Effects of Ultrasonic-Aided Quenching on the Corrosion Resistance of GB 35CrMoV Steel in Seawater Environment
Abstract
:1. Introduction
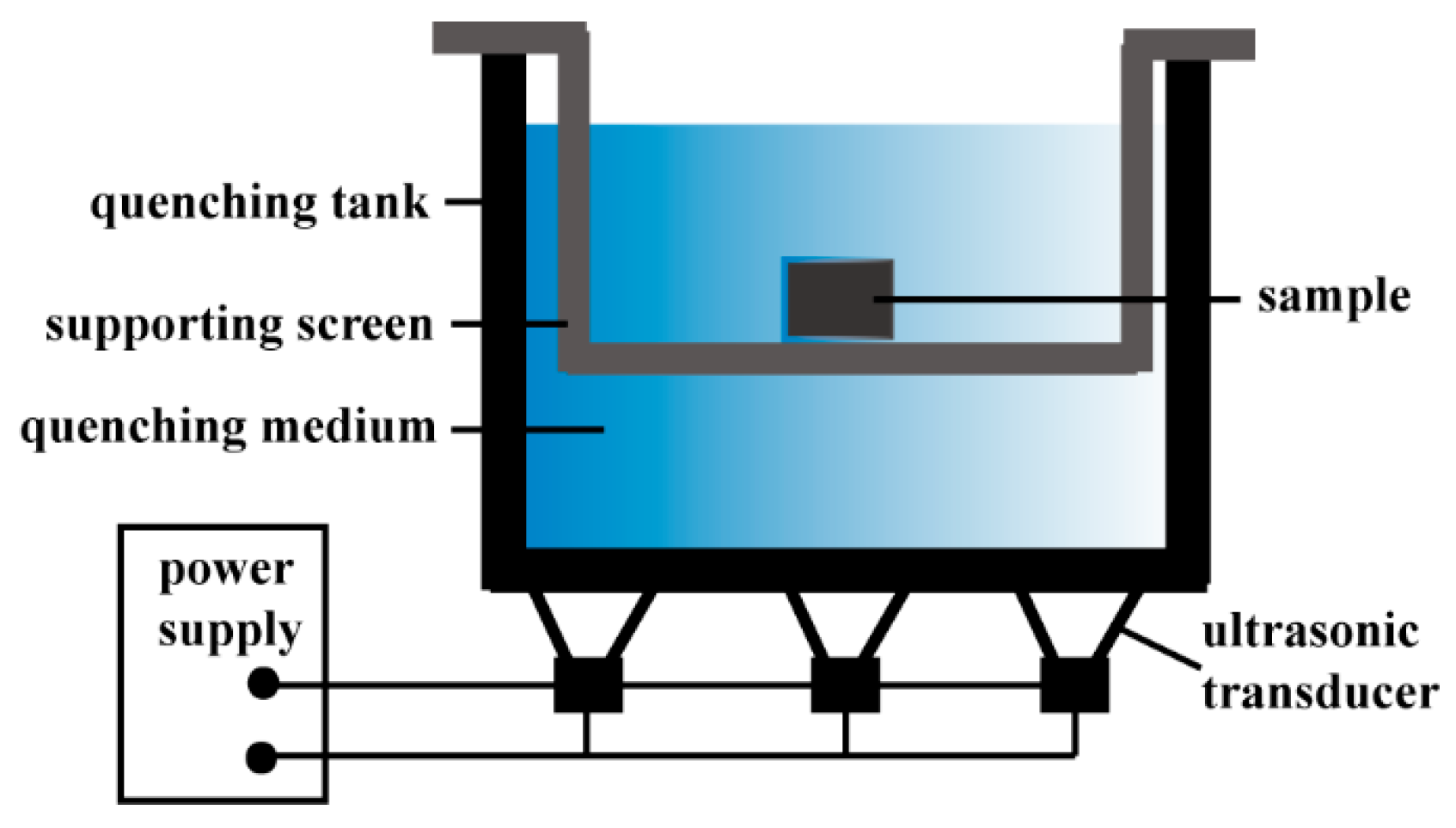
2. Materials and Methods
2.1. Materials
2.2. Microscopic Analysis
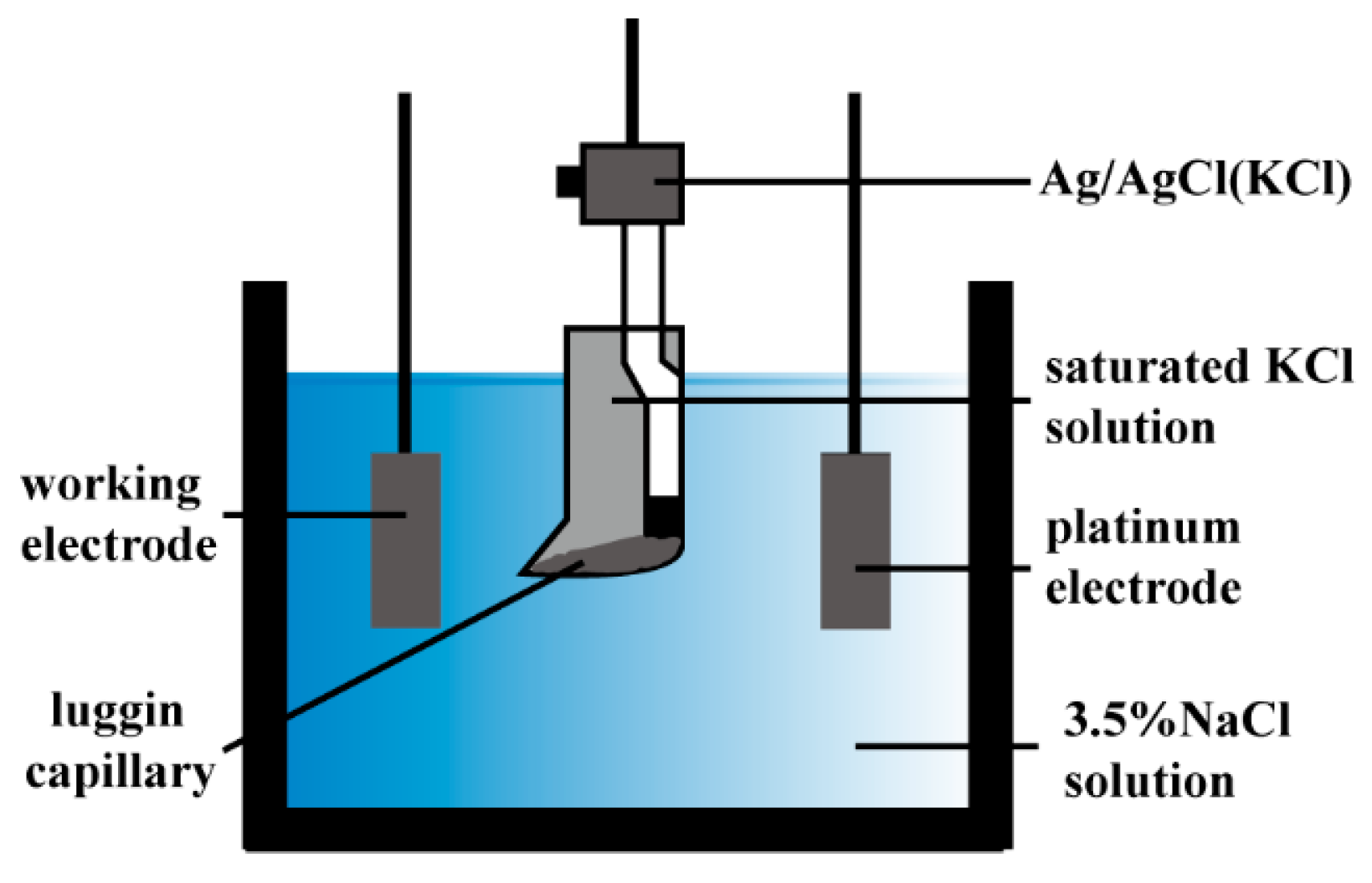
2.3. Electrochemical Measurements
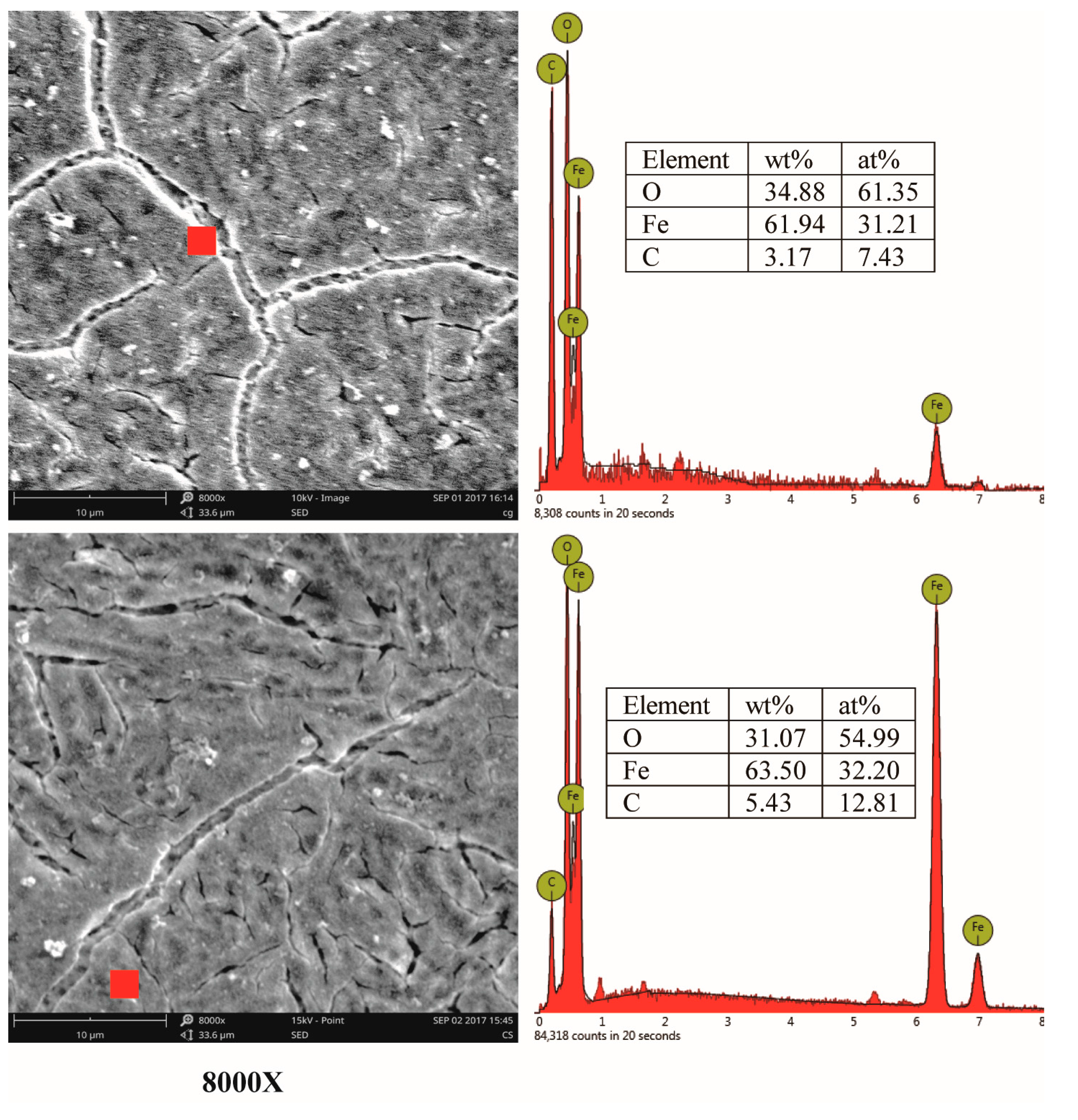
2.4. Corrosion Surface Analysis
3. Results and Discussion
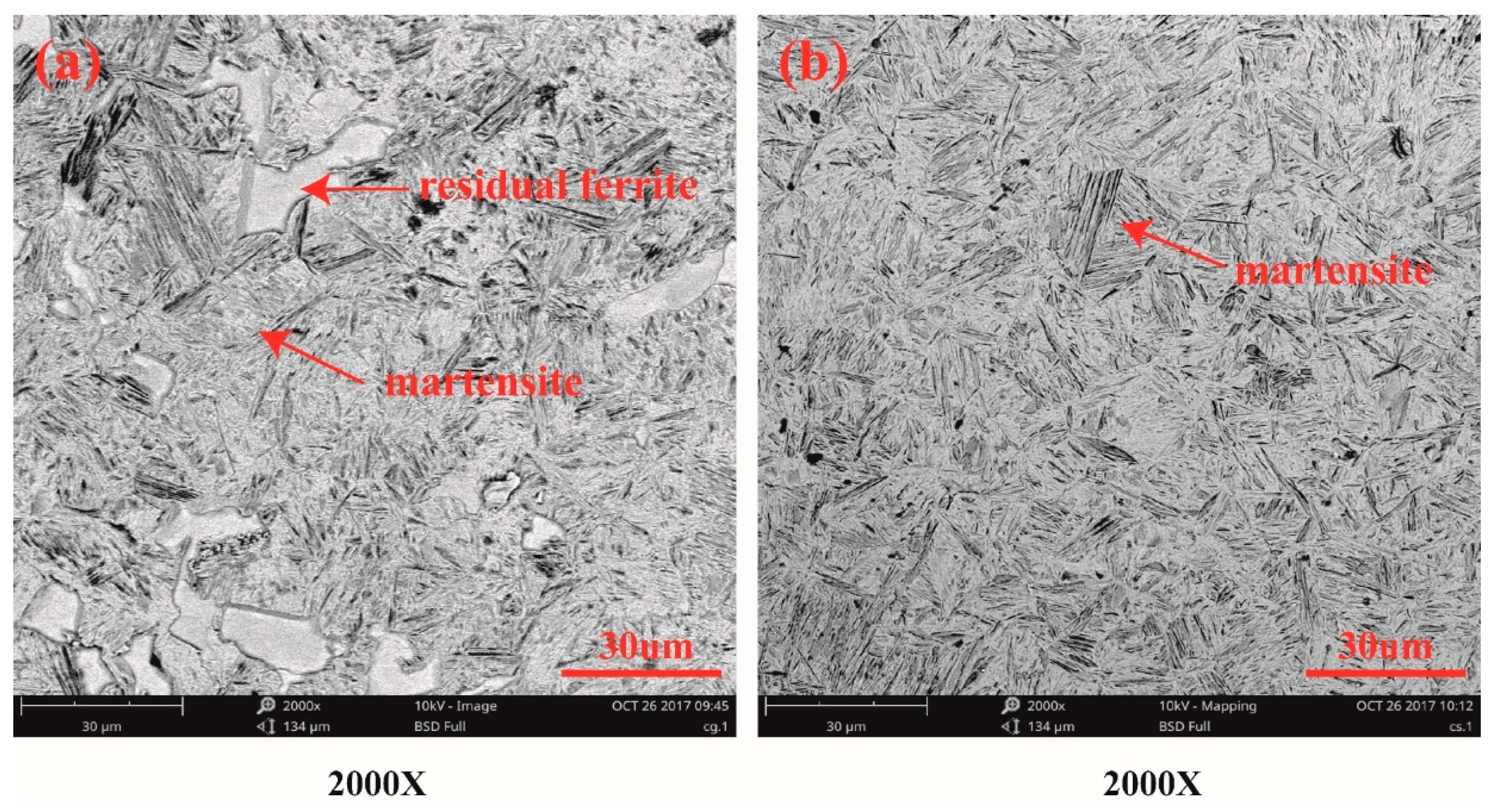
3.1. Microstructure
3.2. Electrochemical Analysis
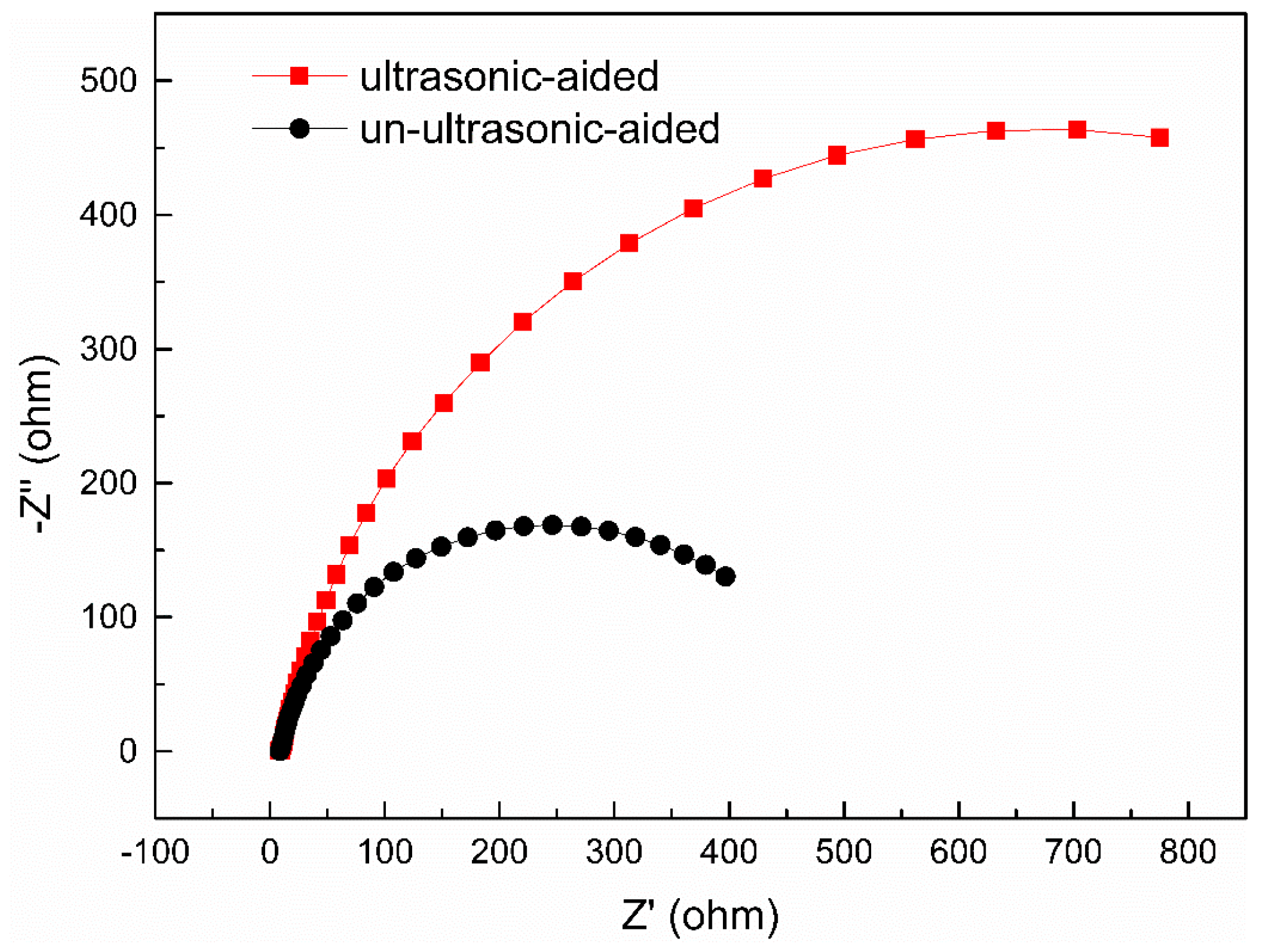
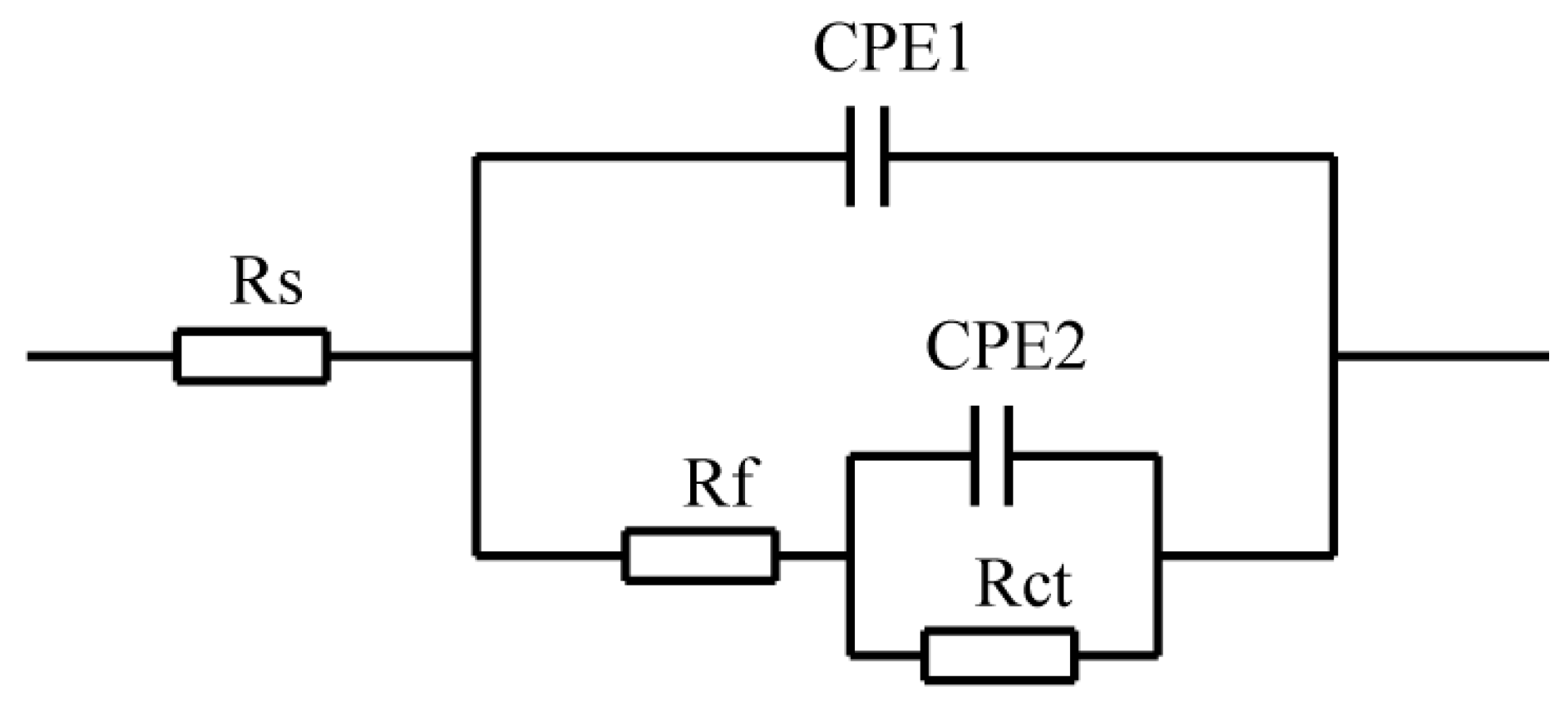
3.2.1. Electrochemical Impedance Spectroscopy
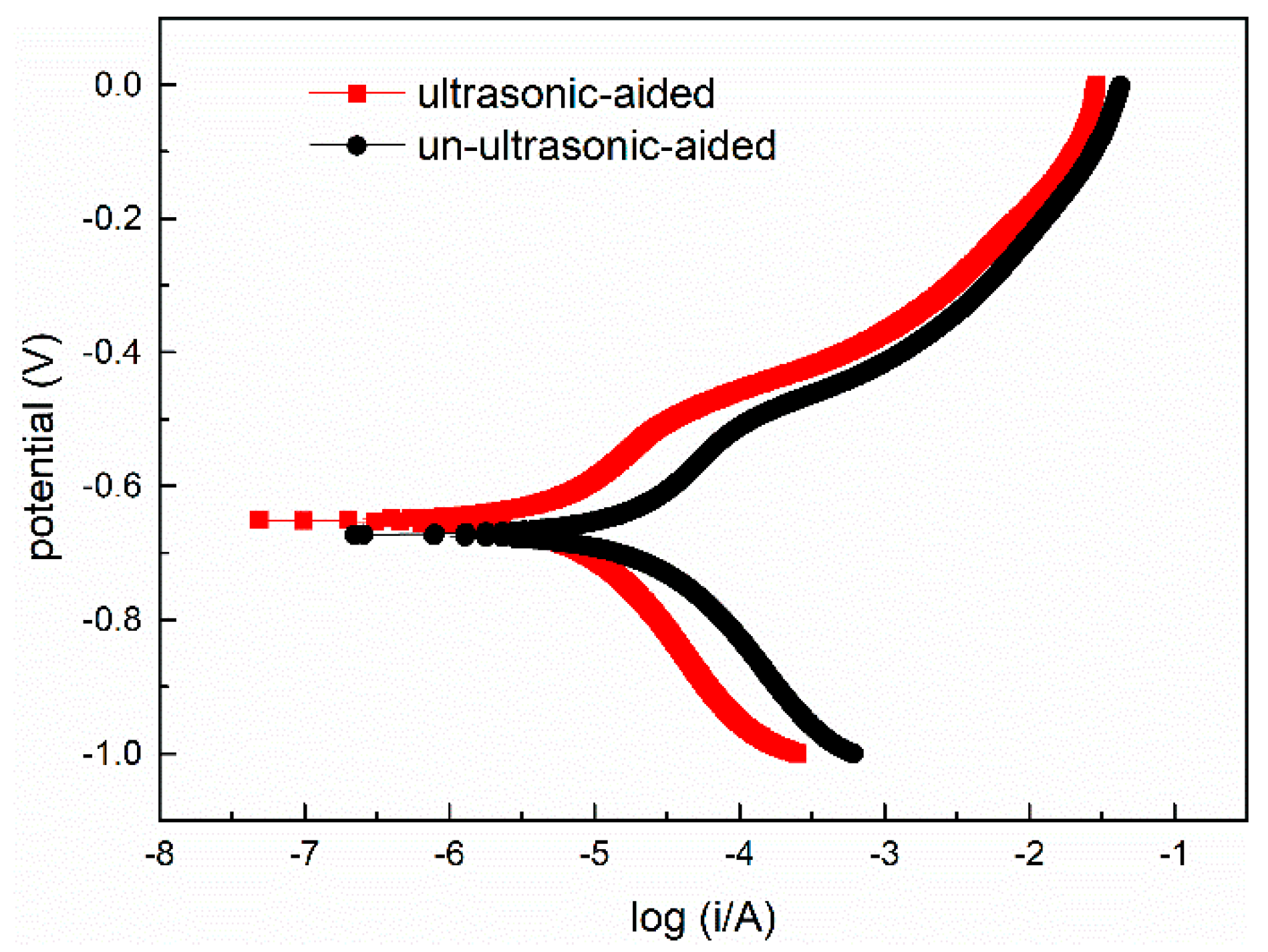
3.2.2. Potentiodynamic Polarization Curve
3.3. Corrosion Products and Corrosion Mechanism Analysis
4. Conclusions
- Ultrasonic waves can increase the quenching intensity of the quenching medium, and helped to reduce residual ferrite and refine the martensite in 35CrMoV steel.
- Compared with the UUSQ sample, the corrosion potential of the USQ sample increased, and the corrosion current density decreased by several orders of magnitude.
- The corrosion cracks of the UUSQ sample were much wider, deeper, and denser than that of the USQ sample, verifying that the USQ sample had a better corrosion resistance in a seawater environment.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.D.; Zhou, Y.; Xiao-Lin, M.A. Corrosion Behaviors of Several Kinds of Arc Sprayed Metal Coatings in 3.5%NaCl Solution. Surf. Technol. 2016, 45, 71–75. [Google Scholar]
- Hossain, K.M.A.; Easa, S.M.; Lachemi, M. Evaluation of the effect of marine salts on urban built infrastructure. Build. Environ. 2009, 44, 713–722. [Google Scholar] [CrossRef]
- Meng, H.; Hu, X.; Neville, A. A systematic erosion-corrosion study of two stainless steels in marine conditions via experimental design. Wear 2007, 263, 355–362. [Google Scholar] [CrossRef]
- Wu, X.; Tao, N.; Hong, Y.; Xu, B.; Lu, J.; Lu, K. Microstructure and evolution of mechanically-induced ultrafine grain in surface layer of AL-alloy subjected to USSP. Acta Mater. 2002, 50, 2075–2084. [Google Scholar] [CrossRef]
- Amanov, A.; Penkov, O.V.; Pyun, Y.; Kim, D. Effects of ultrasonic nanocrystalline surface modification on the tribological properties of AZ91D magnesium alloy. Tribol. Int. 2012, 54, 106–113. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, J.; Zhang, L.; Pyoun, Y.; Murakami, R. Effect of ultrasonic nanocrystal surface modification on surface and fatigue properties of quenching and tempering S45C steel. Appl. Surf. Sci. 2014, 321, 318–330. [Google Scholar] [CrossRef]
- Matsuoka, S.; Imai, H. Direct welding of different metals used ultrasonic vibration. J. Mater. Process. Technol. 2009, 209, 954–960. [Google Scholar] [CrossRef]
- Yang, J.; Cao, B. Investigation of resistance heat assisted ultrasonic welding of 6061 aluminum alloys to pure copper. Mater. Des. 2015, 74, 19–24. [Google Scholar] [CrossRef]
- Barbosa, J.; Puga, H. Ultrasonic melt processing in the low pressure investment casting of Al alloys. J. Mater. Process. Technol. 2017, 244, 150–156. [Google Scholar] [CrossRef]
- Kessler, R.R.O. Ultrasonic assisted water quenching of aluminium and steel cylinders. Int. Heat Treat. Surf. Eng. 2013, 2013, 115–121. [Google Scholar]
- Mordyuk, B.N.; Prokopenk, G.I.; Vasylyev, M.A.; Iefimov, M.O. Effect of structure evolution induced by ultrasonic peening on the corrosion behavior of AISI-321 stainless steel. Mater. Sci. Eng. A 2007, 458, 253–261. [Google Scholar] [CrossRef]
- Komarov, S. Cavitation Phenomena in Ultrasonic Casting and Their Industrial Application. Tetsu-to-Hagane 2016, 102, 179–185. [Google Scholar] [CrossRef]
- Chen, Y.; Orazem, M.E. Impedance analysis of ASTM A416 tendon steel corrosion in alkaline simulated pore solutions. Corros. Sci. 2016, 104, 26–35. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Orazem, M.E.; Pebere, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Corrosion of the Heat-Affected Zones (HAZs) of API-X100 pipeline steel in dilute bicarbonate solutions at 90 °C—An electrochemical evaluation. Corros. Sci. 2013, 74, 297–307. [Google Scholar] [CrossRef]
- Ping, H.; Rui, S.; Kuaishe, W.; Fan, Y.; Boliang, H.; Yu, C.Z. Electrochemical Corrosion Behavior of Titanium-Zirconium-Molybdenum Alloy. Rare Metal Mater. Eng. 2017, 46, 1225–1230. [Google Scholar] [CrossRef]
- Lei, L.; Liang, Y.; Jiang, Y. Effect of Quench Rate on the High Cycle Fatigue Property of 60Si2CrVAT Spring Steels. Trans. Mater. Heat Treat. 2017, 31, 65–73. [Google Scholar]
- Huang, X.L.; He, Y.H.; Zhang, Q.; Shen, W.; Zhang, H. Effect of heat treatment on the structure and mechanical properties of 18%Cr martensitic stainless steel. Mater. Sci. Eng. Powder Metall. 2017, 22, 503–509. [Google Scholar]
- Hao, X.C.; Su, P.; Xiao, K. Influence of NaCl Concentration on Corrosion Products of Weathering Steel. Corros. Prot. 2009, 30, 297–299. [Google Scholar]
- Kimura, M.; Kihira, H.; Ohta, N.; Hashimoto, M.; Senuma, T. Control of Fe(O,OH)6 nano-network structures of rust for high atmospheric-corrosion resistance. Corros. Sci. 2005, 47, 2499–2509. [Google Scholar] [CrossRef]

| Chemical Composition | C | Si | Mn | Cr | Mo | V | S | P | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Measured | 0.34 | 0.25 | 0.58 | 1.09 | 0.23 | 0.13 | 0.008 | 0.026 | Bal. |
| Sample | Rs (Ω·cm2) | Y1 (Ω·cm−2·sn) | n | Rf (Ω·cm2) | Y2 (Ω·cm−2·sn) | n | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| Ultrasonic-aided | 7.494 | 2.74 × 10−4 | 0.3255 | 9.613 | 6.795 × 10−4 | 0.8678 | 1504 |
| Un-ultrasonic-aided | 8.621 | 9.07 × 10−4 | 0.8441 | 419.8 | 1.815 × 10−2 | 1 | 79.08 |
| Sample | Ecorr/V | Rp/(Ω·cm2) | Icorr/(A·cm−2) | ba/(V/Decade) | bc/(V/Decade) |
|---|---|---|---|---|---|
| Ultrasonic-aided | −0.651 | 5947.6 | 6.91 × 10−6 | 0.054 | 0.051 |
| Un-ultrasonic-aided | −0.673 | 1954.0 | 2.085 × 10−5 | 0.050 | 0.056 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zhou, Y.; Shi, C.; Mao, D. Effects of Ultrasonic-Aided Quenching on the Corrosion Resistance of GB 35CrMoV Steel in Seawater Environment. Metals 2018, 8, 104. https://doi.org/10.3390/met8020104
Jiang X, Zhou Y, Shi C, Mao D. Effects of Ultrasonic-Aided Quenching on the Corrosion Resistance of GB 35CrMoV Steel in Seawater Environment. Metals. 2018; 8(2):104. https://doi.org/10.3390/met8020104
Chicago/Turabian StyleJiang, Xin, Yajun Zhou, Chen Shi, and Daheng Mao. 2018. "Effects of Ultrasonic-Aided Quenching on the Corrosion Resistance of GB 35CrMoV Steel in Seawater Environment" Metals 8, no. 2: 104. https://doi.org/10.3390/met8020104




