The Effect of Interlayer Materials on the Joint Properties of Diffusion-Bonded Aluminium and Magnesium
Abstract
:1. Introduction
2. Experimental Section
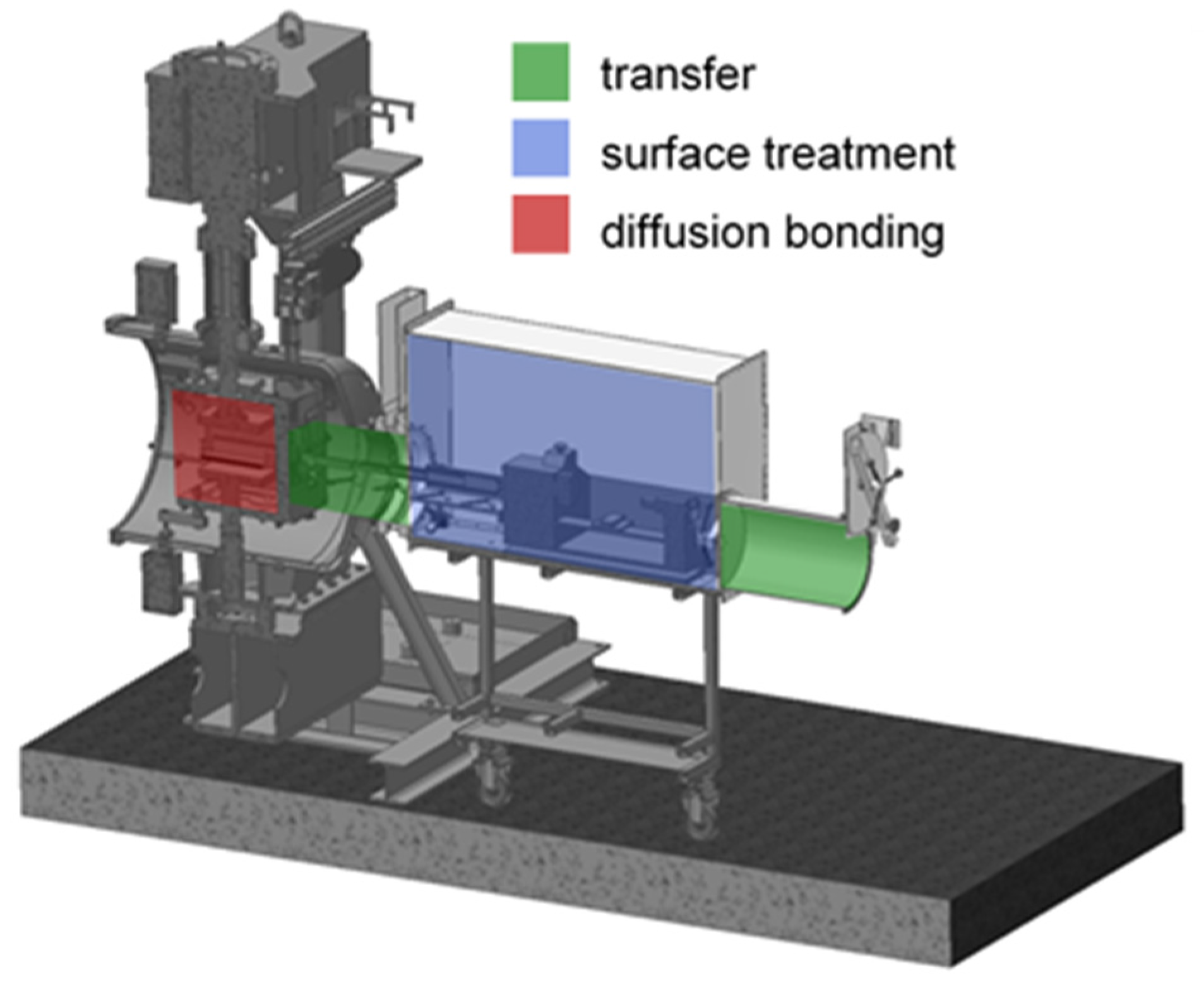
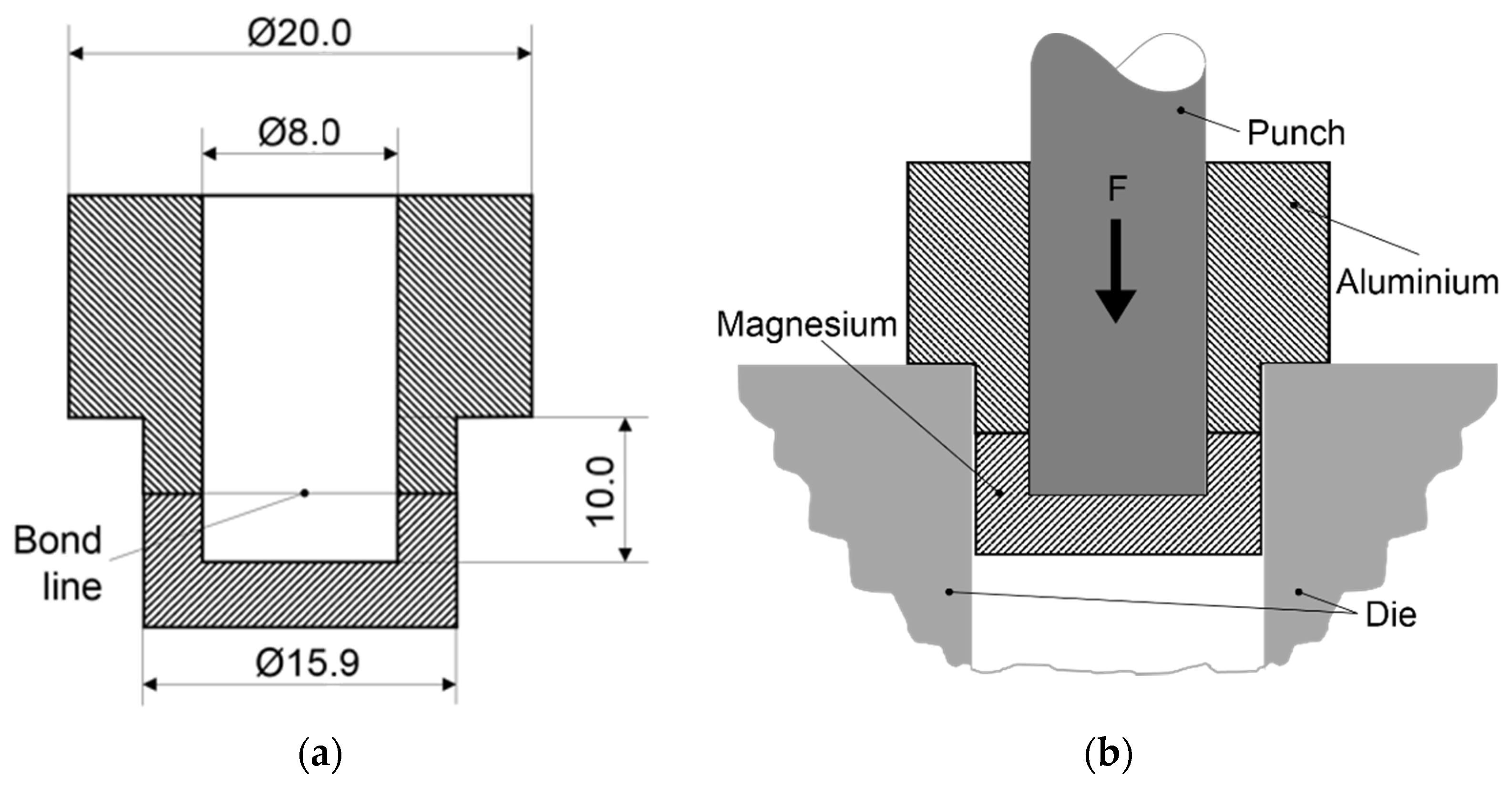
2.1. Materials, Equipment and Process Parameters
2.2. Surface Preparation
2.3. Diffusion Bonding Procedure
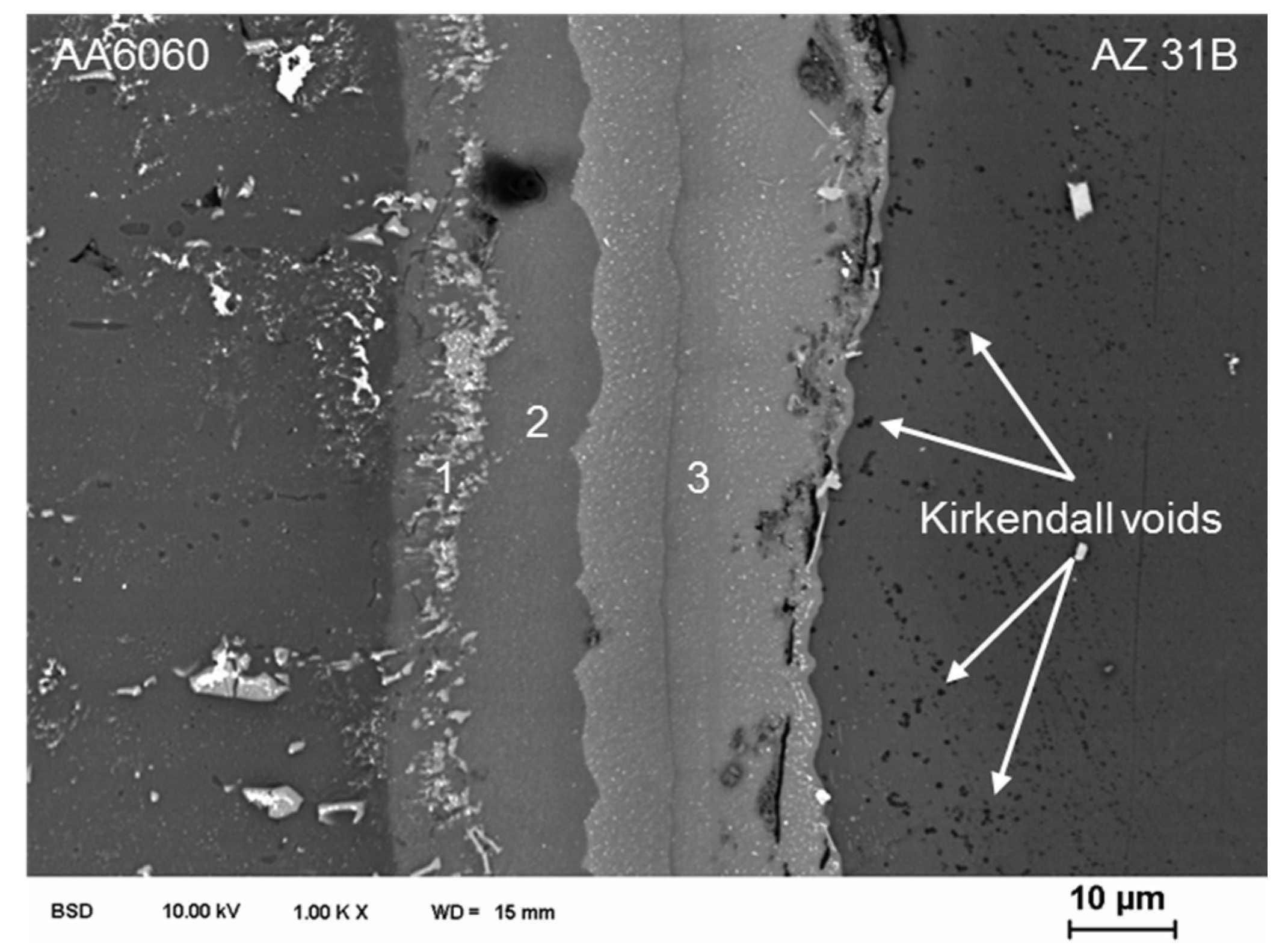
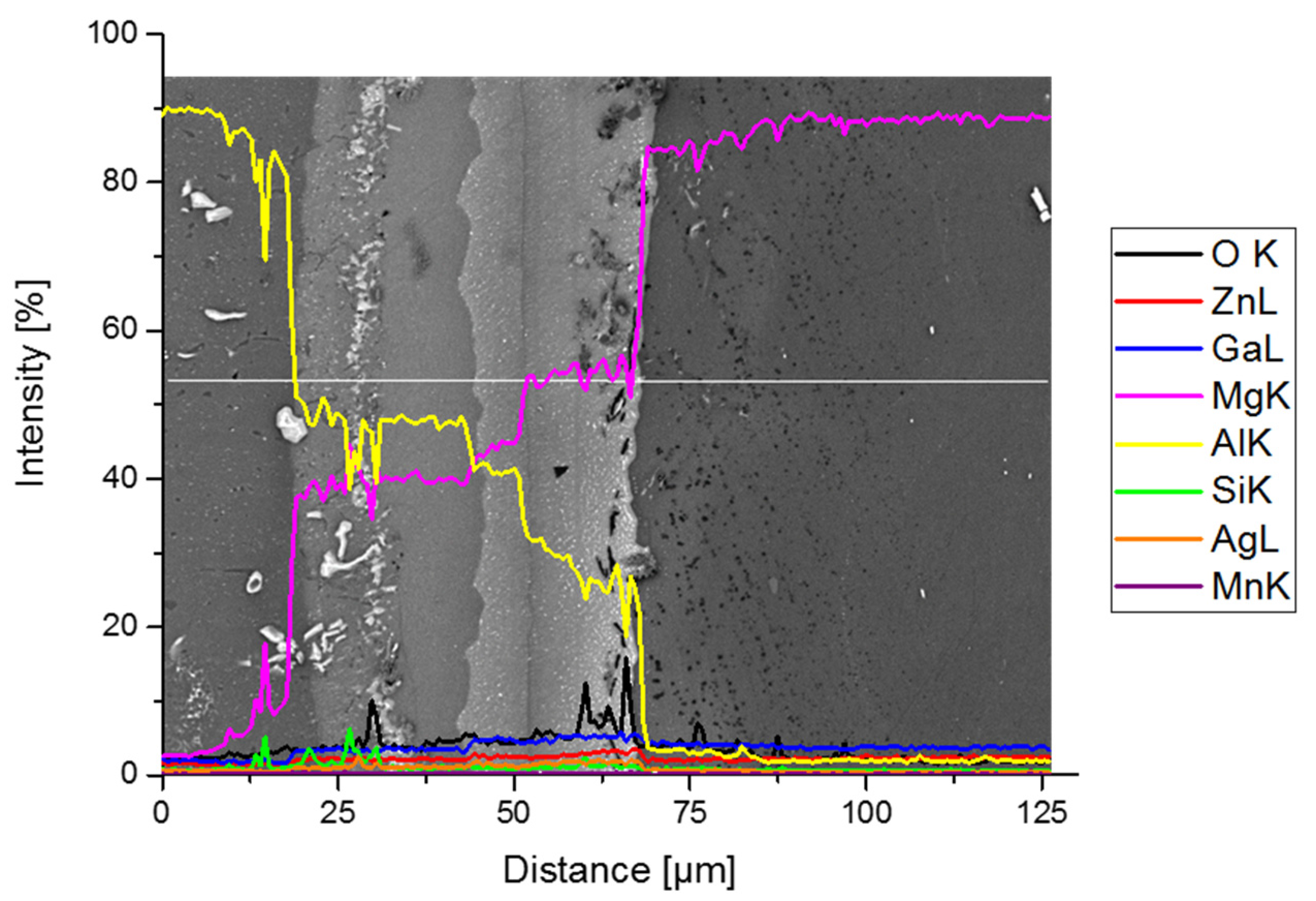
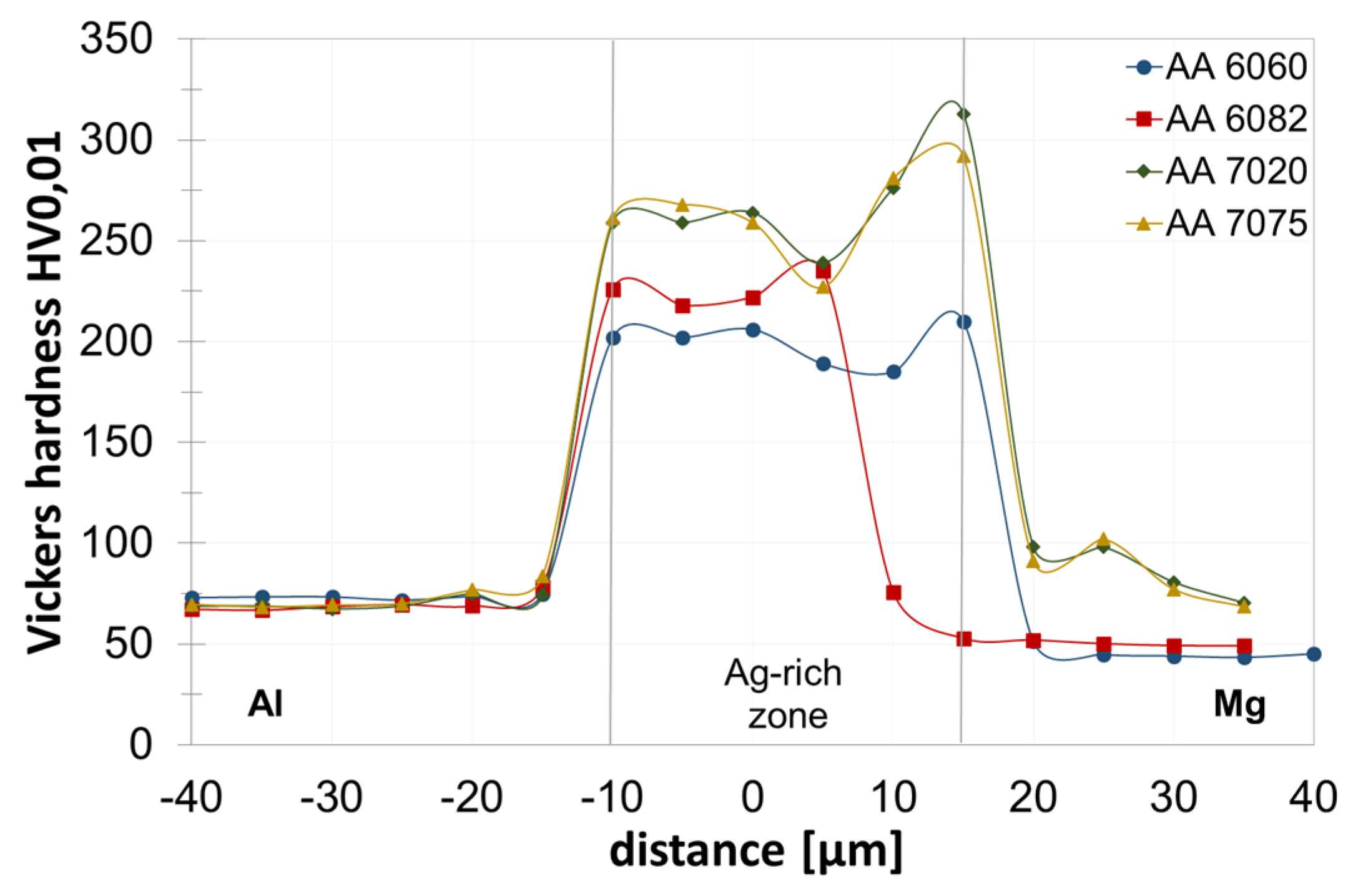
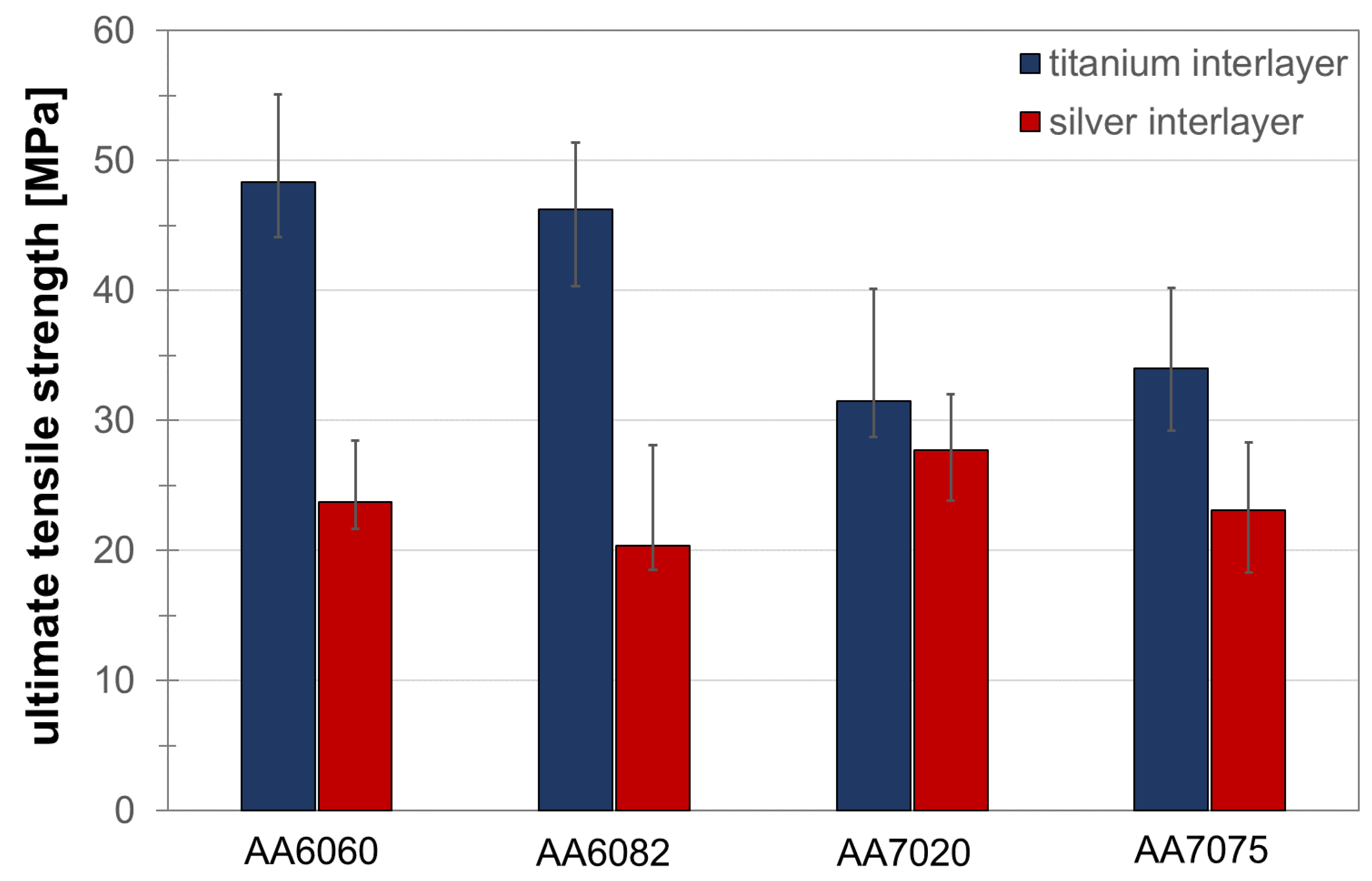
3. Results and Discussion
3.1. Joining Surfaces
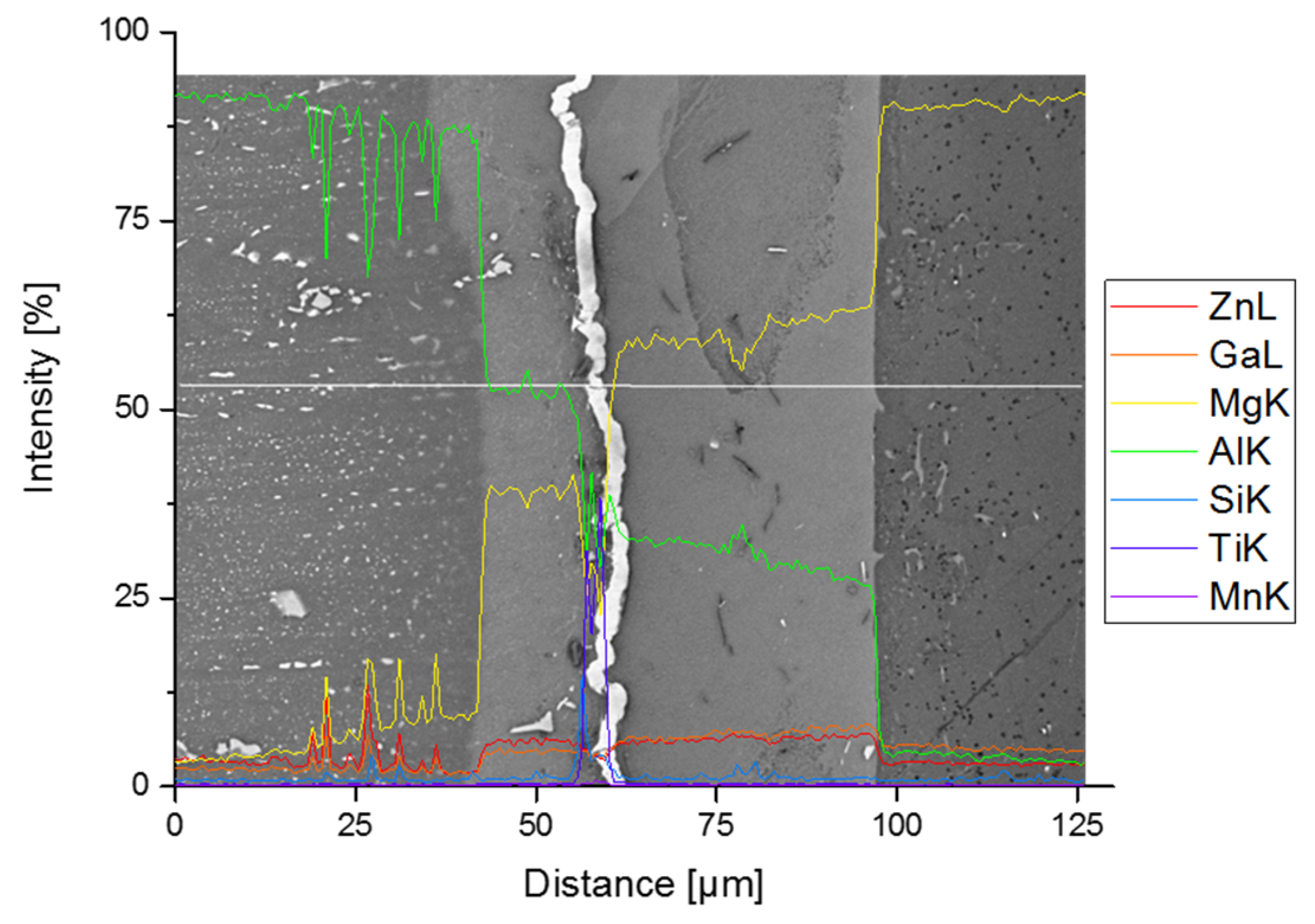
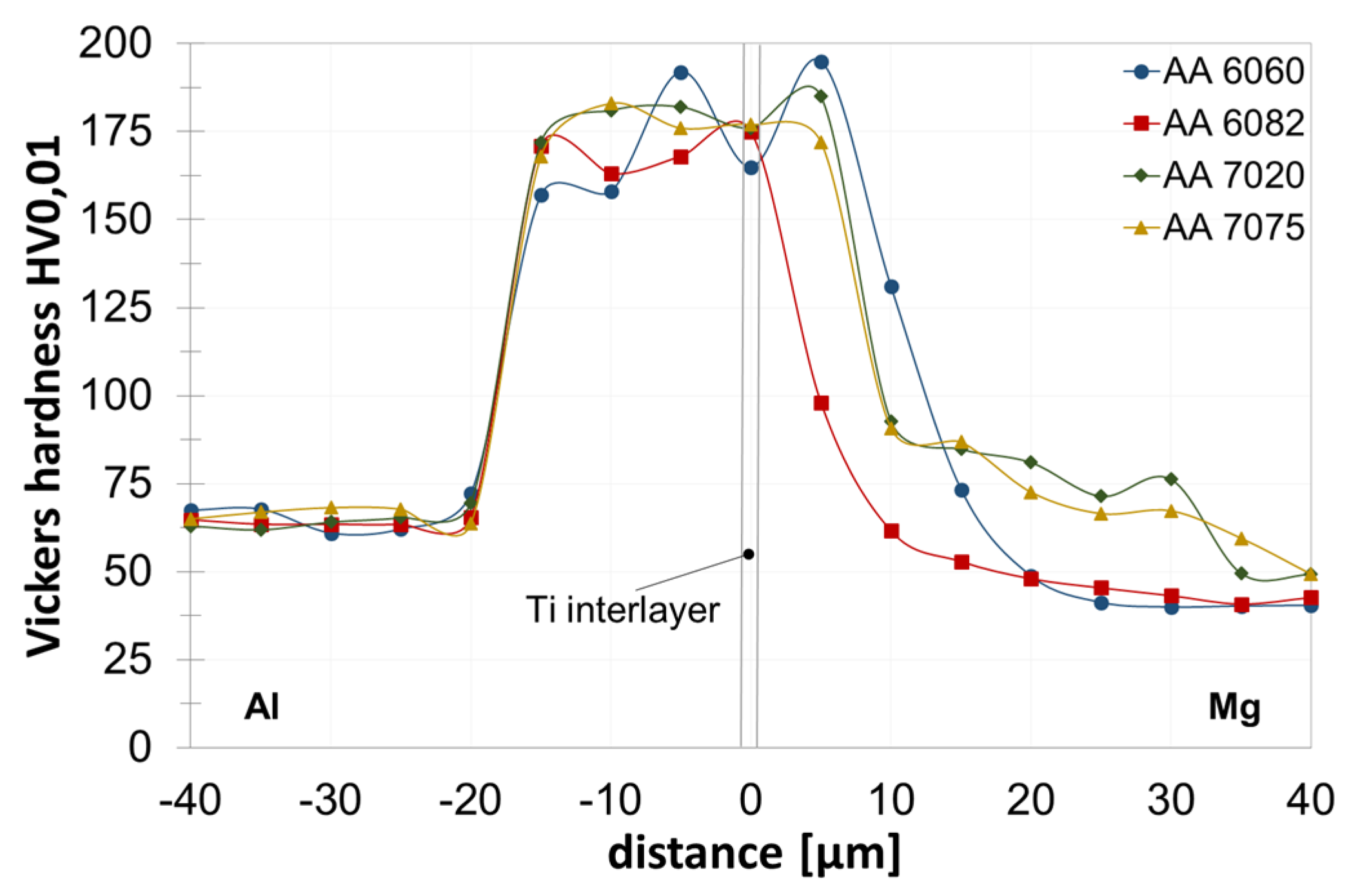
3.2. Joint Properties with Titanium Interlayer
3.3. Joint Properties with Silver Interlayer
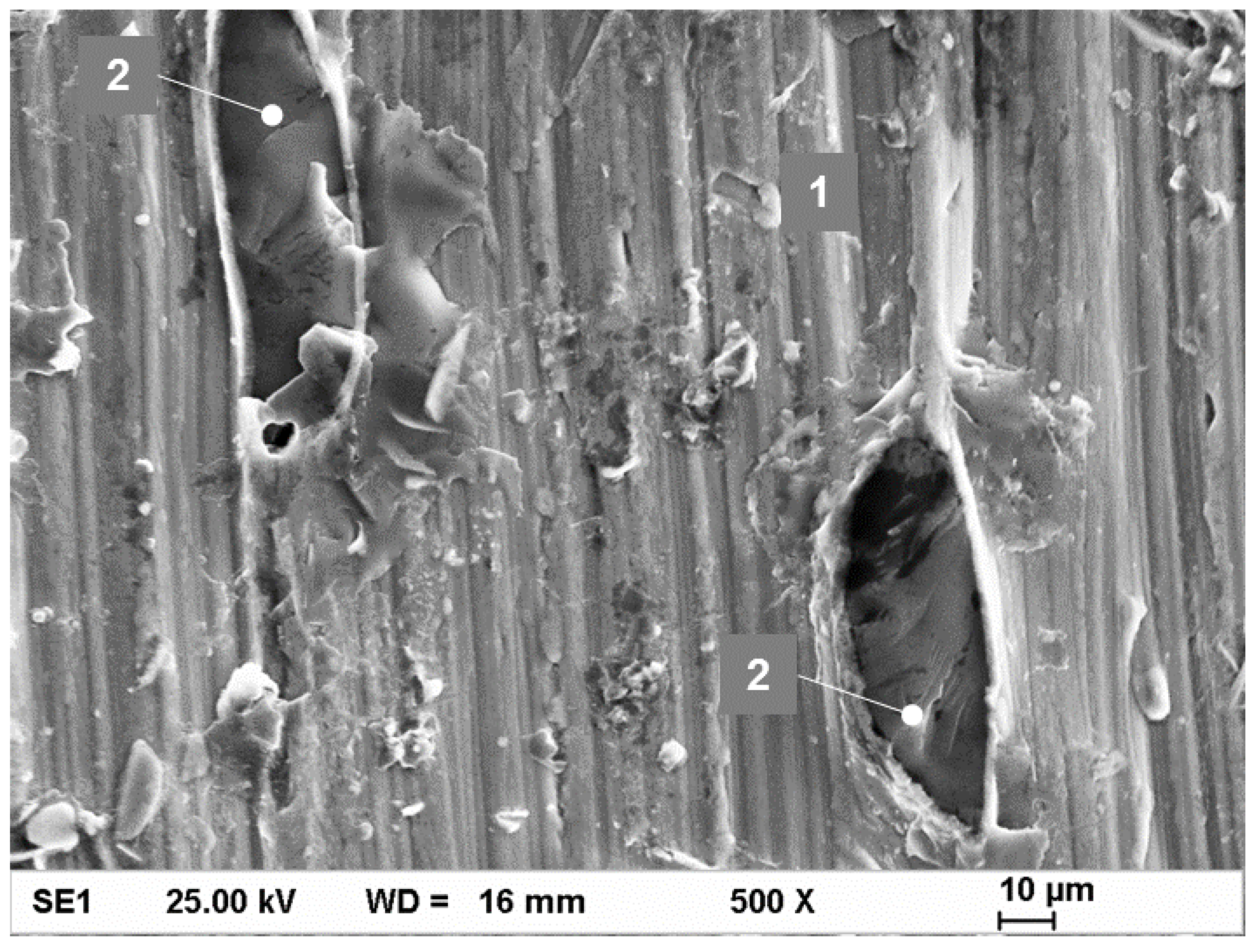
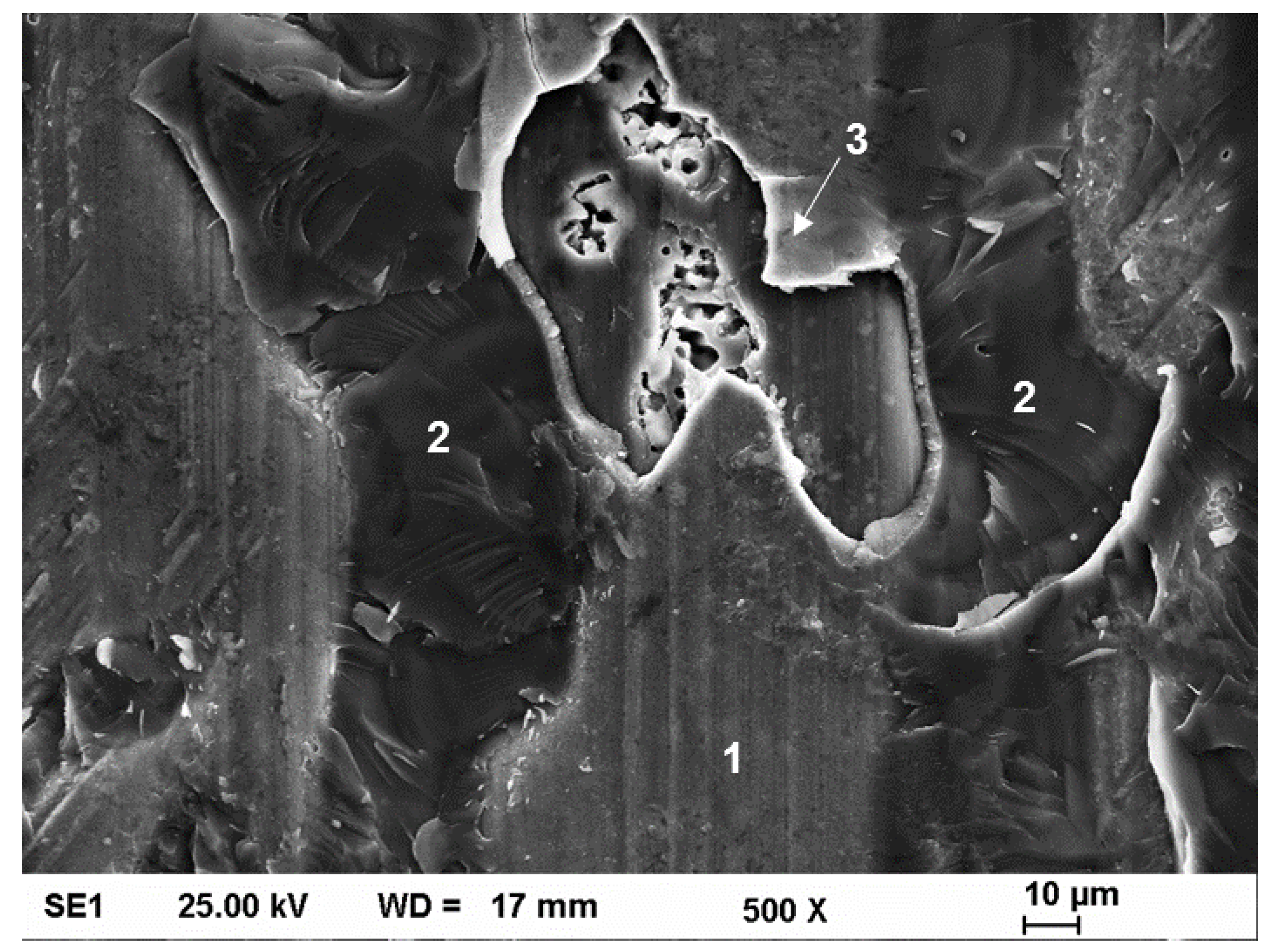
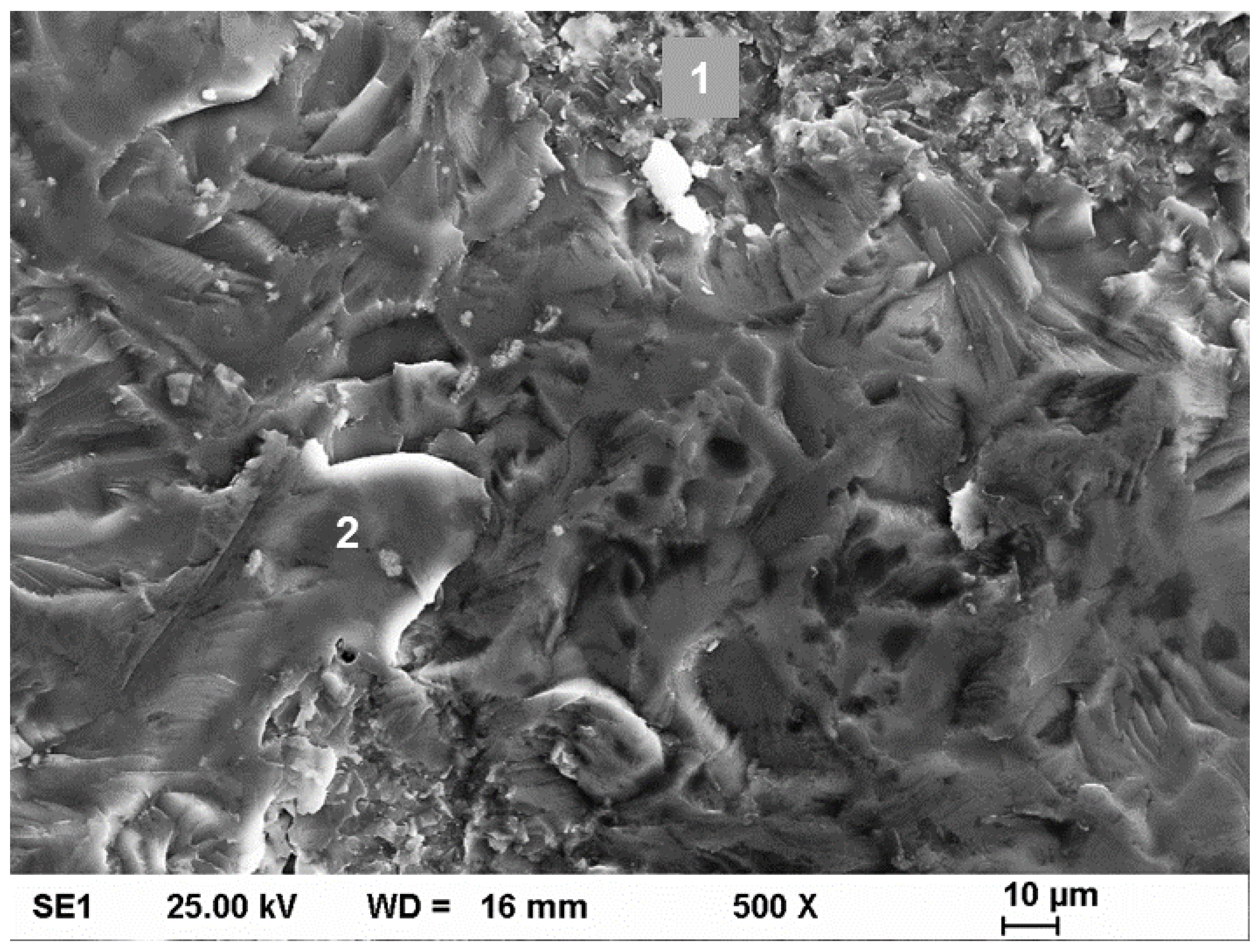
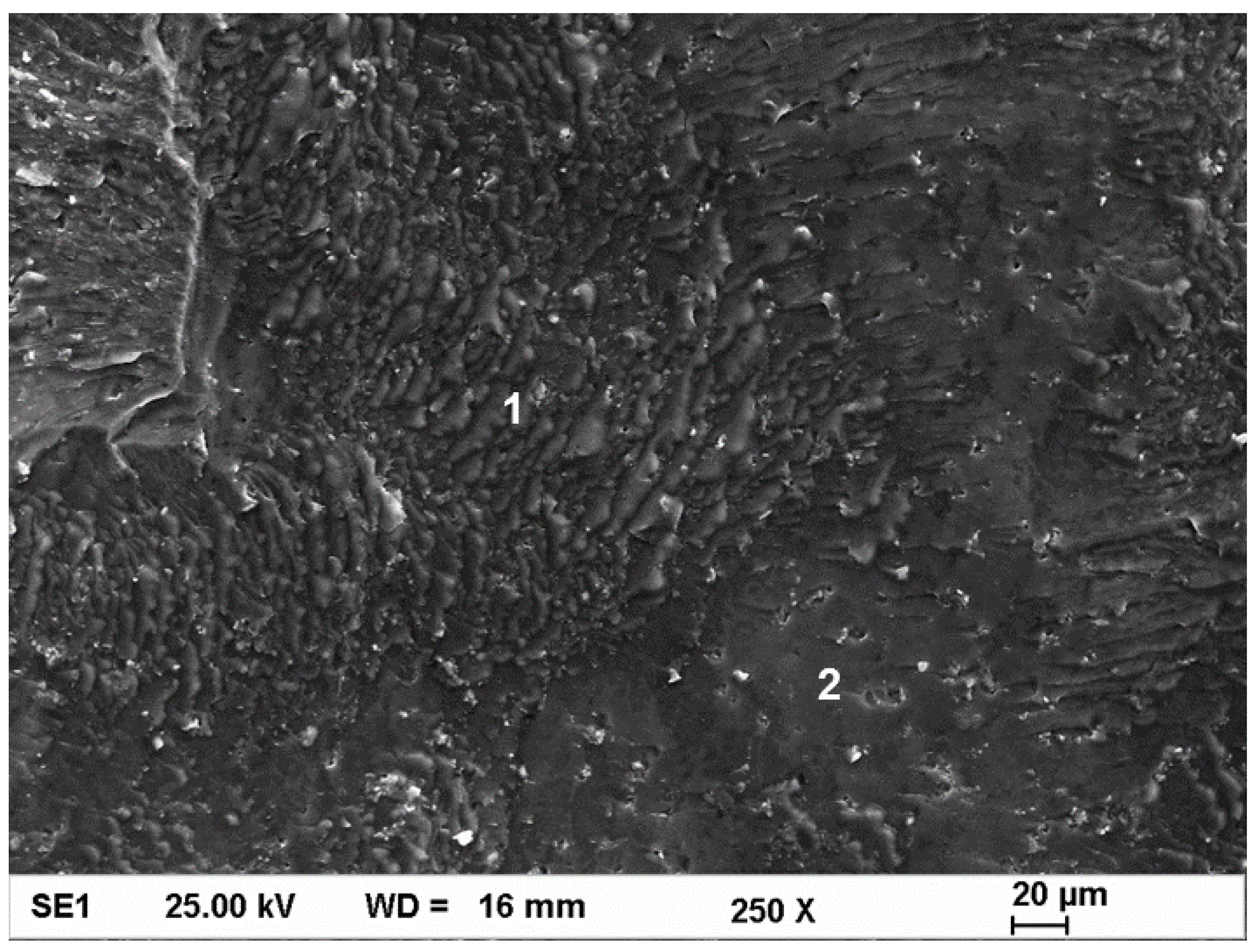
3.4. Tensile Testing and Fractography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shirzadi, A.A.; Assadi, H.; Wallach, E.R. Interface evolution and bond strength when diffusion bonding materials with stable oxide films. Surf. Interface Anal. 2001, 31, 609–618. [Google Scholar] [CrossRef]
- Huang, Y.; Humphreys, F.J.; Ridley, N.; Wang, C. Diffusion bonding of hot rolled 7075 aluminium alloy. Mater. Sci. Technol. 1998, 14, 405–410. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Shen, T.H.; Esposito, A.P.; Tillotson, T.M. Characterization of an effective cleaning procedure for aluminum alloys: Surface enhanced Raman spectroscopy and zeta potential analysis. J. Colloid Interface Sci. 2005, 282, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Reichelt, S.; Schmidtchen, M.; Schwarz, F.; Kawalla, R.; Krüger, L. Effect of inter-metallic phases on the bonding strength and forming properties of Al/Mg sandwiched composite. Key Eng. Mater. 2014, 622, 467–475. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, G.; Wang, Y.; Shen, Q.; Zhang, L. An investigation on diffusion bonding of aluminium and magnesium using a Ni interlayer. Mater. Lett. 2012, 83, 189–191. [Google Scholar] [CrossRef]
- Liu, L.M.; Zhao, L.M.; Xu, R.Z. Effect of interlayer composition on the microstructure and strength of diffusion bonded Mg/Al joint. Mater. Des. 2009, 30, 4548–4551. [Google Scholar] [CrossRef]
- Liu, L.M.; Zhao, L.M.; Wu, Z.H. Influence of holding time on microstructure and shear strength of Mg–Al alloys joint diffusion bonded with Zn–5Al interlayer. Mater. Sci. Technol. 2011, 27, 1372–1376. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Q.; Shen, Q.; Wang, C.; Zhang, L. Effect of Holding Time on Microstructure and Mechanical Properties of Diffusion-Bonded Mg1/Pure Ag Foil/1060Al Joints. Key Eng. Mater. 2014, 616, 280–285. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Q.; Zhang, J.; Shen, Q.; Zhang, L. Microstructure and mechanical properties of diffusion-bonded Mg–Al joints using silver film as interlayer. Mater. Sci. Eng. A 2013, 559, 868–874. [Google Scholar] [CrossRef]
- Jafarian, M.; Khodabandeh, A.; Manafi, S. Evaluation of diffusion welding of 6061 aluminum and AZ31 magnesium alloys without using an interlayer. Mater. Des. 2015, 65, 160–164. [Google Scholar] [CrossRef]
- Magnesium—Otto Fuchs GmbH. Available online: https://www.otto-fuchs.com/en/competence/materials/magnesium.html (accessed on 29 November 2017).
- Bikar Aluminium GmbH. Available online: http://www.bikar.com/aluminium-round-bars.html (accessed on 19 January 2018).
- Mayr, P.; Habisch, S.; Haelsig, A.; Georgi, W. Challenges of joining lightweight materials for dissimilar joints. In Proceedings of the 10th International Conference on Trends in Welding Research and 9th International Welding Symposium of Japan Welding Society (9WS), Tokyo, Japan, 11–14 October 2016. [Google Scholar]
- Zatorski, Z. Evaluation of steel clad plate weldability using ram tensile test method. Mater. Trans. 2007, 3, 229–238. [Google Scholar]
- Dietrich, D.; Nickel, D.; Krause, M. ; Lampke; T.; Coleman, M.P.; Randle, V. Formation of intermetallic phases in diffusion-welded joints of aluminium and magnesium alloys. J. Mater. Sci. 2011, 46, 357354. [Google Scholar] [CrossRef]


| Materials | Chemical Composition in wt % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Mg | Zn | Si | Cu | Mn | Fe | Ti | Cr | |
| AA 6060 | bal. | 0.35–0.6 | 0.15 | 0.3–0.6 | 0.1 | 0.1 | 0.1–0.3 | 0.1 | 0.05 |
| AA 6082 | bal. | 0.6–1.2 | 0.2 | 0.7–1.3 | 0.1 | 0.4–1.0 | 0.5 | 0.1 | 0.25 |
| AA 7020 | bal. | 1.0–1.4 | 4.0–5.0 | 0.35 | 0.2 | 0.05–0.5 | 0.4 | - | 0.1–0.35 |
| AA 7075 | bal. | 2.1–2.9 | 5.1–6.1 | 0.4 | 1.2–2.0 | 0.3 | 0.5 | 0.2 | 0.18–0.28 |
| AZ 31 B | 2.8–3.0 | bal. | 1.0 | - | - | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habisch, S.; Böhme, M.; Peter, S.; Grund, T.; Mayr, P. The Effect of Interlayer Materials on the Joint Properties of Diffusion-Bonded Aluminium and Magnesium. Metals 2018, 8, 138. https://doi.org/10.3390/met8020138
Habisch S, Böhme M, Peter S, Grund T, Mayr P. The Effect of Interlayer Materials on the Joint Properties of Diffusion-Bonded Aluminium and Magnesium. Metals. 2018; 8(2):138. https://doi.org/10.3390/met8020138
Chicago/Turabian StyleHabisch, Stefan, Marcus Böhme, Siegfried Peter, Thomas Grund, and Peter Mayr. 2018. "The Effect of Interlayer Materials on the Joint Properties of Diffusion-Bonded Aluminium and Magnesium" Metals 8, no. 2: 138. https://doi.org/10.3390/met8020138





