Effects of Zr Additive on Microstructure, Mechanical Properties, and Fractography of Al-Si Alloy
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
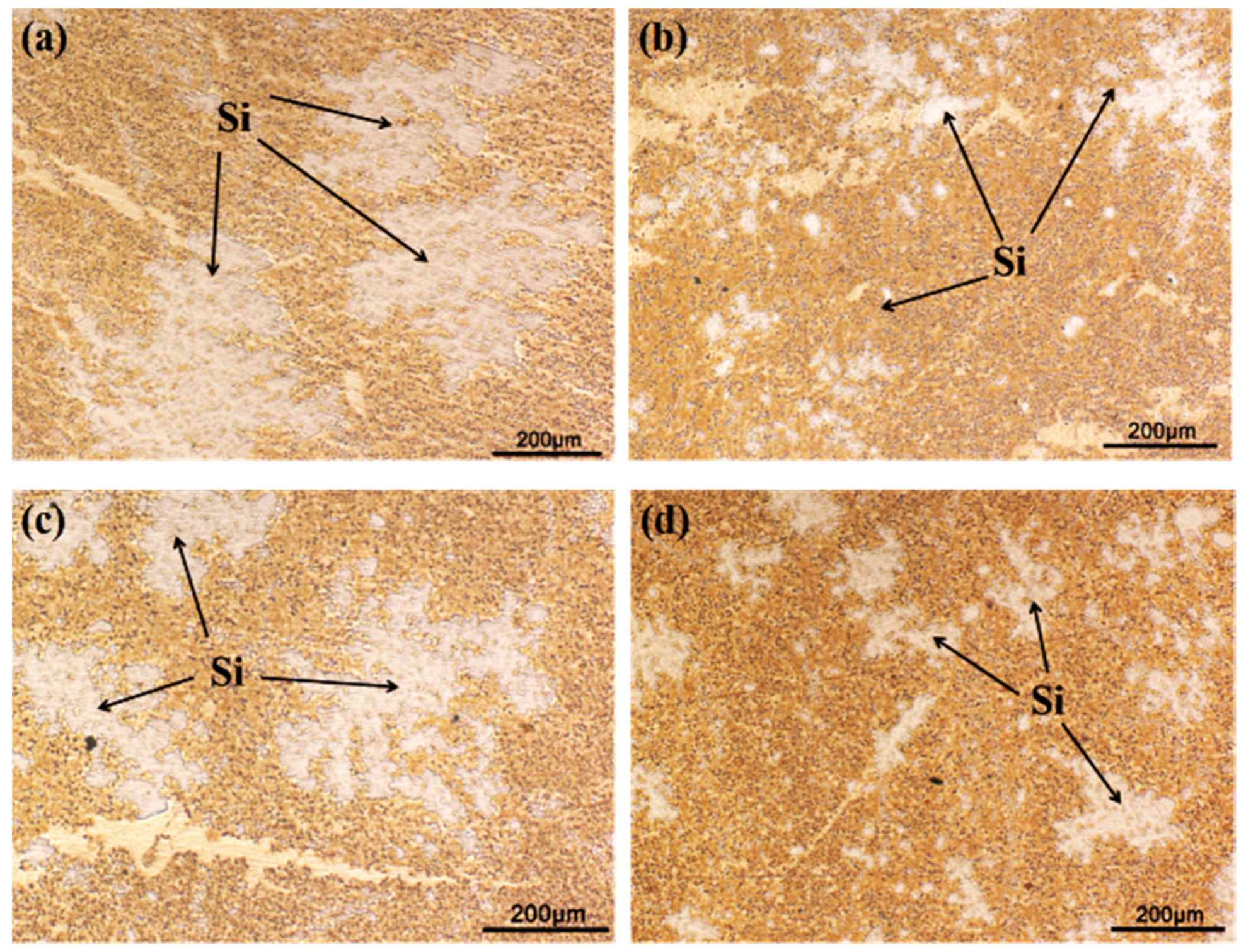
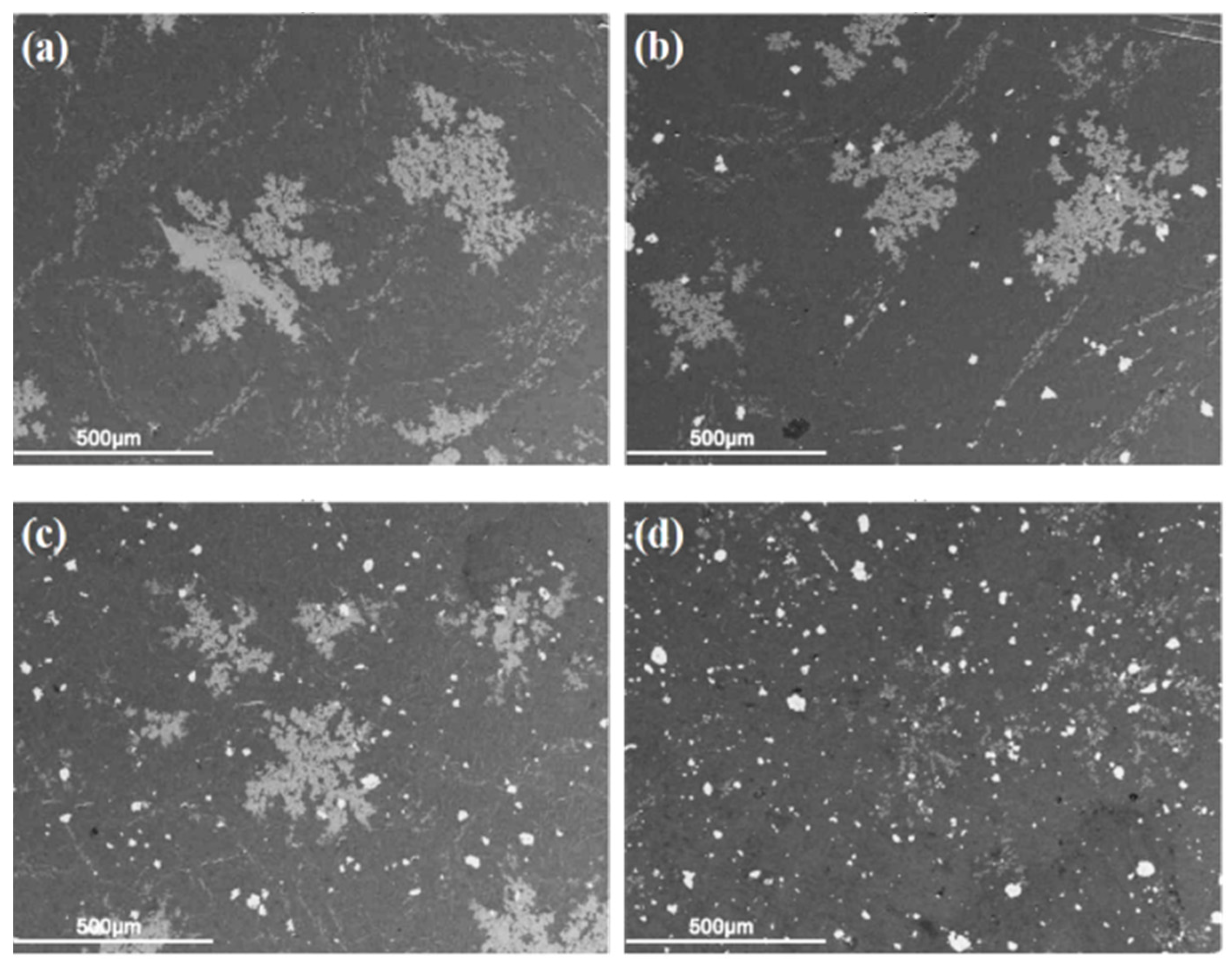
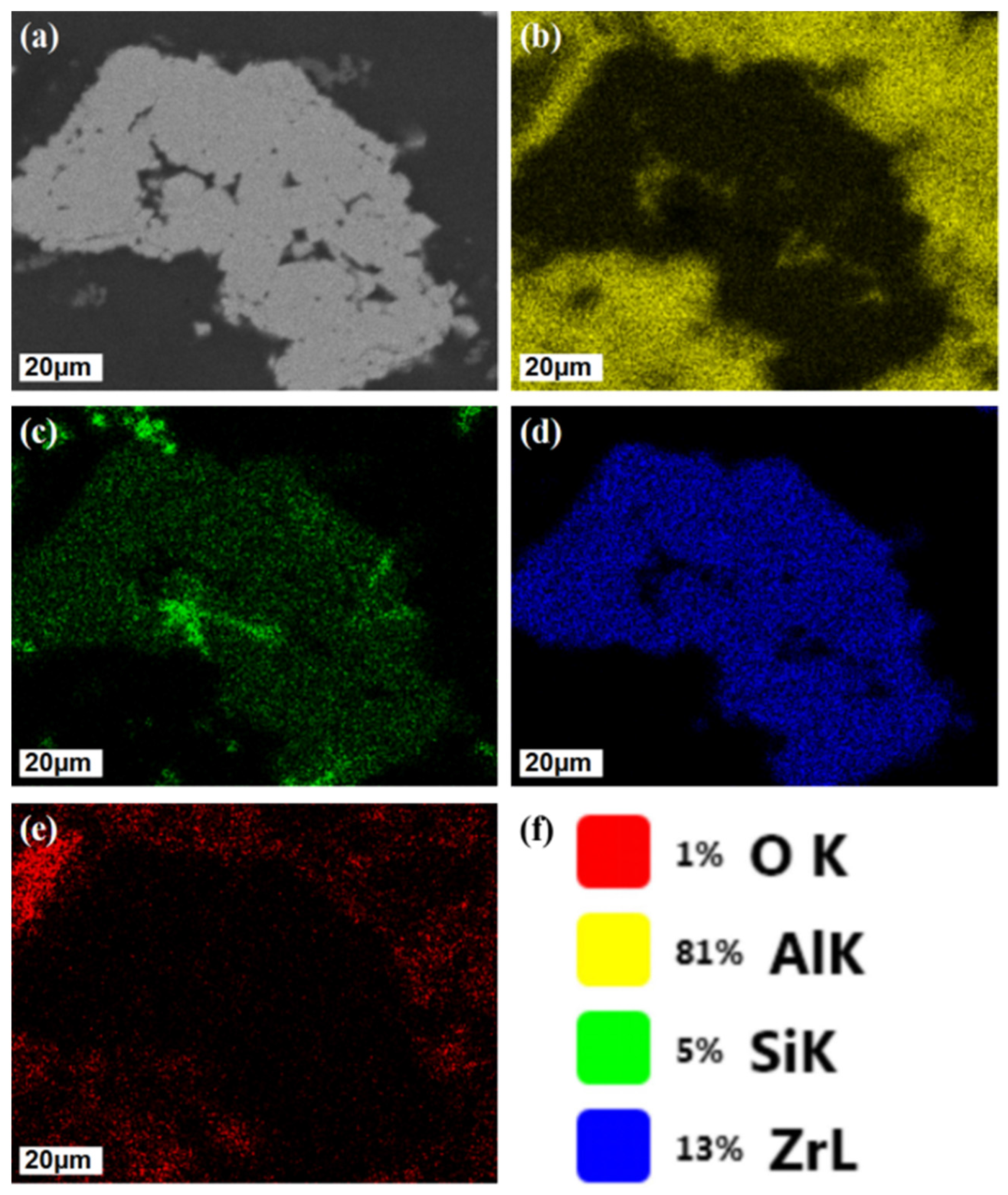
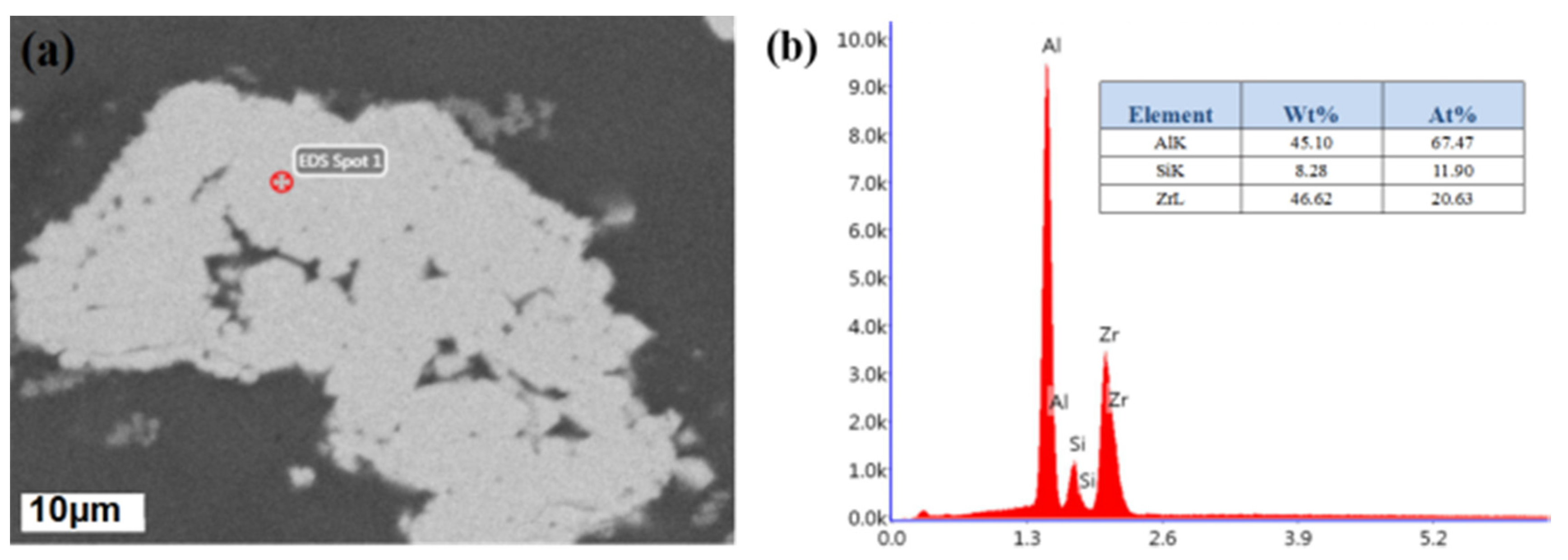
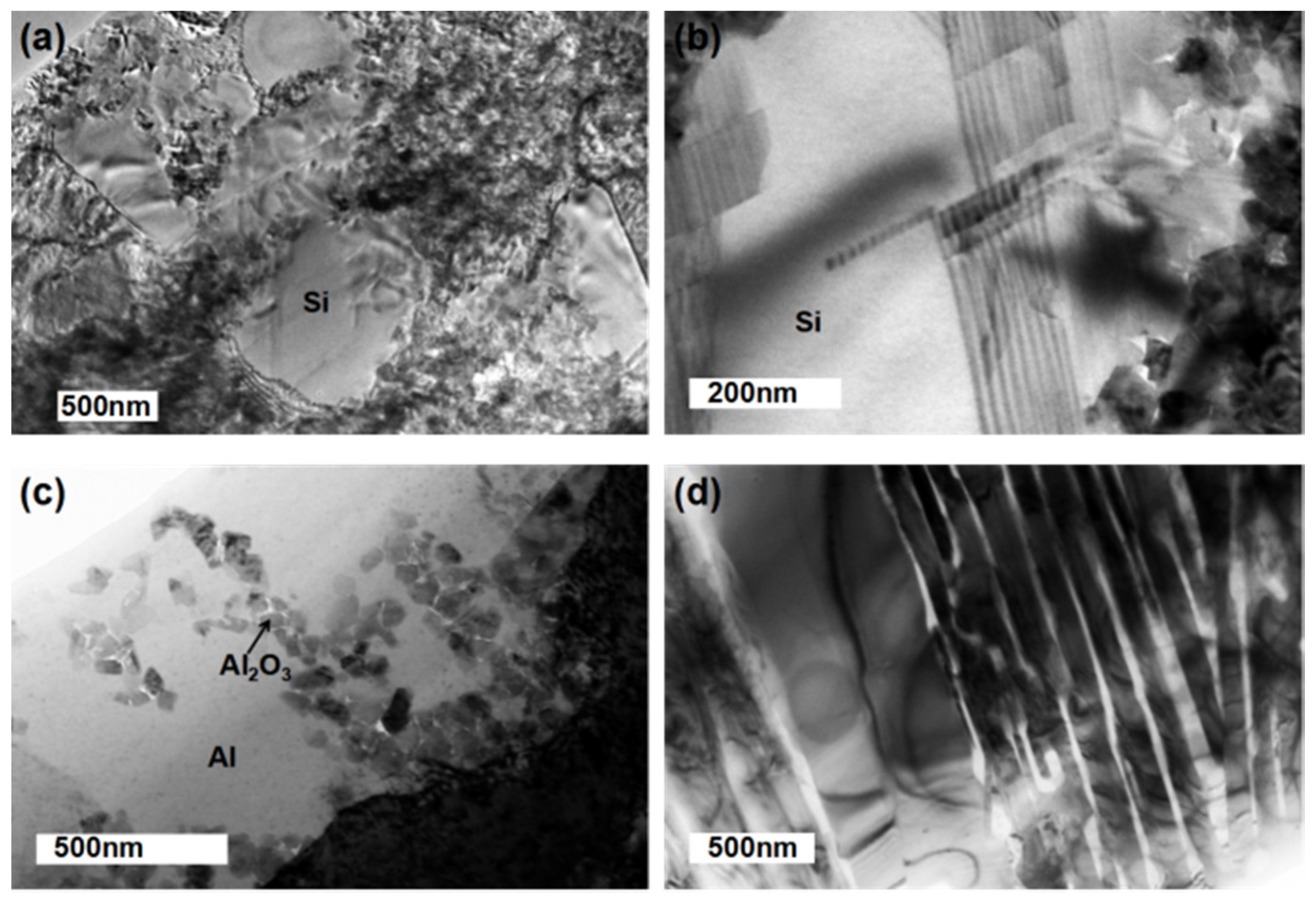
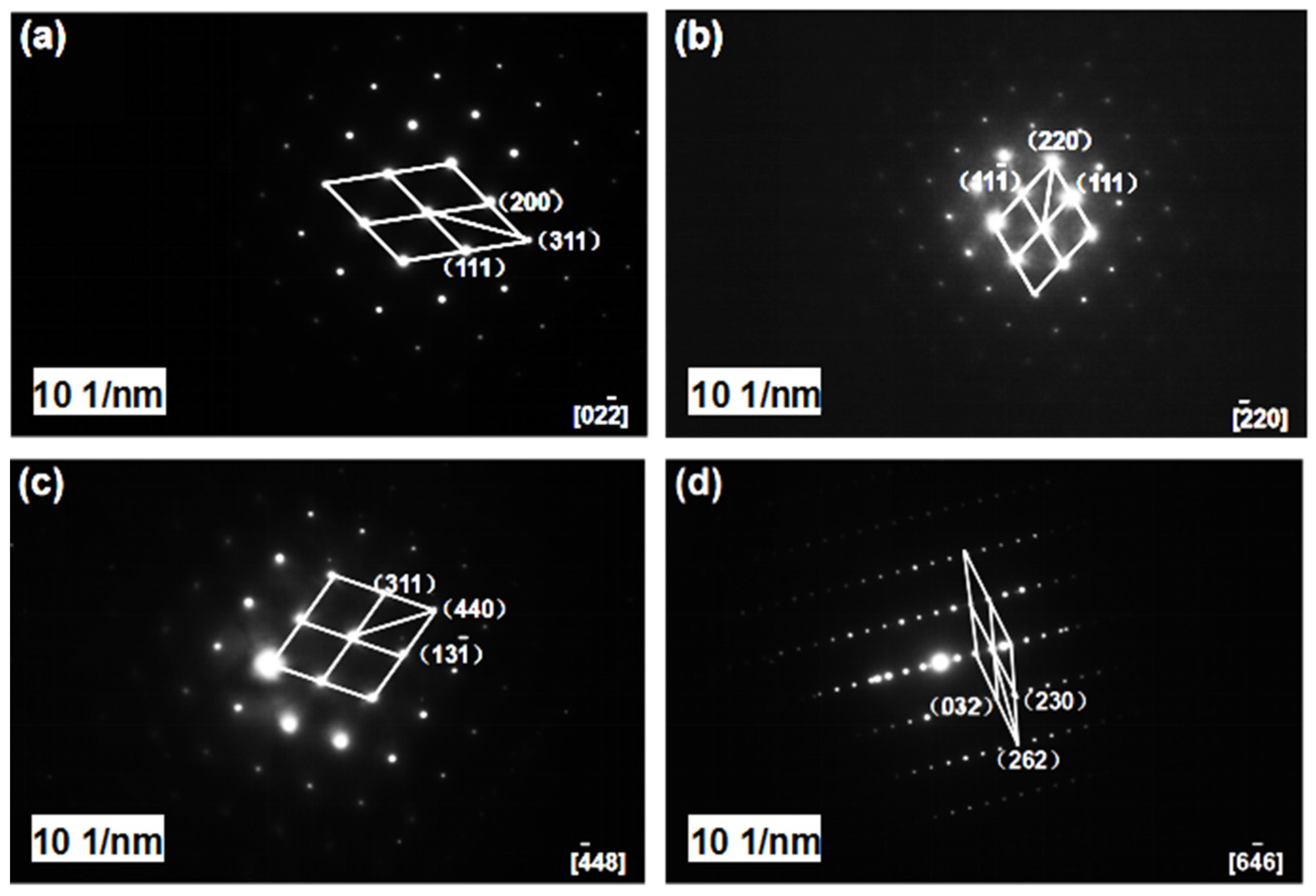
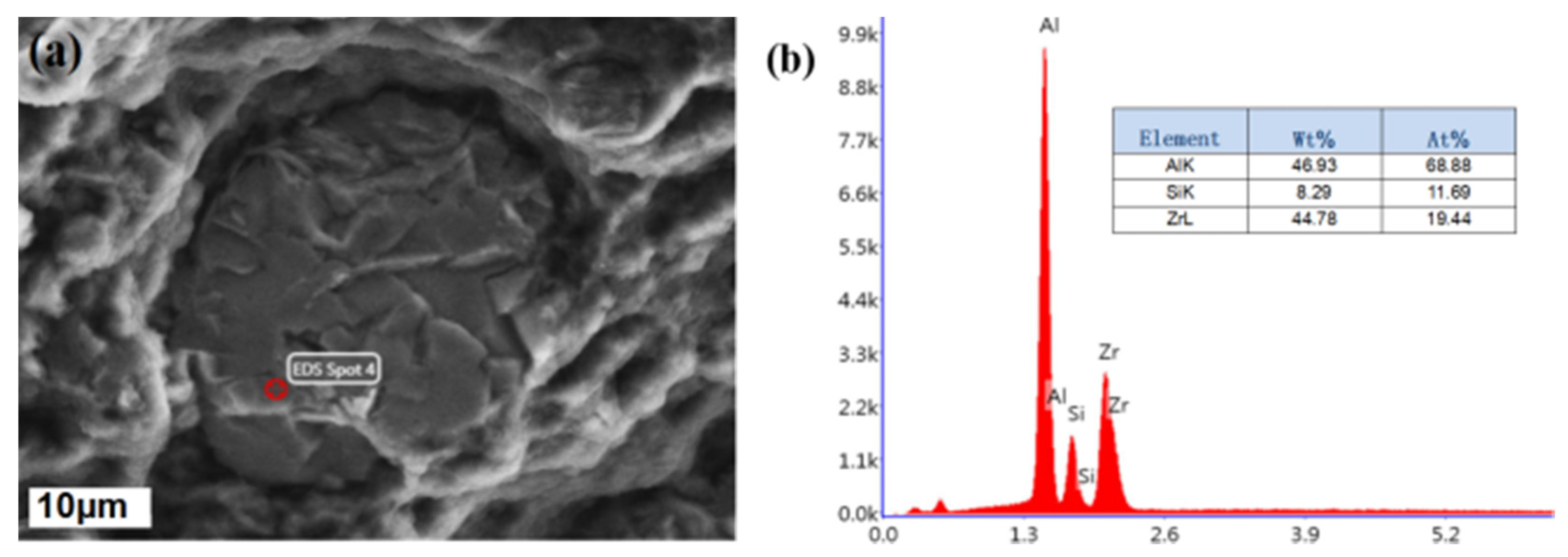
3.1. Micro-Structure Characterization
3.2. Mechanical Properties
3.3. Fractography
4. Conclusions
- (1)
- The size of Si particles was significantly reduced and Si was distributed more homogeneously within Al-Si alloy.
- (2)
- The mechanical properties were improved significantly by adding Zr. The best results were for Zr content of 2.4 wt %. The tensile strength, compressive strength, shear strength, and hardness of Al-Si alloy with 2.4 wt % Zr reached 231.1 MPa, 343.1 MPa, 174.9 MPa, and 85.5 HV, respectively.
- (3)
- The fracture surface of Al-Si alloy without Zr additive showed a brittle fracture mode and no pits in the surface. When Zr was added, the Al-Si alloys exhibited a typical plastic deformation and a large amount of pits were formed.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miracle, D.B. Metal matrix composites–From science to technological significance. Compos. Sci. Technol. 2005, 65, 2526–2540. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.D.; Kainer, K.U. Intermetallics in Magnesium Alloys. Adv. Eng. Mater. 2006, 8, 235–240. [Google Scholar] [CrossRef]
- Guo, Z.X.; Derby, B. Solid-state fabrication and interfaces of fibre reinforced metal matrix composites. Prog. Mater. Sci. 1995, 39, 411–495. [Google Scholar] [CrossRef]
- Harrigan, W.C. Commercial processing of metal matrix composites. Mater. Sci. Eng. A 1998, 244, 75–79. [Google Scholar] [CrossRef]
- Alizadeh, A.; Abdollahi, A.; Rafar, M.J. Processing, characterization, room temperature mechanical properties and fracture behavior of hot extruded multi-scale B4C reinforced 5083 aluminum alloy based composites. Trans. Nonferr. Met. Soc. China 2017, 27, 1233–1247. [Google Scholar] [CrossRef]
- ElSebaie, O.; Samuel, A.M.; Samuel, F.H.; Doty, H.W. The effects of mischmetal, cooling rate and heat treatment on the eutectic Si particle characteristics of A319.1, A356.2 and A413.1 Al–Si casting alloys. Mater. Sci. Eng. A 2008, 480, 342–355. [Google Scholar] [CrossRef]
- Chomsaeng, N.; Haruta, M.; Chairuangsri, T.; Kurata, H.; Isoda, S.; Shiojiri, M. HRTEM and ADF-STEM of precipitates at peak-ageing in cast A356 aluminum alloy. J. Alloys Compd. 2010, 496, 478–487. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chou, C.Y.; Lee, S.L.; Lin, C.K.; Lin, J.C.; Lim, S.W. Effect of trace La addition on the microstructures and mechanical properties of A356 (Al–7Si–0.35Mg) aluminum alloys. J. Alloys Compd. 2009, 487, 157–162. [Google Scholar] [CrossRef]
- Dobrzanski, L.A.; Weodarzyk, A.; Adamiak, M. The structure and properties of PM composite materials based on EN AW-2124 aluminum alloy reinforced with the BN or Al2O3 ceramic particles. J. Mater. Process. Technol. 2006, 175, 186–191. [Google Scholar] [CrossRef]
- Turralba, J.M.; Dacost, C.E.; Velasco, F. P/M aluminum matrix composites: An overview. J. Mater. Process. Technol. 2003, 133, 203–206. [Google Scholar] [CrossRef]
- Kang, H.S.; Yoon, W.Y.; Kim, K.H.; Kim, M.H.; Yoon, Y.P.; Cho, I.S. Effective parameter for the selection of modifying agent for Al–Si alloy. Mater. Sci. Eng. A 2007, 449, 334–337. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Jenn, M.Y. Selective laser melting of TiC reinforced 316L stainless steel matrix nanocomposites: Influence of starting TiC particle size and volume content. Mater. Des. 2016, 104, 141–151. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Jenn, M.Y. Rapid fabrication of bulk-form TiB2/316L stainless steel nanocomposites with novel reinforcement architecture and improved performance by selective laser melting. J. Alloys Compd. 2016, 680, 480–493. [Google Scholar] [CrossRef]
- Emadi, D.; Rao, A.K.P.; Mahfoud, M. Influence of scandium on the microstructure and mechanical properties of A319 alloy. Mater. Sci. Eng. A 2010, 527, 6123–6132. [Google Scholar] [CrossRef]
- Prukkanon, W.; Srisukhumbowornchai, N.; Limmaneevichitr, C. Modification of hypoeutectic Al–Si alloys with scandium. J. Alloys Compd. 2009, 477, 454–460. [Google Scholar] [CrossRef]
- Pandee, P.; Patakharm, U.; Limmaneevichitc, C. Microstructural evolution and mechanical properties of Al-7Si-0.3Mg alloys with erbium additions. J. Alloys Compd. 2017, 728, 844–853. [Google Scholar] [CrossRef]
- Gao, Z.H.; Li, H.Y.; Lai, Y.Q. Effects of minor Zr and Er on microstructure and mechanical properties of pure aluminum. Mater. Sci. Eng. A 2013, 580, 92–98. [Google Scholar] [CrossRef]
- Sepehrband, P.; Mahmudi, R.; Khomamizadeh, F. Effect of Zr addition on the aging behavior of A319 aluminum cast alloy. Scr. Mater. 2005, 52, 253–257. [Google Scholar] [CrossRef]
- Liu, W.Y.; Xiao, W.L.; Xu, C. Synergistic effects of Gd and Zr on grain refinement and eutectic Si modification of Al-Si cast alloy. Mater. Sci. Eng. A 2017, 693, 93–100. [Google Scholar] [CrossRef]
- Slipenyuk, A.; Kuprin, V.; Milman, Y.; Goncharuk, V.; Eckert, J. Properties of P/M processed particle reinforced metal matrix composites specified by reinforcement concentration and matrix-to-reinforcement particle size ratio. Acta Mater. 2006, 54, 157–166. [Google Scholar] [CrossRef]
- Smagorinski, M.E.; Tsantrizos, P.G.; Grenier, S.; Cavasin, A.; Brzezinski, T.; Kim, G. The properties and microstructure of Al-based composites reinforced with ceramic particles. Mater. Sci. Eng. A 1998, 244, 86–90. [Google Scholar] [CrossRef]
- Ryum, N. Precipitation and recrystallization in an A1-0.5 wt % Zr-alloy Precipitation et recristallisation dans un alliage Al-0.5% Zr (en poids) Ausscheidung und rekristallisation in einer AL-0.5gew.% zr-legierung. Acta Metall. 1969, 17, 269–278. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Dispersoid precipitation and process modelling in zirconium containing commercial aluminum alloys. Acta Metall. 2001, 49, 599–613. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, D.; Liu, Z.L. The grain refinement mechanism of cast aluminum by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]


| Alloy | wt % (Al) | wt % (SiO2) | wt % (Zr) |
|---|---|---|---|
| Alloy 1 | 90.4 | 9.6 | 0 |
| Alloy 2 | 89.5 | 9.5 | 1.0 |
| Alloy 3 | 89.1 | 9.4 | 1.5 |
| Alloy 4 | 88.2 | 9.4 | 2.4 |
| Alloy | Theoretical Density (g/cm3) | Actual Density (g/cm3) |
|---|---|---|
| Alloy 1 | 2.78 | 2.78 ± 0.02 |
| Alloy 2 | 2.80 | 2.82 ± 0.02 |
| Alloy 3 | 2.80 | 2.82 ± 0.02 |
| Alloy 4 | 2.81 | 2.81 ± 0.02 |
| Alloy | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | Compressive Strength (MPa) | Shear Strength (MPa) | Hardness (HV) |
|---|---|---|---|---|---|---|
| Alloy 1 | 211.2 ± 7.2 | 89.1 ± 7.9 | 5.7 ± 0.3 | 299.6 ± 22.6 | 151.6 ± 3.7 | 65.2 ± 7.2 |
| Alloy 2 | 224.2 ± 10.2 | 99.1 ± 8.5 | 6.5 ± 0.3 | 337.8 ± 28.8 | 171.3 ± 4.5 | 82.5 ± 10.2 |
| Alloy 3 | 221.6 ± 9.0 | 98.4 ± 6.7 | 6.7 ± 0.2 | 330.6 ± 32.3 | 174.7 ± 7.7 | 79.3 ± 9.4 |
| Alloy 4 | 231.1 ± 15.4 | 114.6 ± 9.7 | 7.4 ± 0.7 | 343.1 ± 24.4 | 174.9 ± 5.5 | 85.5 ± 11.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, H.; Zhu, D.; Hu, C.; Jiang, X. Effects of Zr Additive on Microstructure, Mechanical Properties, and Fractography of Al-Si Alloy. Metals 2018, 8, 124. https://doi.org/10.3390/met8020124
Qian H, Zhu D, Hu C, Jiang X. Effects of Zr Additive on Microstructure, Mechanical Properties, and Fractography of Al-Si Alloy. Metals. 2018; 8(2):124. https://doi.org/10.3390/met8020124
Chicago/Turabian StyleQian, Hui, Degui Zhu, Chunfeng Hu, and Xiaosong Jiang. 2018. "Effects of Zr Additive on Microstructure, Mechanical Properties, and Fractography of Al-Si Alloy" Metals 8, no. 2: 124. https://doi.org/10.3390/met8020124





