxidized steel. Metall. Mater. Trans. A 2001, 32, 1519–1525. [Google Scholar] [CrossRef]
- Wang, K.P.; Jiang, M.; Wang, X.H.; Wang, Y.; Zhao, H.Q.; Cao, Z.M. Formation Mechanism of SiO2-Type Inclusions in Si-Mn-Killed Steel Wires Containing Limited Aluminum Content. Metall. Mater. Trans. B 2015, 46, 2198–2207. [Google Scholar] [CrossRef]
| Method | Functions | Anode | Cathode | Electrolyte Solution | Electric Current |
|---|---|---|---|---|---|
| N-S electrolytic | To extract inclusion | Specimen | Stainless steel tube | Organic solution | <200 mA/cm2 |
| RTO | To wrap and cut inclusion | Copper plate | Copper plate | Organic solution | <500 mA |
| Steel Grade | C | Si | Mn | P | S | Al | Ti | Cr | Ni | Nb | RE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.004 | 0.02 | 0.15 | 0.01 | 0.005 | 0.04 | 0.06 | - | - | - | - |
| B | 0.045 | 0.046 | 0.204 | 0.0097 | 0.006 | 0.041 | - | - | - | - | - |
| C | 0.10 | 0.35 | 1.48 | 0.017 | 0.007 | - | - | - | - | 0.01 | - |
| D | 0.05 | 0.50 | 1.10 | 0.011 | 0.003 | - | - | 18.22 | 8.10 | - | - |
| E | 4..20 | 1.07 | 0.20 | 0.005 | 0.003 | - | - | - | - | - | 9.32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Fang, K.; Guo, H.; Luo, Y.; Duan, S.; Shi, X.; Yang, W. Determination of Three-Dimensional Morphology and Inner Structure of Second-Phase Inclusions in Metals by Non-Aqueous Solution Electrolytic and Room Temperature Organic Methods. Metals 2018, 8, 68. https://doi.org/10.3390/met8010068
Guo J, Fang K, Guo H, Luo Y, Duan S, Shi X, Yang W. Determination of Three-Dimensional Morphology and Inner Structure of Second-Phase Inclusions in Metals by Non-Aqueous Solution Electrolytic and Room Temperature Organic Methods. Metals. 2018; 8(1):68. https://doi.org/10.3390/met8010068
Chicago/Turabian StyleGuo, Jing, Keming Fang, Hanjie Guo, Yiwa Luo, Shengchao Duan, Xiao Shi, and Wensheng Yang. 2018. "Determination of Three-Dimensional Morphology and Inner Structure of Second-Phase Inclusions in Metals by Non-Aqueous Solution Electrolytic and Room Temperature Organic Methods" Metals 8, no. 1: 68. https://doi.org/10.3390/met8010068




