Exothermic Reaction Kinetics in High Energy Density Al-Ni with Nanoscale Multilayers Synthesized by Cryomilling
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Processes
2.2. Characterization
3. Results and Discussion
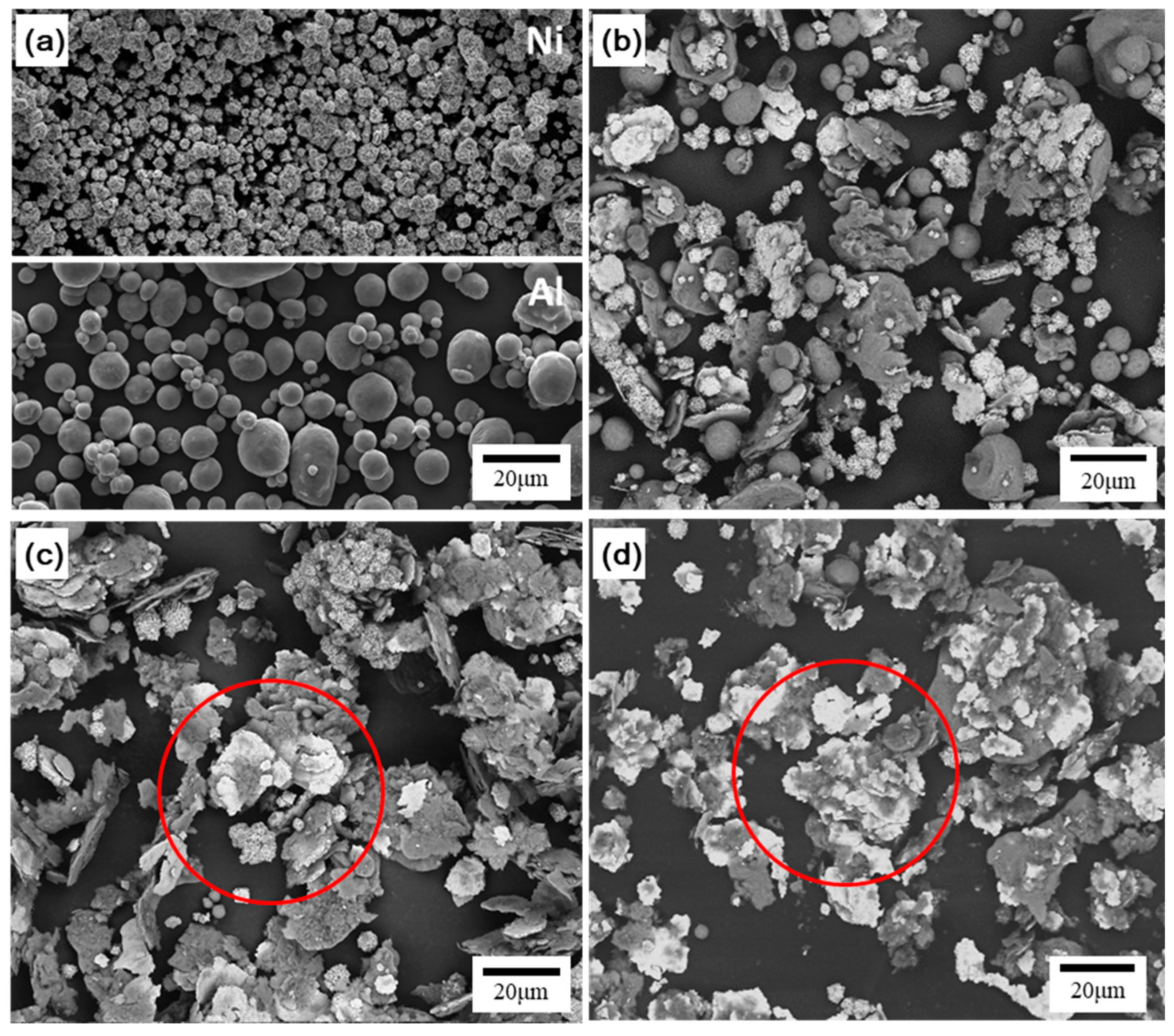
3.1. Surface Morphology
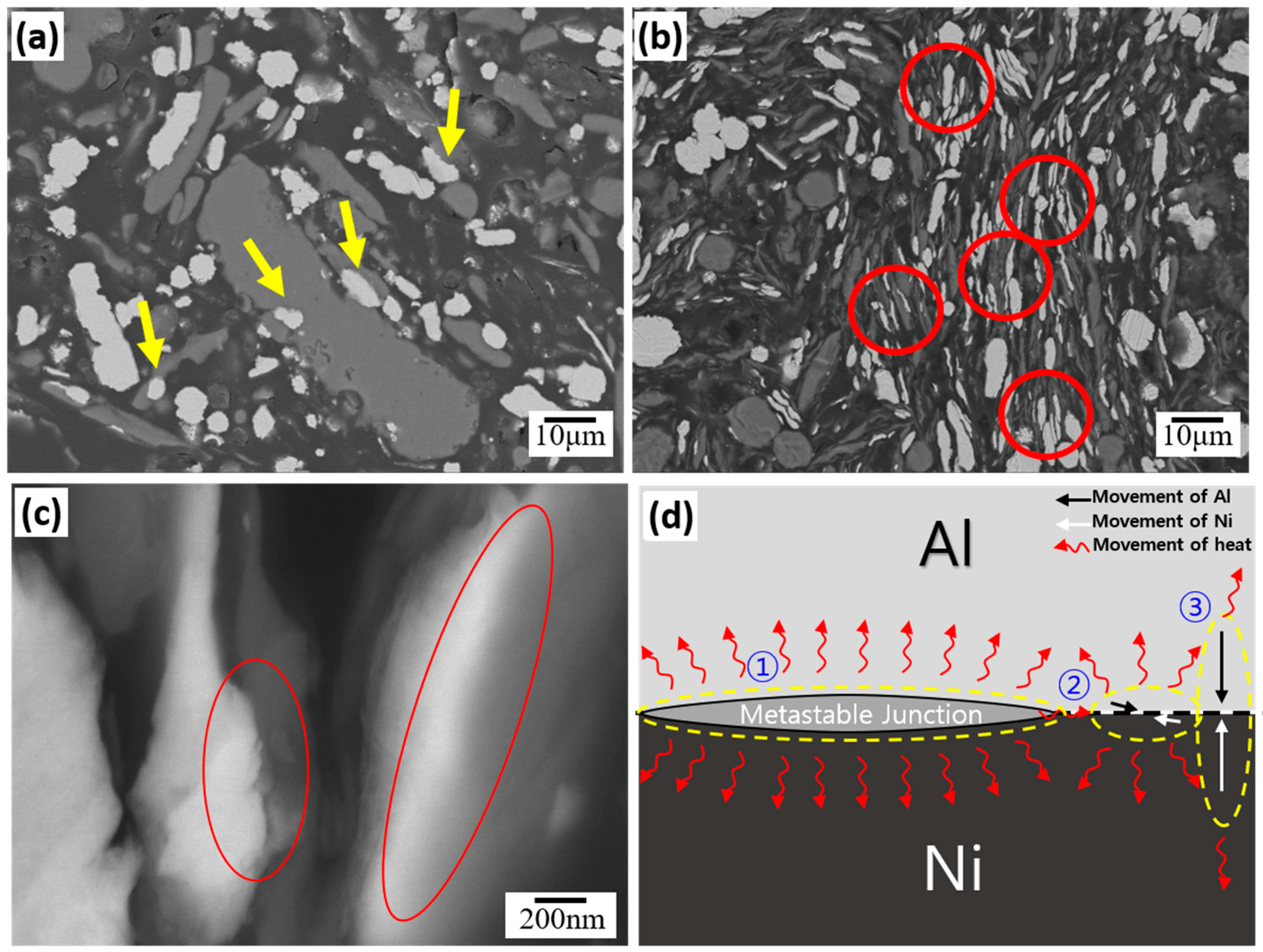
3.2. Microstructural Evolution
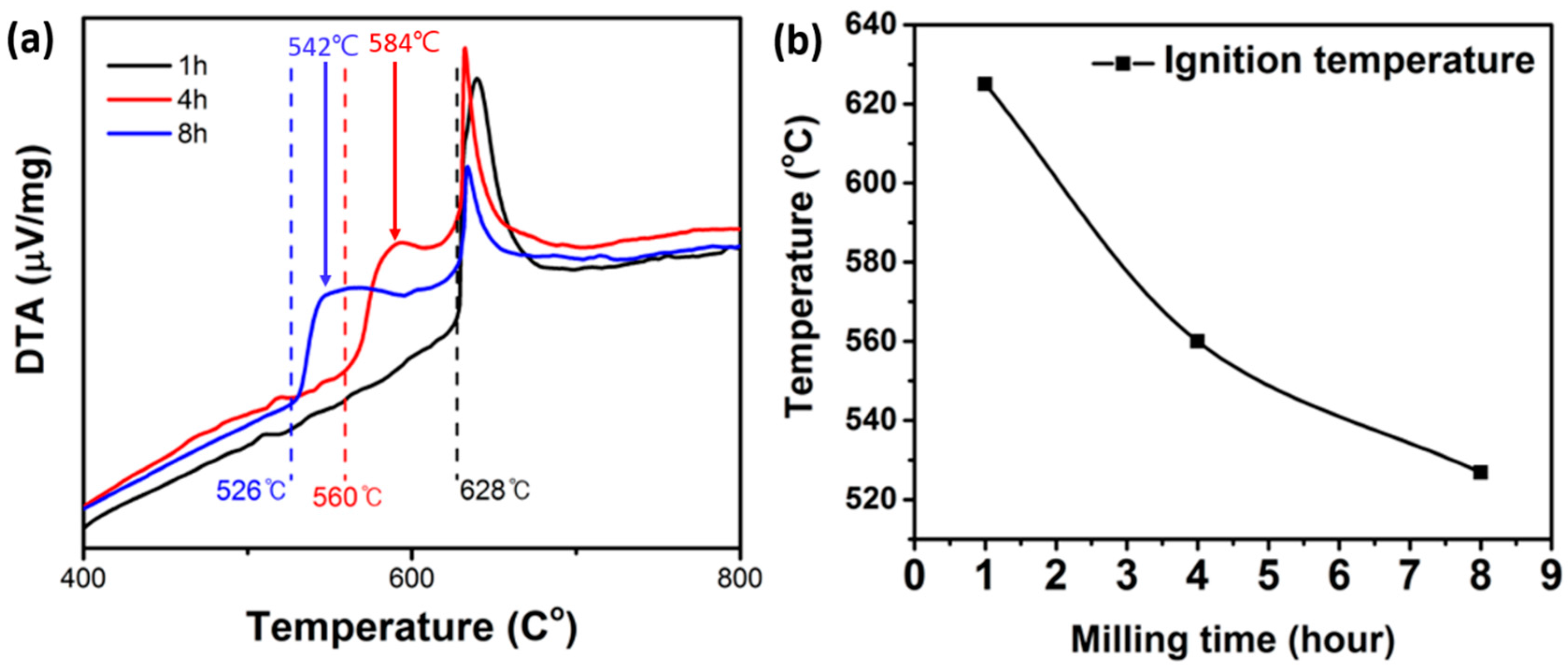
3.3. Thermal Property
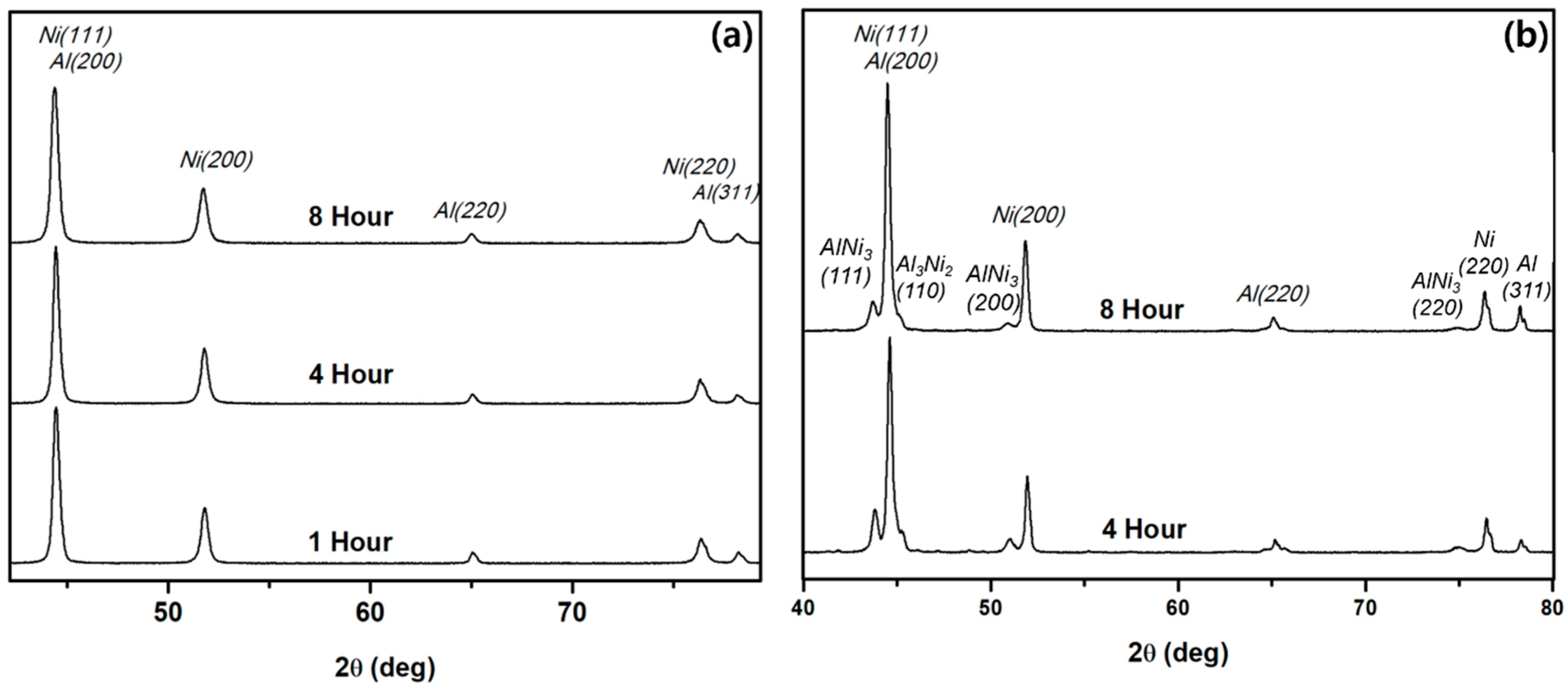
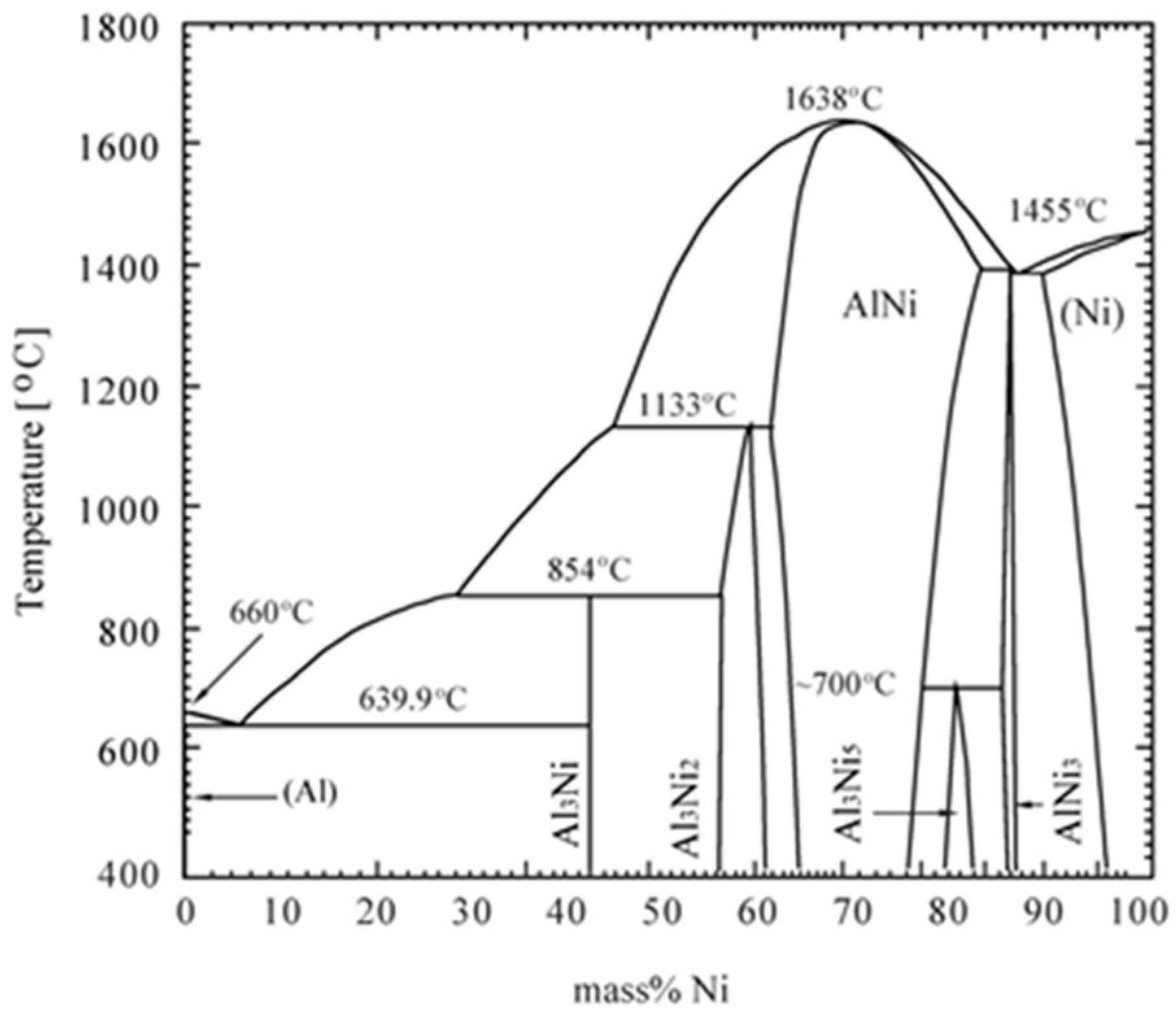
3.4. Phase Evolution
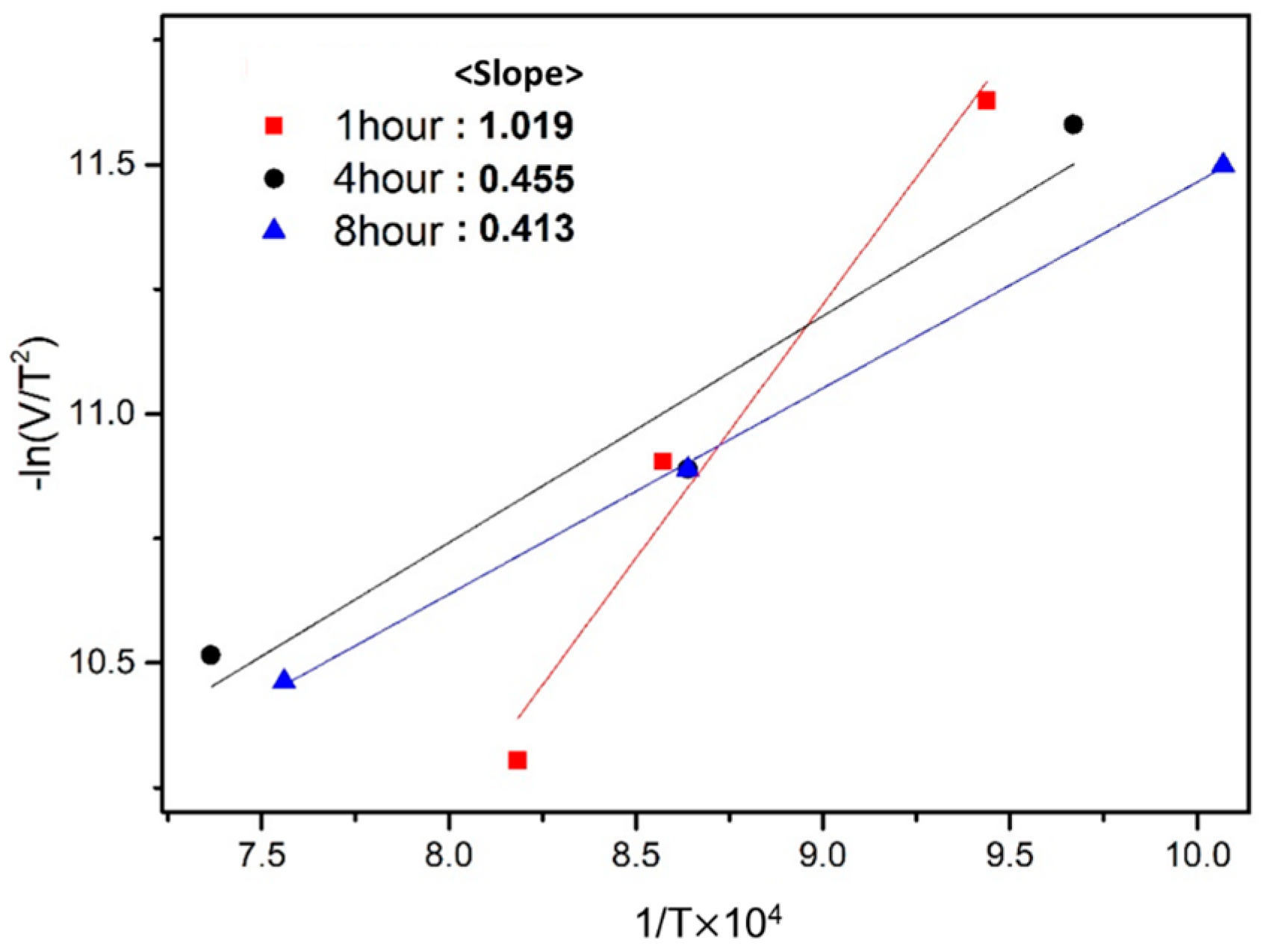
3.5. Activation Energy for Exothermic Reaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adams, D.P. Reactive multilayers fabricated by vapor deposition: A critical review. Thin Solid Films 2015, 576, 98–128. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Mukasyan, A.S. Combustion of heterogeneous nanostructural systems (review). Combust. Explos. Shock Waves 2010, 46, 243–266. [Google Scholar] [CrossRef]
- Floro, J.A. Propagation of explosive crystallization in thin Rh-Si multilayer films. J. Vac. Sci. Technol. 1986, 4, 631–637. [Google Scholar] [CrossRef]
- Gavens, A.J.; Van Heerden, D.; Mann, A.B.; Reiss, M.E.; Weihs, T.P. Effect of intermixing on self-propagating reactions in Al/Ni nanolaminate foils. J. Appl. Phys. 2000, 87, 1255–1263. [Google Scholar] [CrossRef]
- Mann, A.B.; Gavens, A.J.; Reiss, M.E.; Heerden, D.V.; Bao, G.; Weihs, T.P. Modeling and characterizing the propagation velocity of exothermic reactions in multilayer foils. J. Appl. Phys. 1997, 82, 1178–1188. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskaya, I.P. Historical retrospective of SHS: An autoreview. Int. J. Self-Propag. High-Temp. Synth. 2008, 17, 242–265. [Google Scholar] [CrossRef]
- Hilpert, K.; Kobertz, D.; Venugopal, V.; Miller, M.; Gerads, H.; Bremer, F.J.; Nickel, H. Phase diagram studies on the Al-Ni system. Z. Naturforsch. A 1987, 42, 1327–1332. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.C.M.; Liu, C.T. Reaction mechanism of combustion synthesis of NiAl. Mater. Sci. Eng. A 2002, 329–331, 57–68. [Google Scholar] [CrossRef]
- Mason, B.A.; Sippel, T.R.; Groven, L.J.; Gunduz, I.E.; Son, S.F. Combustion of mechanically activated Ni/Al reactive composites with microstructural refinement tailored using two-step milling. Intermetallics 2015, 66, 88–95. [Google Scholar] [CrossRef]
- Mason, B.A.; Groven, L.J.; Son, S.F. The role of microstructure refinement on the impact ignition and combustion behavior of mechanically activated Ni/Al reactive composites. J. Appl. Phys. 2013, 114, 113501. [Google Scholar] [CrossRef]
- Anselmi-Tamburini, U.; Munir, Z.A. The propagation of a solid-state combustion wave in Ni-Al foils. J. Appl. Phys. 1989, 66, 5039–5045. [Google Scholar] [CrossRef]
- Hunt, E.M.; Granier, J.J.; Plantier, K.B.; Pantoya, M.L. Nickel aluminum superalloys created by the self-propagating high-temperature synthesis of nanoparticle reactants. J. Mater. Res. 2004, 19, 3028–3036. [Google Scholar] [CrossRef]
- Gibbins, J.D.; Stover, A.K.; Krywopusk, N.M.; Woll, K.; Weihs, T.P. Properties of reactive Al: Ni compacts fabricated by radial forging of elemental and alloy powders. Combust. Flame 2015, 162, 4408–4416. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Tan, W.; Deboer, R.J.; Stech, E.J.; Aprahamian, A.; Wiescher, M.; Rouvimov, S.; Overdeep, K.R.; Shuck, C.E.; Weihs, T.P.; et al. Irradiation-enhanced reactivity of multilayer Al/Ni nanomaterials. ACS Appl. Mater. Interfaces 2015, 7, 11272–11279. [Google Scholar] [CrossRef] [PubMed]
- Sraj, I.; Specht, P.E.; Thadhani, N.N.; Weihs, T.P.; Knio, O.M. Numerical simulation of shock initiation of Ni/Al multilayered composites. J. Appl. Phys. 2014, 115, 023515. [Google Scholar] [CrossRef]
- Battezzati, L.; Pappalepore, P.; Durbiano, F.; Gallino, I. Solid state reactions in Al/Ni alternate foils induced by cold rolling and annealing. Acta Mater. 1999, 47, 1901–1914. [Google Scholar] [CrossRef]
- Duckham, A.; Spey, S.J.; Wang, J.; Reiss, M.E.; Weihs, T.P.; Besnoin, E.; Knio, O.M. Reactive nanostructured foil used as a heat source for joining titanium. J. Appl. Phys. 2004, 96, 2336–2342. [Google Scholar] [CrossRef]
- Noro, J.; Ramos, A.S.; Vieira, M.T. Intermetallic phase formation in nanometric Ni/Al multilayer thin films. Intermetallics 2008, 16, 1061–1065. [Google Scholar] [CrossRef]
- Andrzejak, T.A.; Shafirovich, E.; Varma, A. Ignition mechanism of nickel-coated aluminum particles. Combust. Flame 2007, 150, 60–70. [Google Scholar] [CrossRef]
- Simoes, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Anisothermal solid-state reactions of Ni/Al nanometric multilayers. Intermetallics 2011, 19, 350–356. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Mason, B.A.; Groven, L.J.; Lin, Y.C.; Cherukara, M.; Son, S.F.; Strachan, A.; Mukasyan, A.S. Tailored reactivity of Ni + Al nanocomposites: Microstructural correlations. J. Phys. Chem. C 2012, 116, 21027–21038. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Koch, C.C. Mechanical Milling and Alloying. In Materials Science and Technology; Cahn, R.W., Haasen, P., Kramer, E.J., Eds.; VCH Verlagsgesellschaft: Weinheim, Germany, 1991; Volume 15, pp. 193–246. [Google Scholar]
- Koch, C.C. Nanostructured Materials: Processing, Properties and Potential Applications; William Andrew Publishing: Norwich, NY, USA, 2002. [Google Scholar]
- White, J.D.; Reeves, R.V.; Son, S.F.; Mukasyan, A.S. Thermal explosion in Al-Ni system: Influence of mechanical activation. J. Phys. Chem. A 2009, 113, 13541–13547. [Google Scholar] [CrossRef] [PubMed]
- Lagoviyer, O.S.; Schoenitz, M.; Dreizin, E.L. Effect of milling temperature on structure and reactivity of Al-Ni composites. J. Mater. Sci. 2018, 53, 1178–1190. [Google Scholar] [CrossRef]
- Huang, B.L.; Vallone, J.; Luton, M.J. Formation of nanocrystalline B2 NiAl through cryomilling of Ni-50 at. % Al at 87 K. Nanostruct. Mater. 1995, 5, 411–424. [Google Scholar] [CrossRef]
- Reeves, R.V.; Mukasyan, A.S.; Son, S.F. Thermal and impact reaction initiation in Ni/Al heterogeneous reactive systems. J. Phys. Chem. C 2010, 114, 14772–14780. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, M.; Oh, M.C.; Han, D.; Jung, S.-H.; Ahn, B. Exothermic Reaction Kinetics in High Energy Density Al-Ni with Nanoscale Multilayers Synthesized by Cryomilling. Metals 2018, 8, 121. https://doi.org/10.3390/met8020121
Oh M, Oh MC, Han D, Jung S-H, Ahn B. Exothermic Reaction Kinetics in High Energy Density Al-Ni with Nanoscale Multilayers Synthesized by Cryomilling. Metals. 2018; 8(2):121. https://doi.org/10.3390/met8020121
Chicago/Turabian StyleOh, Minseok, Min Chul Oh, Deokhyun Han, Sang-Hyun Jung, and Byungmin Ahn. 2018. "Exothermic Reaction Kinetics in High Energy Density Al-Ni with Nanoscale Multilayers Synthesized by Cryomilling" Metals 8, no. 2: 121. https://doi.org/10.3390/met8020121




