Figure 1.
Leaching reactor (A) and tank for solution neutralization and cementation (B).
Figure 2.
Overview of upscaling procedure.
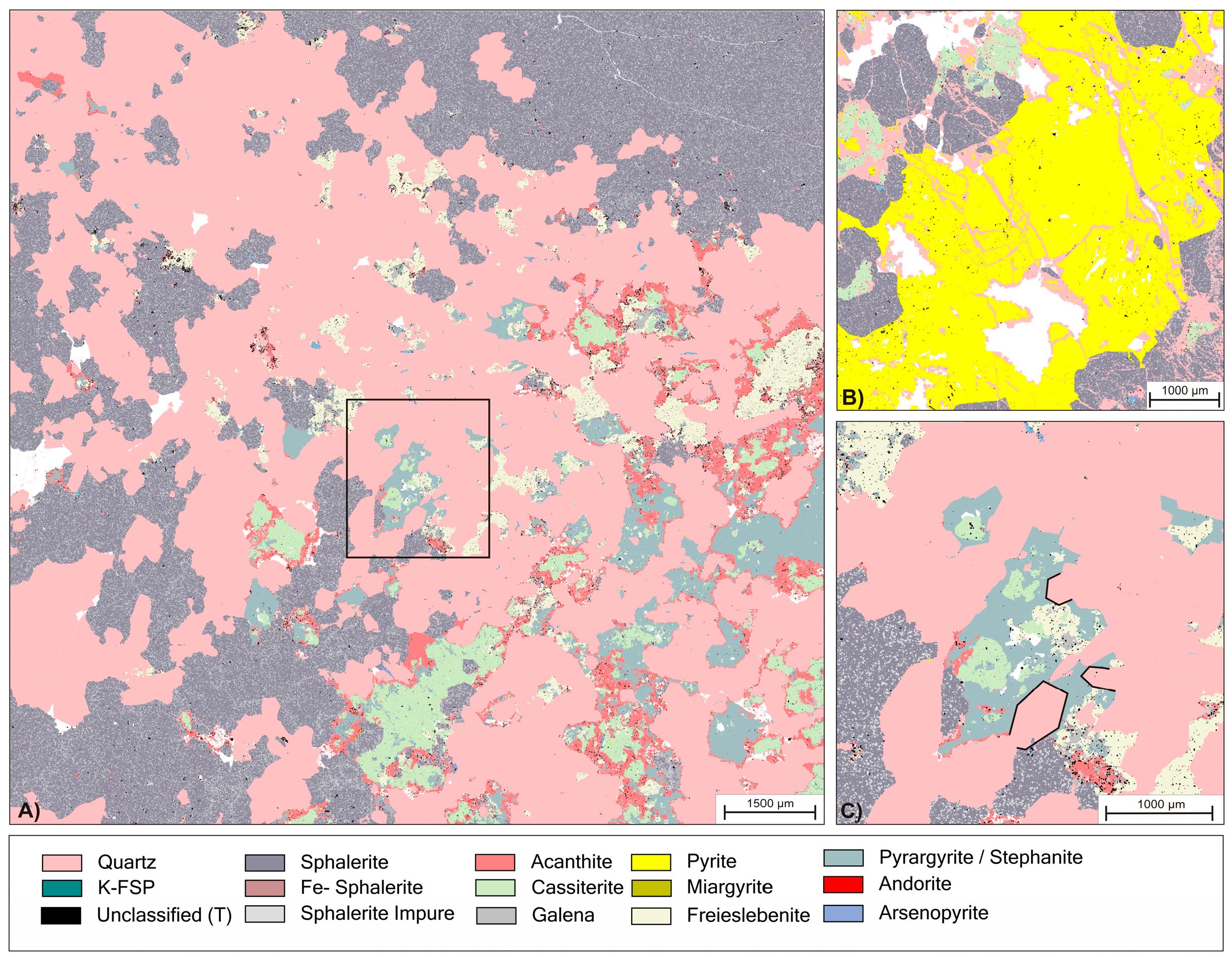
Figure 3.
(A) Overview phase map of initial ore bearing vein; (B) Detailed view of different examples for hydraulic brecciation of sphalerite and pyrite. Fractures has been healed by quartz precipitation; (C) Detailed view of box in (A) with highlighted examples of idiomorphic textures at grain boundaries of quartz (pink) against an agglomerate of ore minerals.
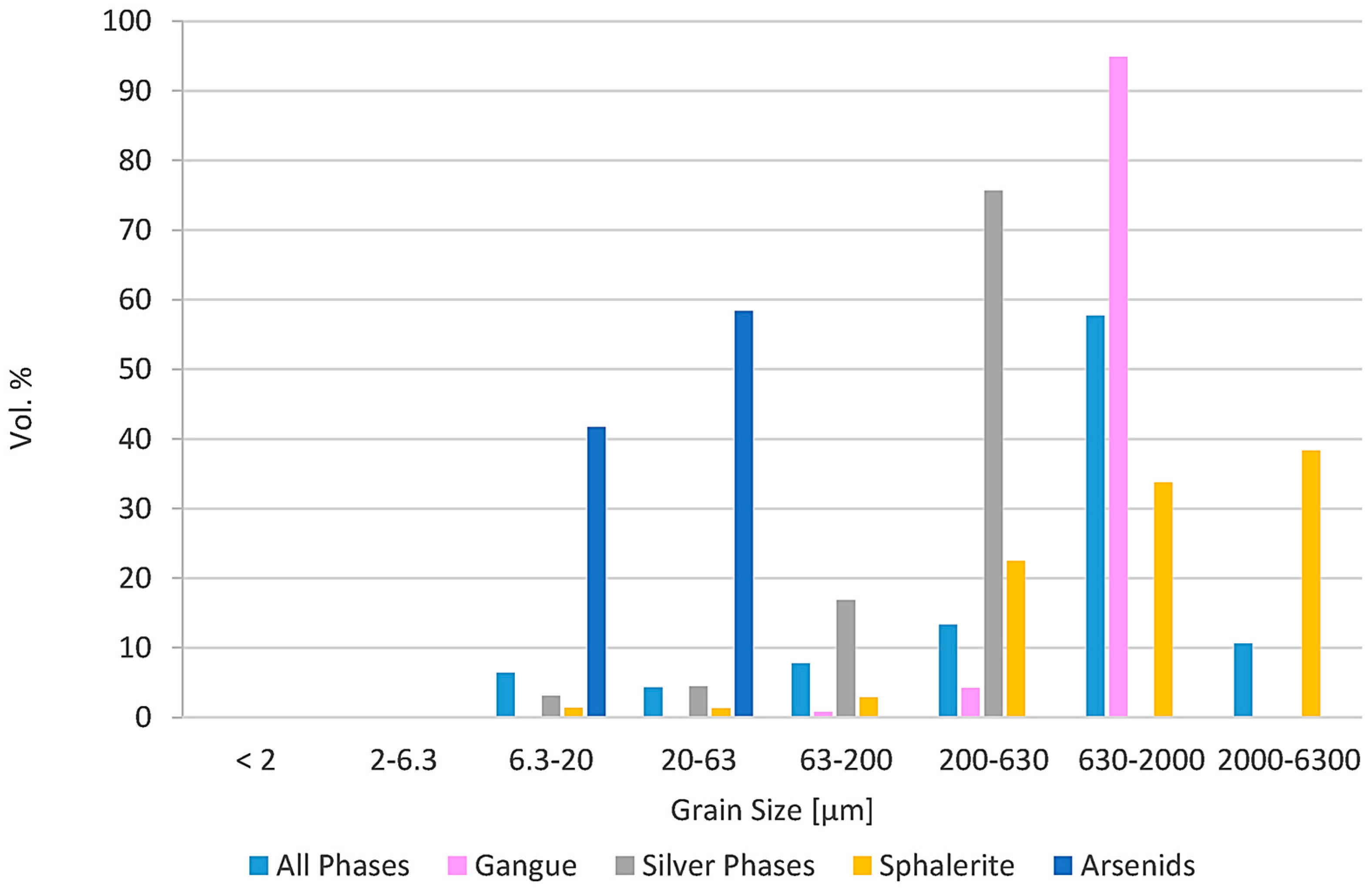
Figure 4.
Grain Size of different phases from thin-section analysis. Based on this analysis, the target grain size was optimized before grinding. Silver phases were handled together due to their similar chemical character, except acanthite.
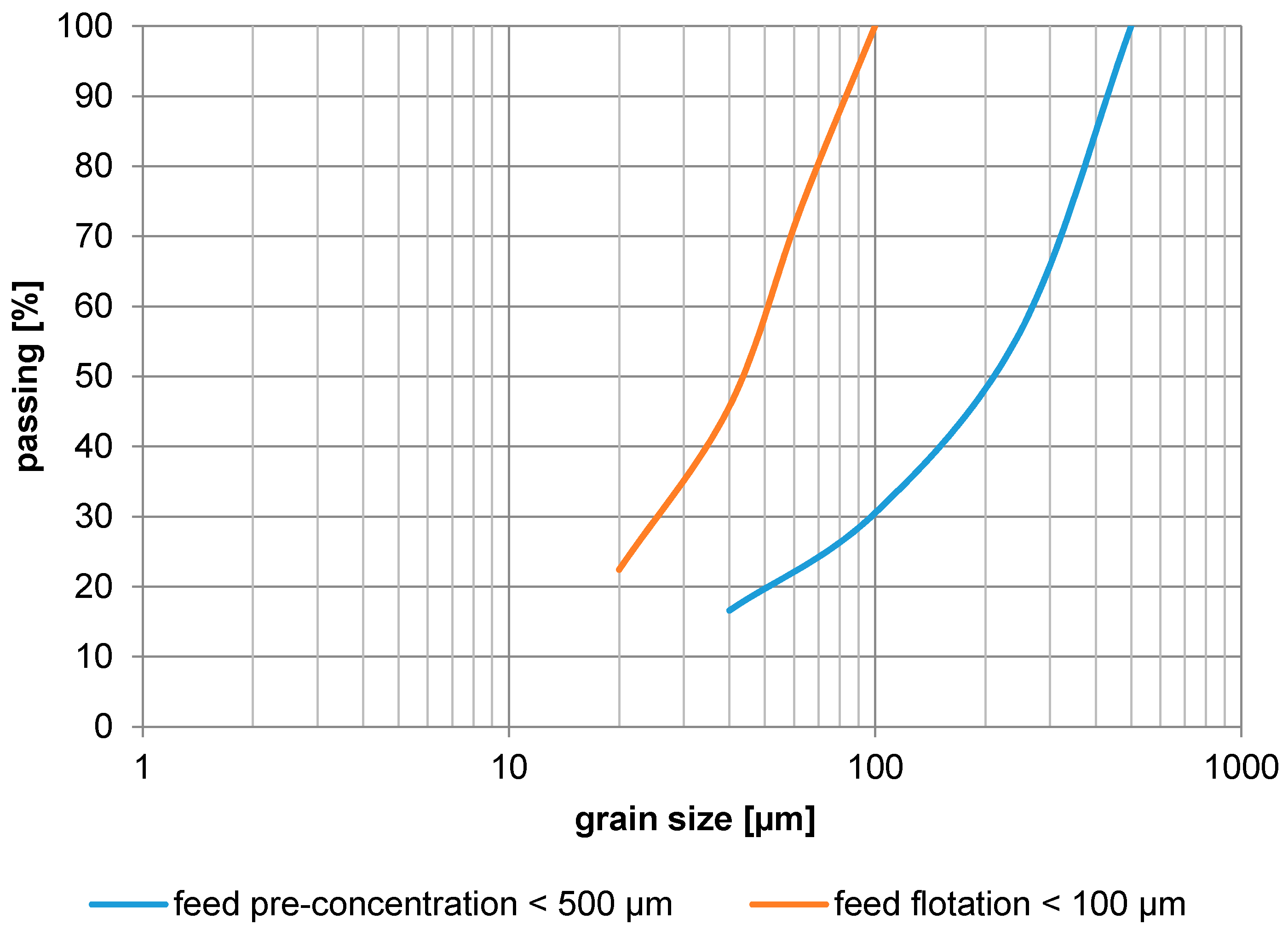
Figure 5.
Grain size distribution (feed pre-concentration, feed flotation).
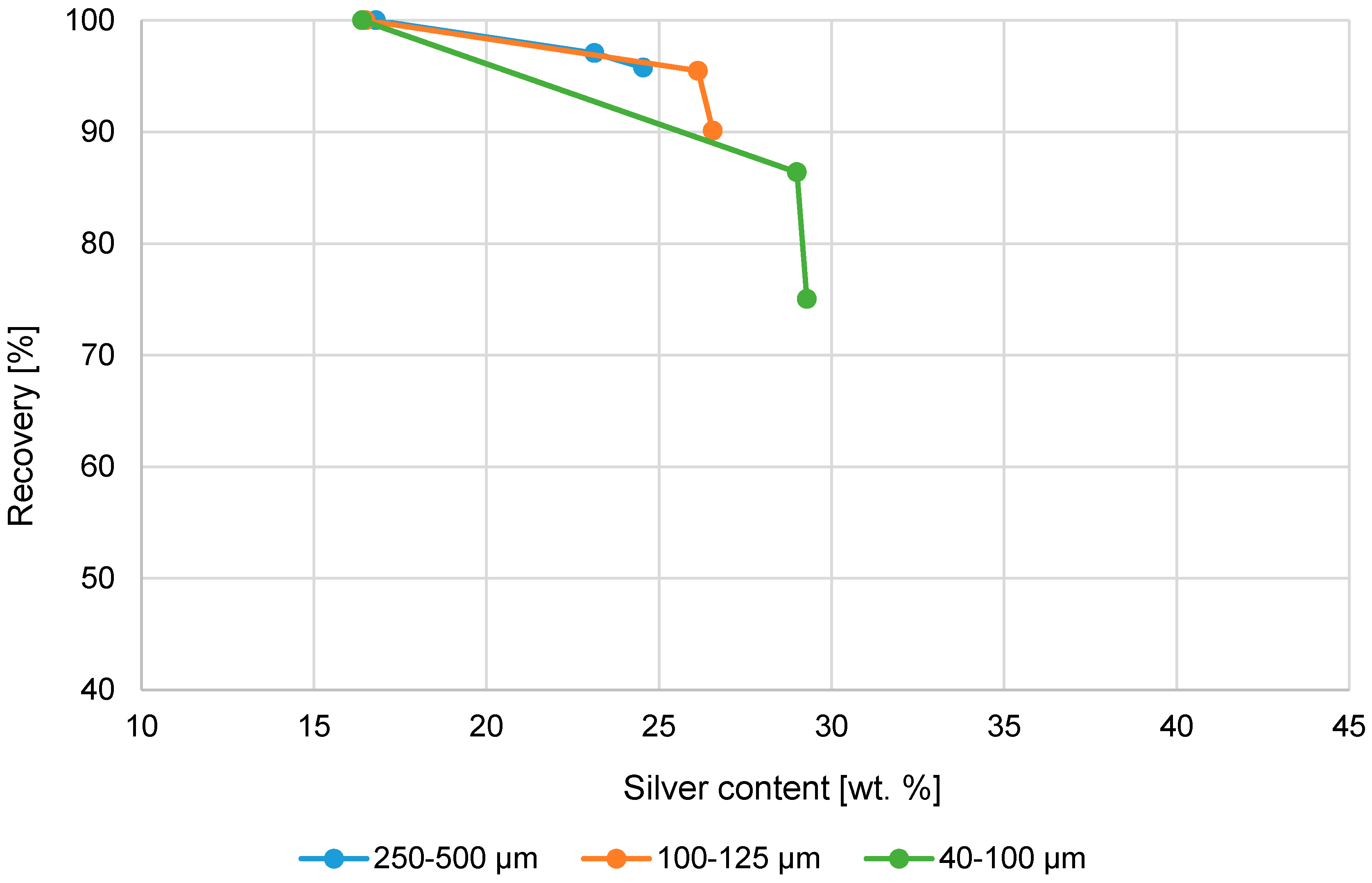
Figure 6.
Cumulative silver recovery (%) and Ag-content (wt. %) in product using magnetic separation.
Figure 7.
Cumulative silver recovery (%) and Ag-content (wt. %) in product using electrostatic separation.
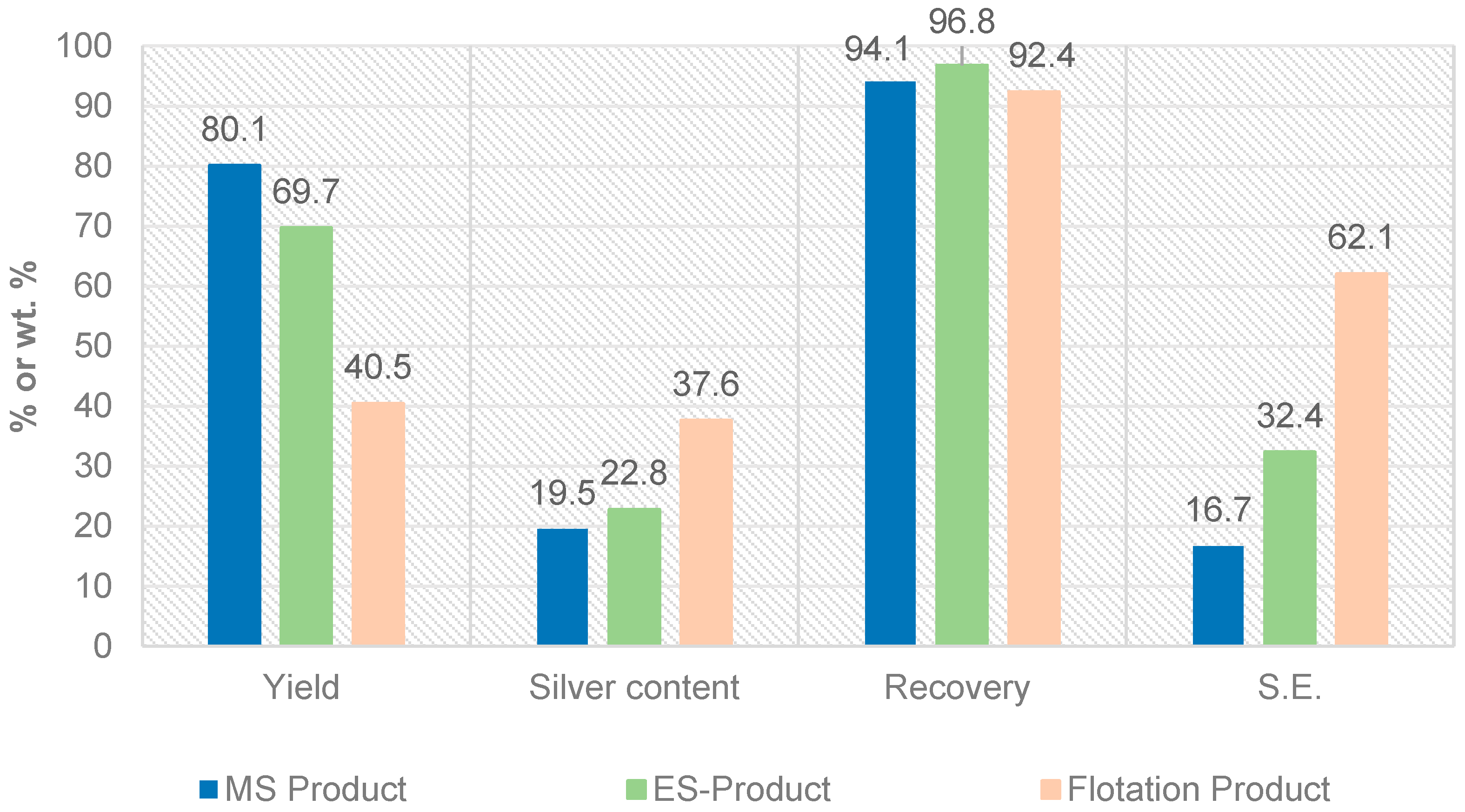
Figure 8.
Comparison of the results of magnetic-, electrostatic separation and flotation (yield: mass output in relation to input, S.E.: separation efficiency).
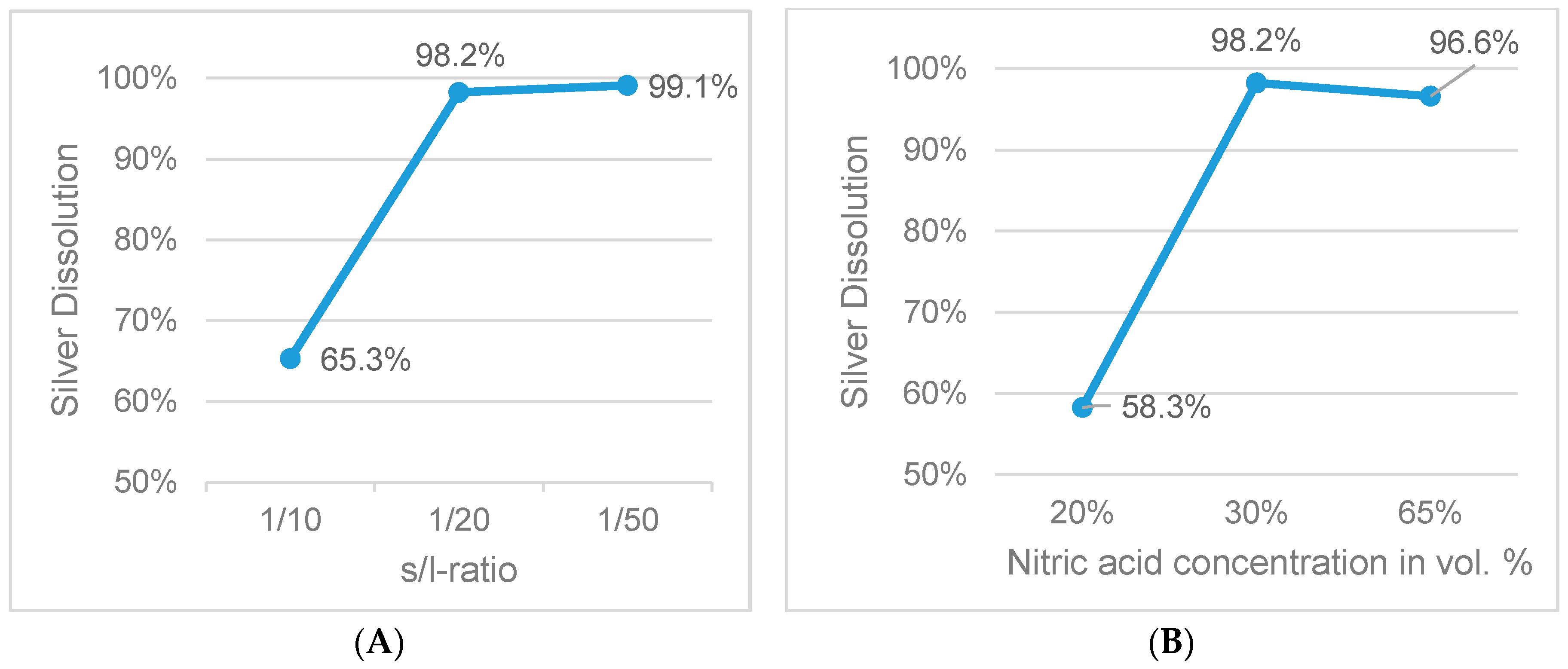
Figure 9.
Influence of s/l-ratio (A) and acid concentration (B) on silver dissolution (fixed parameters: 1 h leaching duration, 65 °C).
Figure 10.
Influence of temperature and leaching duration on silver dissolution (fixed parameters: 30% HNO3, s/l = 1/20).
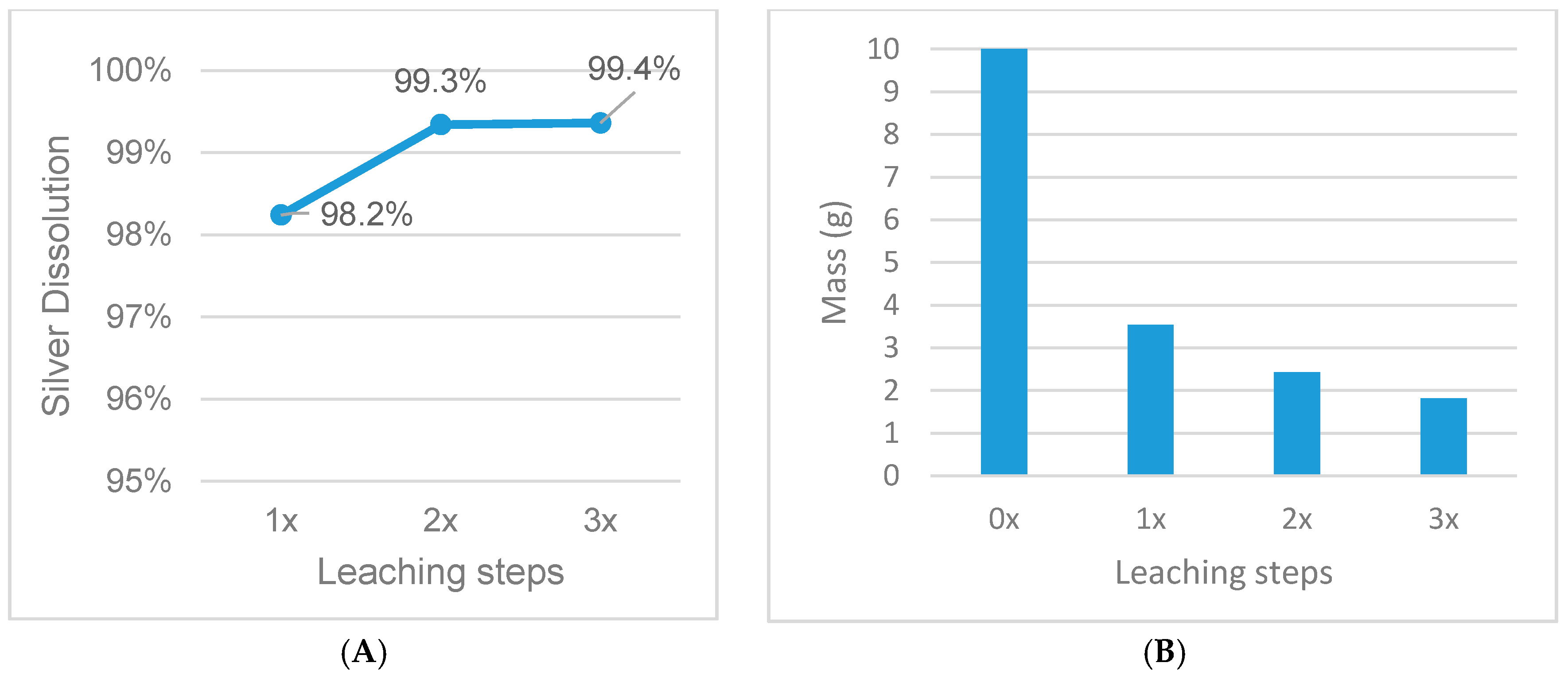
Figure 11.
Influence of multiple-step leaching on silver dissolution (A) and mass reduction (B), fixed parameters: 30% HNO3, s/l = 1/20, 1 h leaching duration, 65 °C.
Figure 12.
Silver cementation with copper wire in a stirred beaker.
Figure 13.
Copper impurities in silver cementate (magnification 500×).
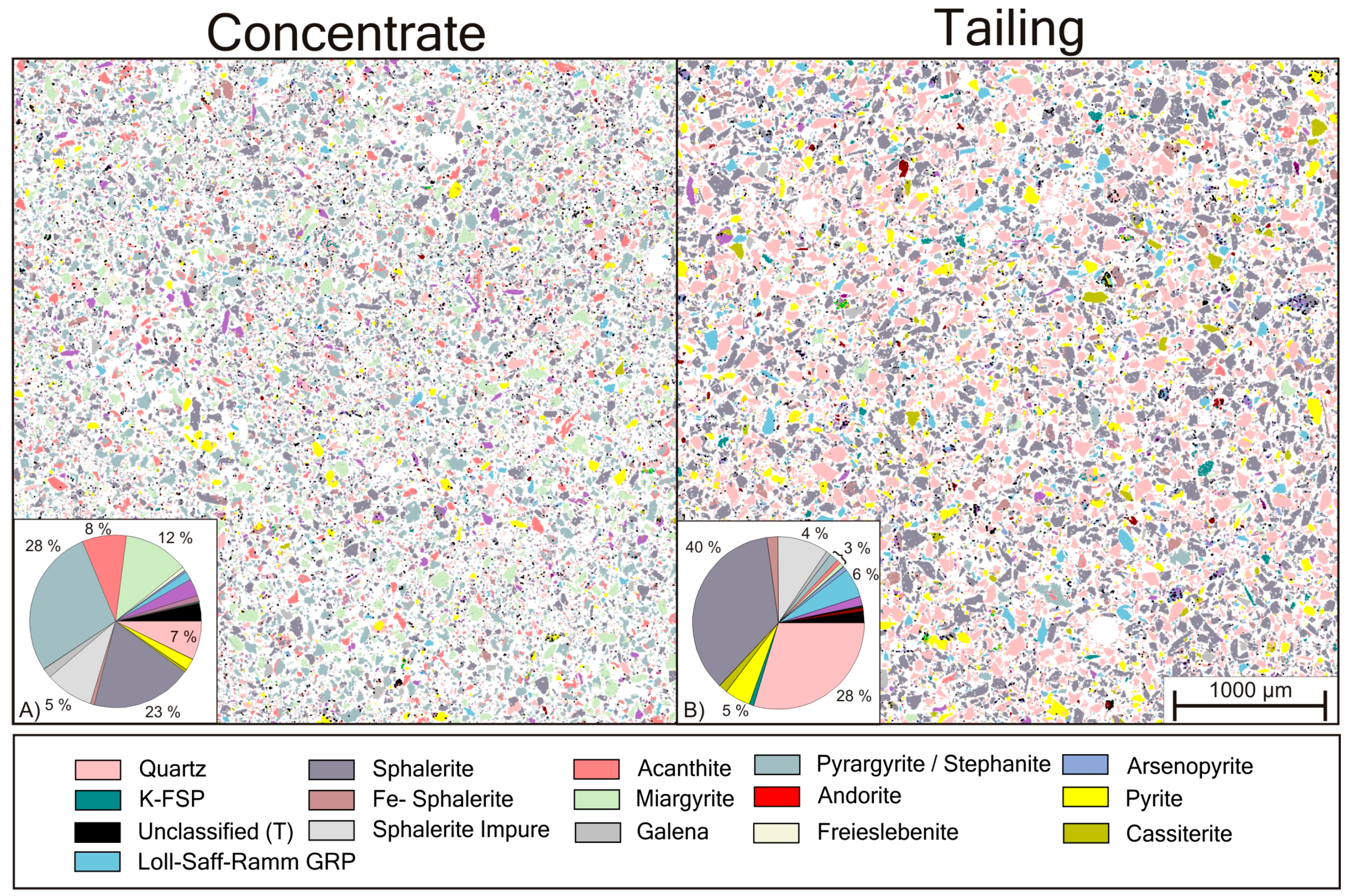
Figure 14.
Detailed section view and modal composition (vol. %) of the minerals processing concentrate (A) and tailings (B).
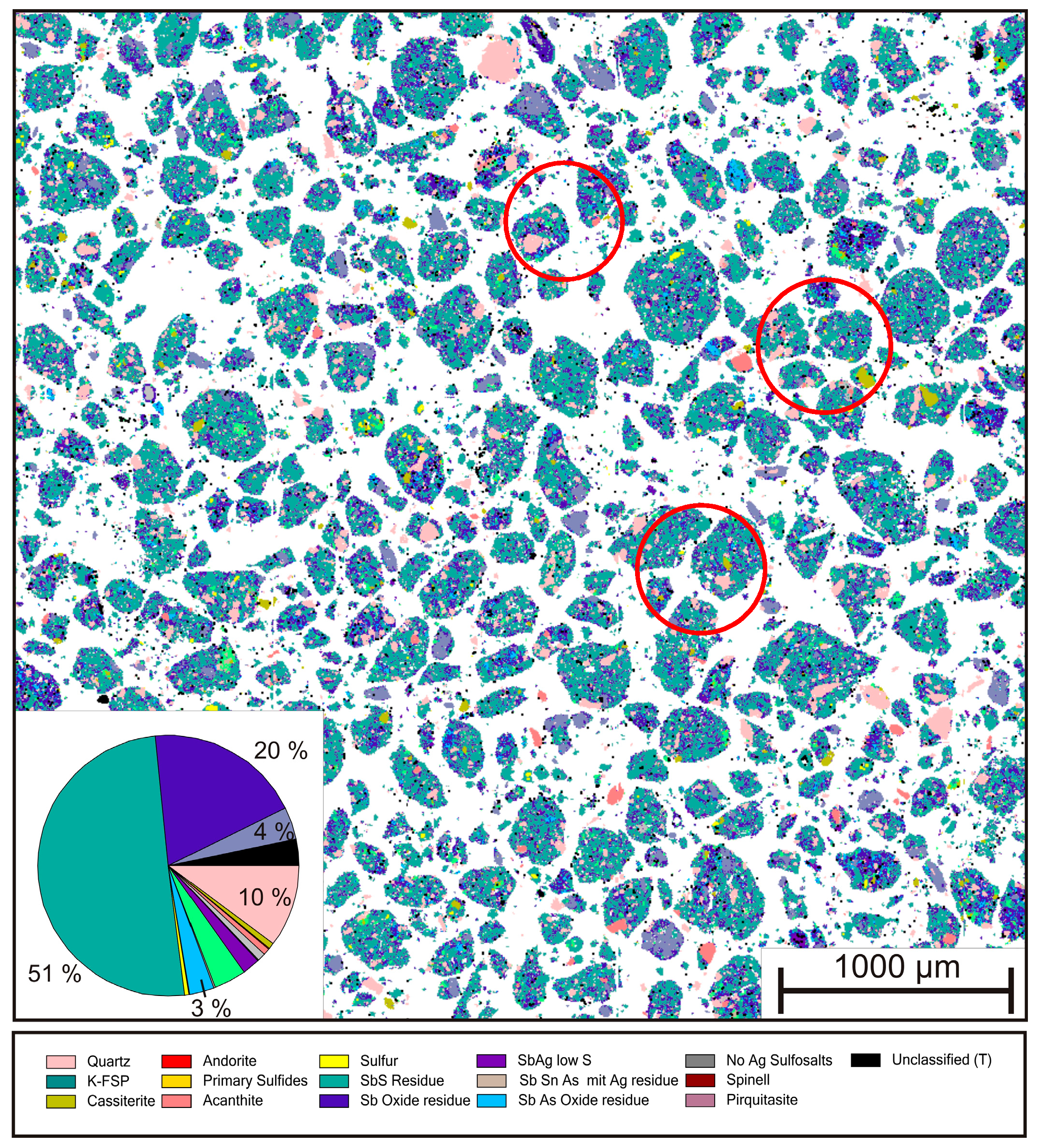
Figure 15.
Section overview and modal distribution of leaching residue.
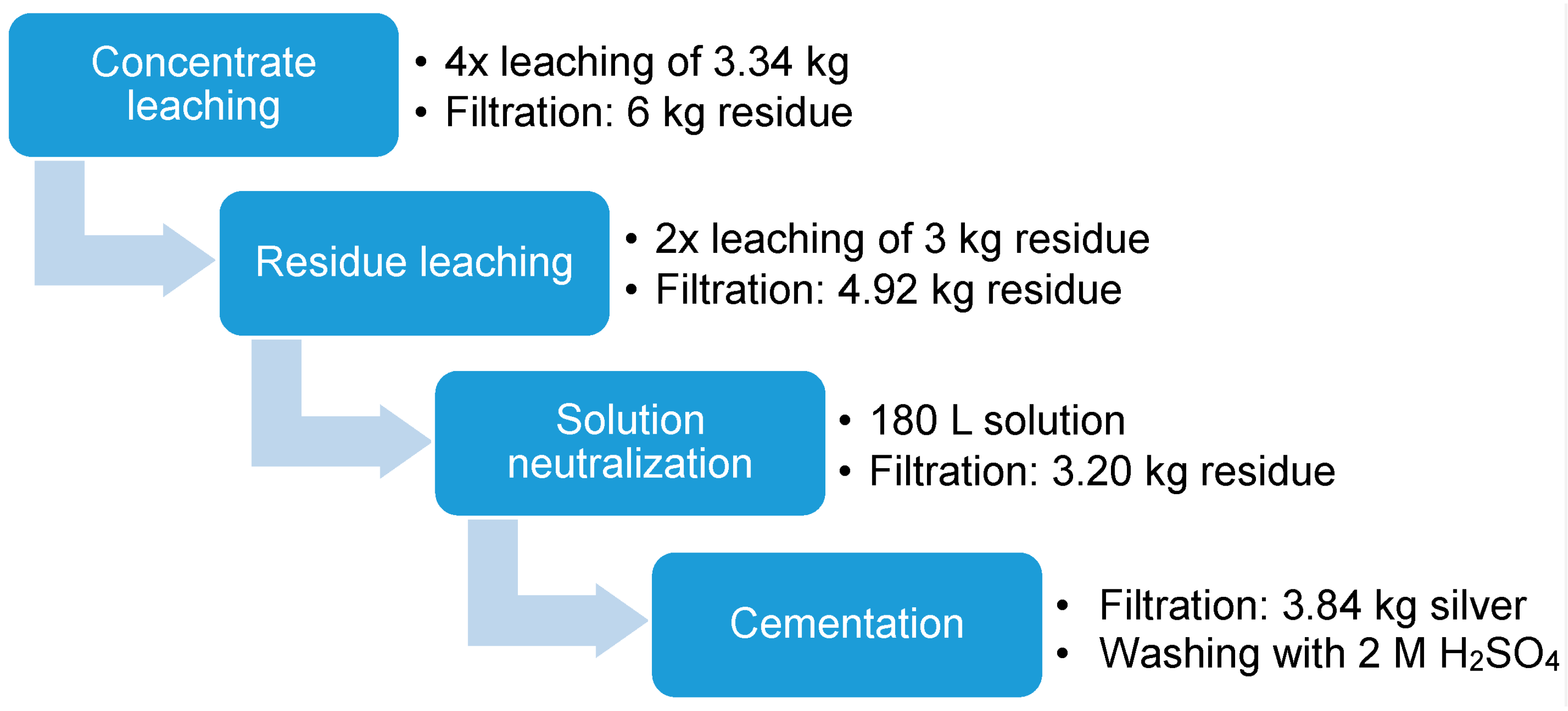
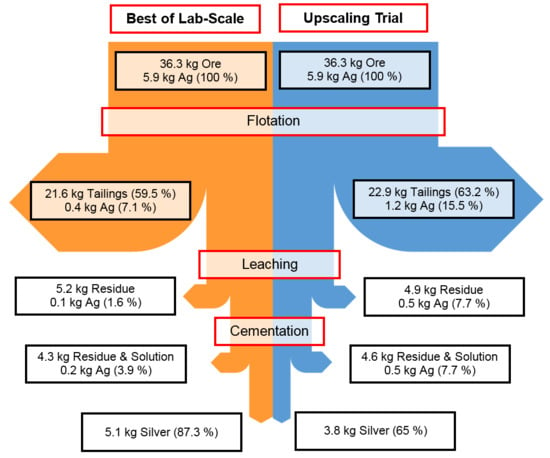
Figure 16.
Mass flow diagram comparing best results of lab scale experiments (left) with the upscaling trial (right).
Table 1.
Composition of silver concentrate used in small scale experiments in (wt. %).
| Sample | Ag | As | S | Pb | Zn | Sb | Other |
|---|
| Preliminary Concentrate | 26.0 | 24.0 | 12.3 | 10.9 | 10.5 | 5.7 | 10.6 |
Table 2.
Parameters of nitric acid leaching tests (parameter of investigation is set bold).
| Test | s/l Ratio | c(HNO3), vol. % | Stoich. Ratio of Ag–HNO3 | T, °C | Time, h | Leach. Steps |
|---|
| 1 | 1/20 | 30 | 35× | 65 | 1 | 1 |
| 2 | 1/20 | 30 | 35× | 65 | 1 | 1 |
| 3 | 1/10 | 30 | 17.5× | 65 | 1 | 1 |
| 4 | 1/50 | 30 | 87.5× | 65 | 1 | 1 |
| 5 | 1/20 | 20 | 23.3× | 65 | 1 | 1 |
| 6 | 1/20 | 65 | 75.7× | 65 | 1 | 1 |
| 7 | 1/20 | 30 | 35× | 65 | 0.5 | 1 |
| 8 | 1/20 | 30 | 35× | 65 | 2 | 1 |
| 9 | 1/20 | 30 | 35× | 65 | 24 | 1 |
| 10 | 1/20 | 30 | 35× | 45 | 0.5 | 1 |
| 11 | 1/20 | 30 | 35× | 45 | 1 | 1 |
| 12 | 1/20 | 30 | 35× | 45 | 2 | 1 |
| 13 | 1/20 | 30 | 35× | 45 | 24 | 1 |
| 14 | 1/20 | 30 | 35× | 25 | 1 | 1 |
| 15 | 1/20 | 30 | 35× | 65 | 1 | 2 |
| 16 | 1/20 | 30 | 35× | 65 | 1 | 3 |
Table 3.
Electrochemical potential of selected elements.
| Reaction | Potential in V |
|---|
| −0.762 |
| −0.126 |
| −0.037 |
| 0.000 |
| 0.342 |
| 0.521 |
| 0.771 |
| 0.800 |
| 1.498 |
Table 4.
Effect of pH value and type of collector on the selective froth flotation of silver sulfide minerals (Yield = mass output in relation to input, S.E.: Separation efficiency).
| pH | Collector (100 g/t) + Frother (25 g/t) | Addition of Collector and Flotation | Depressant, kg/t | Yield, wt. % | Ag-Content, wt. % | Silver Recovery, % | S.E., % |
|---|
| 5.5 | Hostaflot PEB | stepwise | - | 89.2 | 18.33 | 99.1 | 11.9 |
| 7 | Hostaflot PEB | stepwise | - | 47.2 | 30.6 | 85.9 | 46.5 |
| 8.25 | Hostaflot PEB | stepwise | - | 26.7 | 28.1 | 41.3 | 18.5 |
| 9.5 | Hostaflot PEB | stepwise | - | 7.6 | 21.7 | 10.2 | 3.1 |
| 7 | Hostaflot PEB | normal | - | 42.8 | 35.1 | 90.6 | 57.5 |
| 7 | Hostaflot PEB | normal | 5 | 40.5 | 37.6 | 92.4 | 62.1 |
| 7 | PAX | normal | 5 | 47.4 | 31.6 | 90.2 | 51.2 |
| 7 | Hostaflot LBS | normal | 5 | 42.0 | 29.0 | 74.8 | 39.1 |
| 7 | Ekofol ANT33 | normal | 5 | 20.5 | 27.3 | 34.0 | 16,2 |
Table 5.
Composition ranges (wt. %) of metal and slag phases generated in several oxidizing melting experiments of silver ore.
| Sample | Ag | As | Pb | Zn | Sb |
|---|
| Metal phase | 72.3–75.3 | 0.0–0.0 | 0.2–2.2 | 0.0–0.1 | 22.8–23.2 |
| Slag phase | 14.7–31.0 | 15.6–41.3 | 8.9–10.2 | 6.2–11.4 | 3.0–4.7 |
Table 6.
Silver precipitate composition in wt. %.
| Sample | Ag | Cu | Sb | Pb | Fe | Zn | As |
|---|
| Cementation at pH 4 | 96.67 | 2.098 | <LOD | 0.16 | <LOD | <LOD | <LOD |
| Cementation at pH 5 | 97.70 | 1.32 | <LOD | 0.04 | <LOD | 0.03 | <LOD |
| H2SO4-washed Precipitate | 99.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Table 7.
Composition (wt. %) of reference trial leaching residue.
| Sample | Ag | As | S | Pb | Zn | Sb |
|---|
| Preliminary Concentrate | 26.0 | 24.0 | 12.3 | 10.9 | 10.5 | 5.7 |
| Leaching residue | 0.0 | 0.2 | 37.0 | 0.0 | 0.7 | 0.4 |
| Dissolution in % | 98.2 | 93.1 | -5.5 | 99.8 | 97.8 | 35.5 |
Table 8.
Silver recovery of the upscaling process steps.
| Sample | kg | % | Best of Lab (%) |
|---|
| Metallic silver in concentrate | 4.748 | 100.00 | 100.00 |
| Silver in leaching residue | 0.453 | 9.55 | 1.76 |
| PLS | 4.295 | 90.45 | 98.24 |
| Leach purification sludge at pH 5 | 0.353 | 7.43 | 3.52 |
| Silver in solution after cementation | 0.099 | 2.09 | 0.83 |
| Metallic silver product | 3.843 | 80.94 | 93.89 |
Table 9.
Composition of input and output streams (wt. %).
| Sample | Ag | Sb | Pb | Fe | Zn | As |
|---|
| Concentrate | 35.6 | 13.2 | 4.7 | 3.4 | 14.9 | 2.0 |
| Leaching residue | 9.2 | 35.2 | 0.4 | 0.4 | 0.0 | 4.4 |
| Neutralization Residue | 11.0 | 7.6 | 18.1 | 10.0 | 2.0 | 2.5 |
| Silver cementate | 99.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |