An Efficient Approach to Address Issues of Graphene Nanoplatelets (GNPs) Incorporation in Aluminium Powders and Their Compaction Behaviour
Abstract
:1. Introduction
2. Materials and Methods
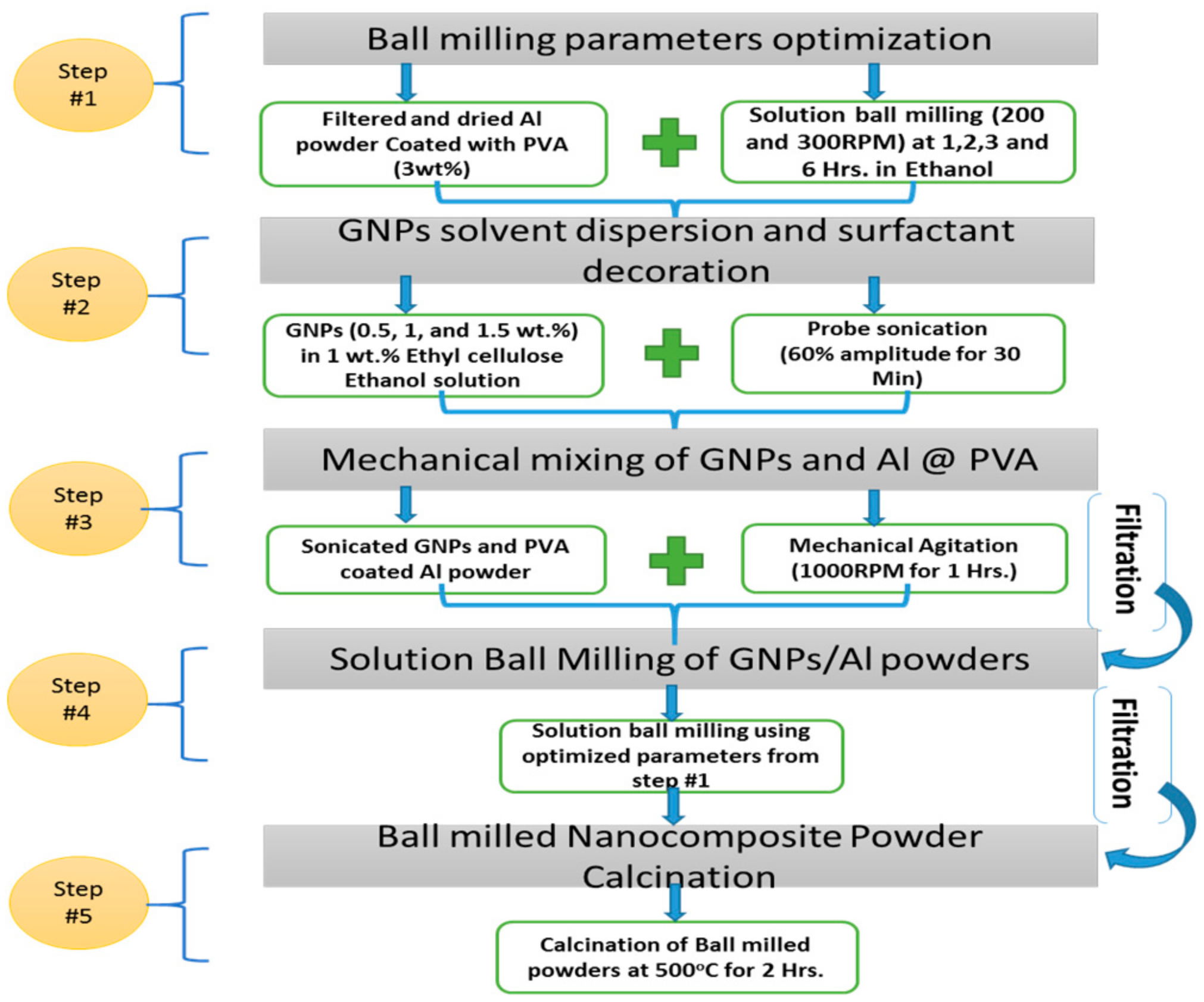
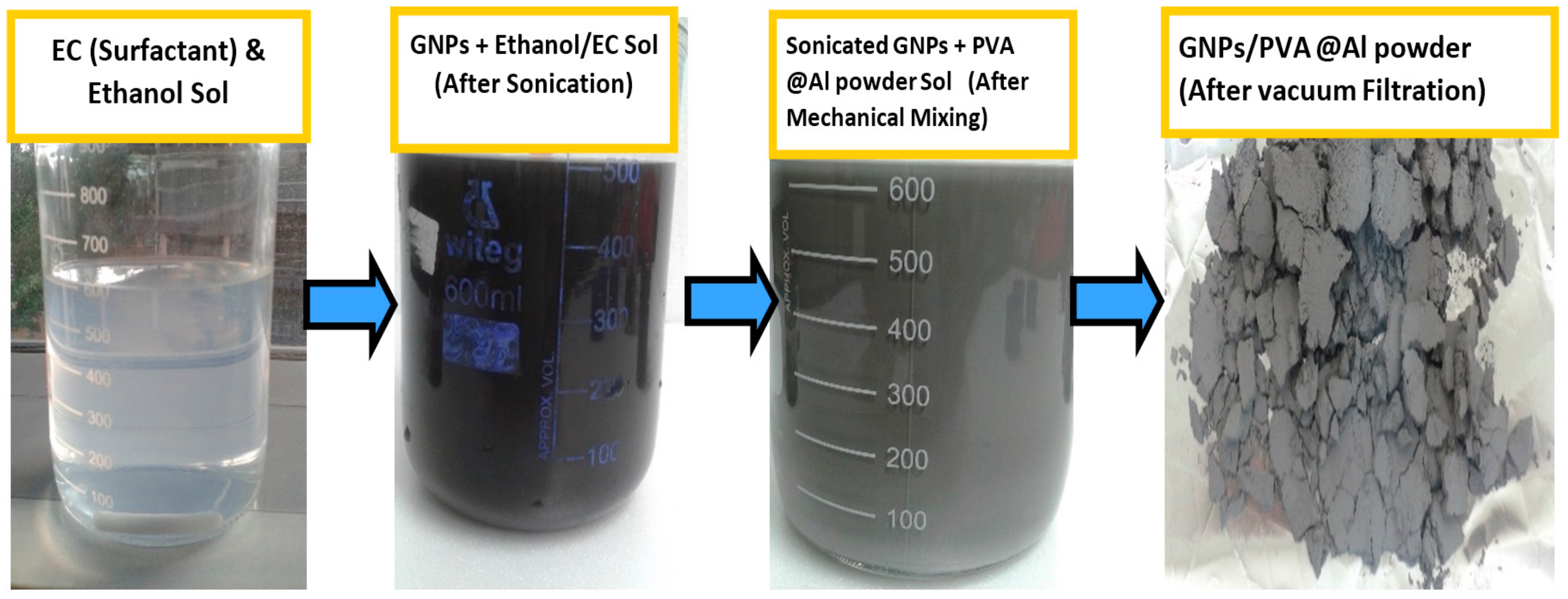
2.1. GNPs/Al Nanocomposite Powder and Samples Formation
2.2. GNPs/Al Nanocomposite Characterization
3. Results and Discussion
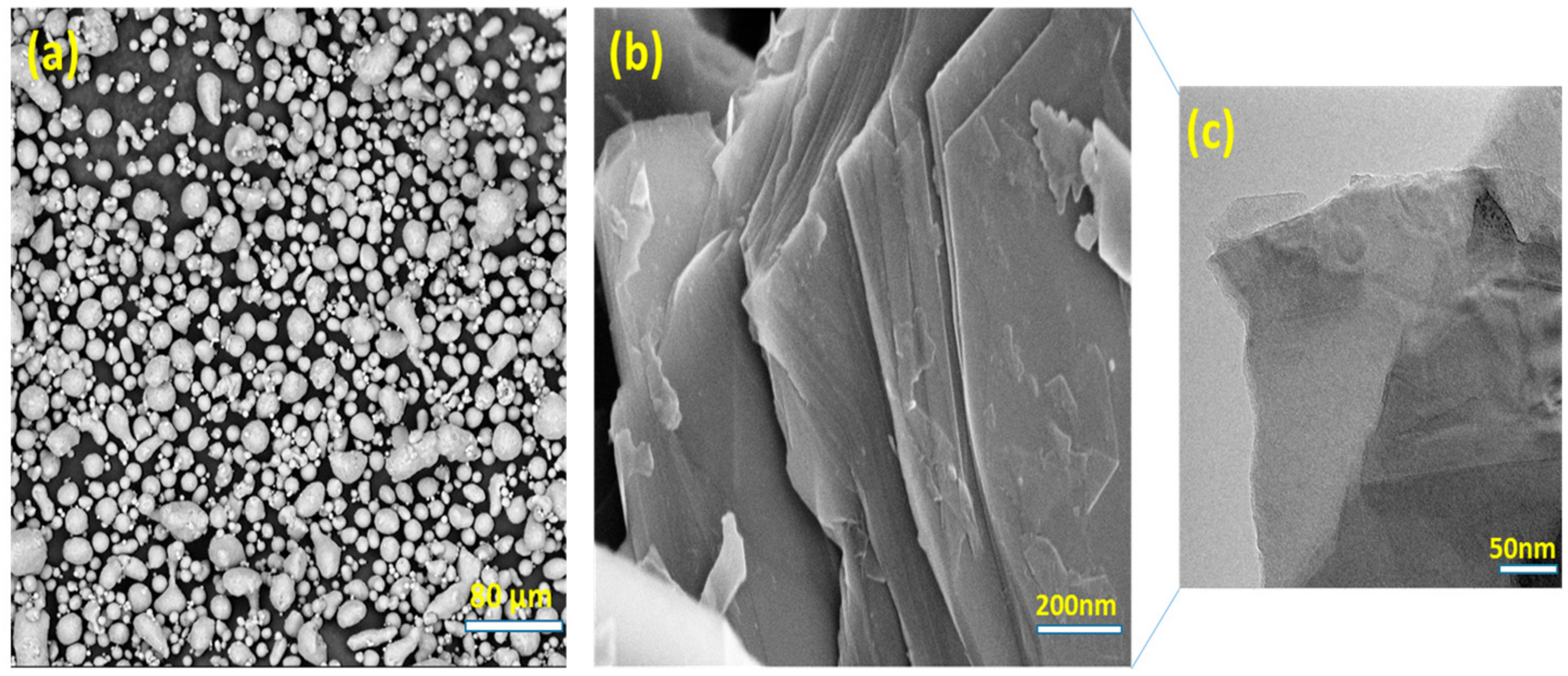
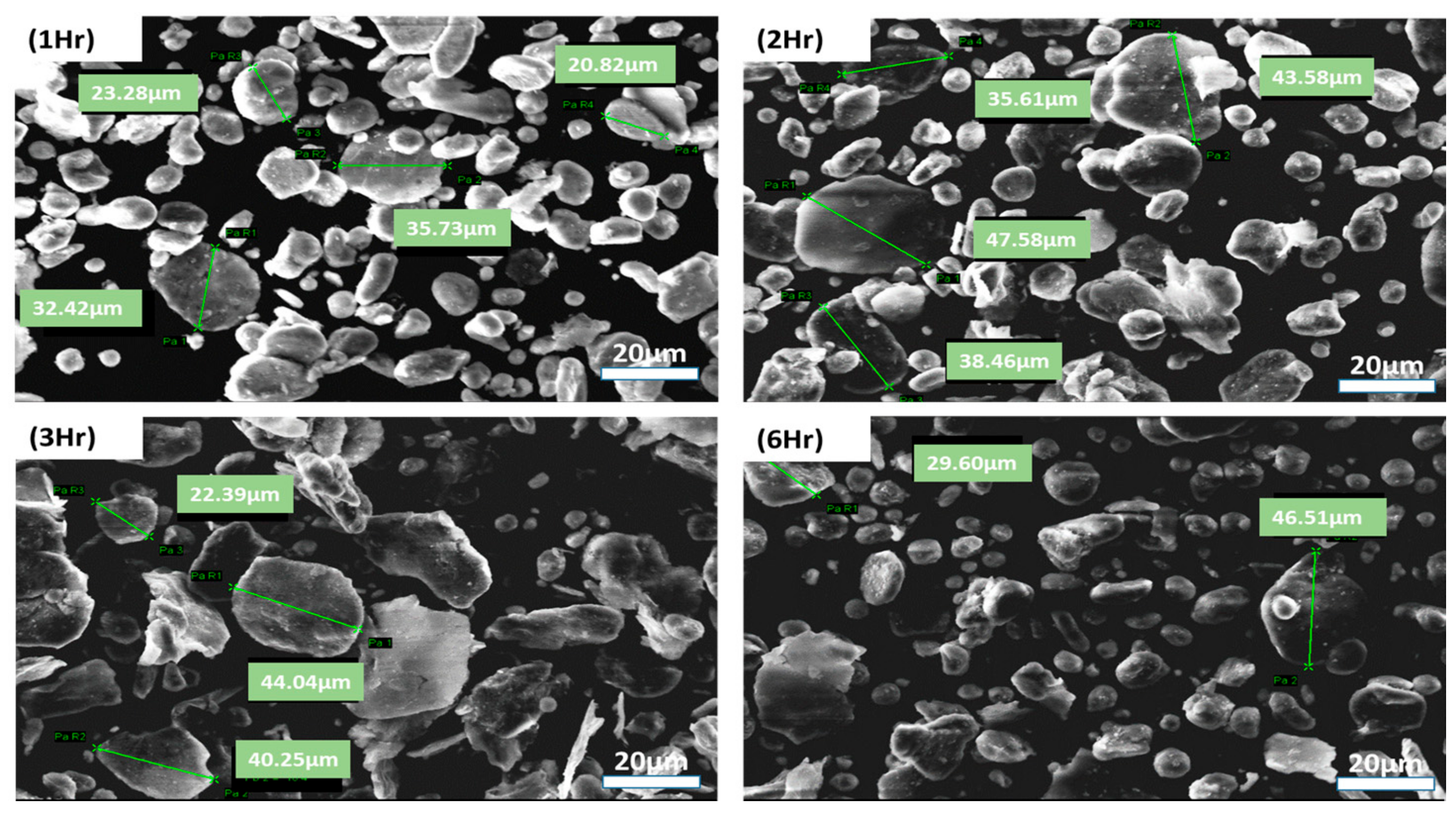
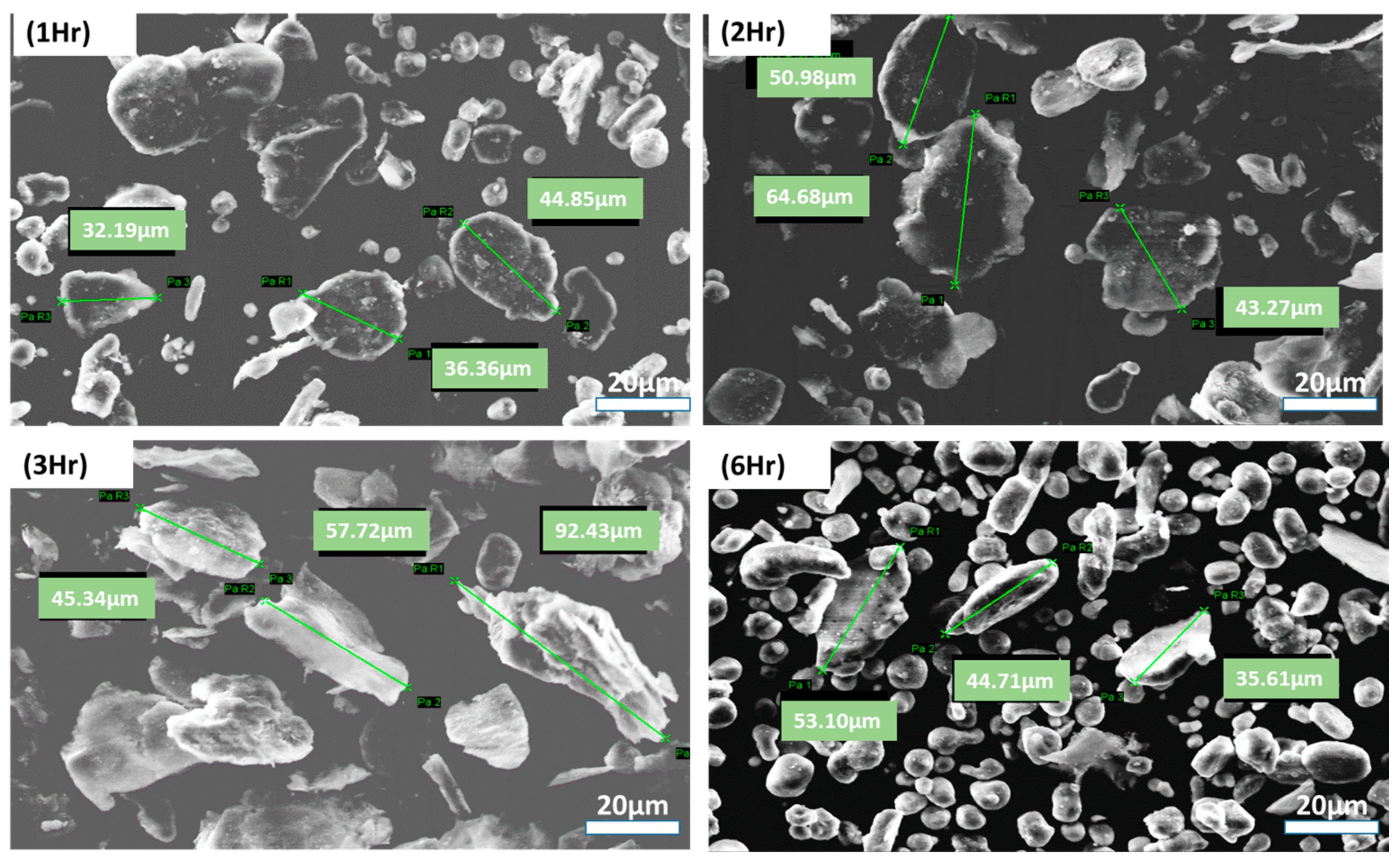
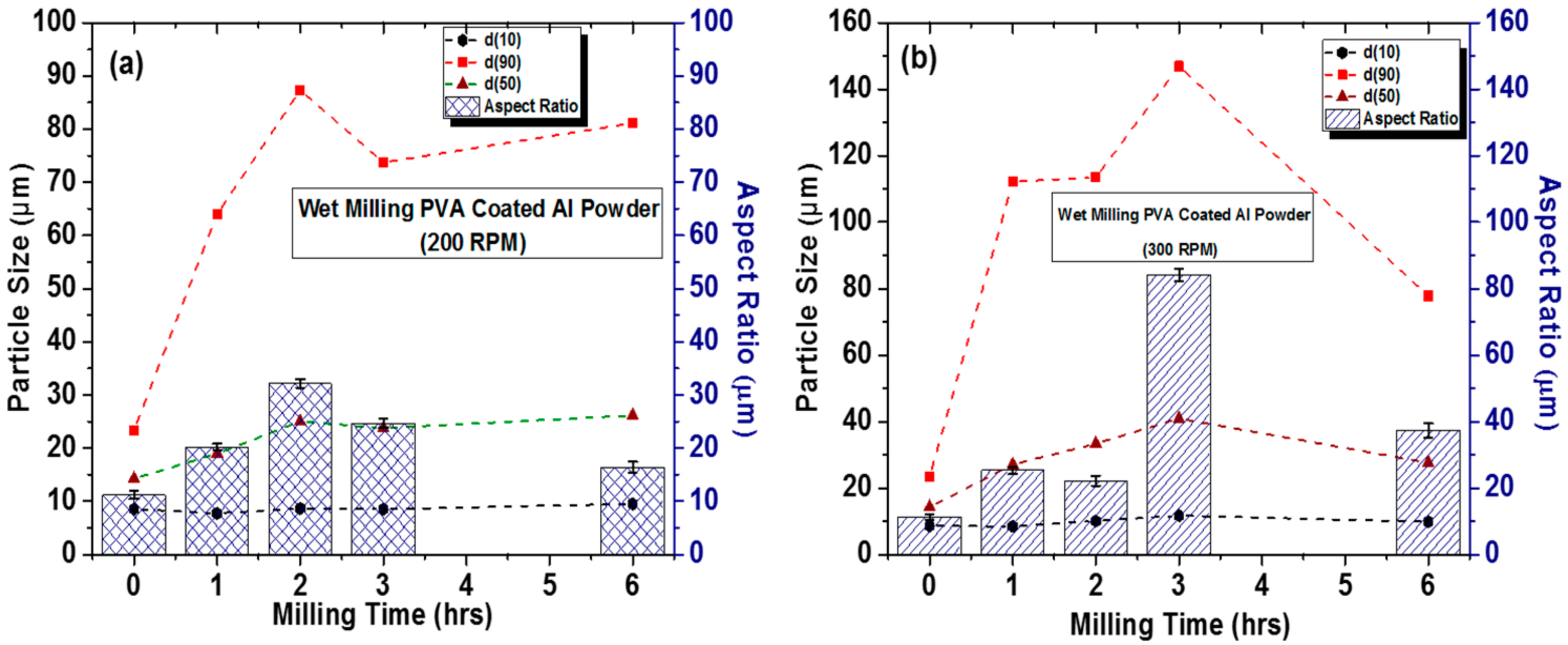
3.1. Optimizing Solution Ball Milling Parameters for PVA @ Al Powder and Morphological Analysis
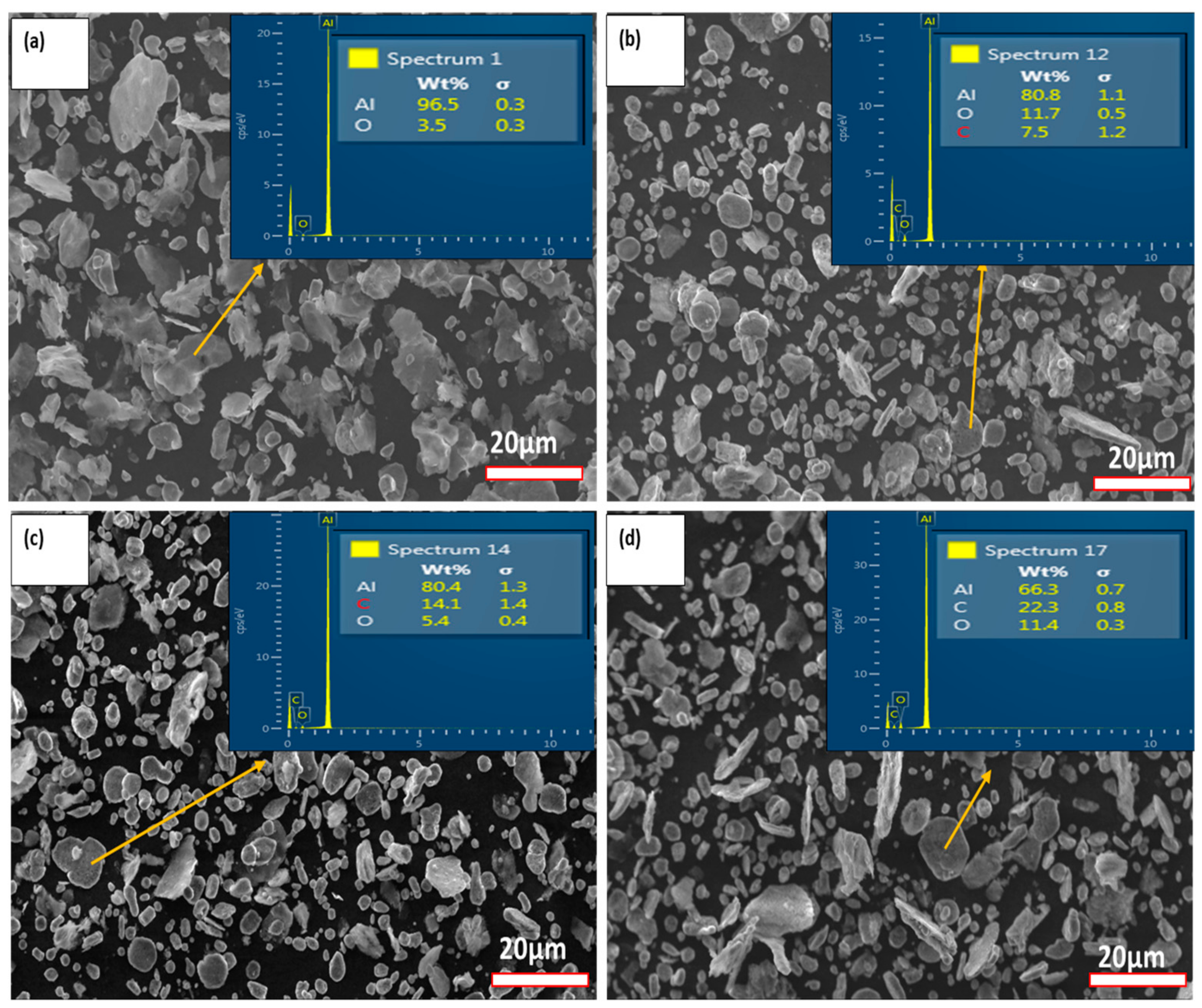
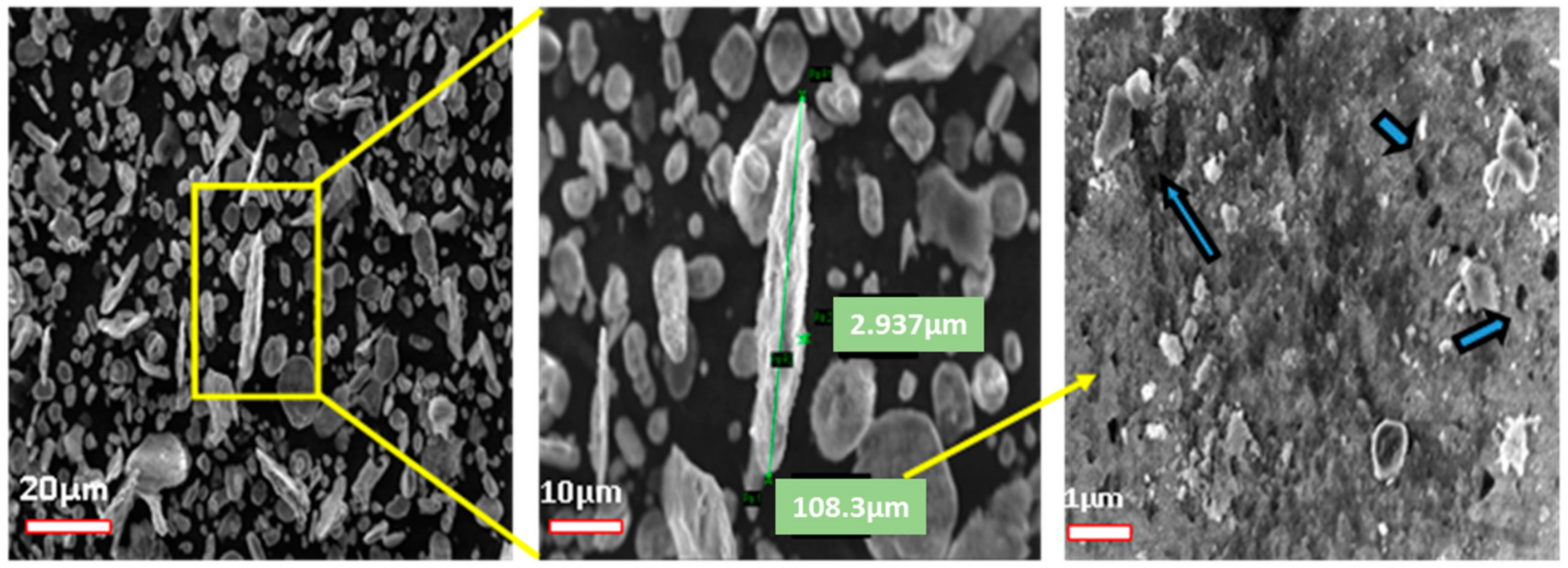
3.2. GNPs/Al Nanocomposite Powder Formation
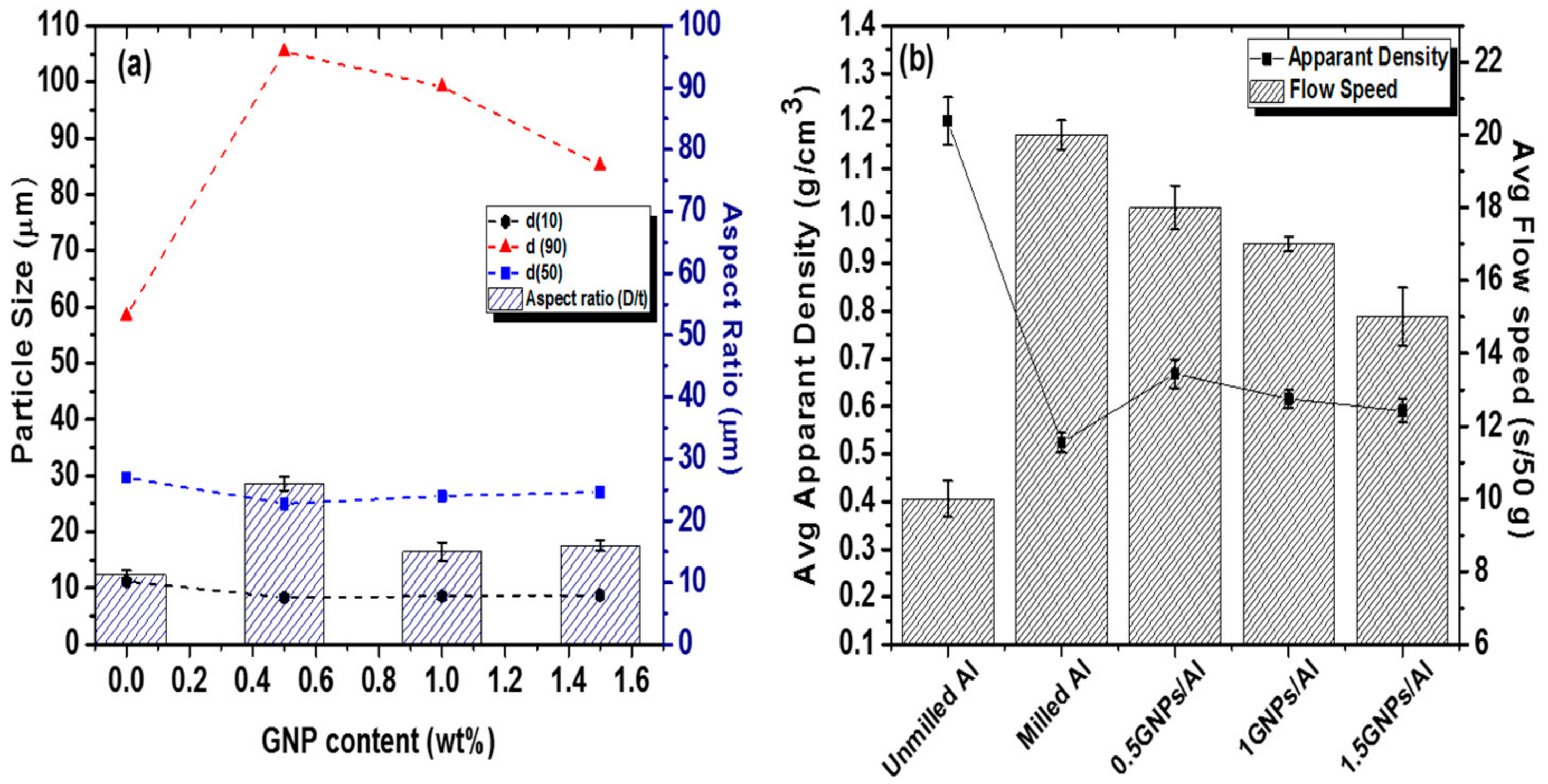
3.2.1. Particle Size and Powder Characteristics Analysis
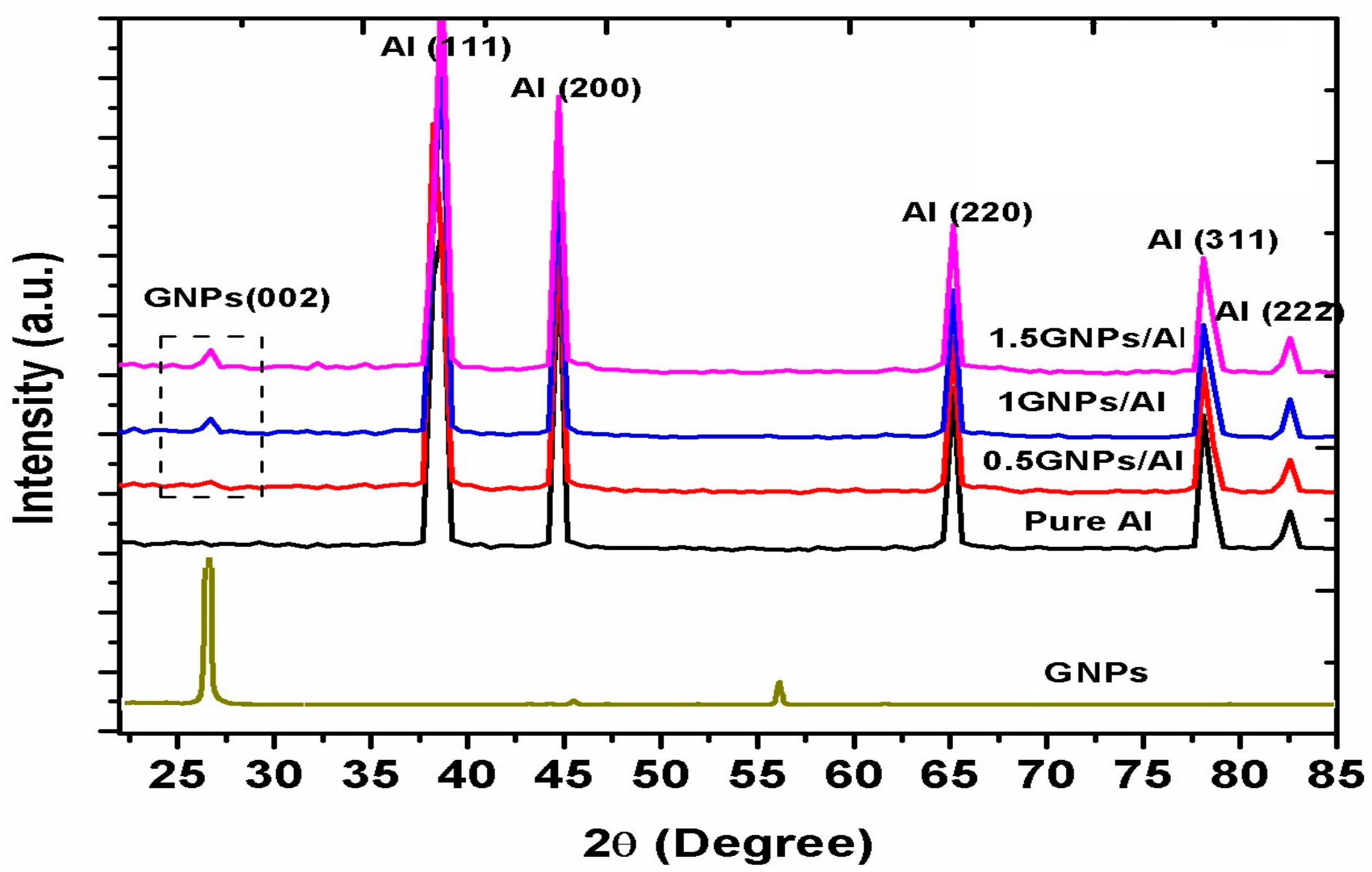
3.2.2. FTIR Spectroscopy and XRD Analysis of the Powders
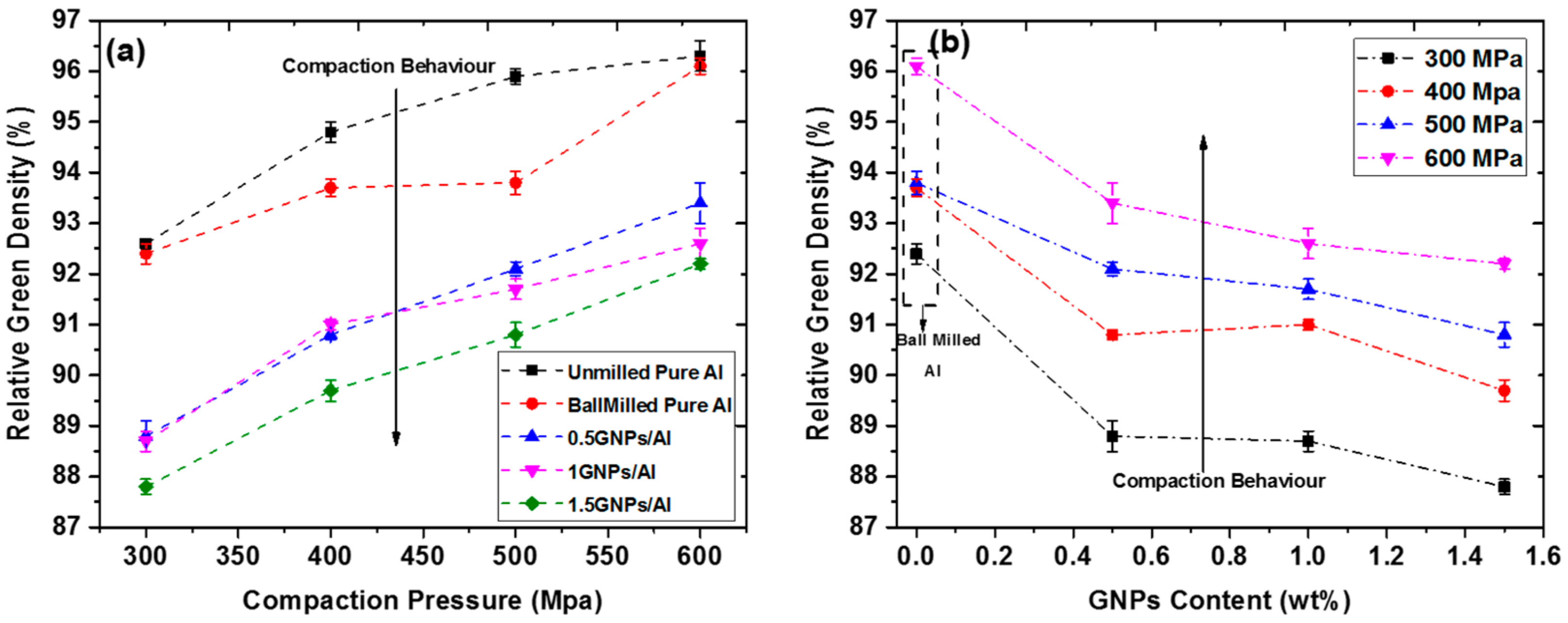
4. Compaction Behavior of GNPs/Al Nanocomposite
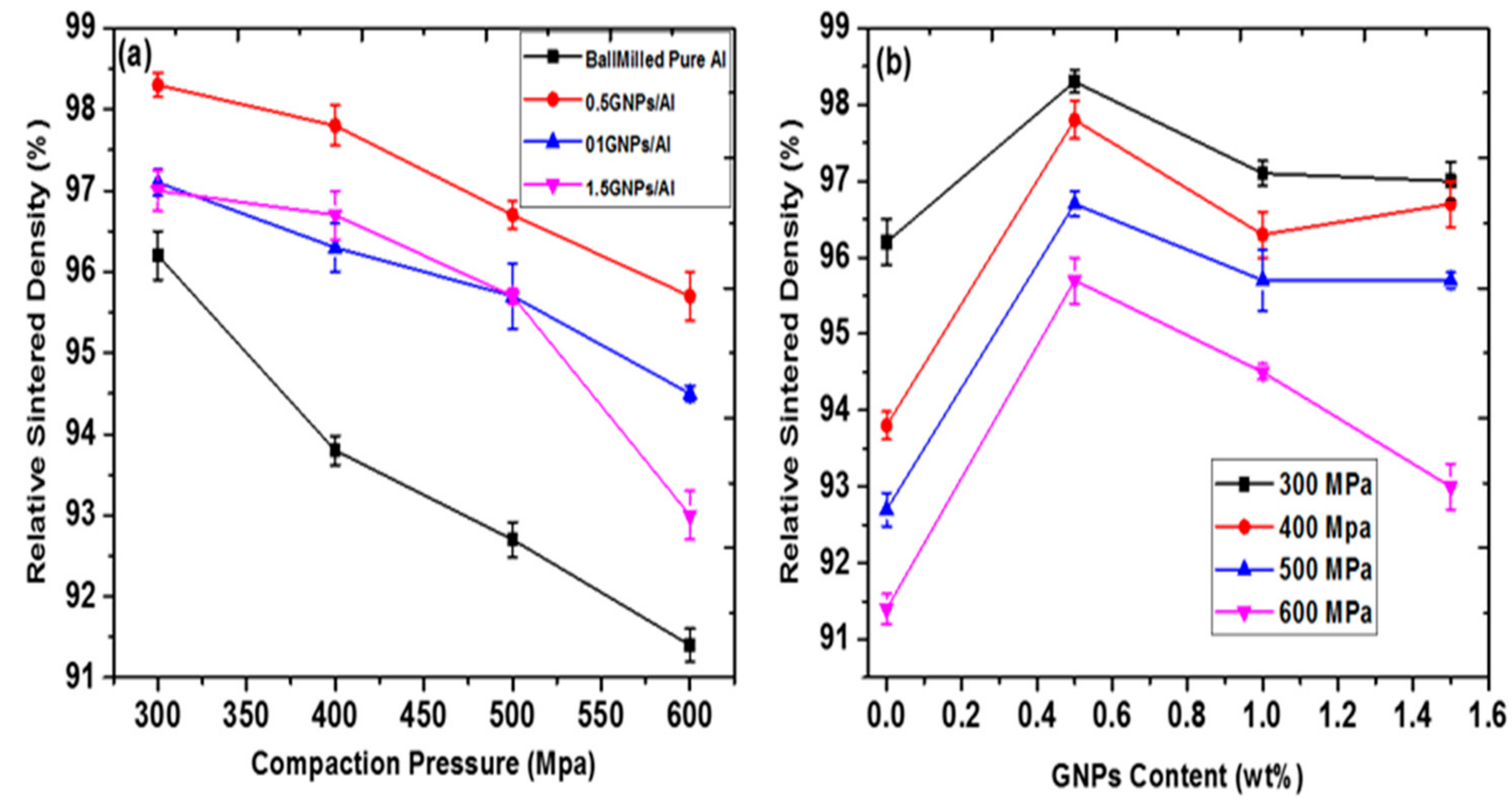
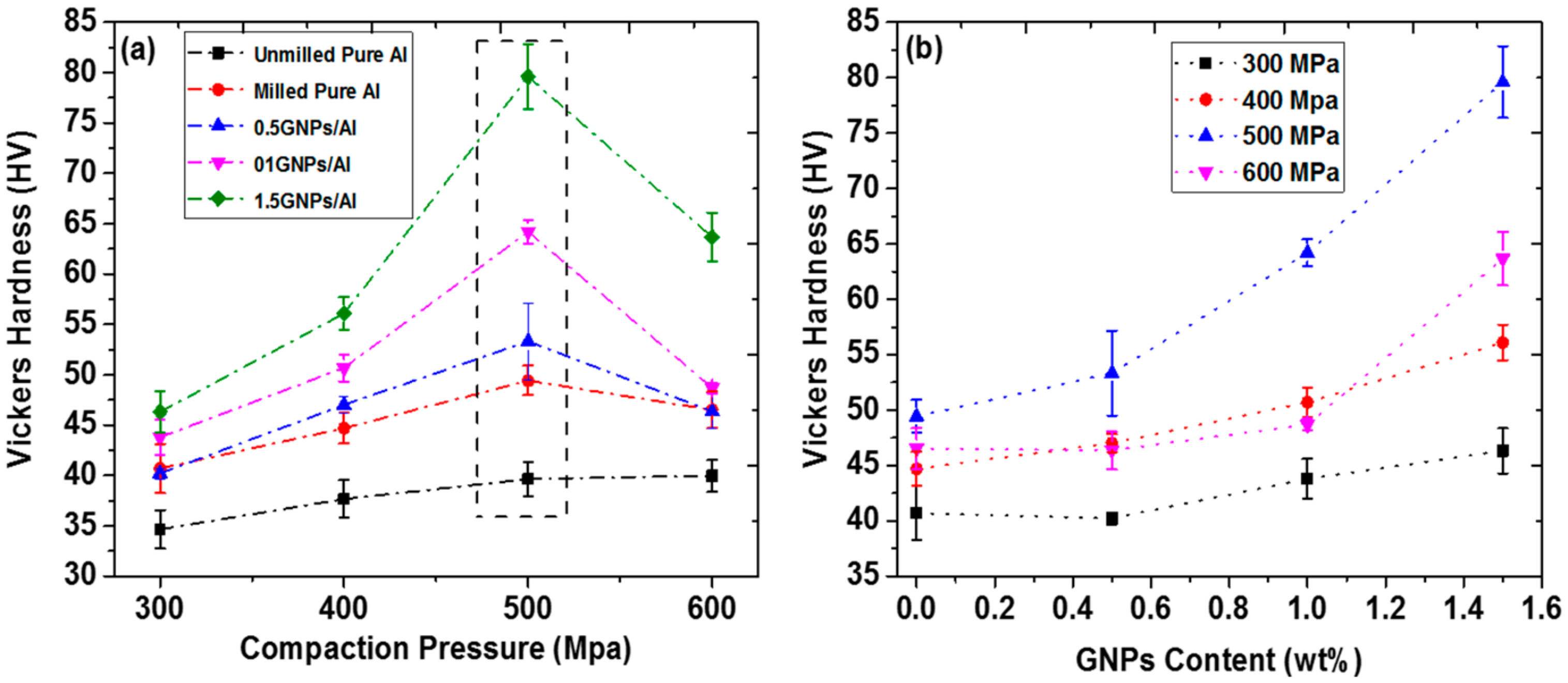
5. Physical and Mechanical Properties of the Sintered GNPs/Al Nanocomposites
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tjong, S.C. Recent progress in the development and properties of novel metal matrix nanocomposites reinforced with carbon nanotubes and graphene nanosheets. Mater. Sci. Eng. R Rep. 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Baig, Z.; Mamat, O.; Mustapha, M. Recent Progress on the Dispersion and the Strengthening Effect of Carbon Nanotubes and Graphene Reinforced Metal Nanocomposites: A Review. Crit. Rev. Solid State Mater. Sci. 2016, 41, 1–46. [Google Scholar] [CrossRef]
- Li, G.; Xiong, B. Effects of graphene content on microstructures and tensile property of graphene-nanosheets/aluminum composites. J. Alloys Compd. 2017, 697, 31–36. [Google Scholar] [CrossRef]
- Huang, H.; Fan, G.; Tan, Z.; Xiong, D.; Guo, Q.; Guo, C.; Li, Z.; Zhang, D. Superplastic Behavior of Carbon Nanotube Reinforced Aluminum Composites Fabricated by Flake Powder Metallurgy. Mater. Sci. Eng. A 2017, 699, 55–61. [Google Scholar] [CrossRef]
- Bartolucci, S.F.; Paras, J.; Rafiee, M.A.; Rafiee, J.; Lee, S.; Kapoor, D.; Koratkar, N. Graphene–aluminum nanocomposites. Mater. Sci. Eng. A 2011, 528, 7933–7937. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M. Effect of Graphene Nanoplatelets addition on mechanical properties of pure aluminum using a semi-powder method. Prog. Nat. Sci. Mater. Int. 2014, 24, 101–108. [Google Scholar] [CrossRef]
- Gao, X.; Yue, H.; Guo, E.; Zhang, H.; Lin, X.; Yao, L.; Wang, B. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites. Mater. Des. 2016, 94, 54–60. [Google Scholar] [CrossRef]
- Yan, S.; Dai, S.; Zhang, X.; Yang, C.; Hong, Q.; Chen, J.; Lin, Z. Investigating aluminum alloy reinforced by graphene nanoflakes. Mater. Sci. Eng. A 2014, 612, 440–444. [Google Scholar] [CrossRef]
- Rikhtegar, F.; Shabestari, S.; Saghafian, H. Synthesis of Carbon Nanotube-Reinforced Al2024 Matrix Nanocomposite Using Flake Powder Metallurgy Method. Metall. Mater. Trans. A 2016, 47, 6428–6437. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, R.; Fan, G.; Chen, K.; Tan, Z.; Xiong, D.-B.; Li, Z.; Kaloshkin, S.D.; Zhang, D. Reinforcement with in-situ synthesized carbon nano-onions in aluminum composites fabricated by flake powder metallurgy. J. Alloys Compd. 2015, 650, 217–223. [Google Scholar] [CrossRef]
- Varol, T.; Canakci, A. Microstructure, electrical conductivity and hardness of multilayer graphene/copper nanocomposites synthesized by flake powder metallurgy. Met. Mater. Int. 2015, 21, 704–712. [Google Scholar] [CrossRef]
- Baig, Z.; Mamat, O.; Mustapha, M.; Sarfraz, M. Influence of surfactant type on the dispersion state and properties of graphene nanoplatelets reinforced Aluminium matrix nanocomposites. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 545–557. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Xiao, W.; Ameyama, K.; Ma, C. Enhanced mechanical properties of Al5083 alloy with graphene nanoplates prepared by ball milling and hot extrusion. Mater. Sci. Eng. A 2016, 658, 8–15. [Google Scholar] [CrossRef]
- Pérez-Bustamante, R.; Bolaños-Morales, D.; Bonilla-Martínez, J.; Estrada-Guel, I.; Martínez-Sánchez, R. Microstructural and hardness behavior of graphene-nanoplatelets/aluminum composites synthesized by mechanical alloying. J. Alloys Compd. 2014, 615, S578–S582. [Google Scholar] [CrossRef]
- Bisht, A.; Srivastava, M.; Kumar, R.M.; Lahiri, I.; Lahiri, D. Strengthening mechanism in graphene nanoplatelets reinforced aluminum composite fabricated through spark plasma sintering. Mater. Sci. Eng. A 2017, 695, 20–28. [Google Scholar] [CrossRef]
- Khan, M.; Amjad, M.; Khan, A.; Ud-Din, R.; Ahmad, I.; Subhani, T. Microstructural evolution, mechanical profile, and fracture morphology of aluminum matrix composites containing graphene nanoplatelets. J. Mater. Res. 2017, 32, 2055–2066. [Google Scholar] [CrossRef]
- Liu, J.; Khan, U.; Coleman, J.; Fernandez, B.; Rodriguez, P.; Naher, S.; Brabazon, D. Graphene oxide and graphene nanosheet reinforced aluminium matrix composites: Powder synthesis and prepared composite characteristics. Mater. Des. 2016, 94, 87–94. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Imai, H.; Jia, L.; Umeda, J.; Takahashi, M.; Kondoh, K. An Approach for Homogeneous Carbon Nanotube Dispersion in Al Matrix Composites. Mater. Des. 2015, 72, 1–8. [Google Scholar] [CrossRef]
- Pérez-Bustamante, R.; Pérez-Bustamante, F.; Estrada-Guel, I.; Licea-Jiménez, L.; Miki-Yoshida, M.; Martínez-Sánchez, R. Effect of milling time and CNT concentration on hardness of CNT/Al 2024 composites produced by mechanical alloying. Mater. Charact. 2013, 75, 13–19. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Xiao, B.; Xue, P.; Wang, W.; Ma, Z. Effect of ball-milling time on mechanical properties of carbon nanotubes reinforced aluminum matrix composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2161–2168. [Google Scholar] [CrossRef]
- Rikhtegar, F.; Shabestari, S.; Saghafian, H. The homogenizing of carbon nanotube dispersion in aluminium matrix nanocomposite using flake powder metallurgy and ball milling methods. Powder Technol. 2015, 280, 26–34. [Google Scholar] [CrossRef]
- Hesabi, Z.; Kamrani, S.; Simchi, A.; Reihani, S. Effect of nanoscaled reinforcement particles on the structural evolution of aluminium powder during mechanical milling. Powder Metall. 2009, 52, 151–157. [Google Scholar] [CrossRef]
- Fogagnolo, J.; Velasco, F.; Robert, M.; Torralba, J. Effect of mechanical alloying on the morphology, microstructure and properties of aluminium matrix composite powders. Mater. Sci. Eng. A 2003, 342, 131–143. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.A.; Ostovan, F.; Abdul Aziz, S.; Mamat, M.S. Effect of Milling Time on the Microstructure, Physical and Mechanical Properties of Al-Al2O3 Nanocomposite Synthesized by Ball Milling and Powder Metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Varol, T.; Canakci, A. The effect of type and ratio of reinforcement on the synthesis and characterization Cu-based nanocomposites by flake powder metallurgy. J. Alloys Compd. 2015, 649, 1066–1074. [Google Scholar] [CrossRef]
- Asgharzadeh, H.; Sedigh, M. Synthesis and mechanical properties of Al matrix composites reinforced with few-layer graphene and graphene oxide. J. Alloys Compd. 2017, 728, 47–62. [Google Scholar] [CrossRef]
- Shin, S.; Bae, D. Deformation behavior of aluminum alloy matrix composites reinforced with few-layer graphene. Compos. Part A Appl. Sci. Manuf. 2015, 78, 42–47. [Google Scholar] [CrossRef]
- Bastwros, M.; Kim, G.-Y.; Zhu, C.; Zhang, K.; Wang, S.; Tang, X.; Wang, X. Effect of ball milling on graphene reinforced Al6061 composite fabricated by semi-solid sintering. Compos. Part B Eng. 2014, 60, 111–118. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T. Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, G.; Li, Z.; Kai, X.; Zhang, D.; Chen, Z.; Humphries, S.; Heness, G.; Yeung, W.Y. An approach to the uniform dispersion of a high volume fraction of carbon nanotubes in aluminum powder. Carbon 2011, 49, 1965–1971. [Google Scholar] [CrossRef]
- Hesabi, Z.R.; Hafizpour, H.; Simchi, A. An investigation on the compressibility of aluminum/nano-alumina composite powder prepared by blending and mechanical milling. Mater. Sci. Eng. A 2007, 454, 89–98. [Google Scholar] [CrossRef]
- Varol, T.; Canakci, A.; Ozkaya, S.; Erdemir, F. Determining the effect of flake matrix size and Al2O3 content on microstructure and mechanical properties of Al2O3 nanoparticle reinforced Al matrix composites. Part. Sci. Technol. 2016, 1–12. [Google Scholar] [CrossRef]
- Varo, T.; Canakci, A. Effect of the CNT Content on Microstructure, Physical and Mechanical Properties of Cu-Based Electrical Contact Materials Produced by Flake Powder Metallurgy. Arab. J. Sci. Eng. 2015, 40, 2711–2720. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Z.; Fan, G.; Cao, L.; Zhang, D. The use of flake powder metallurgy to produce carbon nanotube (CNT)/aluminum composites with a homogenous CNT distribution. Carbon 2012, 50, 1993–1998. [Google Scholar] [CrossRef]
- He, C.-N.; Feng, C.; Lin, J.-C.; Liu, E.-Z.; Shi, C.-S.; Li, J.-J.; Zhao, N.-Q. Fabrication of Carbon Nanotube-Reinforced 6061Al Alloy Matrix Composites by an In Situ Synthesis Method Combined with Hot Extrusion Technique. Acta Metall. Sin. 2016, 29, 188–198. [Google Scholar] [CrossRef]
- Xiang, S.; Gupta, M.; Wang, X.; Wang, L.; Hu, X.; Wu, K. Enhanced overall strength and ductility of magnesium matrix composites by low content of graphene nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2017, 100, 183–193. [Google Scholar] [CrossRef]
- Saboori, A.; Novara, C.; Pavese, M.; Badini, C.; Giorgis, F.; Fino, P. An investigation on the sinterability and the compaction behavior of aluminum/graphene nanoplatelets (GNPs) prepared by powder metallurgy. J. Mater. Eng. Perform. 2017, 26, 993–999. [Google Scholar] [CrossRef]
- Seo, H.Y.; Jiang, L.R.; Kang, C.G.; Jin, C.K. Effect of Compression Process of MWCNT-Reinforced Al6061 Powder on Densification Characteristics and Its Mechanical Properties. Metals 2017, 7, 437. [Google Scholar] [CrossRef]
- Höganäs AB. Höganäs Handbook for Sintered Components; Höganäs AB: Höganäs, Sweden, 2004. [Google Scholar]
- Rashad, M.; Pan, F.; Yu, Z.; Asif, M.; Lin, H.; Pan, R. Investigation on microstructural, mechanical and electrochemical properties of aluminum composites reinforced with graphene nanoplatelets. Prog. Nat. Sci. Mater. Int. 2015, 25, 460–470. [Google Scholar] [CrossRef]
- Shin, S.; Choi, H.; Shin, J.; Bae, D. Strengthening behavior of few-layered graphene/aluminum composites. Carbon 2015, 82, 143–151. [Google Scholar] [CrossRef]
- Gürbüz, M.; Can Şenel, M.; Koç, E. The effect of sintering time, temperature, and graphene addition on the hardness and microstructure of aluminum composites. J. Compos. Mater. 2017. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, Z.; Mamat, O.; Mustapha, M.; Mumtaz, A.; Sarfraz, M.; Haider, S. An Efficient Approach to Address Issues of Graphene Nanoplatelets (GNPs) Incorporation in Aluminium Powders and Their Compaction Behaviour. Metals 2018, 8, 90. https://doi.org/10.3390/met8020090
Baig Z, Mamat O, Mustapha M, Mumtaz A, Sarfraz M, Haider S. An Efficient Approach to Address Issues of Graphene Nanoplatelets (GNPs) Incorporation in Aluminium Powders and Their Compaction Behaviour. Metals. 2018; 8(2):90. https://doi.org/10.3390/met8020090
Chicago/Turabian StyleBaig, Zeeshan, Othman Mamat, Mazli Mustapha, Asad Mumtaz, Mansoor Sarfraz, and Sajjad Haider. 2018. "An Efficient Approach to Address Issues of Graphene Nanoplatelets (GNPs) Incorporation in Aluminium Powders and Their Compaction Behaviour" Metals 8, no. 2: 90. https://doi.org/10.3390/met8020090



