Thermodynamic Alloy Design of High Strength and Toughness in 300 mm Thick Pressure Vessel Wall of 1.25Cr-0.5Mo Steel
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
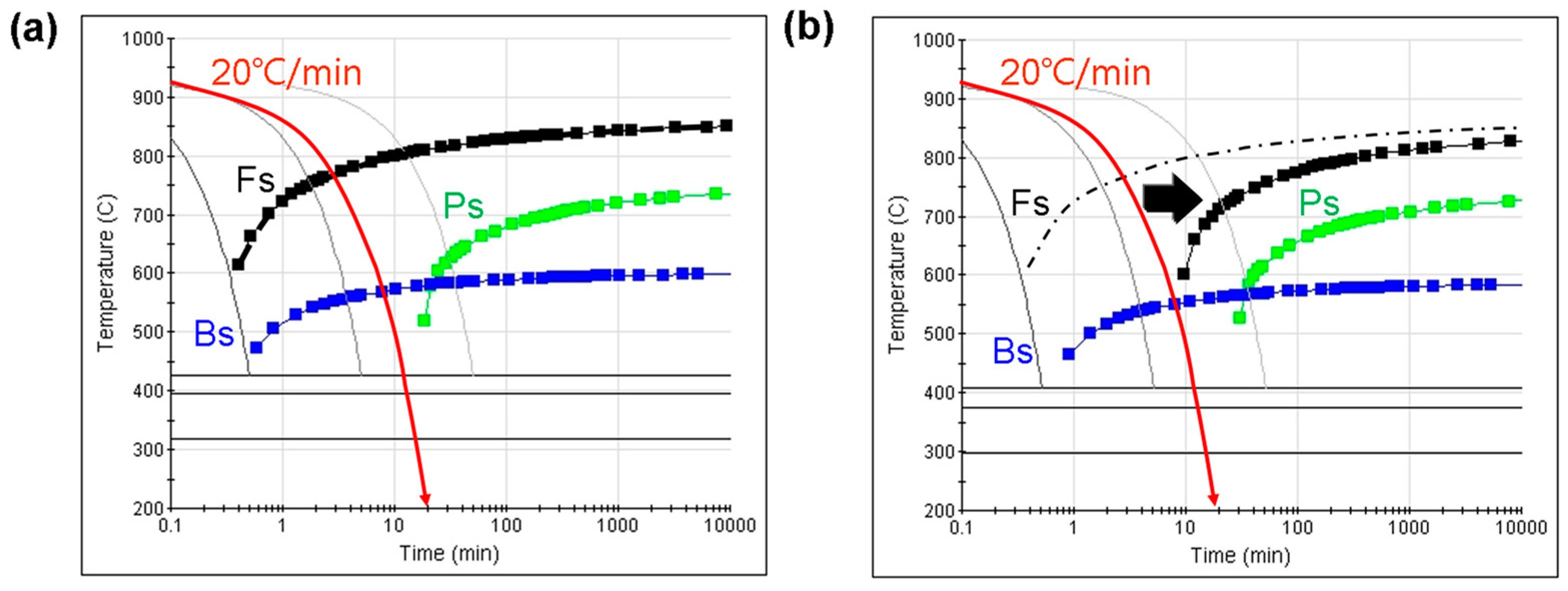
3.1. Problems from the External and Central Cooling Rates
3.2. Hardenability Design Using Thermodynamic Calculation (JMatPro)
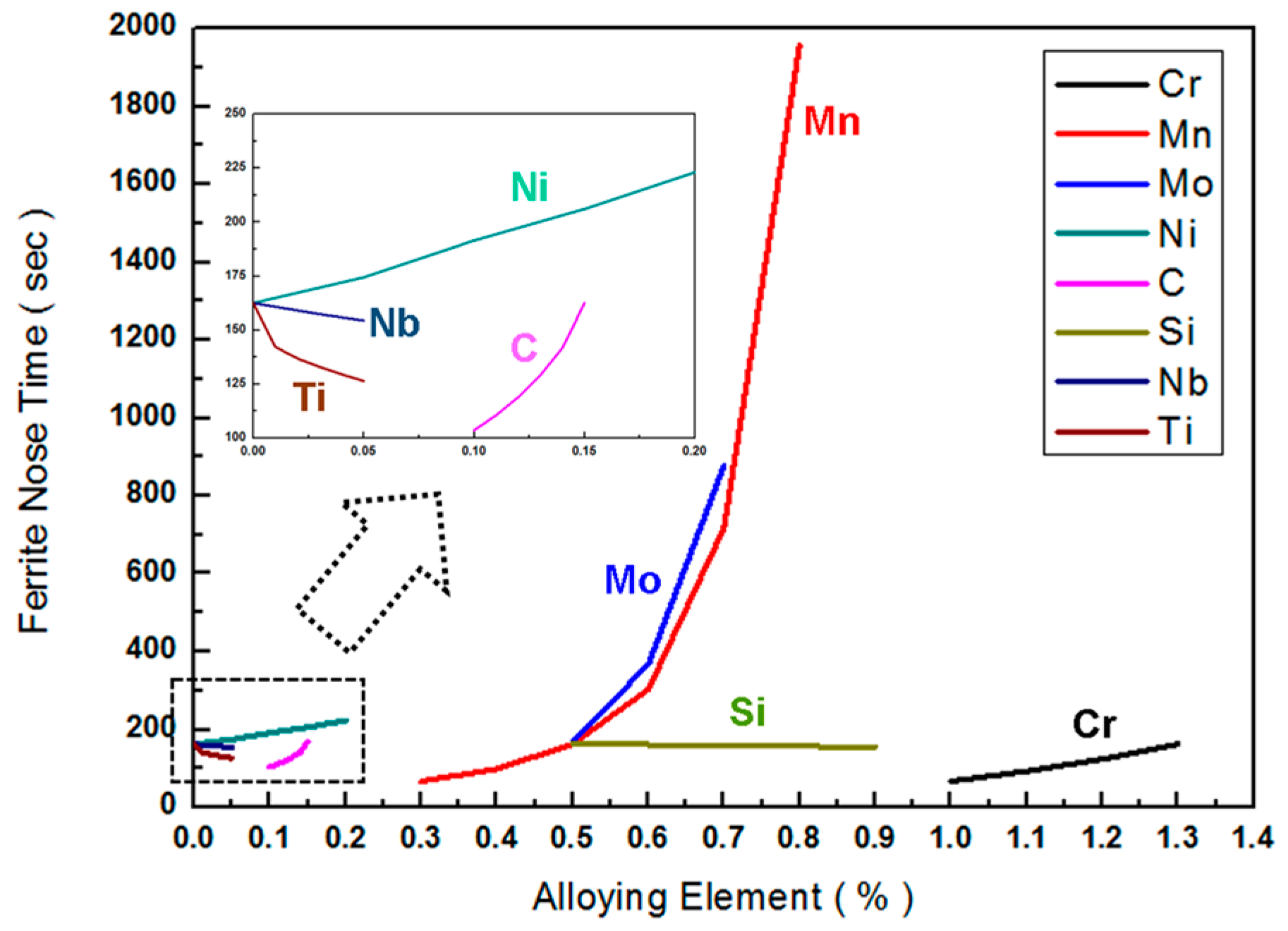
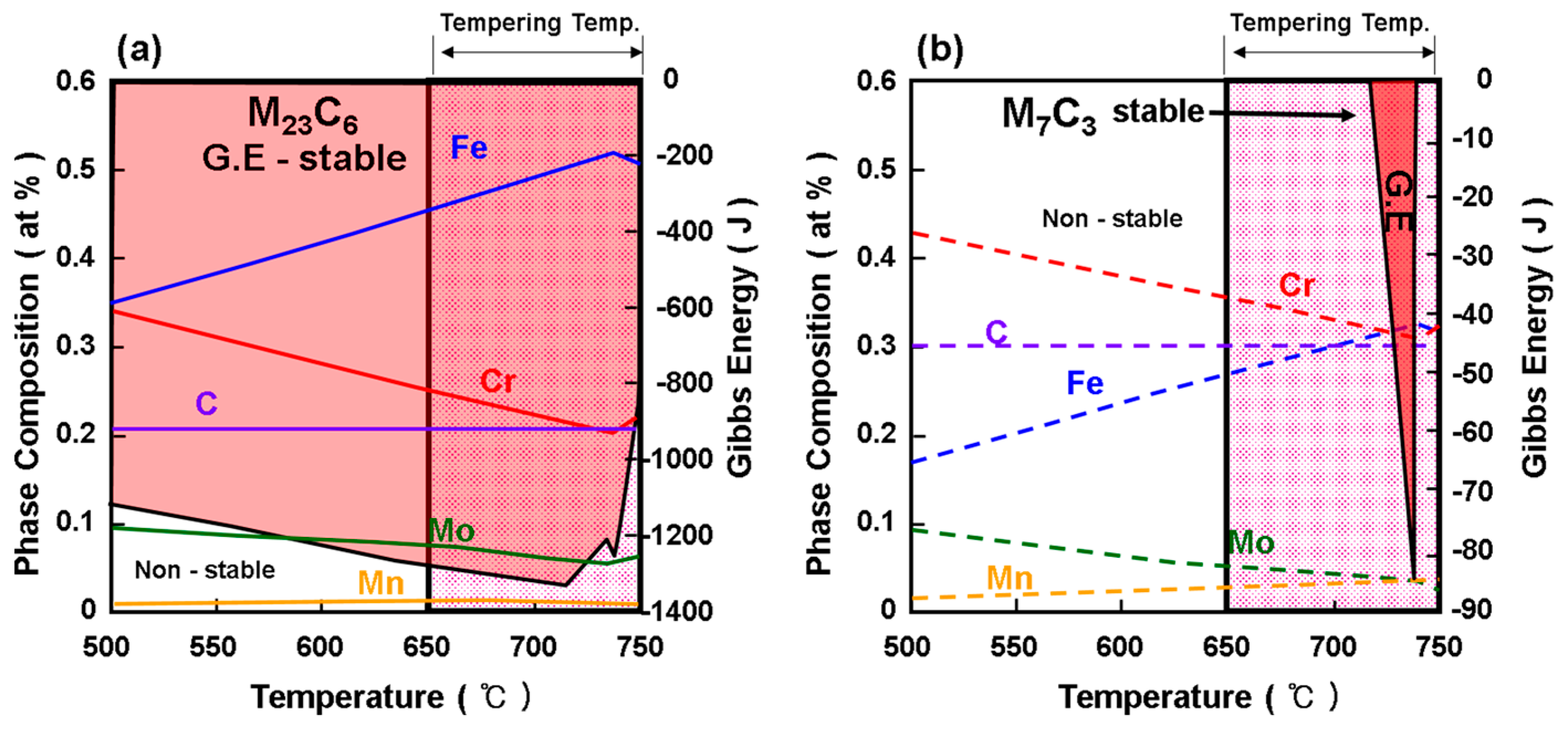
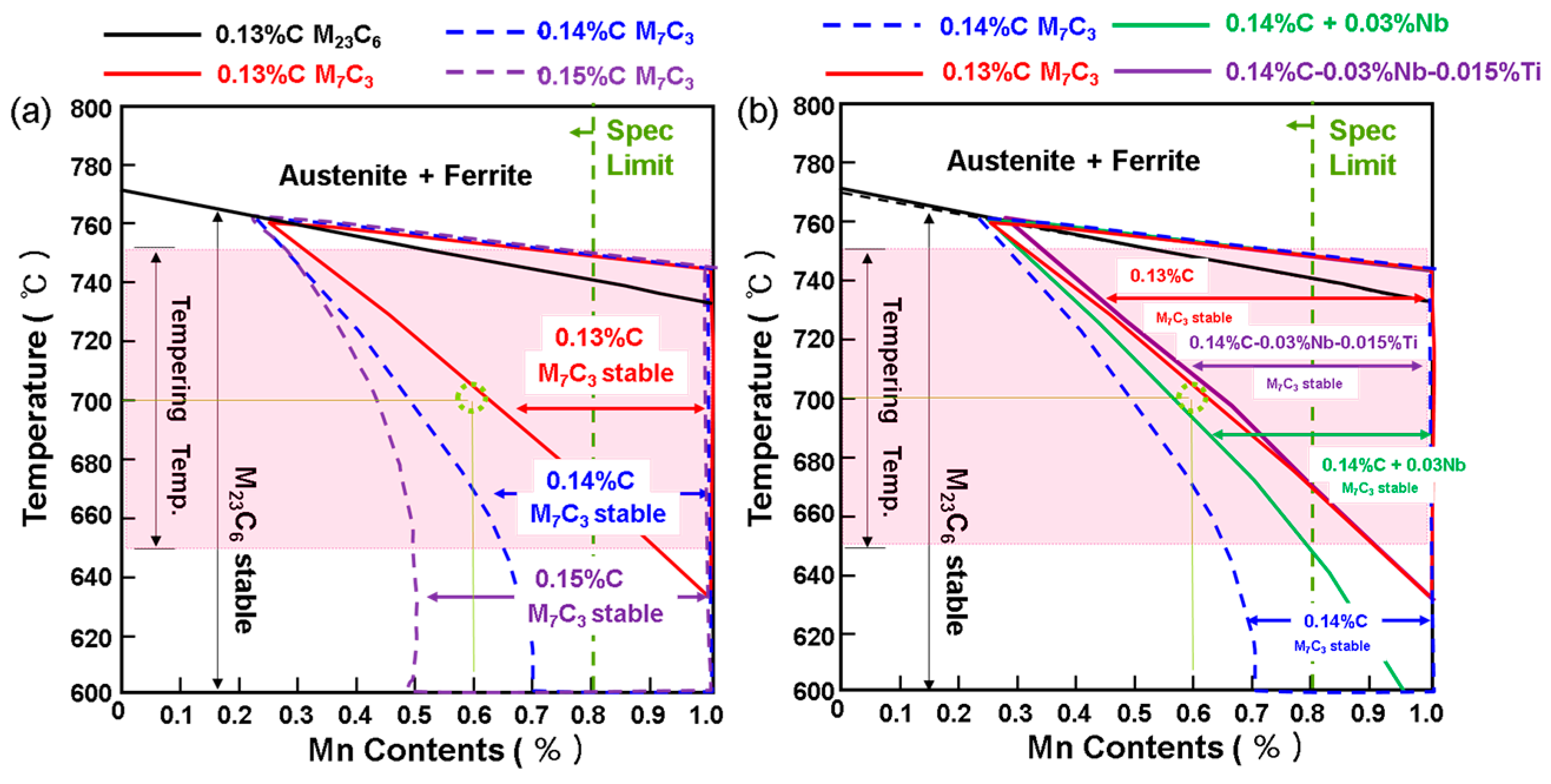
3.3. Strengthening Design by Grain Refinement and Hardening Using Thermodynamic Calculations (Thermo-Calc)
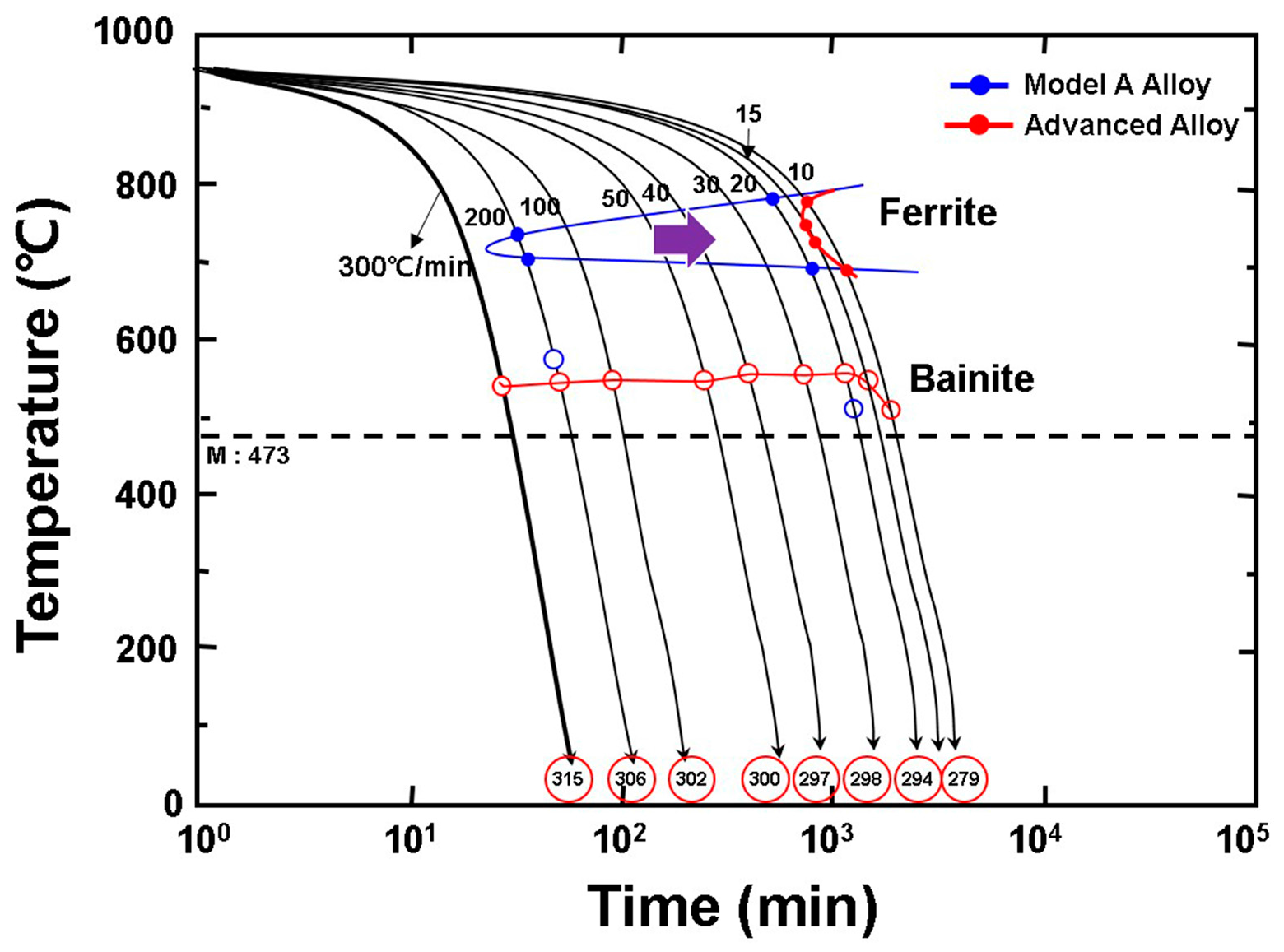
3.4. Hardenability and Mechanical Properties of Advanced Alloy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McMullan, J.T.; Williams, B.C.; Sloan, E.P. Clean coal technologies. Proc. Inst. Mech. Eng. Part A J. Power Energy 1997, 211, 95–107. [Google Scholar] [CrossRef]
- Guillermo, O.-G.; Douglas, P.; Croiset, E.; Zheng, L. Technoeconomic evaluation of IGCC power plants for CO2 avoidance. Energy Convers. Manag. 2006, 47, 2250–2259. [Google Scholar]
- Descamps, C.; Bouallou, C.; Kanniche, M. Efficiency of an integrated gasification combined cycle (IGCC) power plant including CO2 removal. Energy 2008, 33, 874–881. [Google Scholar] [CrossRef]
- Wang, T. An overview of IGCC systems. Integr. Gasif. Comb. Cycle Technol. 2017, 1–80. [Google Scholar] [CrossRef]
- Barnes, I. Recent Operating Experience and Improvement of Commercial IGCC; IEA Clean Coal Center: London, UK, 2013; pp. 8–11. [Google Scholar]
- Brust, F.W.; Dong, P. Welding residual stresses and effects on fracture in pressure vessel and piping components: A millennium review and beyond. J. Press. Vessel Technol. 2000, 122, 329–338. [Google Scholar]
- Bouchard, P.J.; Withers, P.J.; McDonald, S.A.; Heenan, R.K. Quantification of creep cavitation damage around a crack in a stainless steel pressure vessel. Acta Mater. 2004, 52, 23–34. [Google Scholar] [CrossRef]
- Brust, F.W.; Paul, M.S. Weld residual stresses and primary water stress corrosion cracking in bimetal nuclear pipe welds. In Proceedings of the ASME 2007 Pressure Vessels and Piping Conference, San Antonio, TX, USA, 22–26 July 2007. [Google Scholar]
- Davis, J.R. (Ed.) ASM Specialty Handbook: Heat-Resistant Materials; ASM International: Geauga, OH, USA, 1997; pp. 89–122. [Google Scholar]
- Das, S.K.; Joarder, A.; Mitra, A. Magnetic Barkhausen emissions and microstructural degradation study in 1.25 Cr-0.50 Mo steel during high temperature exposure. NDT E Int. 2004, 37, 243–248. [Google Scholar] [CrossRef]
- Mitchell, D.R.G.; Moss, C.J.; Griffiths, R.R. Optimisation of post-weld heat treatment of a 1.25 Cr-0.5 Mo pressure vessel for high temperature hydrogen service. Int. J. Press. Vessels Pip. 1999, 76, 259–266. [Google Scholar] [CrossRef]
- Pickering, F.B. Physical Metallurgy and the Design of Steels; Applied Science Publishers: Rotherham, UK, 1978. [Google Scholar]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strength bainitic steels: Part 2. Mater. Sci. Technol. 2001, 17, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Grajcar, A.; Zalecki, W.; Burian, W.; Kozłowska, A. Phase Equilibrium and Austenite Decomposition in Advanced High-Strength Medium-MnBainitic Steels. Metals 2016, 6, 248. [Google Scholar] [CrossRef]
- Wang, H.-S.; Hsieh, P.-J. Establishment of Heat Treatment Process for Modified 440A Martensitic Stainless Steel Using Differential Scanning Calorimetry and Thermo-Calc Calculation. Metals 2016, 6, 4. [Google Scholar] [CrossRef]
- Knežević, V.; Balun, J.; Sauthoff, G.; Inden, G.; Schneider, A. Design of martensitic/ferritic heat-resistant steels for application at 650 C with supporting thermodynamic modelling. Mater. Sci. Eng. A 2008, 477, 334–343. [Google Scholar] [CrossRef]
- Haidemenopoulos, G.N.; Grujicic, M.; Olson, G.B.; Cohen, M. Thermodynamics-based alloy design criteria for austenite stabilization and transformation toughening in the Fe Ni Co system. J. Alloys Compd. 1995, 220, 142–147. [Google Scholar] [CrossRef]
- Saunders, N.; Guo, U.K.Z.; Li, X.; Miodownik, A.P.; Schillé, J.P. Using JMatPro to model materials properties and behavior. JOM 2003, 55, 60–65. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- Saunders, N.; Guo, Z.; Li, X.; Miodownik, A.P.; Schillé, J.P. The Calculation of TTT and CCT Diagrams for General Steels. JMatPro Software Literature, 2004. Available online: https://www.sentesoftware.co.uk/ (accessed on 5 January 2018).
- Lee, S.; Na, H.; Kim, B.; Kim, D.; Kang, C. Effect of Niobium on the Ferrite Continuous-Cooling-Transformation (CCT) Curve of Ultrahigh-Thickness Cr-Mo Steel. Metall. Mater. Trans. A 2013, 44, 2523–2532. [Google Scholar] [CrossRef]
- Lee, S.-H.; Na, H.S.; Park, G.D.; Kim, B.H.; Song, S.W.; Kang, C.Y. Effects of titanium on ferrite continuous cooling transformation curves of high-thickness Cr-Mo steels. Met. Mater. Int. 2013, 19, 907. [Google Scholar] [CrossRef]
- Shinozaki, T.; Komura, T.; Fujitsuna, N.; Nakashima, H.; Yamada, M.; Nakanishi, T. Fabrication and Properties of Forged Rings MADE of Modified 9Cr-1Mo-V Steel for High-Temperature and High-Pressure Reactor. Kobelco Technol. Rev. 2015, 33, 39–43. [Google Scholar]
- Chipman, J. Thermodynamics and phase diagram of the Fe-C system. Metall. Mater. Trans. B 1972, 3, 55–64. [Google Scholar] [CrossRef]
- Trzaska, J.; Jagiełło, A.; Dobrzański, L.A. The calculation of CCT diagrams for engineering steels. Arch. Sci. Eng. 2009, 39, 13–20. [Google Scholar]
- Naudin, C.; Frund, J.M.; Pineau, A. Intergranular fracture stress and phosphorus grain boundary segregation of a Mn-Ni-Mo steel. Scr. Mater. 1999, 40, 1013–1019. [Google Scholar] [CrossRef]
- Enomoto, M.; Aaronson, H.I. Nucleation kinetics of proeutectoid ferrite at austenite grain boundaries in Fe-CX alloys. Metall. Mater. Trans. A 1986, 17, 1385–1397. [Google Scholar] [CrossRef]
- Kowalski, M.; Spencer, P.J.; Granat, K.; Drzeniek, H.; Lugscheider, E. Phase relations in the C-Cr-Fe system in the vicinity of the/liquid + bcc + M23C6 + M7C3/invariant equilibrium: Experimental determinations and thermodynamic modelling. Z. Metall. 1994, 85, 359–364. [Google Scholar]
- Zhang, Y.; Miao, L.; Wang, X.; Zhang, H.; Li, J. Evolution behavior of carbides in 2.25Cr-1Mo-0.25V steel. Mater. Trans. 2009, 50, 2507–2511. [Google Scholar]


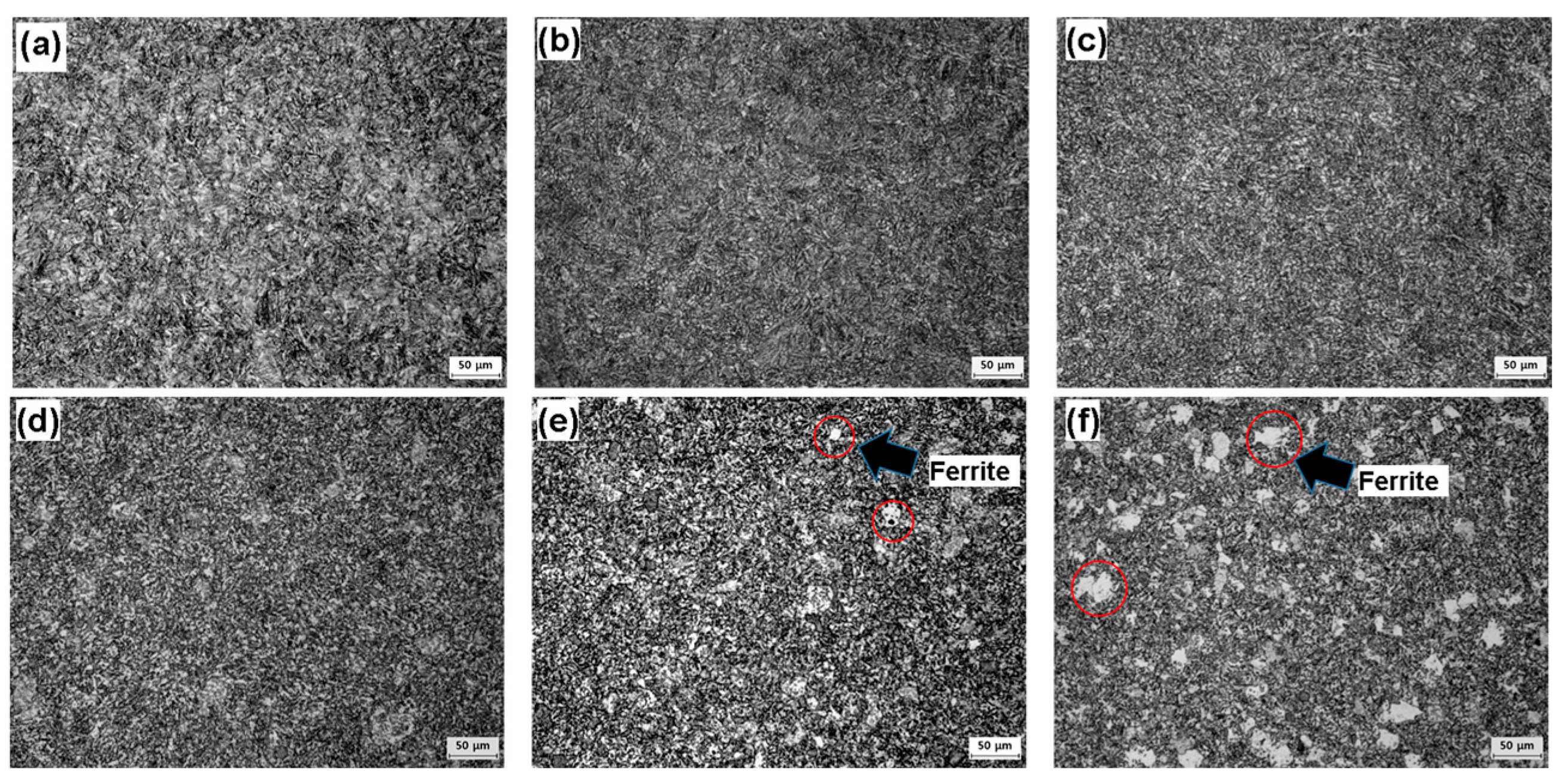
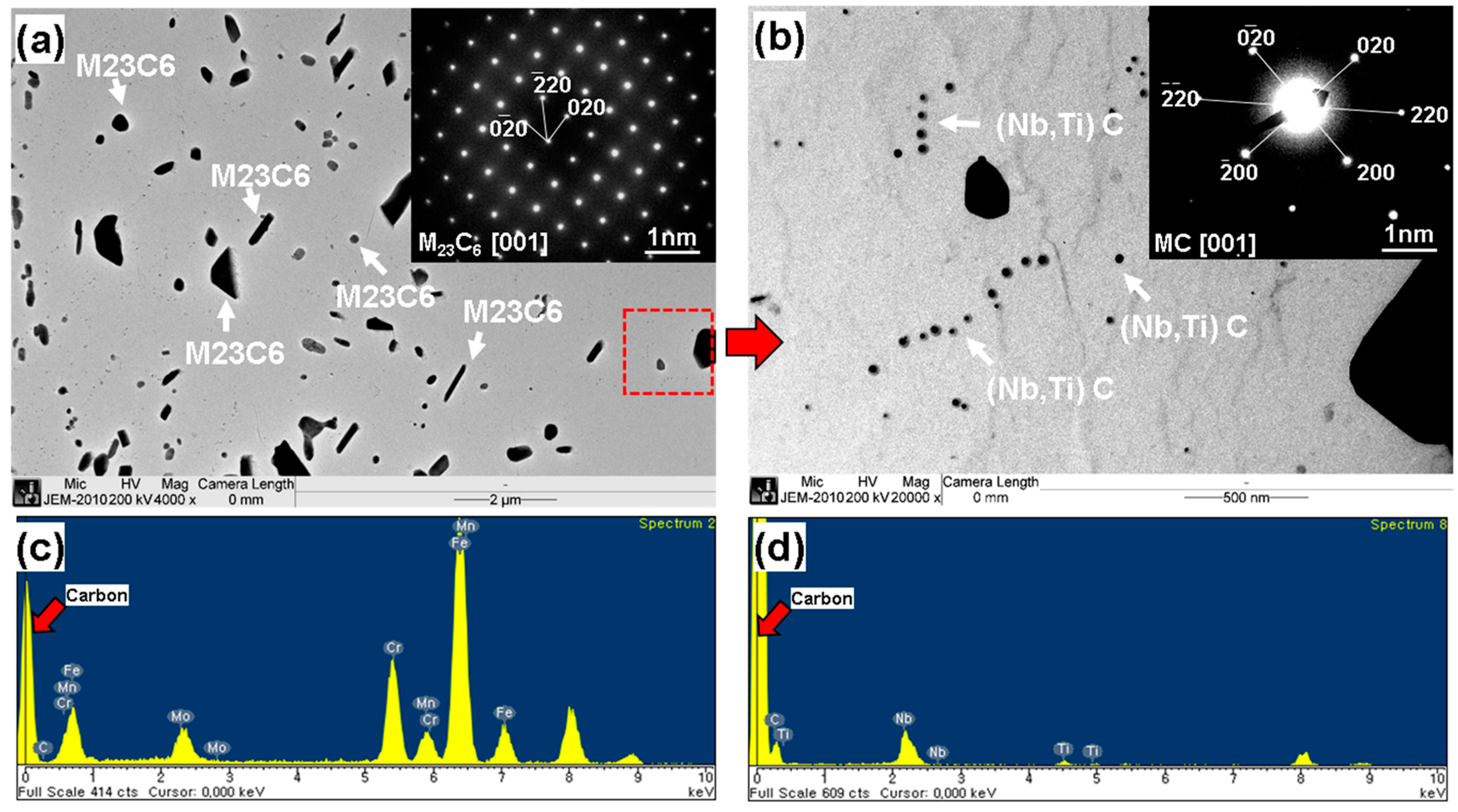
| Chemical Composition (wt %) | C | Cr | Mo | Mn | Si | Ni | Nb | Ti | Etc. |
|---|---|---|---|---|---|---|---|---|---|
| Model A | 0.15 | 1.3 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0 | P: 0.004 S: 0.004 N: 0.005 |
| Addition range | 0.1–0.15 | 1.0–1.3 | 0.5–0.7 | 0.3–0.8 | 0.5–0.9 | 0–0.2 | 0–0.05 | 0–0.05 |
| Alloying | Range | Equation | R2 |
|---|---|---|---|
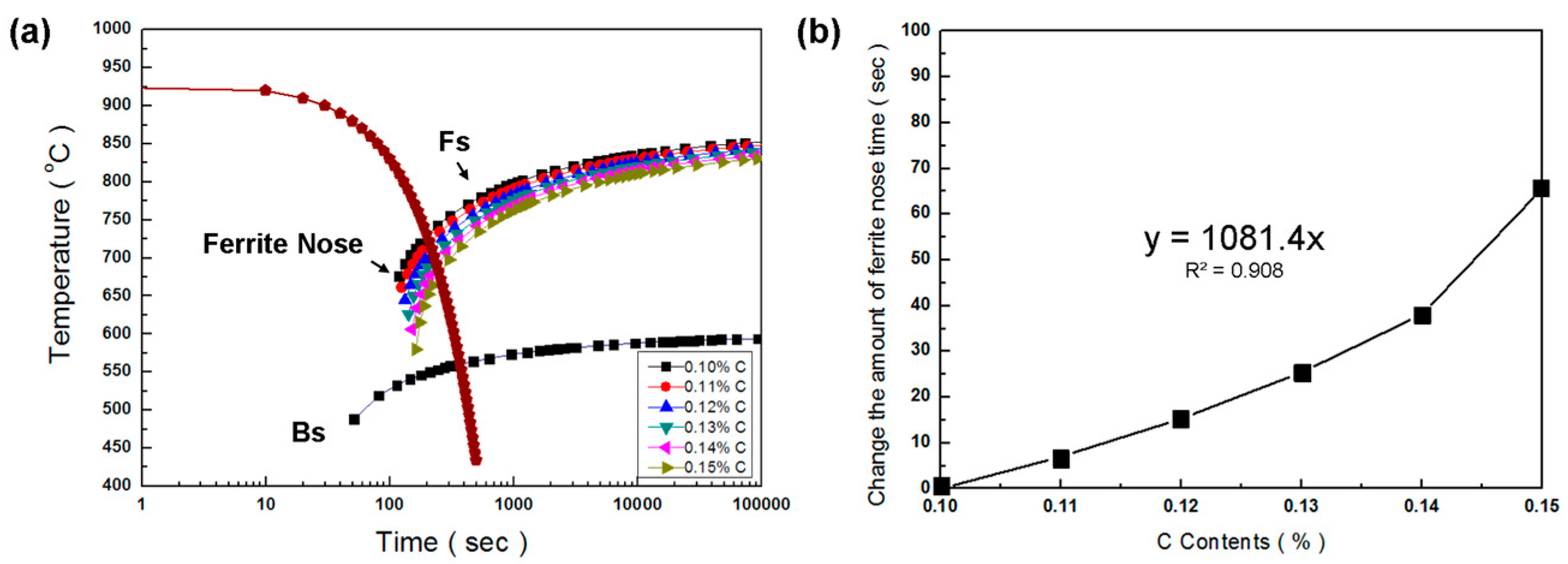
| C | (0.1–0.15) | y = 1081.4x | 0.908 |
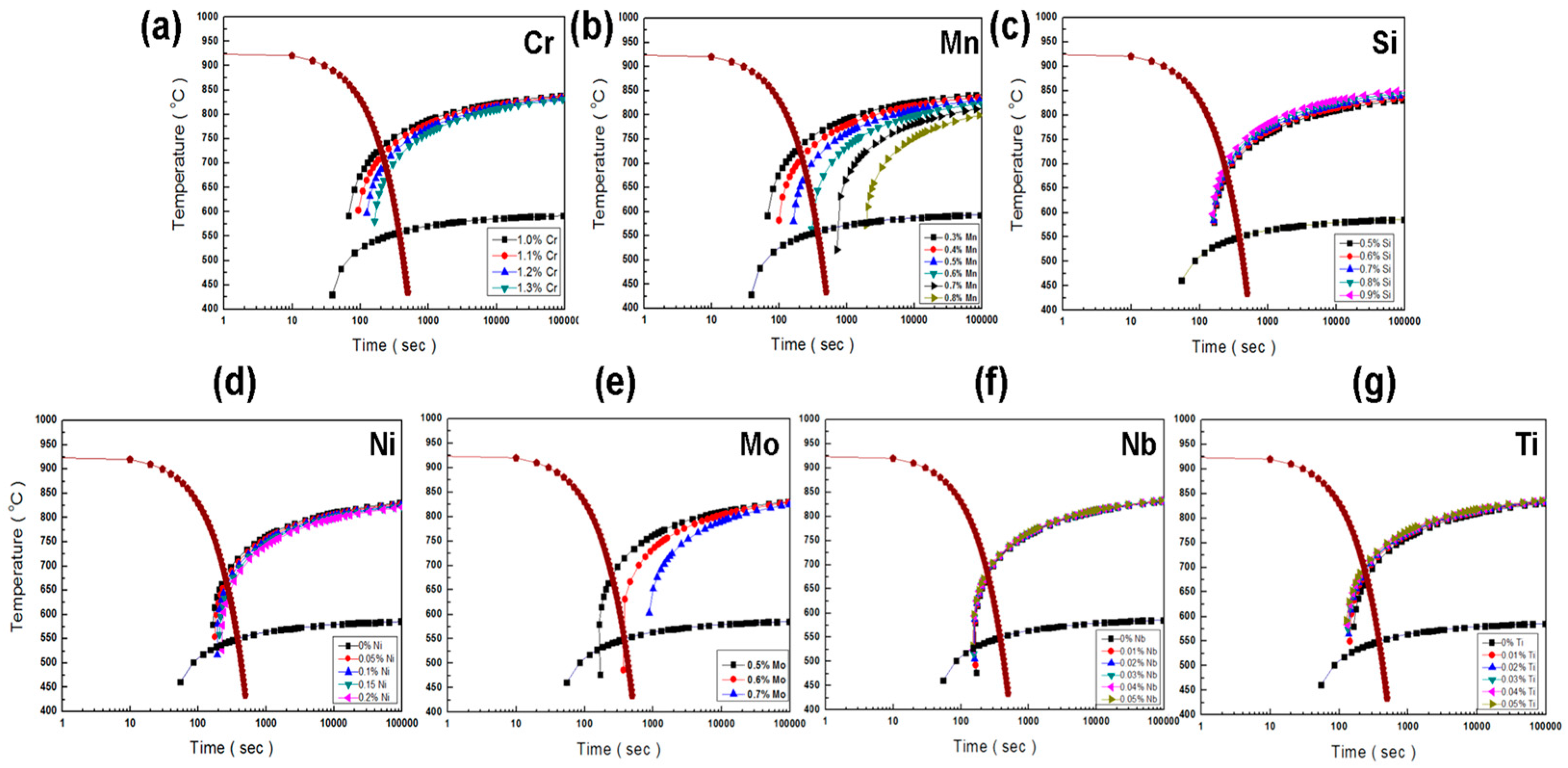
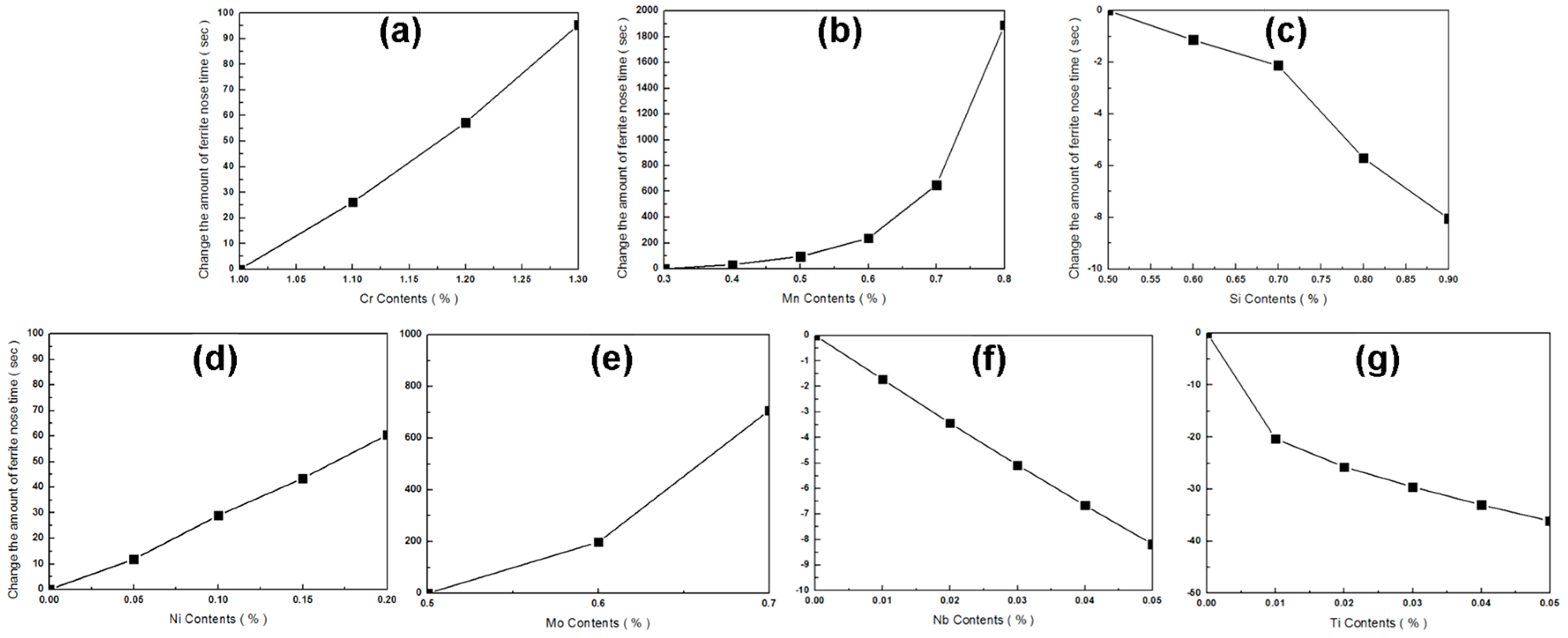
| Cr | (1.0–1.3) | y = 304.88x | 0.9904 |
| Mo | (0.5–0.7) | y = 3228.3x | 0.9274 |
| Mn | (0.3–0.5) | y = 446.32x | 0.9589 |
| (0.6–0.8) | y = 8864.9x | 0.9153 | |
| Si | (0.5–0.9) | y = −18.283x | 0.9244 |
| Ni | (0–0.2) | y = 294.99x | 0.9951 |
| Nb | (0–0.05) | y = −166.15x | 0.9991 |
| Ti | (0–0.05) | y = −860.02x | 0.6789 |
| Chemical Composition | C | Mn | Si | Cr | Mo | Ni | Nb | Ti | |
|---|---|---|---|---|---|---|---|---|---|
| ASME Spec.* | 0.10–0.20 | 0.30–0.80 | 0.50–1.00 | 1.00–1.50 | 0.45–0.65 | <0.20 | <0.07 | <0.05 | |
| Advanced Alloy | 0.15 | 0.6 | 0.5 | 1.3 | 0.5 | 0.18 | 0.03 | 0.015 | |
Specification
| Advanced alloy
| ||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, H.-s.; Kim, B.-h.; Lee, S.-h.; Kang, C.-y. Thermodynamic Alloy Design of High Strength and Toughness in 300 mm Thick Pressure Vessel Wall of 1.25Cr-0.5Mo Steel. Metals 2018, 8, 70. https://doi.org/10.3390/met8010070
Na H-s, Kim B-h, Lee S-h, Kang C-y. Thermodynamic Alloy Design of High Strength and Toughness in 300 mm Thick Pressure Vessel Wall of 1.25Cr-0.5Mo Steel. Metals. 2018; 8(1):70. https://doi.org/10.3390/met8010070
Chicago/Turabian StyleNa, Hye-sung, Byung-hoon Kim, Sang-hoon Lee, and Chung-yun Kang. 2018. "Thermodynamic Alloy Design of High Strength and Toughness in 300 mm Thick Pressure Vessel Wall of 1.25Cr-0.5Mo Steel" Metals 8, no. 1: 70. https://doi.org/10.3390/met8010070




